Reparación laparoscópica para la úlcera péptica perforada
Resumen
Antecedentes
La úlcera péptica perforada es un trastorno abdominal frecuente que se trata mediante cirugía. El desarrollo de la cirugía laparoscópica ha cambiado la forma de tratar tales urgencias quirúrgicas abdominales. Los resultados de algunos ensayos clínicos indican que la cirugía laparoscópica podría ser una mejor estrategia que la cirugía abierta para la corrección de la úlcera péptica perforada, pero la evidencia no favorece ni se opone firmemente a esta intervención.
Objetivos
Medir el efecto del tratamiento quirúrgico laparoscópico versus el tratamiento quirúrgico abierto en pacientes con diagnóstico de úlcera péptica perforada, en relación con complicaciones abdominales sépticas, la infección de la herida, las complicaciones extraabdominales, la duración de la estancia hospitalaria y los costes directos.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) en la Cochrane Library (2004, Número 2), PubMed/MEDLINE (1966 a julio de 2004), EMBASE (1985 a noviembre de 2004) y LILACS (1988 a noviembre de 2004), así como en las listas de referencias de los artículos pertinentes. Las búsquedas en todas las bases de datos se actualizaron en diciembre de 2009 y enero de 2012. La búsqueda no se limitó a publicaciones en idioma inglés.
Criterios de selección
Ensayos clínicos aleatorizados que compararan cirugía laparoscópica versus cirugía abierta para la reparación de la úlcera péptica perforada y utilizaran cualquier método mecánico de cierre (sutura, parche epiploico o sellador de fibrina).
Obtención y análisis de los datos
Las medidas de resultado primarias incluyeron la proporción de complicaciones abdominales sépticas y de otro tipo (infección del sitio quirúrgico, filtración en la sutura, absceso intraabdominal, íleo posoperatorio) y de complicaciones extraabdominales (pulmonares). Los resultados secundarios incluyeron mortalidad, tiempo para regresar a la dieta normal, tiempo de aspiración nasogástrica, duración de la estancia hospitalaria y costes. Los resultados se resumieron mediante los odds ratios (OR) y los intervalos de confianza (IC) del 95%, con el modelo de efectos fijos.
Resultados principales
Se incluyeron tres ensayos clínicos aleatorizados de calidad aceptable. No se encontraron diferencias estadísticamente significativas entre la cirugía laparoscópica y la cirugía abierta en la proporción de complicaciones abdominales sépticas (OR 0,66; IC del 95%: 0,30 a 1,47), las complicaciones pulmonares (OR 0,52; IC del 95%: 0,08 a 3,55) o el número de complicaciones abdominales sépticas (OR 0,60; IC del 95%: 0,32 a 1,15). La heterogeneidad fue significativa para las complicaciones pulmonares y la duración de la cirugía.
Conclusiones de los autores
Esta revisión sistemática indica que puede existir una disminución de las complicaciones abdominales sépticas cuando se utiliza la cirugía laparoscópica para corregir la úlcera péptica perforada. Sin embargo, para confirmar tal suposición es necesario desarrollar más ensayos controlados aleatorizados que incluyan a un número mayor de pacientes, lo que garantiza una curva de aprendizaje prolongada para los cirujanos participantes. Con la información proporcionada se podría decir que los resultados de la cirugía laparoscópica no son clínicamente diferentes de los de la cirugía abierta.
PICO
Resumen en términos sencillos
Reparación laparoscópica (mínimamente invasiva) para la úlcera péptica perforada
La úlcera péptica perforada se puede reparar mediante cirugía abierta o laparoscopia, una técnica quirúrgica mínimamente invasiva a veces conocida como cirugía "de mínimo acceso". Se identificaron tres ensayos controlados aleatorizados que compararon los dos métodos. Estos ensayos incluyeron a pacientes con sospecha clínica de úlcera péptica perforada que se confirmó en la cirugía. Las reparaciones laparoscópicas y abiertas se realizaron con un parche de epiplón o sellador de fibrina. Los resultados primarios evaluados fueron las complicaciones sépticas abdominales y extraabdominales. Los resultado secundarios evaluados fueron la mortalidad, la duración de la cirugía y la duración de la estancia hospitalaria. La calidad de los ensayos fue aceptable. No se observaron diferencias estadísticamente significativas en las complicaciones abdominales sépticas entre la reparación laparoscópica y la reparación abierta de la úlcera péptica perforada. Para confirmar tal suposición se necesitan más ensayos controlados aleatorizados, con un número mayor de pacientes, lo que garantizará una curva de aprendizaje prolongada para los cirujanos participantes.
Authors' conclusions
Summary of findings
| Laparoscopic surgery versus open surgery for perforated peptic ulcer disease | ||||||
| Patient or population: patients with perforated peptic ulcer disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Laparoscopic surgery versus open surgery | |||||
| Septic abdominal complications (presence or absence) | 155 per 1000 | 108 per 1000 | OR 0.66 | 214 | ⊕⊕⊝⊝ | |
| Pulmonary complications (presence or absence) | 86 per 1000 | 46 per 1000 | OR 0.52 | 315 | ⊕⊕⊝⊝ | |
| Surgical site infection | 72 per 1000 | 21 per 1000 | OR 0.28 | 315 | ⊕⊕⊕⊝ | |
| Suture dehiscence | 13 per 1000 | 20 per 1000 | OR 1.52 | 315 | ⊕⊕⊝⊝ | |
| Postoperative ileus | Study population | OR 0.54 | 315 | ⊕⊕⊝⊝ | ||
| 46 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 19 per 1000 | |||||
| Intra‐abdominal abscess | 20 per 1000 | 23 per 1000 | OR 1.15 | 315 | ⊕⊝⊝⊝ | |
| Operative time | The mean operative time in the control groups was | The mean operative time in the intervention groups was | 214 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision is probably because the small sample size of the studies and the lack of reporting form the largest one. | ||||||
Background
Description of the condition
The appearance of laparoscopy in the late 1980s marked a milestone in surgery. Its advantages of diminished pain, surgical wound complications, hospital stay and global costs in uncomplicated cases of gallbladder disease (Gadacz 2000) led to the expansion of its use to other intra‐abdominal organs such as the distal oesophagus, the proximal stomach (Chekan 1999; Consensus 1997; Horgan 1997; Klingler 1999) and the colon (Rickard 2001; Tisminezky 2000).
Most of the early laparoscopic approaches were confined to elective surgery. However, with the improvement of technology and the gaining of experience the laparoscopic approach for acute intra‐abdominal pathologies can be applied more widely (Bergamaschi 2000; Pamoukian 2001; Sauerland 2004).
Peptic ulcer perforation is the second most frequent abdominal perforation that requires surgery, following perforated appendicitis. Peptic ulcer is a common disease in the general population. It is estimated that almost 10% of American men will suffer from duodenal ulcer in their lifetime, although its incidence varies within a country (Paimela 1991) as it is more frequent in men and the incidence increases with age. Peptic ulcer disease has been associated with many etiological factors such as Helicobacter pylori infection, non‐steroidal anti‐inflammatory drug (NSAID) use, stress, cigarette smoking, diet and genetics but multifactorial hypotheses are widely accepted. Complications of peptic ulcer include bleeding, obstruction and perforation and they are still treated by general surgeons.
Elective surgery for peptic ulcer disease has decreased significantly over the years due to the introduction of effective medical therapies, first with histamine type 2 (H2)‐receptor antagonists and more recently with proton pump inhibitors. However, the principal complications of perforation and hemorrhage remain indications for surgery. (Paimela 1991; Svanes 1995).
Description of the intervention
Since the first description of surgery for acute perforated peptic ulcer many techniques have been recommended. Ulcers can be repaired by hand suturing the edges of the wound or using surgical stapling devices, covering the defect using an omental patch, or closing it with a fibrin sealant or a gelatin plug product (Darzi 1993; Matsuda 1995; Tate 1993; Walsh 1993).
Since the early 1990s, some authors have suggested that in cases of perforated peptic ulcer the laparoscopic approach may offer theoretical advantages over the open approach. Such advantages include reduced size of the surgical wound and diminished postoperative pain; fewer postoperative complications; less intestinal manipulation, which should diminish postoperative ileus and the long‐term risk of future adhesive obstructive complications; and the global cost savings derived from a shorter hospital stay and an earlier return to daily activities (Benoit 1993; Michelet 2000; Mouret 1990; Naesgaard 1999; Sunderland 1992). Furthermore, it has been suggested that laparoscopic repair could be the best choice for patients with adverse prognostic factors such as advanced age and coexisting cardiopulmonary diseases, or a clinical evaluation delayed beyond 12 hours from the onset of symptoms (Chou 2000; Hermansson 1999). However, some authors have also found that laparoscopic repair presents a somewhat higher incidence of leaks and is a more time‐consuming procedure (Lau 1995; Lee 2001).
Why it is important to do this review
Controlled trials have been carried out trying to evaluate this approach. However, the results are inconclusive because of methodological weaknesses in the trials and the small numbers of participants (Druart 1997; Gomez‐Ferrer 1996; Katkhouda 1999; Kum 1993; Lau 1995; Lau 1996; Lau 1998; Michelet 2000; Ozmen 1995; Robertson 2000; Siu 2002). A systematic review is, therefore, appropriate as meta‐analysis may prove informative as to the comparative efficacy and complication rates for the two surgical approaches.
Thus, the present systematic review was developed to answer the following question: is laparoscopic treatment of perforated peptic ulcer associated with reduced wound complications, postoperative intra‐abdominal sepsis, duration of hospitalization and overall cost compared to the conventional (open) approach?
Objectives
To assess laparoscopic surgical treatment versus open surgical treatment in patients with perforated peptic ulcer diagnosis in terms of abdominal septic complications, surgical wound infection, extra‐abdominal complications, hospital stay and direct costs.
Methods
Criteria for considering studies for this review
Types of studies
The review included randomized controlled trials that were performed after 1988, the date of the first surgical procedure using laparoscopy (Mouret 1990). Restrictions regarding language were not applied.
Studies including patients managed with an open abdomen from the beginning of surgery or that did not have information about relevant clinical outcomes (surgical wound infection, intra‐abdominal infection and hospital length of stay) were excluded.
Types of participants
Adult patients, older than 18 years, with a preoperative clinical diagnosis and intraoperative confirmation of a perforated ulcer (gastric or duodenal) that was corrected by any mechanical method (primary suture, omentum patch, synthetic material patch) by a surgeon.
Types of interventions
Laparoscopic versus open surgery correction of the ulcer with any mechanical method (primary suture, omentum patch, synthetic material patch or resection), with or without insufflation.
Types of outcome measures
Primary outcomes
Septic and other abdominal and extra‐abdominal complications, defined according to the Centers for Disease Control (CDC) classification (Garner 1996) and recorded as 'number of complications' and 'at least one abdominal complication'.
We considered the following abdominal complications: intra‐abdominal abscess; anastomosis leakage; secondary peritonitis; surgical‐site infection; prolonged ileus for more than 72 hours without recovery of bowel movement, clinically‐determined; and incisional hernia.
Secondary outcomes
-
Mortality
-
Number of interventions
-
Conversion rate for the laparoscopic group
-
Nasogastric tube duration
-
Total analgesic dose
-
Time to return to normal diet
-
Overall duration of hospitalisation
-
Operation time
Outcome measures were measured within the period 30 days after surgery.
Search methods for identification of studies
Electronic searches
A search was conducted to identify all published and unpublished randomized controlled trials.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (4th quarter, 2011), PubMed/MEDLINE (1946 to December 2011), EMBASE (1980 to December 2011) and LILACS (1988 to December 2011) as well as reference lists of relevant articles. Searches in all databases were updated in January 2012. We did not confine our search to English language publications.
The Cochrane highly sensitive search strategy for identifying randomized trials in MEDLINE, sensitivity maximising version, Ovid format (Higgins 2008) was combined with the search terms in Appendix 1 to identify randomized controlled trials in MEDLINE. The MEDLINE search strategy was adapted for use in the other databases searched.
Searching other resources
Reference lists from trials selected by electronic searching were screened to identify further relevant trials. Abstracts from the conference proceedings of the American Digestive Disease Week (DDW), published in Gastroenterology and the United European Gastroenterology Week (UEGW) and appearing in Gut, were handsearched.
In addition, members of the Cochrane Upper Gastric and Pancreatic Diseases Group, experts in the field and pharmaceutical companies were contacted and asked to provide details of any outstanding clinical trials or relevant unpublished studies. The websites from ClinicalTrials.gov from the US National Institutes of Health, Current Controlled Trials, International Clinical Trials Registry Platform from the World Health Organization, Clinical Trials Registry‐India (CTRI) and Pan African Clinical Trial Register were reviewed to identify completed or ongoing trials.
Data collection and analysis
Selection of studies
AS, CM and MV reviewed the abstracts of the identified articles independently and selected the studies to be included.
Data extraction and management
CM and MV extracted the data using a previously designed format. They were trained at the beginning of the study on data extraction methods, especially on the non‐imputation of results from the reports. Disagreement between review authors were solved by consensus. The concordance between the extractors was evaluated using an intraclass correlation coefficient and kappa coefficient; the value was 0.99. The studies were centrally blinded by the review authors for the title of the article, authors and publication source. For missing data from the published trials, AS communicated directly with the authors by e‐mail.
A comparison was made between the type of treatment and septic and other complications: abdominal including surgical site infection, intra‐abdominal abscess, leakage from the suture site and prolonged ileus; and extra‐abdominal, specifically pulmonary complications. The number of re‐operations, mortality and hospital length of stay were also compared. Statistical analysis was performed using the Cochrane RevMan 5.0 software.
Assessment of risk of bias in included studies
Assessment of risk of bias was performed by CM and MV. The assessment focused on sequence generation, allocation concealment, blinding, incomplete outcome data, follow‐up losses, intention‐to‐treat method of analysis and selective reporting as recommended by Cochrane Handbook for Systematic Reviews of Interventions Version 5.0 (Higgins 2008).
Measures of treatment effect
The results for each outcome were measured using the crude odds ratio (OR) for categorical variables not related by time; log hazard ratio for time‐to‐event variables; and means differences for continuous variables. The 95% confidence interval (CI) was also estimated.
Dealing with missing data
For missing data, trial authors were contacted via e‐mail. For cases in which the data in an article were in measurement units that differed from the review format, the extractor registered the original data and the review authors performed the necessary conversion.
Assessment of heterogeneity
Heterogeneity analysis was performed using the Q test, with heterogeneity considered significant when P value < 0.1. The I² statistic was used to quantify the presence of heterogeneity in the pooled results.
Assessment of reporting biases
The identification of publication bias with the funnel plot, planned in the protocol, could not be performed because of the small number of articles included in the review.
Data synthesis
The fixed‐effect model and the Mantel‐Haenszel method for the measurement of the global effects outcome were used.
Subgroup analysis and investigation of heterogeneity
If there was significant heterogeneity, a re‐analysis with the random‐effects method was done as well as a sensitivity analysis to try to consider the potential source of the heterogeneity. The origin of the heterogeneity was explained qualitatively. Although a stratified analysis by disease severity was planned in the protocol, as a way to explain any heterogeneity, it was impossible to do because of the lack of information about the severity of the disease in the included studies.
Sensitivity analysis
This was not carried out due to the small number of included studies and insufficient data on items such as surgeon experience or volume of cases, which may be important factors.
Results
Description of studies
Results of the search
Thirteen studies were identified by the primary search. Nine of these were excluded because they were comparative studies and did not use randomization (Bergamaschi 2000; Bhogal 2008; Katkhouda 1999; Lemaitre 2005; Mehendale 2002; Minutolo 2009; Nicolau 2008; Robertson 2000; Vettoretto 2005); another was excluded because of missing and duplicate data (Lau 1998). We tried to contact the authors but received no response.
All studies were identified in MEDLINE and all were in the English language. The one article that was in a language other than English stated clearly in the abstract that it was not a randomized controlled trial, so it was not translated.
Requests to authors and other sources of information did not provide further studies.
Included studies
Three studies were included (Bertleff 2009; Lau 1996; Siu 2002). The updated literature search of January 2012 did not identify any new studies eligible for inclusion. Please see 'Characteristics of included studies'. Inclusion criteria were similar for the three included studies: adult patients with a clinical diagnosis of perforated peptic ulcer (as shown by peritoneal irritation, pneumoperitoneum on chest x‐ray) made by the attending surgeon.
Exclusion criteria were also similar for the included studies:
-
previous abdominal surgery;
-
concomitant bleeding of the ulcer, or gastric outlet obstruction;
-
intraoperative diagnosis of hollow viscus perforation different from peptic ulcer;
-
perforated peptic ulcer that required definitive surgery, criteria not reported;
-
cardiopulmonary severe disease that impeded a long‐duration surgical procedure;
-
clinically sealed ulcer by the omentum at the time of surgery (Lau 1996; Siu 2002);
-
pregnancy.
Design
Bertleff 2009 was a multicenter, parallel design randomized controlled trial (RCT) over 77 months; Lau 1996 was a parallel group RCT, which took place over 28 months; as was Siu 2002 which took place over 41 months.
Sample sizes
The three studies included 315 patients in total (Bertleff 2009; Lau 1996; Siu 2002), 163 in the laparoscopy group (52 in Bertleff 2009, 48 in Lau 1996 and 63 in Siu 2002) and 152 in the open‐surgery group (49 in Bertleff 2009, 45 in Lau 1996 and 58 in Siu 2002).
Setting
The three included studies were carried out in a hospital setting. Bertleff 2009 was a multicenter, parallel design RCT conducted in nine medical centers in the Netherlands over 77 months. Lau 1996 was a parallel group RCT that took place over 28 months in the Prince of Wales Hospital, Shatin, New Territories, Hong Kong. Siu 2002 was also a parallel group RCT from the Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong, Special Administrative Region, China and took place over 41 months.
Participants
The studies did not report on baseline differences in participant age, sex, disease severity (measured with APACHE II score and American Society of Anesthesiologists (ASA) classification), shock, concomitant diseases, ulcer localization or perforation size. Only Bertleff 2009 mentioned the grade of peritonitis, which was similar for both groups.
Interventions
Interventions were as follows. Open surgery was by a midline laparotomy with closure of the ulcer with omentum using the technique of Cellan‐Jones. In the Lau (Lau 1996) study, two more groups were created within the open and laparoscopic surgery groups: one with the Cellan‐Jones technique and the other using sponge and fibrin sealant and peritoneal toilet. Laparoscopic surgery included the creation of a pneumoperitoneum with carbon dioxide at 15 mm Hg using a 10 mm trocar and the insertion of a further two or three 5 mm trocars with closure of the perforation with omentum sutured by the same technique as with open surgery or using a sponge and fibrin sealant.
All patients received antibiotics: one study for a day (Lau 1996), in Siu 2002 five days, and Bertleff 2009 did not specify the type of antibiotic or the time of administration. Postoperative analgesia was maintained with pethidine (1 mg/kg every 4 hours) in Lau 1996 and Siu 2002. Bertleff 2009 used opiates but did not specify the type and doses used.
The included studies mentioned that the surgical procedures were performed by trained surgeons, or by residents accompanied by trained surgeons with experience in open and laparoscopic surgery. However, none of the studies described the number of procedures that the surgeons had previously carried out.
Outcomes
All three included studies reported complications, time of nasogastric aspiration, pain assessed with a Visual Analogue Scale (VAS), operation time, analgesic use, conversion rate for the laparoscopic group and length of hospital stay. Lau 1996 and Siu 2002 also reported duration of intravenous fluid maintenance and time to resume oral diet.
Excluded studies
Ten studies did not meet the inclusion criteria for the review. Please see 'Characteristics of excluded studies'.
Risk of bias in included studies
Participants in the included studies were randomized using computer‐generated random numbers and the concealment of assignment was done using sealed envelopes. Assessment of outcomes was not blinded in either of the studies. In Siu 2002 an independent surgeon made the evaluations for return to normal activities and work.
Follow up was at day 30 but in one study there were no assessments for 27% of the laparoscopy group and 31% of the open surgery group at this date (Lau 1996). However, authors clearly stated that all patients were evaluated at week 8 (Table 2). An intention‐to‐treat analysis for primary outcomes was carried out in all studies.
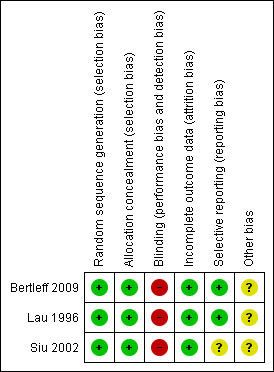
CM and MV differed in their assessment of outcomes related to suture dehiscence and morbidity in the Siu article (Siu 2002) and with blind outcome assessment, operative time and nasogastric aspiration time in the Lau article (Lau 1996). AS reviewed the articles and resolved the differences. Figure 1 and Figure 2 provide summaries of the risk of bias, please also see the Characteristics of included studies table for risk of bias assessments for each included study.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation was adequate in all three included studies, being computer generated. Allocation concealment was also adequate in all three included studies, being achieved by sealed envelopes.
Blinding
Blinding was not possible as the studies compared open surgery with laparoscopy. Blinding probably did not affect the assessment of hard outcomes such as surgical complications, but it was possible that length of stay and pain assessment could have been biased by non‐blind evaluation of outcomes.
Incomplete outcome data
There were no missing outcome data in any of the three included trials.
Selective reporting
Two trials (Bertleff 2009; Lau 1996) adequately reported all outcome data and were judged to be free of selective outcome reporting. There was insufficient information in Siu 2002 to judge this, but analysis of the trial data from this study suggests that any bias due to selective outcome reporting in this study should not greatly bias the results of the analyses.
Other potential sources of bias
In surgical trials, there is always a bias related to the learning curve for the new surgical methods. However, we believe this bias was not present in the included studies because the experience of the surgeons was similar.
In multicenter trials, a bias related to high volume and low volume centers is possible. There was no information about the number of patients by center to investigate this further.
Effects of interventions
Laparoscopic surgery versus open surgery
Primary outcomes
Septic and other abdominal and extra‐abdominal complications
Twelve of 111 patients (10.8%) in the laparoscopy group had an abdominal complication against 16 of 103 (15.5%) in the open‐surgery group (OR 0.66; 95% CI 0.30 to 1.47; Analysis 1.1). The Bertleff 2009 study did record reliable data for this outcome and, when contacted, the authors did not offer any additional data about this outcome. No statistically significant differences or significant heterogeneity were observed.
Septic extra‐abdominal complications, specifically pulmonary complications, occurred in eight patients in Bertleff 2009 (three in the laparoscopic group), in four patients in Lau 1996 (three in the laparoscopic group) and seven patients in Siu 2002 (none in the laparoscopic group) (OR 0.43; 95% CI 0.17 to 1.12; analysis not shown), which was not statistically significant. The heterogeneity test had a P value of 0.09 and the I² was 58%. As stated in the protocol, we performed an analysis using the random‐effects method but without any change in the results (OR 0.52; 95% CI 0.08 to 3.55; Analysis 1.2).
The absolute number of septic abdominal complications was 18 (12.3%) in the laparoscopy group and 26 (17.1%) in the open‐surgery group. The OR was 0.60 (95% CI 0.32 to 1.15; Analysis 1.3) but the difference was not statistically significant. One patient in the laparoscopic group, from the Siu 2002 study, had two concurrent complications (suture leakage and intra‐abdominal abscess). In the Bertleff 2009 study there were patients with more than one complication but it was impossible to get reliable information about the number.
Three patients in the laparoscopic group (1.8%) and 11 (7.2%) in the open‐surgery group had a surgical site infection (OR 0.26; 95% CI 0.08 to 0.9). The analysis made with a random‐effects model showed an OR of 0.28 (95% CI 0.08 to 1.0; Analysis 1.4).
Suture dehiscence was reported in four patients in the laparoscopy group (2.4%) versus two patients in the open group (1.3%) with an OR of 1.70 (95% CI 0.36 to 8.07). As stated in the protocol, we performed an analysis using the random‐effects method (Analysis 1.5) but without any change in the results.
Postoperative ileus occurred in four patients from the laparoscopy group (2.5%) and in seven in the open‐surgery group with an OR of 0.54 (95% CI 0.16 to 1.80; Analysis 1.6). As stated in the protocol, we performed an analysis using the random‐effects method but without any change in the results.
Four patients had intra‐abdominal abscesses in the laparoscopic group and three patients in the open‐surgery group (OR 1.15; 95% CI 0.33 to 4.03; Analysis 1.7) but this difference was not statistically significant.
Incisional hernia incidence was similar between groups, two patients for laparoscopic repair and one for the open‐surgery group (OR 0.93; 95% CI 0.21 to 4.15; Analysis 1.8). None of these comparisons showed a statistically significant difference or heterogeneity.
Secondary outcomes
Mortality
For the secondary outcomes, five patients died in the laparoscopic group (3.0%) and eight patients in the open‐surgery group (5.3%) with an OR of 0.57 (95% CI 0.18 to 1.78; Analysis 1.9). This was not statistically significant.
Number of interventions
Six patients were re‐operated on in the laparoscopic group (5.4%), almost twice as many as in the open‐surgery group (three patients (2.9%)) but this difference was not statistically significant (OR 1.89; 95% CI 0.46 to 7.71; Analysis 1.10). The Bertleff 2009 study did not contain any information about this outcome and in the other trials (Lau 1996; Siu 2002) the investigators did not offer additional data.
Analgesic doses
Bertleff 2009 reported opiate requirements but not the doses used. Data reported in a non‐parametric form are reported in Table 1. The Included trials reported lower analgesic dose or time requirements for the patients in the laparoscopic group.
| Variable | Study | Laparoscopic group | Open surgery group | P value |
| Nasogastric aspiration time (median and range) | 2 (3.0) IQR | 3 (1.3) IQR | 0.33 | |
| 3 (2‐33) | 3(1‐8) | 0.28 | ||
| 2 (1‐4)/ 3 (2‐1) | 2 (1‐13)/ 3(1‐17) | No significant (P value not reported) | ||
| Time to return to oral diet | 4 (3‐35) | 5 (3‐24) | 0.06 | |
| 4 (3‐7)/ 4 (2‐11) | 4 (3‐16)/ 4 (3‐19) | No significant (P value not reported) | ||
| Length of stay | 6.5 (9.3) IQR | 8 (7.3) IQR | 0.23 | |
| 6 (4‐35) | 7 (4‐39) | 0.004 | ||
| 5 (3‐20)/ 6 (3‐11) | 5 (3‐19)/ 5 (2‐21) | No significant (P value not reported) | ||
| Analgesic doses | 0 (0‐11) | 6 (1‐30) | <0.001 | |
| 1 (0‐12)/ 2 (0‐17) | 3 (0‐10)/ 4 (1‐9) | 0.03 | ||
| 1 (1.25) median days of analgesics | 1 (1.0) median days of analgesics | 0.007 |
Time of nasogastric aspiration
Table 1 contains the medians and ranges for the time of nasogastric aspiration. The differences between open surgery and laparoscopy were not statistically significant.
Time to return to oral diet
Bertleff 2009 did not reported time to return to oral diet; the results for Lau 1996 and Siu 2002 are in Table 1. The differences were not statistically significant.
Overall duration of hospitalization
These data were reported non‐parametrically and are reported in Table 1. In Siu 2002, hospital length‐of‐stay was statistically in favour of laparoscopic repair; but not in Lau 1996 and Bertleff 2009.
Operation time
The operation time was different between studies. Siu 2002 reported a lower time for laparoscopic repair (42 ± 25.1 min) than in the open‐surgery group (52.3 ± 24.8 min); the difference was statistically significant (P = 0.02). On the contrary, Bertleff 2009 reported a longer operation time for the laparoscopic group (median 75 min) versus the open‐surgery group (50 min), a non‐statistically significant difference, but did not report standard deviations. Lau 1996 reported a longer operation time for the laparoscopic group (94.3 ± 40.3 min) than the open‐surgery group (53.7 ± 42.6 min); this difference was statistically significant (P = 0.001). The weighted mean difference was 0.77 min (95% CI ‐7.10 to 8.64; Analysis 1.11), which was not statistically significant, but with a highly significant heterogeneity test (P < 0.00001, I² = 96%). The analysis with a random‐effects model did not change the non‐significant result. The conversion rate for the laparoscopy group was 13 patients with a global frequency of 7.9%.
Discussion
Summary of main results
Although there was a tendency for a decrease in septic intra‐abdominal complications, surgical site infection, postoperative ileus, pulmonary complications and mortality with laparoscopic repair compared with open surgery, none of these were statistically significant. However, there was a tendency for an increase in the number of intra‐abdominal abscesses and re‐operations, without statistical significance. This finding could be related to surgeon experience in laparoscopic surgery. It is not possible to draw any conclusions about suture dehiscence and incisional hernia with the two procedures.
Other important variables, time of nasogastric aspiration and time to return to an oral diet, were reported in a non‐parametric format and were not statistically different between groups. In Siu 2002, hospital length of stay was statistically in favour of laparoscopic repair; but not in Lau 1996 and Bertleff 2009. Included trials reported lower analgesic doses or time requirements for the patients in the laparoscopic group. With these findings it is impossible to suggest any beneficial effects in terms of direct costs and this subject should be assessed in other studies specifically designed to assess cost‐effectiveness.
Statistical heterogeneity was found in the frequency of pulmonary complications and in operation times. It is hard to explain heterogeneity for pulmonary complications with the available data. In the case of operation time, the Siu 2002 study showed a shorter operation time for the laparoscopic procedure than did the Lau 1996 and Bertleff 2009 studies. This could be explained by greater experience with minimally‐invasive surgery. The results of this systematic review must be interpreted carefully because of the small sample sizes of the included trials.
Overall completeness and applicability of evidence
Clinical heterogeneity was not identified in the relevant clinical variables of the included studies so it is probable that the patients were similar.
Another important point to discuss is the learning curve for surgeons. It is accepted that surgical procedures are highly dependent on ability and the familiarity that surgeons have with different techniques. Introduction of a new surgical technique has a better prognosis when surgeon experience with it is greater (Solomon 1998). For the included trials, there was no objective information that helped to define the point on the learning curve of the surgeons, which affects the generalizability of the results. However, a conversion rate of 7.9% shows that experience with the technique is enough to consider the laparoscopic approach in these cases. The high number of patients operated on per month and the low conversion rates suggest high experience. It is probable that new trials will provide better outcomes because of greater experience with laparoscopic techniques. In relation to the generalizability of the results, it is important to highlight that two included studies were from Hong Kong, which could be important especially in relation to age, obesity and comorbid conditions of presenting patients. However, the most recent trial from the Netherlands is a multicenter trial with better generalizability.
This systematic review suggests that a decrease in septic abdominal complications may result when laparoscopic surgery is used to correct a perforated peptic ulcer as compared with open surgery. More trials are needed to confirm such an assumption and to assess the effect of the learning curve on outcomes, thus guaranteeing a long learning curve for participating surgeons.
Quality of the evidence
In general terms the quality of the trials was acceptable. There are concerns about blind assessment of outcomes. This quality criterion is difficult to obtain in surgical trials, where it is impossible to blind evaluators to the surgical group. To improve the outcome evaluation and to be closer to ideal blind assessment, it has been proposed that evaluators who are independent from the treating team, or non‐physician evaluators, are equipped with a predesigned data form and used for the evaluations (Solomon 1998; Thomas 2004). Such strategies were applied in some included clinical trials so we consider that outcomes evaluation, although bias susceptible, was strong enough to support the results, even more so considering that outcomes assessed were hard and not dependent on subjective evaluation. Greater than 20% of participants were lost to follow up at 30 days in the study of Lau 1996. However, it is probable that these losses only affected the measures of time to return to work and to normal activities as surgical complications usually occur during the first week when patients are still in hospital.
Potential biases in the review process
None are known.
Agreements and disagreements with other studies or reviews
From the information provided by the clinical trials included in this review, outcomes from laparoscopic surgery for perforated peptic ulcers are not clinically different from those of open surgery, which is the actual gold standard. With the conversion rates reported in this systematic review, laparoscopic surgery could be the first therapeutic option in patients with perforated peptic ulcer after considering other variables such as experience, costs and availability.
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
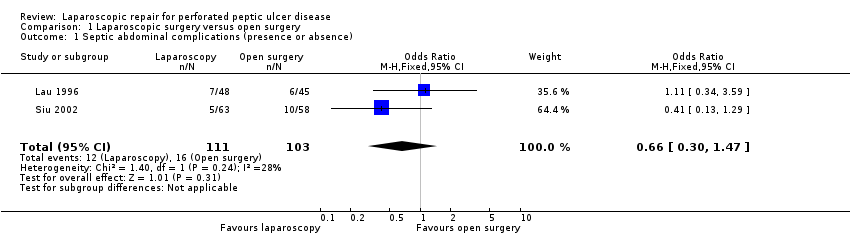
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 1 Septic abdominal complications (presence or absence).

Comparison 1 Laparoscopic surgery versus open surgery, Outcome 2 Pulmonary complications (presence or absence).
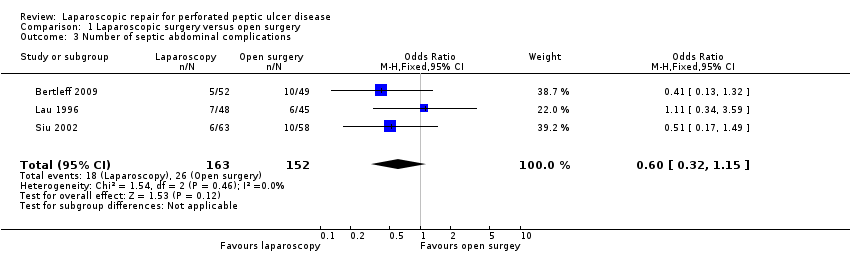
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 3 Number of septic abdominal complications.

Comparison 1 Laparoscopic surgery versus open surgery, Outcome 4 Surgical site infection.
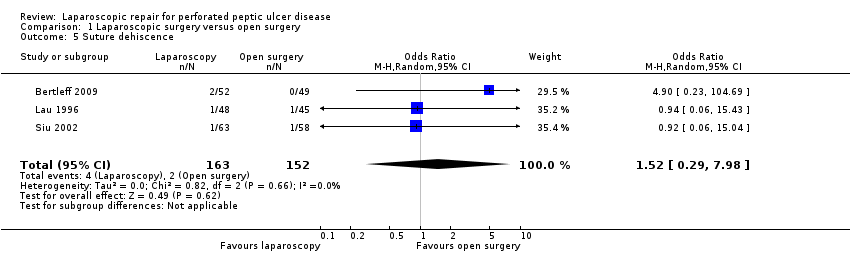
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 5 Suture dehiscence.
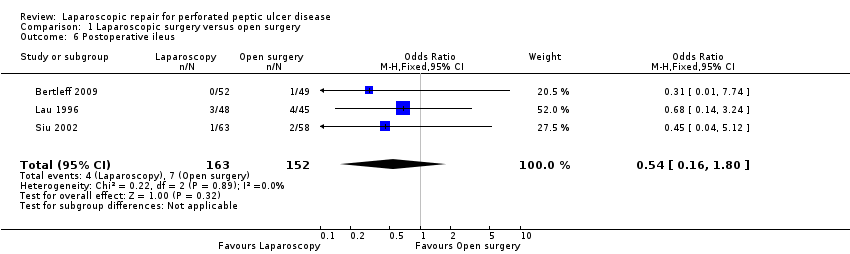
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 6 Postoperative ileus.
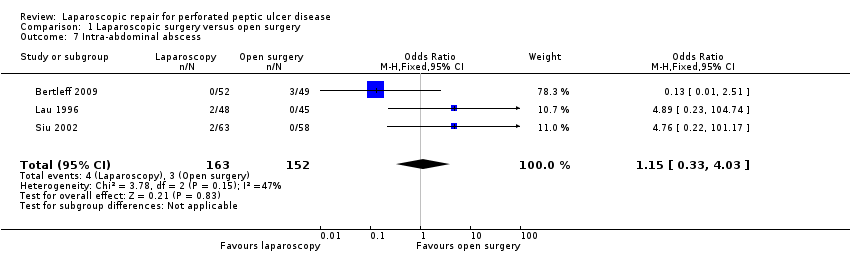
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 7 Intra‐abdominal abscess.
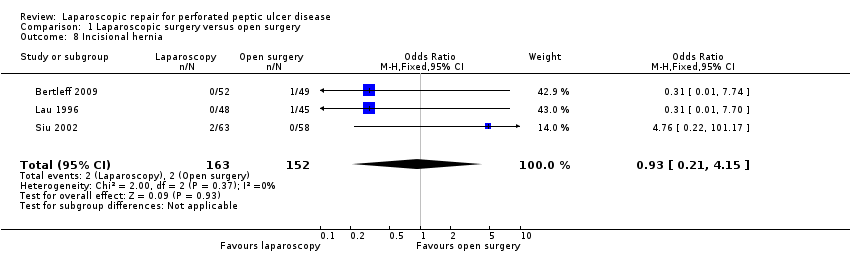
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 8 Incisional hernia.
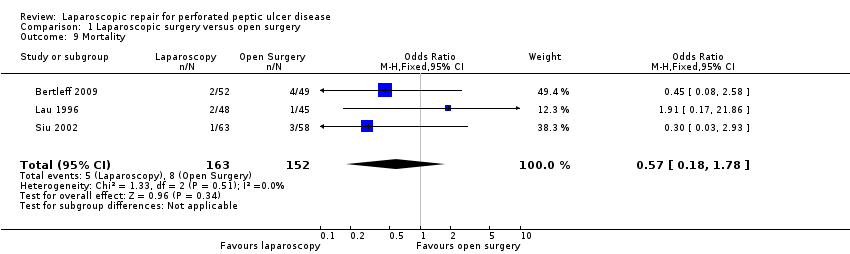
Comparison 1 Laparoscopic surgery versus open surgery, Outcome 9 Mortality.

Comparison 1 Laparoscopic surgery versus open surgery, Outcome 10 Number of reoperations.

Comparison 1 Laparoscopic surgery versus open surgery, Outcome 11 Operative time.
| Laparoscopic surgery versus open surgery for perforated peptic ulcer disease | ||||||
| Patient or population: patients with perforated peptic ulcer disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Laparoscopic surgery versus open surgery | |||||
| Septic abdominal complications (presence or absence) | 155 per 1000 | 108 per 1000 | OR 0.66 | 214 | ⊕⊕⊝⊝ | |
| Pulmonary complications (presence or absence) | 86 per 1000 | 46 per 1000 | OR 0.52 | 315 | ⊕⊕⊝⊝ | |
| Surgical site infection | 72 per 1000 | 21 per 1000 | OR 0.28 | 315 | ⊕⊕⊕⊝ | |
| Suture dehiscence | 13 per 1000 | 20 per 1000 | OR 1.52 | 315 | ⊕⊕⊝⊝ | |
| Postoperative ileus | Study population | OR 0.54 | 315 | ⊕⊕⊝⊝ | ||
| 46 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 19 per 1000 | |||||
| Intra‐abdominal abscess | 20 per 1000 | 23 per 1000 | OR 1.15 | 315 | ⊕⊝⊝⊝ | |
| Operative time | The mean operative time in the control groups was | The mean operative time in the intervention groups was | 214 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Imprecision is probably because the small sample size of the studies and the lack of reporting form the largest one. | ||||||
| Variable | Study | Laparoscopic group | Open surgery group | P value |
| Nasogastric aspiration time (median and range) | 2 (3.0) IQR | 3 (1.3) IQR | 0.33 | |
| 3 (2‐33) | 3(1‐8) | 0.28 | ||
| 2 (1‐4)/ 3 (2‐1) | 2 (1‐13)/ 3(1‐17) | No significant (P value not reported) | ||
| Time to return to oral diet | 4 (3‐35) | 5 (3‐24) | 0.06 | |
| 4 (3‐7)/ 4 (2‐11) | 4 (3‐16)/ 4 (3‐19) | No significant (P value not reported) | ||
| Length of stay | 6.5 (9.3) IQR | 8 (7.3) IQR | 0.23 | |
| 6 (4‐35) | 7 (4‐39) | 0.004 | ||
| 5 (3‐20)/ 6 (3‐11) | 5 (3‐19)/ 5 (2‐21) | No significant (P value not reported) | ||
| Analgesic doses | 0 (0‐11) | 6 (1‐30) | <0.001 | |
| 1 (0‐12)/ 2 (0‐17) | 3 (0‐10)/ 4 (1‐9) | 0.03 | ||
| 1 (1.25) median days of analgesics | 1 (1.0) median days of analgesics | 0.007 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Septic abdominal complications (presence or absence) Show forest plot | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.47] |
| 2 Pulmonary complications (presence or absence) Show forest plot | 3 | 315 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.08, 3.55] |
| 3 Number of septic abdominal complications Show forest plot | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.32, 1.15] |
| 4 Surgical site infection Show forest plot | 3 | 315 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.08, 1.00] |
| 5 Suture dehiscence Show forest plot | 3 | 315 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.29, 7.98] |
| 6 Postoperative ileus Show forest plot | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.16, 1.80] |
| 7 Intra‐abdominal abscess Show forest plot | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.33, 4.03] |
| 8 Incisional hernia Show forest plot | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.21, 4.15] |
| 9 Mortality Show forest plot | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.78] |
| 10 Number of reoperations Show forest plot | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.46, 7.71] |
| 11 Operative time Show forest plot | 2 | 214 | Mean Difference (IV, Random, 95% CI) | 14.62 [‐35.25, 64.49] |

