Profilaxis con betamiméticos orales para la prevención del parto prematuro en mujeres con embarazo gemelar
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004733.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 diciembre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For the 2015 update, Waralak Yamasmit and Surasith Chaithongwongwatthana contributed to assessment the of risk of bias in studies and updated the main text. The final version of the updated review was approved by all review authors.
Sources of support
Internal sources
-
Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Thailand.
-
Chulalongkorn University, Thailand.
-
Khon Kaen University, Thailand.
-
Oregon Health & Sciences University, USA.
-
Global Network for Perinatal and Reproductive Health (GNPRH), USA.
External sources
-
United States Agency for International Development, Child Health Research (USAID‐CHR), USA.
-
The Rockefeller Foundation, USA.
-
National Institute for Health Research (NIHR), UKNIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines (2015 update), UK.
Declarations of interest
Waralak Yamasmit: none known.
Surasith Chaithongwongwatthana: none known.
Jorge E Tolosa: I am a consultant for NIH in the US and part of a scientific review committee of research proposals. This is not directly related to this review.
Sompop Limpongsanurak: none known.
Leonardo Pereira: Although I have grant funding and sources of income form educational and case reviews, these are not directly in the area of this topic review and I have no competing interest which affects my objectivity in this review.
Pisake Lumbiganon: none known.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane programme Grant funding (13/89/05) to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 08 | Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy | Review | Waralak Yamasmit, Surasith Chaithongwongwatthana, Jorge E Tolosa, Sompop Limpongsanurak, Leonardo Pereira, Pisake Lumbiganon | |
| 2012 Sep 12 | Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy | Review | Waralak Yamasmit, Surasith Chaithongwongwatthana, Jorge E Tolosa, Sompop Limpongsanurak, Leonardo Pereira, Pisake Lumbiganon | |
| 2005 Jul 20 | Prophylactic oral betamimetics for reducing preterm birth in women with a twin pregnancy | Review | Waralak Yamasmit, Surasith Chaithongwongwatthana, Jorge E Tolosa, Sompop Limpongsanurak, Leonardo Pereira, Pisake Lumbiganon | |
| 2004 Apr 19 | Prophylactic oral betamimetics for preventing preterm birth in women with a twin pregnancy | Protocol | Waralak Yamasmit, Surasith Chaithongwongwatthana, Jorge E Tolosa, Sompop Limpongsanurak, Leonardo Pereira, Babu Cheku, Pisake Lumbiganon | |
Differences between protocol and review
We have removed the following outcomes from our list of outcomes.
-
Very preterm birth (less than 34 weeks' gestation)
-
Extremely preterm birth (less than 28 weeks' gestation)
-
Very low birthweight (less than 1500 g)
We have added the following outcome to our list of outcomes.
-
Birthweight
We have removed the following planned subgroup from our methods.
-
Dose
-
Duration of therapy
A 'Summary of findings' table has been incorporated in the 2015 update.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Pregnancy, Twin;
- Administration, Oral;
- Adrenergic beta‐Agonists [*administration & dosage];
- Albuterol [administration & dosage];
- Fenoterol [administration & dosage];
- Fetal Membranes, Premature Rupture [prevention & control];
- Gestational Age;
- Isoxsuprine [administration & dosage];
- Premature Birth [*prevention & control];
- Randomized Controlled Trials as Topic;
- Ritodrine [administration & dosage];
- Terbutaline [administration & dosage];
- Tocolytic Agents [*administration & dosage];
Medical Subject Headings Check Words
Adult; Female; Humans; Pregnancy;
PICO
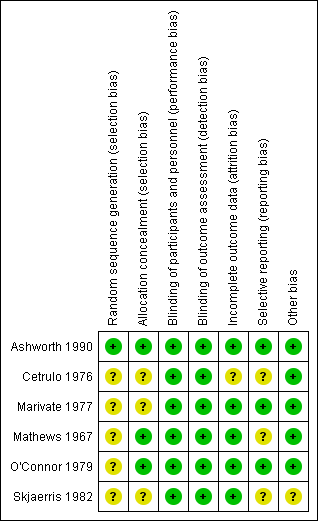
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
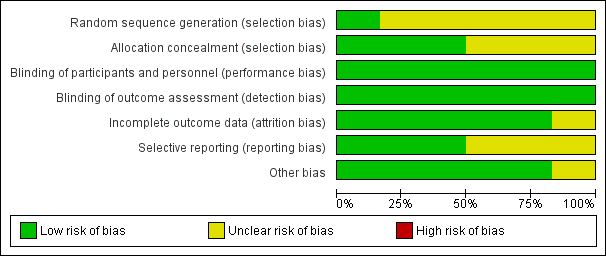
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
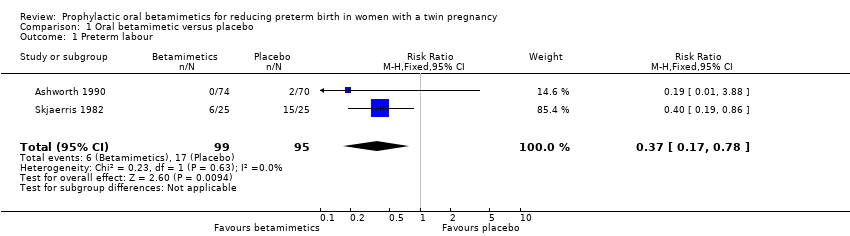
Comparison 1 Oral betamimetic versus placebo, Outcome 1 Preterm labour.
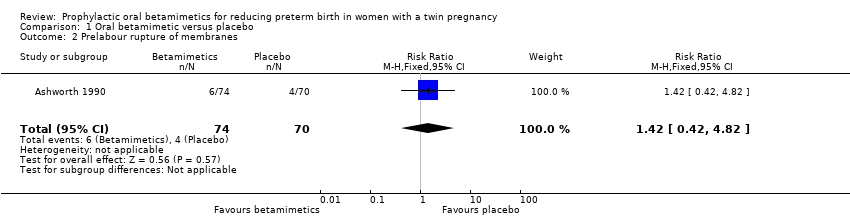
Comparison 1 Oral betamimetic versus placebo, Outcome 2 Prelabour rupture of membranes.

Comparison 1 Oral betamimetic versus placebo, Outcome 3 Preterm birth (less than 37 weeks' gestation).

Comparison 1 Oral betamimetic versus placebo, Outcome 4 Preterm birth (less than 34 weeks' gestation).

Comparison 1 Oral betamimetic versus placebo, Outcome 5 Neonatal mortality.
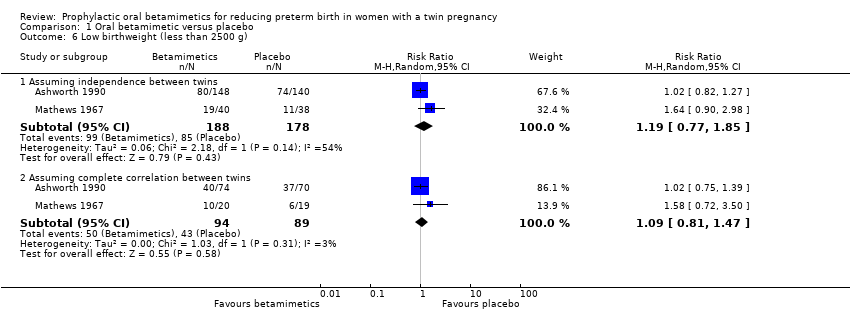
Comparison 1 Oral betamimetic versus placebo, Outcome 6 Low birthweight (less than 2500 g).

Comparison 1 Oral betamimetic versus placebo, Outcome 7 Small‐for‐gestational age (birthweight less than 10th centile).

Comparison 1 Oral betamimetic versus placebo, Outcome 8 Birthweight (not prespecified).

Comparison 1 Oral betamimetic versus placebo, Outcome 9 Respiratory distress syndrome.
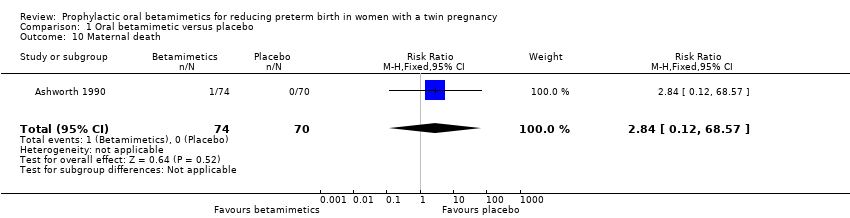
Comparison 1 Oral betamimetic versus placebo, Outcome 10 Maternal death.
| Oral betamimetic versus placebo for pregnant women with a twin pregnancy to prevent preterm birth | ||||||
| Patient or population: pregnant women with a twin pregnancy Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral betamimetic versus placebo | |||||
| Preterm labour | Study population | RR 0.37 | 194 | ⊕⊕⊝⊝ | ||
| 179 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 314 per 1000 | 116 per 1000 | |||||
| Prelabour rupture of membranes | Study population | RR 1.42 | 144 | ⊕⊕⊝⊝ | ||
| 57 per 1000 | 81 per 1000 | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.85 | 276 | ⊕⊕⊝⊝ | ||
| 478 per 1000 | 406 per 1000 | |||||
| Moderate | ||||||
| 427 per 1000 | 363 per 1000 | |||||
| Very preterm birth (less than 34 weeks' gestation) | Study population | RR 0.47 | 144 | ⊕⊕⊝⊝ | ||
| 114 per 1000 | 54 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unclear risk of selection bias (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm labour Show forest plot | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.78] |
| 2 Prelabour rupture of membranes Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.42, 4.82] |
| 3 Preterm birth (less than 37 weeks' gestation) Show forest plot | 4 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.10] |
| 4 Preterm birth (less than 34 weeks' gestation) Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.15, 1.50] |
| 5 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Assuming independence between twins | 3 | 452 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.15, 5.37] |
| 5.2 Assuming complete correlation between twins | 3 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.23, 2.38] |
| 6 Low birthweight (less than 2500 g) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Assuming independence between twins | 2 | 366 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.85] |
| 6.2 Assuming complete correlation between twins | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.47] |
| 7 Small‐for‐gestational age (birthweight less than 10th centile) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Assuming independence between twins | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.41, 1.99] |
| 7.2 Assuming complete correlation between twins | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.42, 2.13] |
| 8 Birthweight (not prespecified) Show forest plot | 3 | 478 | Mean Difference (IV, Fixed, 95% CI) | 111.22 [22.21, 200.24] |
| 9 Respiratory distress syndrome Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Assuming independence between twins | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.77] |
| 9.2 Assuming complete correlation between twins | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.11, 1.16] |
| 10 Maternal death Show forest plot | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.12, 68.57] |

