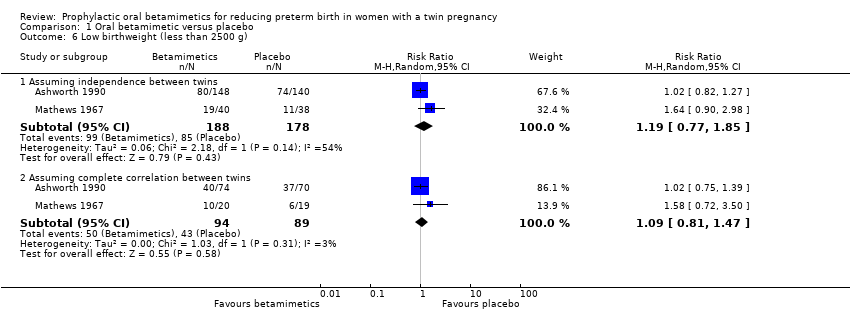
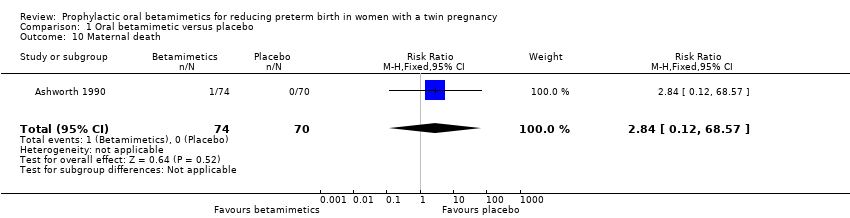
Contenido relacionado
Revisiones y protocolos relacionados
James P Neilson, Helen M West, Therese Dowswell | 5 febrero 2014
Jodie M Dodd, Caroline A Crowther, Philippa Middleton | 12 diciembre 2012
Melissa Whitworth, Siobhan Quenby | 23 enero 2008
Saifon Chawanpaiboon, Malinee Laopaiboon, Pisake Lumbiganon, Ussanee S Sangkomkamhang, Therese Dowswell | 23 marzo 2014
Jodie M Dodd, Rosalie M Grivell, Cecelia M OBrien, Therese Dowswell, Andrea R Deussen | 20 noviembre 2019
Lale Say, A Metin Gülmezoglu, G Justus Hofmeyr | 23 octubre 2001
A Dhanya Mackeen, Jolene Seibel‐Seamon, Jacqueline Muhammad, Jason K Baxter, Vincenzo Berghella | 27 febrero 2014
Diana M Bond, Philippa Middleton, Kate M Levett, David P van der Ham, Caroline A Crowther, Sarah L Buchanan, Jonathan Morris | 3 marzo 2017
Vicki Flenady, Aleena M Wojcieszek, Dimitri NM Papatsonis, Owen M Stock, Linda Murray, Luke A Jardine, Bruno Carbonne | 5 junio 2014
Helen C McNamara, Caroline A Crowther, Julie Brown | 14 diciembre 2015
Respuestas clínicas Cochrane
Ahizechukwu Chigoziem Eke | 7 marzo 2016