Fluoroquinolonas para el tratamiento de la tuberculosis
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Multicentre randomized controlled trial Generation of allocation sequence: randomized in a factorial design; continent and pulmonary cavitation are stratification factors Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants in the final analysis: 59/336 (17.6%) excluded from final analysis Mean duration of follow up: 8 weeks | |
| Participants | Number: 336 randomized; 277 evaluated Inclusion criteria: aged 18 years or older with suspected pulmonary tuberculosis and acid‐fast bacilli in an expectorated sputum sample Exclusion criteria: history of > 7 days of a fluoroquinolone antibiotic or tuberculosis treatment within the previous 6 months; pregnancy or breastfeeding; initial sputum cultures negative for Mycobacterium tuberculosis or resistance to rifampicin, fluoroquinolones, or pyrazinamide (patients whose isolates were resistant to isoniazid were included) | |
| Interventions | Fluoroquinolone (moxifloxacin) substituted into regimen (replacing ethambutol) for 2 months (8 weeks), initial 2 weeks of daily therapy 1. Moxifloxacin (400 mg daily) orally plus basic regimen (5 days a week or thrice a week for both dosing regimens) for 2 months Basic regimen: | |
| Outcomes | 1. Cure (sputum culture negative at 8 weeks) | |
| Notes | Location: Canada, South Africa, Uganda, USA Human immunodeficiency virus (HIV) status: HIV‐positive participants (30/169 randomized ‐ study group, 30/167 randomized ‐ control group) Drug‐resistance status: isoniazid resistance (15/169 randomized ‐ study group, 10/167 randomized ‐ control group); 11 participants with resistance to rifampicin, fluoroquinolone or pyrazinamide ‐ excluded from analysis | |
| Methods | Multicentre randomized controlled trial Generation of allocation sequence: centrally randomized with stratified permuted block randomization; the research unit is a stratification factor Allocation concealment: unclear Blinding: assessors only Inclusion of all randomized participants in the final analysis: adequate for 8 weeks; 39% lost to follow up in continuation phase Mean duration of follow up: 12 months | |
| Participants | Number: 174 randomized; 101 evaluated Inclusion criteria: suspected human immunodeficiency virus (HIV) and pulmonary tuberculosis; age > 18 years in resistant areas or > 13 years in other areas; aspartate aminotransferase (AST) ≤ 10 times upper limit; serum bilirubin < 2.5 times upper limit; serum creatinine ≤ 3 times upper limit or creatinine clearance rate ≥ 50 mL/min Exclusion criteria: history of multiple‐drug‐resistant tuberculosis (MDR‐TB) or close contact with an MDR‐TB patient; > 3 weeks continuous antituberculous treatment immediately prior to enrolment; > 12 weeks antituberculous therapy in the past 2 years; pregnancy; exclusively extrapulmonary tuberculosis | |
| Interventions | Fluoroquinolone (levofloxacin) added to regimen 1. Levofloxacin plus standard regimen 6 weeks (thrice weekly): isoniazid (600 to 900 mg; < 50 to > 50 kg), vitamin B6 (50 mg), rifampicin (600 mg), pyrazinamide (2.0 to 2.5 g; < 50 to > 50 kg), ethambutol (30 mg/kg; rounded to the nearest 400 mg) | |
| Outcomes | 1. Cure (sputum culture negative at 8 weeks and at the end of treatment period; at least 2 consecutive negative cultures with no subsequent positive cultures) | |
| Notes | Location: USA HIV status: suspected HIV‐positive participants (separate data not provided) Drug‐resistance status: resistant areas | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants in the final analysis: no losses Mean duration of follow up: 12 months | |
| Participants | Number: 104 randomized and evaluated Inclusion criteria: multiple‐drug‐resistant tuberculosis (MDR‐TB) patients with positive sputum smear after 1 year conventional antituberculous treatment; sputum culture showing growth of mycobacterium with multiple resistance to at least 2 of streptomycin, isoniazid, rifampicin, pyrazinamide, or ethambutol; no history of allergy to fluoroquinolone; age 16 to 75 years Exclusion criteria: heart, liver or kidney dysfunction, and diabetes | |
| Interventions | Comparison of different fluoroquinolones (sparfloxacin versus ofloxacin) added to regimen 1. Sparfloxacin (0.2 twice daily) plus standard regimen Standard regimen: isoniazid (0.3 g), rifampicin (0.45 g), ethambutol (0.75 g), pyrazinamide (1.5 g), streptomycin (0.75 g intramuscularly) | |
| Outcomes | 1. Sputum smear or culture conversion | |
| Notes | Location: China Human immunodeficiency virus (HIV) status: presumed HIV negative Drug‐resistance status: MDR‐TB | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants in the final analysis: no losses to follow up Mean duration of follow up: 12 months | |
| Participants | Number: 69 randomized (2 new cases; 67 retreatment cases); 69 evaluated Inclusion criteria: multiple‐drug‐resistant tuberculosis (MDR‐TB); sputum‐positive (not reported if culture or smear) inpatients; age 18 to 70 years; new and retreatment cases Exclusion criteria: not reported | |
| Interventions | Comparison of different fluoroquinolones (sparfloxacin versus ofloxacin) added to regimen 1. Sparfloxacin (200 mg/day orally for 2 months) plus standard regimen Standard regimen: isoniazid (0.3 g), pyrazinamide (1.5 g) | |
| Outcomes | 1. Treatment failure | |
| Notes | Location: China Human immunodeficiency virus (HIV) status: not reported Drug‐resistance status: MDR‐TB | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: none Inclusion of all randomized participants in the final analysis: 95.6% included in analysis; 7 (4.4%) excluded Mean duration of follow up: 6 months | |
| Participants | Number: 160 randomized; 153 evaluated Inclusion criteria: "presented with pulmonary TB"; new cases; age > 18 years Exclusion criteria: severe renal, hepatic, or cardiovascular disease | |
| Interventions | Fluoroquinolone (ciprofloxacin) substituted into regimen (replacing pyrazinamide and ethambutol) 1. Ciprofloxacin (750 mg; for the first 4 months) plus basic regimen Basic regimen (daily orally for 6 months): isoniazid (300 mg) and rifampicin (600 mg) | |
| Outcomes | 1. Treatment failure | |
| Notes | Location: Tanzania Human immunodeficiency virus (HIV) status: 37% to 40% HIV‐positive Drug‐resistance status: presumed sensitive | |
| Methods | Randomized controlled trial Generation of allocation sequence: centrally randomized by computer generated allocation sequence in block size of 10 patients Allocation concealment: sealed, opaque envelopes Blinding: only assessors Inclusion of all randomized participants in the final analysis: 6 (9%) lost to follow up Mean duration of follow up: 12 months (6 months after cessation of the 6 months' therapy) | |
| Participants | Number: 200 randomized Inclusion criteria: acid‐fast bacilli present in the sputum on direct fluorescent microscopy Exclusion criteria: history of treatment of tuberculosis or other exposures to any of the study drugs; sputum cultures positive for mycobacteria other than Mycobacterium tuberculosis; isolates of M. tuberculosis resistant to any of the study drugs; severe renal, hepatic, or cardiovascular disease; pregnancy or lactation; history of adverse reaction to any of the study drugs; epilepsy, concomitant treatment with theophylline; severe tuberculosis unlikely to survive | |
| Interventions | Fluoroquinolone (ciprofloxacin) substituted into regimen (replacing pyrazinamide and ethambutol) 1. Ciprofloxacin (750 mg; for the first 4 months) plus basic regimen Basic regimen (daily for 6 months): isoniazid (300 mg) and rifampicin (600 mg) | |
| Outcomes | 1. Treatment failure (clinical at 12 months) | |
| Notes | Location: Tanzania Human immunodeficiency virus (HIV) status: data stratified by HIV status Drug‐resistance status: fully drug‐sensitive tuberculosis A preliminary report on 20 participants with 8 weeks of follow up (no losses) that provided information on outcomes (5), (6), and (7) was published in 1993 | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants in the final analysis: 32 (20% 25%) lost to follow up Mean duration of follow up: 12 months | |
| Participants | Number: 156 randomized; 124 evaluated Inclusion criteria: inpatients previously untreated; sputum smear‐positive or culture‐positive pulmonary tuberculosis, age > 15 years (range 15 to 81 years) Exclusion criteria: not reported | |
| Interventions | Fluoroquinolone (ofloxacin) substituted into regimen (replacing ethambutol) 1. Ofloxacin (600 mg for the initial 2 months; 300 mg for the following 7 months) plus basic regimen Basic regimen (orally, daily for 9 months): isoniazid (300 mg), rifampicin (600 mg) | |
| Outcomes | 1. Relapse (12 months after cessation of therapy) | |
| Notes | Location: Nagasaki, Japan Human immunodeficiency virus (HIV) and drug‐resistance status: not reported | |
| Methods | Randomized controlled trial Generation of allocation sequence: random‐number table Allocation concealment: unclear Blinding: participants blinded; unclear if providers and assessors blinded Inclusion of all randomized participants in the final analysis: 6/144 (4.167%) lost to follow up Mean duration of follow up: 6 months | |
| Participants | Number: 144 randomized; 138 evaluated Inclusion criteria: sputum smear positive and x‐ray confirmed pulmonary tuberculosis, including newly diagnosed (antituberculous treatment ≤ 1 month) or retreatment pulmonary tuberculosis (treatment failure, or after completion of routine chemotherapy with rifampicin or ethambutol for < 6 months, or pyrazinamide < 3 months; or patients relapsed, but never use of fluoroquinolone‐resistant and fluoroquinolone‐sensitive cases); age 15 to 70 years; body weight > 40 kg Exclusion criteria: pregnancy; severe heart, liver, or kidney diseases; other severe complications | |
| Interventions | Comparison of different fluoroquinolones (levofloxacin versus ofloxacin) added to regimen 1. Intervention group: isoniazid (0.3 g), ethambutol (0.75 to 1 g), pyrazinamide (1.5 g), thioacetazone (0.6 g), levofloxacin (0.3 g) | |
| Outcomes | 1. Cure (sputum smear conversion for 2 consecutive months) | |
| Notes | Location: China Human immunodeficiency virus (HIV) status: presumed HIV negative Drug‐resistance status: multiple‐drug‐resistant tuberculosis (MDR‐TB) (presumed 38/73) | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: providers, participants, and radiograph assessors blinded Inclusion of all randomized participants in the final analysis: 25/60 (42%) lost to follow up Mean duration of follow up: 6 months of therapy, and 12 months after cessation of therapy | |
| Participants | Number: 60 randomized; 53 evaluated at 2 months, and 35 at 6 months Inclusion criteria: age > 15 years (age range 15 to > 45 years (9 participants)), sputum smear positive; not previously taken > 3 weeks antituberculous therapy; willing to stay in hospital for initial 2 months' intensive phase of treatment Exclusion criteria: diabetes; human immunodeficiency virus (HIV) infection; hypertension; other concomitant diseases; pregnant women | |
| Interventions | Fluoroquinolone (ciprofloxacin) substituted into regimen (replacing rifampicin) 1. Ciprofloxacin (750 mg orally daily for 6 months) plus basic regimen Basic regimen: streptomycin (0.75 g intramuscularly) and pyrazinamide (1.5 g orally) daily for 2 months; isoniazid (400 mg orally daily) for 6 months | |
| Outcomes | 1. Sputum smear conversion at 2 and 6 months | |
| Notes | Location: India HIV status: all participants HIV‐negative Drug‐resistance status: no data | |
| Methods | Randomized controlled trial Generation of allocation sequence: random‐number table Allocation concealment: unclear Blinding: none Inclusion of all randomized participants in the final analysis: no losses Mean duration of follow up: 12 months | |
| Participants | Number: 31 randomized and evaluated Inclusion criteria: histological evidence of caseating granulomas; sputum positive for acid‐fast bacilli; sputum‐culture positive for Mycobacterium tuberculosis; positive polymerase chain reaction (PCR) for M. tuberculosis in tissues; chronic liver disease informed written consent Exclusion criteria: serum bilirubin > 5 mg/dL; baseline alanine aminotransferase/aspartate aminotransferase (ALT/AST) ALT/AST > 200 international units/L (IU/L); serum creatinine > 2.5 mg/dL; increase in ALT/AST > 2‐fold baseline levels over 1 week before starting the antituberculous drugs | |
| Interventions | Fluoroquinolone (ofloxacin) and pyrazinamide substituted into regimen (replacing rifampicin). 1. Ofloxacin (400 mg orally) and pyrazinamide (World Health Organization (WHO) dose of 25 mg/kg for initial 2 months) daily for 12 months plus basic regimen Basic regimen: (orally, daily for 12 months): isoniazid (WHO dose of 5 mg/kg), ethambutol (WHO dose of 15 mg/kg daily; for the initial 2 months) | |
| Outcomes | 1. Serious adverse events (hepatotoxicity requiring interruption and change of treatment) | |
| Notes | Location: India Human immunodeficiency virus (HIV) and drug‐resistance status: no data | |
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of all randomized participants in the final analysis: no losses Mean duration of follow up: 9 months | |
| Participants | Number: 80 randomized and evaluated Inclusion criteria: multiple‐drug‐resistant tuberculosis (MDR‐TB) with isoniazid and rifampicin resistance; sputum culture positive after 1 year of treatment with streptomycin, isoniazid, rifampicin, pyrazinamide, and ethambutol; advanced pulmonary inflammatory or cavity enlarged; no prior fluoroquinolone treatment; adults (mean age 45.5±5.5 years; range 20 to 71 years) Exclusion criteria: heart, liver, or kidney dysfunction; diabetes | |
| Interventions | Comparison of different fluoroquinolones (sparfloxacin versus ofloxacin) added to regimen 1. Sparfloxacin (0.1 g) 4 times daily plus basic regimen Basic regimen: isoniazid (0.2 g), rifampicin (0.15 g), and protionamide (0.2 g thrice daily) for 6 months | |
| Outcomes | 1. Sputum smear or culture conversion | |
| Notes | Location: China Human immunodeficiency virus (HIV) status: presumed HIV negative Drug‐resistance status: all proven MDR‐TB | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Experimental animal study, plus a small section in healthy human volunteers (tolerability); not a trial report | |
| No randomization or control group | |
| The outcome, early bactericidal activity, not in review | |
| No randomization, and the intervention was a combination of levofloxacin plus capreomycin | |
| Mixed intervention of ciprofloxacin, ofloxacin, or levofloxacin plus kanamycin or amikacin added to the basic regimen in study group versus streptomycin added to the basic regimen in control group | |
| Exclusively urogenital tuberculosis | |
| The outcome, early bactericidal activity, not in review | |
| No randomization; cohort study | |
| The outcome, early bactericidal activity, not in review | |
| No randomization | |
| Study in healthy volunteers, not a trial report, in which the outcome was uric acid concentration in urine samples excreted over 0 to 8 h | |
| Retrospective safety study; not a trial report | |
| Communication to the Editor of Chest; not a trial report | |
| The outcome, early bactericidal activity, not in review | |
| The outcome, early bactericidal activity, not in review | |
| The outcome, early bactericidal activity, not in review | |
| No randomization; cohort study | |
| No randomization; not a controlled study | |
| No control arm, that is, a group treated without the studied fluoroquinolone (ofloxacin), a different fluoroquinolone, or different dose | |
| No randomization and outcomes not reported | |
| The outcome, indices of adrenocortical function, not in review; none of the included outcomes reported, too small (20 participants) | |
| Retrospective study; not a trial report | |
| Retrospective case‐control study; not a trial report | |
| The efficacy of bronchofibrescope and catheter intervention with ofloxacin and amikacin studied in comparison with traditional chemotherapy | |
| The efficacy of rifabutin versus rifapentine containing antituberculous regimens studied, both regimens included levofloxacin; study question not in review | |
| No randomization | |
| Mixed intervention of levofloxacin plus pasiniazide plus Mycobacterium vaccae | |
| The efficacy of rifabutin versus rifapentine containing antituberculous regimens studied, both regimens included levofloxacin; study question not in review |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised comparison of ciprofloxacin, levofloxacin and gatifloxacin for the treatment of adults with tuberculous meningitis |
| Methods | — |
| Participants | Inclusion criteria: aged > 14 years; clinical diagnosis of tuberculous meningitis Exclusion criteria: aged < 15 years; pregnant or breastfeeding; patients in whom the physician believes fluoroquinolones are contraindicated (eg previous adverse reaction); consent of either patient or their relatives not obtained |
| Interventions | 1. Conventional 4‐drug antituberculous chemotherapy (ATC) (comprising of isoniazid, rifampicin, pyrazinamide, and ethambutol) |
| Outcomes | 1. Clinical: 2. Microbiological: |
| Starting date | 1 April 2003 |
| Contact information | Dr Guy Thwaites ([email protected]), Oxford University Clinical Research Unit, Vietnam |
| Notes | Location: Vietnam Registration number: ISRCTN07062956 Source of funding: The Wellcome Trust (UK) |
| Trial name or title | A comparative study of the bactericidal and sterilizing activity of three fluoroquinolones: gatifloxacin, moxifloxacin and ofloxacin substituted for ethambutol in the 2 month initial phase of the standard anti‐tuberculosis treatment regimen also containing rifampicin, isoniazid and pyrazinamide (South Africa) |
| Methods | — |
| Participants | Inclusion criteria: male/female of 18 to 65 years; weight 38 to 80 kg; recently microscopically diagnosed pulmonary tuberculosis; findings in medical history and physical examination not exceeding grade 2; voluntarily signed informed consent; confirmed negative pregnancy test at the screening visit; willing to use effective contraceptive methods during treatment; normal lab values not exceeding grade 2, except haemoglobin < 6.5 g/dL and potassium < 3.0 mEq/L (> grade 1); consent for a pre‐screening biological test to exclude possible multi‐drug‐resistant tuberculosis (MDR‐TB) and negative MDR‐TB screen test will be a check if pre‐screening biological test is done Exclusion criteria: history of tuberculosis within the last 3 years; concomitant infection requiring additional anti‐infectious treatment (especially anti‐retroviral medication (ARV)); human immunodeficiency virus (HIV)‐infected patients at World Health Organization stage 4; diabetes mellitus or non‐insulin dependent diabetes mellitus requiring treatment; drug and alcohol abuse; history of drug hypersensitivity and/or active allergic disease; impaired renal, hepatic or gastric function that may interfere with drug absorption, distribution, metabolism, or elimination |
| Interventions | 1. Standard antituberculous treatment (isoniazid, rifampicin, pyrazinamide, and ethambutol) |
| Outcomes | Bactericidal and sterilizing activity |
| Starting date | 25 November 2004 |
| Contact information | Dr T Kanyok ([email protected]), UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), Switzerland |
| Notes | Location: South Africa Registration number: ISRCTN13670619 Sources of funding: UNICEF‐UNDP‐World Bank‐WHO Special Programme for Research and Training in Tropical Diseases (TDR) |
| Trial name or title | An international multicentre controlled clinical trial to evaluate high dose RIFApentine and a QUINolone in the treatment of pulmonary tuberculosis |
| Methods | — |
| Participants | Inclusion criteria: newly diagnosed pulmonary tuberculosis; 2 sputum specimens positive for tubercle bacilli on direct smear microscopy; either no previous antituberculous chemotherapy, or < 2 weeks of previous chemotherapy; aged 18 years and over; firm home address that is readily accessible for visiting and be intending to remain there during the entire treatment and follow‐up period; willing to agree to participate in the study and to give a sample of blood for HIV testing Exclusion criteria: any condition (except HIV infection) that may prove fatal during the study period; tuberculous meningitis; pre‐existing nontuberculous disease likely to prejudice the response to, or assessment of, treatment (eg insulin‐dependent diabetes, liver or kidney disease, blood disorders, peripheral neuritis); female and known to be pregnant or breastfeeding; suffering from a condition likely to lead to unco‐operative behaviour such as psychiatric illness or alcoholism; contraindications to any medications in the study regimens; requires anti‐retroviral treatment (ART) at diagnosis; history of prolonged QTc syndrome or current or planned therapy with quinidine, procainamide, amiodarone, sotalol, disopyramide, ziprasidone, or terfenadine during the intensive phase of antituberculous therapy; haemoglobin < 7g/L; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 5 times the upper range; creatinine clearance < 30 mL/min; history of seizures; HIV positive with a CD4 count < 200/mm3; weight < 35 kg |
| Interventions | 1. 2 months of daily ethambutol (E), moxifloxacin (M), rifampicin (R), and pyrazinamide (Z) followed by 2 months of twice weekly moxifloxacin and rifapentine (2EMRZ/2P2M2). |
| Outcomes | 1. Combined rate of failure at the end of treatment and relapse, measured at 18 months |
| Starting date | 31 July 2007 |
| Contact information | Dr Amina Jindani ([email protected]), Centre for Infection Department of Cellular and Molecular Medicine St. George’s University of London, UK |
| Notes | Location: South Africa, Mozambique, Zimbabwe, Zambia Registration number: ISRCTN44153044 Source of funding: European and Developing Countries Clinical Trials Partnership (EDCTP) (The Netherlands) |
| Trial name or title | Controlled comparison of two moxifloxacin containing treatment shortening regimens in pulmonary tuberculosis |
| Methods | — |
| Participants | Inclusion criteria: signed written consent or witnessed oral consent in the case of illiteracy, before undertaking any trial related activity; 2 sputum specimens positive for tubercle bacilli on direct smear microscopy at the local laboratory; no previous antituberculous chemotherapy; aged 18 years and over; firm home address that is readily accessible for visiting and willingness to inform the study team of any change of address during the treatment and follow‐up period; agreement to participate in the study and to give a sample of blood for human immunodeficiency virus (HIV) testing; laboratory parameters performed up to 14 days before enrolment; serum aspartate aminotransferase (AST) activity < 3 times the upper limit of normal (ULN); serum total bilirubin level < 2.5 times ULN; creatinine clearance level > 30 mL/min; haemoglobin level of at least 7.0 g/dL; platelet count of at least 50 x 10^9 cells/L; serum potassium > 3.5 mmol/L; negative pregnancy test (women of childbearing potential); pre‐menopausal women must be using a barrier form of contraception or be surgically sterilized or have an intra‐uterine contraceptive device in place Exclusion criteria: unable to take oral medication; previously enrolled in this study; received any investigational drug in the past 3 months; received an antibiotic active against Mycobacterium tuberculosis in the last 14 days (fluoroquinolones, macrolides, standard antituberculous drugs); any condition that may prove fatal during the first two months of the study period; tuberculous meningitis or other forms of severe tuberculosis with high risk of a poor outcome; pre‐existing non‐tuberculosis disease likely to prejudice the response to, or assessment of, treatment (eg insulin‐dependent diabetes, liver or kidney disease, blood disorders, peripheral neuritis, chronic diarrhoeal disease); pregnant or breast feeding; suffering from a condition likely to lead to unco‐operative behaviour (eg psychiatric illness or alcoholism); contraindications to any medications in the study regimens; known to have congenital or sporadic syndromes of QTc prolongation or receiving concomitant medication reported to increase the QTc interval (eg amiodarone, sotalol, disopyramide, quinidine, procainamide, terfenadine); end stage liver failure (class Child‐Pugh C); uncorrected hypokalaemia; weight < 35 kg; known allergy to any fluoroquinolone antibiotic or history of tendinopathy associated with quinolones; HIV infection with CD4 count < 250 x 10^9/L; patients already receiving anti‐retroviral therapy; patients whose initial isolate is shown to be multiple drug resistant |
| Interventions | 1. 8 weeks of chemotherapy with ethambutol, isoniazid, rifampicin, and pyrazinamide plus the moxifloxacin placebo, followed by 9 weeks of isoniazid and rifampicin plus the moxifloxacin placebo, followed by 9 weeks of isoniazid and rifampicin only Dosages dependent on patient weight category (all drugs taken orally): |
| Outcomes | 1. Combined failure of bacteriological cure and relapse within 1 year of completion of therapy |
| Starting date | 1 June 2007 |
| Contact information | Prof Stephen Gillespie, Centre for Medical Microbiology, Royal Free and University College Medical School, UK |
| Notes | Location: Kenya, South Africa, Tanzania, Zambia Registration number: ISRCTN85595810 Sources of funding: European and Developing Countries Clinical Trials Partnership (EDCTP) (The Netherlands); TB Alliance (USA); Bayer HealthCare Pharmaceuticals (USA); Sanofi‐Aventis (France) |
| Trial name or title | TBTC Study 28: Evaluation of a moxifloxacin‐based, isoniazid‐sparing regimen for tuberculosis treatment |
| Methods | — |
| Participants | Inclusion criteria: suspected pulmonary tuberculosis with acid‐fast bacilli in a stained smear of expectorated or induced sputum. Patients whose sputum cultures do not grow M. tuberculosis and those having an M. tuberculosis isolate resistant to (one or more) isoniazid, rifampin, fluoroquinolones, will be discontinued from the study, but followed for 14 days to detect late toxicities from study therapy. Patients having extra‐pulmonary manifestations of tuberculosis, in addition to smear‐positive pulmonary disease, are eligible for enrolment. Sputum must be expectorated or induced; smear results from respiratory secretions obtained by bronchoalveolar lavage or bronchial wash may not be used for assessment of study eligibility; willingness to have HIV testing performed, if HIV serostatus is not known or if the last documented negative HIV test was more than 6 months before enrolment. HIV testing does not need to be repeated if there is written documentation of a positive test (positive ELISA and Western Blot or a plasma HIV‐RNA level > 5000 copies/mL) at any time in the past; 7 or fewer days of multidrug therapy for tuberculosis disease in the 6 months preceding enrolment; 7 or fewer days of fluoroquinolone therapy in the 3 months preceding enrolment; age > 18 years; Karnofsky score of at least 60 (requires occasional assistance but is able to care for most of his/her needs); signed informed consent; women with child‐bearing potential must agree to practice an adequate (barrier) method of birth control or to abstain from heterosexual intercourse during study therapy; serum amino aspartate transferase (AST) activity ≤ 3 times upper limit of normal; serum total bilirubin level ≤ 2.5 times upper limit of normal; serum creatinine level ≤ 2 times upper limit of normal; complete blood count with hemoglobin level of at least 7.0 g/dL; complete blood count with platelet count of at least 50,000/mm 3 ; serum potassium > 3.5 meq/L; negative pregnancy test (women of childbearing potential) Exclusion criteria: breastfeeding; known intolerance to any of the study drugs; known allergy to any fluoroquinolone antibiotic; concomitant disorders or conditions for which moxifloxacin (MXF), isoniazid (INH), rifampin (RIF), pyrazinamide (PZA), or ethambutol (EMB) are contraindicated (including severe hepatic damage, acute liver disease of any cause, and acute uncontrolled gouty arthritis); current or planned therapy during the intensive phase of therapy using drugs having unacceptable interactions with rifampin (rifabutin can be substituted for rifampin during the continuation phase of therapy); current or planned antiretroviral therapy during intensive phase of therapy; history of prolonged QT syndrome or current or planned therapy with quinidine, procainamide, amiodarone, sotalol, disopyramide, ziprasidone, or terfenadine during the intensive phase of therapy; pulmonary silicosis; central nervous system tuberculosis |
| Interventions | 1. Moxifloxacin (with rifampin, pyrazinamide, and ethambutol) |
| Outcomes | 1. Culture‐conversion rate at the end of the intensive phase of therapy |
| Starting date | February 2006 |
| Contact information | Richard E Chaisson (Study Chair), Johns Hopkins University, USA |
| Notes | Location: Brazil, Canada (Manitoba, Quebec), South Africa, Spain, Uganda, USA (Arkansas, California, Colorado, Washington DC, Georgia, Illinois, Maryland, Massachusetts, New Jersey, New York, North Carolina, Tennessee, Texas, Washington) Registration number: NCT00144417 Sponsors and collaborators: Centers for Disease Control and Prevention; Global Alliance for TB Drug Development; Bayer |
| Trial name or title | A controlled trial of a 4‐month quinolone‐containing regimen for the treatment of pulmonary tuberculosis |
| Methods | — |
| Participants | Inclusion criteria: male or female; aged 18 to 65 years; currently suffering from recently diagnosed microscopically proven pulmonary tuberculosis and providing informed consent for inclusion in the study Exclusion criteria: history of tuberculosis treatment within the last 3 years; history of diabetes mellitus or noninsulin dependent diabetes mellitus requiring treatment; concomitant infection requiring additional anti‐infective treatment (especially anti‐retroviral therapy); HIV‐ infected patients with WHO stage 3 infection ‐ except those presenting with only the "loss of weight>10% body weight" criterion ‐ and all HIV infected patients at WHO stage 4 |
| Interventions | 1. 4‐month gatifloxacin‐containing antituberculous regimen |
| Outcomes | 1. Percentage of relapses by 24 months following treatment cure |
| Starting date | January 2005 |
| Contact information | Christian Lienhardt (Study Director), Institut de Recherche pour le Developpement, France |
| Notes | Location: Benin, Guinea, Kenya, Senegal, South Africa Registration number: NCT00216385 Sponsors and collaborators: Institut de Recherche pour le Developpement; World Health Organization; |
| Trial name or title | Randomized, open label, multiple dose Phase I study of the early bactericidal activity of linezolid, gatifloxacin, levofloxacin, and moxifloxacin in HIV‐non‐infected adults with Initial episodes of sputum smear‐positive pulmonary tuberculosis (DMID 01‐553) |
| Methods | — |
| Participants | Inclusion criteria: adults, male or female, aged 18 to 65 years; women with child‐bearing potential (not surgically sterilized or postmenopausal for < 1 year) must be using or agree to use an adequate method of birth control (condom: intravaginal spermicide (foams, jellies, sponge) and diaphragm: cervical cap or intrauterine device) during study drug treatment; newly diagnosed sputum smear‐positive pulmonary tuberculosis as confirmed by sputum AFB smear and chest x‐ray findings consistent with pulmonary tuberculosis; willing and able to provide informed consent; reasonably normal hemoglobin (≥ 8 gm/dL), renal function (serum creatinine < 2 mg/dL), hepatic function (serum AST < 1.5 times the upper limit of normal for the testing laboratory and total bilirubin < 1.3 mg/dL), and random blood glucose < 150 mg/dL Exclusion criteria: HIV infection; weight < 75% of ideal body weight; presence of significant hemoptysis; patients who cough up frank blood (more than blood streaked sputum); pregnant or breastfeeding women and those who are not practicing birth control; significant respiratory impairment (respiratory rate > 35/min); clinical suspicion of dissemated tuberculosis or tuberculosis meningitis; presence of serious underlying medical illness (eg such as liver failure, renal failure, diabetes mellitus, chronic alcoholism, decompensated heart failure, haematologic malignancy) or patients receiving myelosuppressive chemotherapy; patients receiving any of monoamine oxidase inhibitors (phenelzine, tranylcypromine), adrenergic/serotonergic agonists such as pseudoephedrine and phenylpropanolamine (frequently found in cold and cough remedies), tricyclic antidepressants (amitriptyline, nortriptyline, protriptyline, doxepin, amoxapine, etc), antipsychotics (eg chlorpromazine and buspirone), serotonin re‐uptake inhibitors (fluoxetine, paroxetine, sertaline, etc), buproprion, agents known to prolong the QTc interval [erythromycin, clarithromycin, astemizole, type Ia (quinidine, procainamide, disopyramide) and III (amiodarone, sotalol) anti‐arrhythmics, carbamazepine, insulin, sulfonylureas, and meperidine; presence of QTc prolongation (> 450 msec) on baseline EKG; allergy or contraindication to use of study drugs; treatment with antituberculous medications or other antibiotics with known activity against Mycobacterium tuberculosis during the preceding 6 months; inability to provide informed consent; total white blood cell count < 3000/mm3; platelet count < 150,000/mm3; patients with suspected drug‐resistant tuberculosis (eg contact to source patient with drug‐resistant tuberculosis, patients who have relapsed after previous treatment for tuberculosis); patients likely, in the opinion of the local investigator, to be unable to comply with the requirements of the study protocol |
| Interventions | Participants will be randomized to receive gatifloxacin, levofloxacin, moxifloxacin, or isoniazid (control), and after these arms are enrolled, they will be randomized to receive either linezolid (600 mg once daily) or linezolid (600 mg twice daily) or isoniazid (control). After the initial treatment, all participants will receive 6 months of standard antituberculous treatment outside of the hospital |
| Outcomes | 1. Early bactericidal activity |
| Starting date | February 2004 |
| Contact information | John Johnson ([email protected]) |
| Notes | Location: University of Espírito Santo, Vitória, Brazil Registration number: NCT00396084 Sponsors: National Institute of Allergy and Infectious Diseases (NIAID) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion) at 8 weeks Show forest plot | 3 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| Analysis 1.1  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 1 Cure (sputum culture conversion) at 8 weeks. | ||||
| 1.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.32] |
| 1.2 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.42, 1.09] |
| 1.3 Moxifloxacin vs ethambutol | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 2 Treatment failure at 12 months Show forest plot | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.71, 6.42] |
| Analysis 1.2  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 2 Treatment failure at 12 months. | ||||
| 2.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 2.2 Ciprofloxacin vs ethambutol plus pyrazinamide | 2 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.63, 6.58] |
| 3 Relapse Show forest plot | 3 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.17 [1.33, 38.58] |
| Analysis 1.3  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 3 Relapse. | ||||
| 3.1 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.72 [0.91, 270.96] |
| 3.2 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 3.3 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Relapse: by HIV status Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 4 Relapse: by HIV status. | ||||
| 4.1 HIV‐positive participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 HIV‐negative participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to sputum culture conversion (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 5 Time to sputum culture conversion (months). | ||||
| 5.1 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time to sputum culture conversion (months): by HIV status Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 6 Time to sputum culture conversion (months): by HIV status. | ||||
| 6.1 HIV‐positive participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 HIV‐negative participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Clinical or radiological improvement at 8 weeks Show forest plot | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.49, 1.59] |
| Analysis 1.7  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 7 Clinical or radiological improvement at 8 weeks. | ||||
| 7.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.32] |
| 7.2 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.08] |
| 8 Serious adverse events Show forest plot | 5 | 743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.56, 1.72] |
| Analysis 1.8  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 8 Serious adverse events. | ||||
| 8.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 8.2 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.47, 3.57] |
| 8.3 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.69] |
| 8.4 Ofloxacin vs rifampicin | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.79] |
| 8.5 Moxifloxacin vs ethambutol | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.05] |
| 9 Total number of adverse events Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.96, 1.43] |
| Analysis 1.9  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 9 Total number of adverse events. | ||||
| 9.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.56] |
| 9.2 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 9.3 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.70, 5.44] |
| 9.4 Moxifloxacin vs ethambutol | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.00, 1.66] |
| 10 Total number of adverse events, substitutions for ethambutol Show forest plot | 2 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.05, 1.72] |
| Analysis 1.10  Comparison 1 Fluoroquinolone substituted into regimen, Outcome 10 Total number of adverse events, substitutions for ethambutol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion) at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Fluoroquinolone added to regimen, Outcome 1 Cure (sputum culture conversion) at 8 weeks. | ||||
| 1.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment failure at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Fluoroquinolone added to regimen, Outcome 2 Treatment failure at 12 months. | ||||
| 2.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Clinical or radiological improvement at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Fluoroquinolone added to regimen, Outcome 3 Clinical or radiological improvement at 8 weeks. | ||||
| 3.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Death from any cause Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Fluoroquinolone added to regimen, Outcome 4 Death from any cause. | ||||
| 4.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Tuberculosis‐related death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Fluoroquinolone added to regimen, Outcome 5 Tuberculosis‐related death. | ||||
| 5.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Fluoroquinolone added to regimen, Outcome 6 Serious adverse events. | ||||
| 6.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion) within 2 to 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 1 Cure (sputum culture conversion) within 2 to 3 weeks. | ||||
| 2 Treatment failure at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 2 Treatment failure at 12 months. | ||||
| 3 Clinical or radiological improvement at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 3 Clinical or radiological improvement at 8 weeks. | ||||
| 4 Total number of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 4 Total number of adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion within 2 to 3 weeks) Show forest plot | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.77, 5.71] |
| Analysis 4.1  Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 1 Cure (sputum culture conversion within 2 to 3 weeks). | ||||
| 2 Treatment failure at 12 months Show forest plot | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.47] |
| Analysis 4.2  Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 2 Treatment failure at 12 months. | ||||
| 3 Clinical or radiological improvement at 8 weeks Show forest plot | 3 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| Analysis 4.3  Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 3 Clinical or radiological improvement at 8 weeks. | ||||
| 4 Total number of adverse events Show forest plot | 3 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.64] |
| Analysis 4.4  Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 4 Total number of adverse events. | ||||

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 1 Cure (sputum culture conversion) at 8 weeks.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 2 Treatment failure at 12 months.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 3 Relapse.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 4 Relapse: by HIV status.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 5 Time to sputum culture conversion (months).
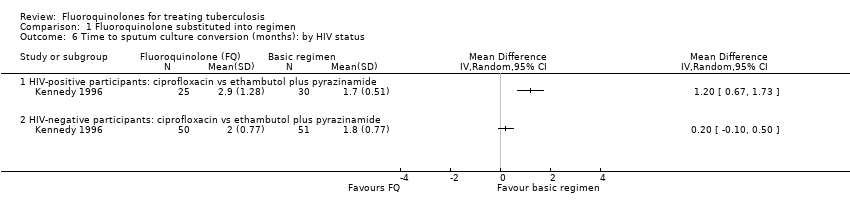
Comparison 1 Fluoroquinolone substituted into regimen, Outcome 6 Time to sputum culture conversion (months): by HIV status.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 7 Clinical or radiological improvement at 8 weeks.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 8 Serious adverse events.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 9 Total number of adverse events.

Comparison 1 Fluoroquinolone substituted into regimen, Outcome 10 Total number of adverse events, substitutions for ethambutol.

Comparison 2 Fluoroquinolone added to regimen, Outcome 1 Cure (sputum culture conversion) at 8 weeks.

Comparison 2 Fluoroquinolone added to regimen, Outcome 2 Treatment failure at 12 months.

Comparison 2 Fluoroquinolone added to regimen, Outcome 3 Clinical or radiological improvement at 8 weeks.

Comparison 2 Fluoroquinolone added to regimen, Outcome 4 Death from any cause.

Comparison 2 Fluoroquinolone added to regimen, Outcome 5 Tuberculosis‐related death.

Comparison 2 Fluoroquinolone added to regimen, Outcome 6 Serious adverse events.
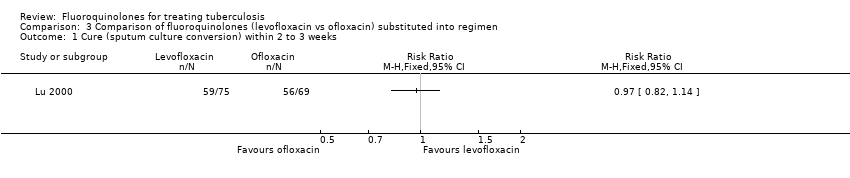
Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 1 Cure (sputum culture conversion) within 2 to 3 weeks.

Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 2 Treatment failure at 12 months.
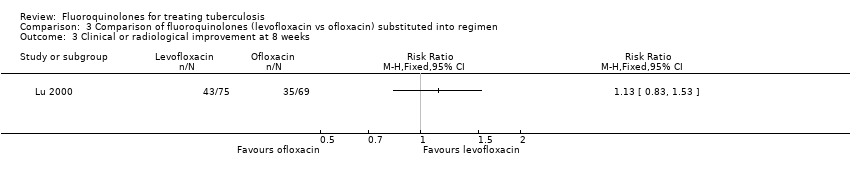
Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 3 Clinical or radiological improvement at 8 weeks.

Comparison 3 Comparison of fluoroquinolones (levofloxacin vs ofloxacin) substituted into regimen, Outcome 4 Total number of adverse events.

Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 1 Cure (sputum culture conversion within 2 to 3 weeks).
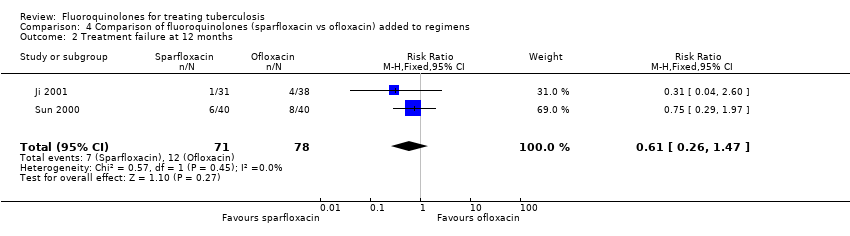
Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 2 Treatment failure at 12 months.

Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 3 Clinical or radiological improvement at 8 weeks.

Comparison 4 Comparison of fluoroquinolones (sparfloxacin vs ofloxacin) added to regimens, Outcome 4 Total number of adverse events.
| Location | New case | Previously treated case |
| Estonia | 14.1 | 18.1 |
| Henan Province, China | 10.8 | 15.1 |
| Latvia | 9.0 | 12.0 |
| Ivanovo Oblast, Russian Federation | 9.0 | 12.3 |
| Tomsk Oblast, Russian Federation | 6.5 | 13.7 |
| aSource: Loddenkemper 2002. | ||
| Trial | Allocation sequence generation | Allocation concealment | Blinding | Inclusiona |
| Unclear | Unclear | Unclear | Inadequate | |
| Adequate | Unclear | Assessors only | Adequate for 8 weeks | |
| Unclear | Unclear | Unclear | Adequate | |
| Unclear | Unclear | Unclear | Adequate | |
| Unclear | Unclear | None | Adequate | |
| Adequate | Adequate | Assessors only | Adequate | |
| Unclear | Unclear | Unclear | Inadequate | |
| Adequate | Unclear | Participants: yes | Adequate | |
| Unclear | Unclear | Providers, participants, and radiograph assessors: yes | Inadequate | |
| Adequate | Unclear | None | Adequate | |
| Unclear | Unclear | Unclear | Adequate | |
| aInclusion of all randomized participants in the final analysis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion) at 8 weeks Show forest plot | 3 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.17] |
| 1.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.32] |
| 1.2 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.42, 1.09] |
| 1.3 Moxifloxacin vs ethambutol | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 2 Treatment failure at 12 months Show forest plot | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.71, 6.42] |
| 2.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.83] |
| 2.2 Ciprofloxacin vs ethambutol plus pyrazinamide | 2 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.63, 6.58] |
| 3 Relapse Show forest plot | 3 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.17 [1.33, 38.58] |
| 3.1 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.72 [0.91, 270.96] |
| 3.2 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 3.3 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Relapse: by HIV status Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 HIV‐positive participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 HIV‐negative participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to sputum culture conversion (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time to sputum culture conversion (months): by HIV status Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 HIV‐positive participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 HIV‐negative participants: ciprofloxacin vs ethambutol plus pyrazinamide | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Clinical or radiological improvement at 8 weeks Show forest plot | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.49, 1.59] |
| 7.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.32] |
| 7.2 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.08] |
| 8 Serious adverse events Show forest plot | 5 | 743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.56, 1.72] |
| 8.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 8.2 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.47, 3.57] |
| 8.3 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.69] |
| 8.4 Ofloxacin vs rifampicin | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.79] |
| 8.5 Moxifloxacin vs ethambutol | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.05] |
| 9 Total number of adverse events Show forest plot | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.96, 1.43] |
| 9.1 Ciprofloxacin vs rifampicin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.56] |
| 9.2 Ciprofloxacin vs ethambutol plus pyrazinamide | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 9.3 Ofloxacin vs ethambutol | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.70, 5.44] |
| 9.4 Moxifloxacin vs ethambutol | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.00, 1.66] |
| 10 Total number of adverse events, substitutions for ethambutol Show forest plot | 2 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.05, 1.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion) at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment failure at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Clinical or radiological improvement at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Death from any cause Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Tuberculosis‐related death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Levofloxacin vs no levofloxacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion) within 2 to 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Clinical or radiological improvement at 8 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Total number of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cure (sputum culture conversion within 2 to 3 weeks) Show forest plot | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.77, 5.71] |
| 2 Treatment failure at 12 months Show forest plot | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.47] |
| 3 Clinical or radiological improvement at 8 weeks Show forest plot | 3 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 4 Total number of adverse events Show forest plot | 3 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.64] |

