Fármacos antiinflamatorios no esteroideos para el dolor en mujeres con endometriosis
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Trial design: 2‐period, 4‐treatment cross‐over trial | |
| Participants | 24 women randomised; 18 analysed Mean age: 33 (22‐43) years Inclusion criteria: women with symptomatic endometriosis (stage and severity not described). Endometriosis was diagnosed by laparoscopy (n = 13) and by pelvic examination (n = 5). Setting: Finland Timing: unclear | |
| Interventions | Group 1: indomethacin 25 mg given 3 × daily for 2 menstrual cycles, then cross‐over to acetylsalicylic acid, tolfenamic acid and placebo for 2 menstrual cycles each (n = 6) Group 2: acetylsalicylic acid 500 mg given 3 × daily for 2 menstrual cycles, then cross‐over to tolfenamic acid, placebo and indomethacin for 2 menstrual cycles each (n = 6) Group 3: tolfenamic acid 200 mg given 3 × daily for 2 menstrual cycles, then cross‐over to placebo, indomethacin and acetylsalicylic acid for 2 menstrual cycles each (n = 6) Group 4: placebo given 3 × daily for 2 menstrual cycles, then cross‐over to indomethacin, acetylsalicylic acid and tolfenamic acid for 2 menstrual cycles each (n = 6) | |
| Outcomes | These were self‐reported by questionnaire, which was completed by the participant immediately after each menstrual cycle. Pain relief: pelvic pain, lower back pain, pain in walking, dyspareunia, pain on defecation, headache; number not reported but described as more common with placebo and indomethacin | |
| Notes | Drugs for use in the trial were provided by Medica Ltd, Helsinki, Finland. "The authors wish to thank Medica Ltd, Helsinki, Finland, for the drugs." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | "placebo‐controlled double‐blind trial" |
| Incomplete outcome data (attrition bias) | Low risk | "Twenty‐four patients...volunteered for this study. Eighteen women completed the trial; the remaining six terminated treatment for a variety of personal reasons." |
| Selective reporting (reporting bias) | Unclear risk | Prespecified primary and secondary outcomes were not clearly defined. |
| Other bias | Unclear risk | Insufficient information was provided to enable a judgement of low risk of bias. |
| Methods | Trial design: 2‐period, 2‐treatment cross‐over trial | |
| Participants | 24 women randomised; 20 analysed Mean age: group 1, 32 years; group 2, 35 years Inclusion criteria: women with endometriosis classified by the American Fertility Society (mild endometriosis, n = 7; moderate endometriosis, n = 8; severe endometriosis, n = 6). Endometriosis was diagnosed by pelvic examination, history of menstrual distress and direct visualisation of pelvic regions at laparoscopy or laparotomy. Timing: unclear | |
| Interventions | Group 1: naproxen sodium (nonsteroidal anti‐inflammatory drug (NSAID)) 275 mg (102 tablets) 4 × daily for 2 menstrual cycles, then cross‐over to placebo for 2 menstrual cycles (n = 12) Group 2: placebo given for 2 menstrual cycles, then cross‐over to naproxen sodium (NSAID) for 2 menstrual cycles (n = 12) Additional interventions: Additional analgesia was allowed if no relief was noted after first 2 doses. | |
| Outcomes | All were self‐reported by questionnaire, which was completed by the participant immediately after each menstrual cycle. Pain relief: measured after each menstrual cycle (score 3 to ‐1) | |
| Notes | Drugs used in the trial were supplied by Syntex Research, Maidenhead, England. "The study drugs were kindly supplied by Syntex Research, Maidenhead, England." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "The study was conducted according to a randomized, double‐blind, four‐period crossover design." |
| Incomplete outcome data (attrition bias) | Low risk | "Twenty‐four patients...entered the present study...One patient became pregnant before the first treatment, one patient had psychiatric problems that rendered her responses unreliable, and two patients were lost to follow‐up for unknown reasons during the trial." |
| Selective reporting (reporting bias) | Unclear risk | Prespecified primary and secondary outcomes were not clearly defined. |
| Other bias | Unclear risk | Information provided was insufficient to enable a judgement of low risk of bias. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| This trial assessed use of the cyclo‐oxygenase (COX)‐2‐specific inhibitor (rofecoxib) for management of pain related to endometriosis. However, this drug was withdrawn from the marketplace in November 2004 on safety grounds; therefore, it is inappropriate to assess the efficacy of the product in this review. If the drug is re‐launched, we will review this decision at the time the review would be updated. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
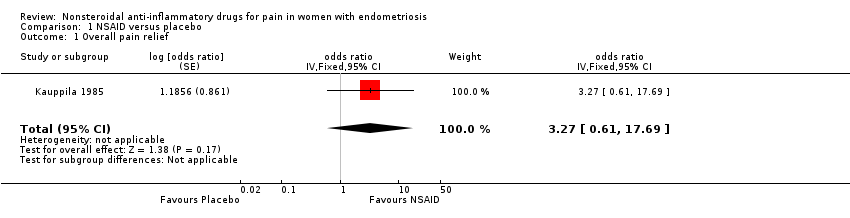
| 1 Overall pain relief Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 3.27 [0.61, 17.69] | |
| Analysis 1.1  Comparison 1 NSAID versus placebo, Outcome 1 Overall pain relief. | ||||
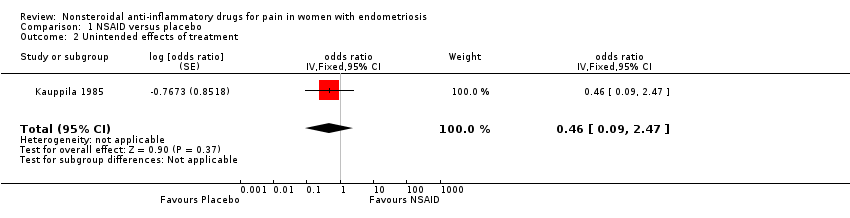
| 2 Unintended effects of treatment Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 0.46 [0.09, 2.47] | |
| Analysis 1.2 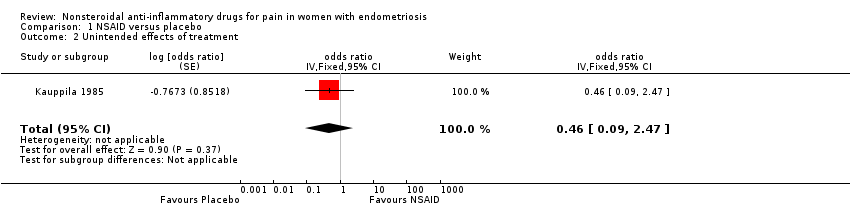 Comparison 1 NSAID versus placebo, Outcome 2 Unintended effects of treatment. | ||||
| 3 Requirements for additional medication Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 0.12 [0.01, 1.29] | |
| Analysis 1.3 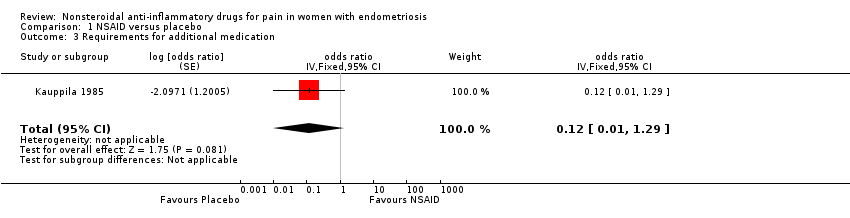 Comparison 1 NSAID versus placebo, Outcome 3 Requirements for additional medication. | ||||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Comparison 1 NSAID versus placebo, Outcome 1 Overall pain relief.
Comparison 1 NSAID versus placebo, Outcome 2 Unintended effects of treatment.

Comparison 1 NSAID versus placebo, Outcome 3 Requirements for additional medication.
| NSAID compared with placebo for pain in women with endometriosis | ||||||
| Patient or population: women with endometriosis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with NSAID | |||||
| Pain relief assessed with: overall pain relief score follow‐up: median 2 months | 50 per 100 | 77 per 100 | OR 3.27 | 24 | ⊕⊝⊝⊝ | |
| Unintended effects from treatment follow‐up: median 2 months | 58 per 100 | 39 per 100 | OR 0.46 | 24 | ⊕⊝⊝⊝ | |
| Quality of life: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Effects on daily activities: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Absence from work or school: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Number of women requiring more invasive treatment: not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Requirements for additional medication follow‐up: median 2 months | 83 per 100 | 38 per 100 | OR 0.12 | 24 | ⊕⊝⊝⊝ | |
| Participant satisfaction with treatment: not reported | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level owing to overall unclear risk of bias for included trial. bDowngraded two levels for imprecision because confidence interval is wide, consistent with benefit and harm and evidence based on a single small trial. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall pain relief Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 3.27 [0.61, 17.69] | |
| 2 Unintended effects of treatment Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 0.46 [0.09, 2.47] | |
| 3 Requirements for additional medication Show forest plot | 1 | odds ratio (Fixed, 95% CI) | 0.12 [0.01, 1.29] | |

