Tratamiento antimicótico sistémico para la tiña capitis en niños
Resumen
Antecedentes
La tiña capitis es una micosis contagiosa frecuente del cuero cabelludo de los niños. El tratamiento sistémico es necesario para prevenir la diseminación. Ésta es una actualización de la revisión Cochrane original.
Objetivos
Evaluar los efectos de los fármacos antimicóticos sistémicos para la tiña capitis en niños.
Métodos de búsqueda
Se actualizaron las búsquedas de las siguientes bases de datos hasta noviembre 2015: registro especializado del Grupo Cochrane de Piel (Cochrane Skin Group), CENTRAL (2015, número 10), MEDLINE (desde 1946), EMBASE (desde 1974), LILACS (desde 1982) y en CINAHL (desde 1981). Se hicieron búsquedas en cinco registros de ensayos y se verificaron las listas de los estudios para obtener referencias de ensayos controlados aleatorios (ECA) relevantes. Se obtuvieron ensayos no publicados, en curso y de la literatura gris mediante la correspondencia con expertos en el campo y de las compañías farmacéuticas.
Criterios de selección
ECA de tratamiento antimicótico sistémico en niños con inmunidad normal menores de 18 años de edad con tiña capitis confirmada por microscopía, crecimiento de hongos (dermatófitos) en el cultivo o ambos.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane.
Resultados principales
Se incluyeron 25 estudios (N = 4449); cuatro estudios (n = 2637) fueron nuevos en esta actualización.
La terbinafina durante cuatro semanas y la griseofulvina durante ocho semanas mostraron una eficacia similar para el resultado primario curación completa (es decir, clínica y micológica) en tres estudios que incluyeron a 328 participantes con infecciones por especies de Trichophyton (84,2% versus 79,0%; cociente de riesgos [CR] 1,06; intervalo de confianza [IC] del 95%: 0,98 a 1,15; pruebas de baja calidad).
La curación completa con el itraconazol (dos a seis semanas) y la griseofulvina (seis semanas) fue similar en dos estudios (83,6% versus 91,0%; CR 0,92; IC del 95%: 0,81 a 1,05; N = 134; pruebas de muy baja calidad). En dos estudios no hubo diferencias entre el itraconazol y la terbinafina durante dos a tres semanas de tratamiento (73,8% versus 78,8%; CR 0,93; IC del 95%: 0,72 a 1,19; N = 160; pruebas de baja calidad). En tres estudios hubo una proporción similar que logró la curación completa con dos a cuatro semanas de fluconazol o seis semanas de griseofulvina (41,4% versus 52,7%; CR 0,92; IC del 95%: 0,81 a 1,05; N = 615; pruebas de calidad moderada). Las pruebas actuales para ketoconazol versus griseofulvina fueron limitadas. Un estudio favoreció a la griseofulvina (12 semanas) porque el ketoconazol (12 semanas) pareció ser menos efectivo para la curación completa (CR 0,76; IC del 95%: 0,62 a 0,94; pruebas de baja calidad). Sin embargo, sus efectos parecieron ser similares cuando el tratamiento duró 26 semanas (CR 0,95; IC del 95%: 0,83 a 1,07; pruebas de baja calidad). Otro estudio indicó que la curación completa fue similar con el ketoconazol (12 semanas) y la griseofulvina (12 semanas) (CR 0,89; IC del 95%: 0,57 a 1,39; pruebas de baja calidad). En un ensayo no hubo diferencias significativas en la curación completa entre el fluconazol (durante dos a tres semanas) y la terbinafina (durante dos a tres semanas) (82,0% versus 94,0%; CR 0,87; IC del 95%: 0,75 a 1,01; N = 100; pruebas de baja calidad). Para la curación completa no se encontraron diferencias significativas entre el fluconazol (durante dos a tres semanas) y el itraconazol (durante dos a tres semanas) (82,0% versus 82,0%; CR 1,00; IC del 95%: 0,83 a 1,20; pruebas de baja calidad).
Esta actualización aporta nuevos datos: en los niños con infecciones por Microsporum, un metanálisis de dos estudios encontró que la curación completa fue inferior con la terbinafina (seis semanas) que con la griseofulvina (seis a 12 semanas) (34,7% versus 50,9%; CR 0,68; IC del 95%: 0,53 a 0,86; N = 334; pruebas de calidad moderada). En la revisión original no hubo diferencias significativas en la curación completa entre la terbinafina (cuatro semanas) y la griseofulvina (ocho semanas) en los niños con infecciones por Microsporum en un estudio pequeño (27,2% versus 60,0%; CR 0,45; IC del 95%: 0,15 a 1,35; N = 21; pruebas de baja calidad).
Un estudio proporciona nuevas pruebas de que la terbinafina y la griseofulvina durante seis semanas muestran una eficacia similar (49,5% versus 37,8%; CR 1,18; IC del 95%: 0,74 a 1,88; N = 1006; pruebas de baja calidad). Sin embargo, en los niños infectados por T. tonsurans, la terbinafina fue mejor que la griseofulvina (52,1% versus 35,4%; CR 1,47; IC del 95%: 1,22 a 1,77; pruebas de calidad moderada). En los niños infectados por T. violaceum,, estos dos regímenes tienen efectos similares (41,3% versus 45,1%; CR 0,91; IC del 95%: 0,68 a 1,24; pruebas de baja calidad). Además, tres semanas de fluconazol fueron similares a seis semanas de fluconazol en un estudio en 491 participantes infectados por T. tonsurans y M. canis (30,2% versus 34,1%; CR 0,88; IC del 95%: 0,68 a 1,14; pruebas de baja calidad).
La frecuencia de eventos adversos atribuidos a los fármacos en estudio fue similar para la terbinafina y la griseofulvina (9,2% versus 8,3%; CR 1,11; IC del 95%: 0,79 a 1,57; pruebas de calidad moderada) y los eventos adversos graves fueron poco frecuentes (0,6% versus 0,6%; CR 0,97; IC del 95%: 0,24 a 3,88; pruebas de calidad moderada). Los eventos adversos de la terbinafina, la griseofulvina, el itraconazol, el ketoconazol y el fluconazol fueron leves y reversibles.
Todos los estudios incluidos tuvieron riesgo alto o incierto de sesgo en al menos un dominio. Al utilizar GRADE para valorar la calidad general de las pruebas, las pruebas de calidad inferior dieron lugar a una confianza inferior en la estimación del efecto.
Conclusiones de los autores
Los tratamientos más nuevos que incluyen terbinafina, itraconazol y fluconazol son al menos similares a la griseofulvina en los niños con tiña capitis causada por especies de Trichophyton. Pruebas limitadas indican que la terbinafina, el itraconazol y el fluconazol tienen efectos similares, mientras que el ketoconazol puede ser menos efectivo que la griseofulvina en los niños infectados por Trichophyton. Con algunas intervenciones la proporción que logró la curación clínica completa fue mayor del 90% (p.ej. un estudio de terbinafina o griseofulvina para infecciones por Trichophyton), pero en muchas de las comparaciones estudiadas la proporción de pacientes que se curaron fue mucho menor.
Nuevas pruebas de esta actualización indican que la terbinafina es más efectiva que la griseofulvina en los niños con infección por T. tonsurans.
Sin embargo, en los niños con infecciones por Microsporum, nuevas pruebas indican que el efecto de la griseofulvina es mejor que el de la terbinafina. No se encontraron pruebas que apoyen una diferencia en cuanto a la adherencia entre cuatro semanas de terbinafina versus ocho semanas de griseofulvina. No todos los tratamientos para la tiña capitis están disponibles en formulaciones pediátricas, pero todos tienen perfiles de seguridad razonables.
PICO
Resumen en términos sencillos
Fármacos antimicóticos para el tratamiento de los niños con tiña
Antecedentes
La tiña capitis, o tiña, es una micosis del cuero cabelludo causada principalmente por dos especies de hongos llamados Trichophyton y Microsporum. Esta enfermedad es frecuente en los niños. La mayoría de las micosis se pueden tratar con cremas antimicóticas aplicadas directamente a la piel (tratamientos tópicos). Sin embargo, debido a que la micosis se encuentra en la raíz de los folículos del pelo, que puede no ser alcanzada por los tratamientos tópicos, la tiña capitis siempre requiere medicación administrada por vía oral para que el tratamiento se propague a todo el cuerpo (tratamientos sistémicos). Hay varios tipos diferentes de fármacos antimicóticos disponibles.
Pregunta de la revisión
¿Qué fármaco antimicótico es mejor para tratar la tiña del cuero cabelludo en los niños?
Características de los estudios
En noviembre de 2015 se buscaron los estudios que utilizaron el diseño estándar para los ensayos clínicos (ensayos controlados aleatorios) de tratamientos antimicóticos administrados por vía oral. Se encontraron 25 estudios en los que participaron 4449 niños menores de 18 años (cuatro estudios con 2637 niños fueron nuevos en esta actualización).
Resultados clave
Con respecto a la curación completa (curación de la infección y curación visible, es decir, curación micótica y clínica) pruebas de calidad baja a moderada indican que los tratamientos más nuevos como la terbinafina, el itraconazol y el fluconazol son al menos tan buenos como la griseofulvina, el tratamiento habitual en los niños con tiña capitis provocada por infecciones por Trichophyton. Sin embargo, nuevas pruebas en esta actualización indican que la terbinafina puede tener mejores efectos que la griseofulvina para curar completamente a los niños con infección por T. tonsurans. Por el contrario, en los niños con infecciones por Microsporum, nuevas pruebas parecen indicar que la griseofulvina es más efectiva que la terbinafina.
La terbinafina, el itraconazol y el fluconazol parecen tener efectos similares en cuanto a la proporción de participantes que logran la curación completa, mientras que el ketoconazol parece ser menos efectivo que la griseofulvina para los niños con tiña capitis causada por especies de Trichophyton. Sin embargo, la calidad de estas pruebas es baja. Con algunas intervenciones, la proporción de pacientes con curación clínica completa fue mayor del 90% (p.ej. un estudio de terbinafina versus griseofulvina para infecciones por Trichophyton), pero en muchas de las comparaciones estudiadas la proporción de pacientes curados fue mucho menor.
Los estudios incluidos informaron efectos secundarios negativos, que fueron de igual manera leves y reversibles para la terbinafina, la griseofulvina, el itraconazol, el ketoconazol y el fluconazol. Incluyeron efectos cutáneos específicos como prurito, así como molestias abdominales, cefalea y náuseas.
Calidad de la evidencia
La calidad de las pruebas de esta revisión fue en general baja a moderada, de manera que es probable que los estudios de investigación adicionales tengan un efecto importante sobre la confianza en estos resultados. Algunas pruebas fueron incluso de muy baja calidad. Todavía se necesitan más y mejores pruebas para poder comprender la efectividad y los eventos adversos de los fármacos antimicóticos sistémicos para la tiña capitis en los niños.
Conclusiones de los autores
Summary of findings
| Terbinafine versus griseofulvin for children with tinea capitis | ||||||
| Patient or population: children with tinea capitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Griseofulvin | Terbinafine | |||||
| Proportion of participants with complete cure | 790 per 1000 | 837 per 1000 | RR 1.06 | 328 | ⊕⊕⊝⊝ | This outcome was for children infected with Trichophyton, terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration |
| Proportion of participants with complete cure | 378 per 1000 | 446 per 1000 | RR 1.18 | 1006 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton (T. tonsurans and T. violaceum) Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 354 per 1000 | 521 per 1000 | RR 1.47 | 764 | ⊕⊕⊕⊝ | This outcome was for children infected with T. tonsurans Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 451 per 1000 | 411 per 1000 | RR 0.91 | 242 | ⊕⊕⊝⊝ | This outcome was for children infected with T. violaceum Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 509 per 1000 | 346 per 1000 | RR 0.68 | 334 | ⊕⊕⊕⊝ | This outcome was for children infected with Microsporum. Terbinafine medium‐ (6 to 8 weeks) and long‐term (10 to 12 weeks) treatment versus griseofulvin |
| Proportion of participants with complete cure | 600 per 1000 | 270 per 1000 (90 to 810) | RR 0.45 (0.15 to 1.35) | 21 (1 study) | ⊕⊝⊝⊝ | This outcome was for children infected with Microsporum. Terbinafine short‐term (4 weeks) versus griseofulvin |
| Adverse events attributed to the study drugs | 83 per 1000 | 92 per 1000 | RR 1.11 | 1549 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton and Microsporum Terbinafine (6 weeks) versus griseofulvin (6 weeks), medium treatment duration |
| Severe adverse events | 6 per 1000 | 6 per 1000 | RR 0.97 | 1549 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton and Microsporum Terbinafine (6 weeks) versus griseofulvin (6 weeks), medium treatment duration |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because 1 of the 3 studies was at high risk of bias, the other two studies were at unclear risk of bias. | ||||||
| Itraconazole versus griseofulvin for children infected with Trichophyton and Microsporum | |||||
| Patient or population: children infected with Trichophyton and Microsporum | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Griseofulvin | Itraconazole | ||||
| Proportion of participants with complete cure | 910 per 1000 | 838 per 1000 | RR 0.92 | 134 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because both studies were at unclear risk of bias. | |||||
| Itraconazole versus terbinafine in children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Terbinafine | Itraconazole | ||||
| Proportion of participants with complete cure | 788 per 1000 | 732 per 1000 | RR 0.93 | 160 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because both studies were at unclear risk to bias. | |||||
| Ketoconazole versus griseofulvin in children infected with Trichophyton | ||||||
| Patient or population: children infected with Trichophyton | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Griseofulvin | Ketoconazole | |||||
| Proportion of participants with complete cure Follow‐up: 12 weeks | 964 per 1000 | 733 per 1000 (598 to 906) | RR 0.76 (0.62 to 0.94) | 62 | ⊕⊕⊝⊝ | Ketoconazole (12 weeks) versus griseofulvin (12 weeks) |
| Proportion of participants with complete cure Follow‐up: 26 weeks | 1000 per 1000 | 920 per 1000 (810 to 1000) | RR 0.92 (0.81 to 1.03) | 62 (1 study) | ⊕⊕⊝⊝ | Ketoconazole (up to 26 weeks) versus griseofulvin (up to 26 weeks) |
| Proportion of participants with complete cure Follow‐up: 12 weeks | 543 per 1000 | 484 per 1000 (310 to 755) | RR 0.89 (0.57 to 1.39) | 79 (1 study) | ⊕⊕⊝⊝ | Ketoconazole (12 weeks) versus griseofulvin (12 weeks) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because the study was at high risk of bias. | ||||||
| Fluconazole versus griseofulvin in children with tinea capitis | |||||
| Patient or population: children with tinea capitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Griseofulvin | Fluconazole | ||||
| Proportion of participants with complete cure | 449 per 1000 | 413 per 1000 | RR 0.92 | 615 | ⊕⊕⊕⊝ |
| Proportion of participants with complete cure | 322 per 1000 | 341 per 1000 | RR 1.06 | 361 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because one of the three studies was at high risk of bias, the other two were at unclear risk of bias. | |||||
| Fluconazole versus terbinafine for children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Terbinafine | Fluconazole | ||||
| The proportion of participants with complete cure | 940 per 1000 | 818 per 1000 | RR 0.87 | 100 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Fluconazole versus itraconazole in children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Itraconazole | Fluconazole | ||||
| Proportion of participants with complete cure Follow‐up:12 weeks | 820 per 1000 | 820 per 1000 | RR 1.00 | 100 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Different durations of fluconazole in children infected with T. tonsurans and M. canis | |||||
| Patient or population: children infected with T. tonsurans and M. canis Comparison: fluconazole (6 weeks duration) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Fluconazole ( 6 weeks duration) | Fluconazole ( 3 weeks duration) | ||||
| Proportion of participants with complete cure Follow‐up: 8‐12 weeks | 341 per 1000 | 300 per 1000 | RR 0.88 | 491 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
Antecedentes
Ésta es una versión actualizada de una revisión Cochrane original (Gonzalez 2007).
Descripción de la afección
Tiña capitis es el término médico para una infección del cuero cabelludo (también conocida como tiña del cuero cabelludo) que incluye la piel y el pelo (Higgins 2000). Es causada por hongos (dermatófitos), principalmente por especies de Trichophyton o de Microsporum que invaden el eje del pelo (Higgins 2000). El sello clínico distintivo es uno o más parches de alopecia, a veces con un patrón de puntos negros (placas con pelos partidos), que se puede acompañar de inflamación, escamas, pústulas y prurito (Chan 2004; Higgins 2000).
La tiña capitis es poco frecuente en adultos y se observa principalmente en niños preadolescentes de comunidades desfavorecidas en países de todos los niveles de ingresos (Chan 2004; Ginter‐Hanselmayer 2007). Durante los últimos 30 años, la incidencia informada de tiña capitis ha aumentado significativamente, ya que los viajes y la migración se han asociado con cambios en la epidemiología y la distribución de las especies de hongos que es probable que causen la tiña capitis (Aly 1999).
Hay varias especies de dermatófitos asociados de forma distintiva con la tiña capitis. Las infecciones por Trichophyton son muy frecuentes en Centroamérica, los Estados Unidos y en partes de Europa occidental. Los casos de infecciones por Microsporum se observan principalmente en América del Sur, Europa del sur y central, África y el Medio Oriente (Havlickova 2008).
La tiña capitis es contagiosa y se puede transmitir por los seres humanos, los animales o por objetos que transportan el hongo(Yu 2005). Los estados de portador también existen, en los que el hongo está presente en el cuero cabelludo pero no hay una infección clínica (Pomeranz 1999). Aunque no es potencialmente mortal en los pacientes con inmunidad normal, si no se trata puede haber síntomas persistentes (Elewski 2000). La forma inflamatoria, el querion, puede dar lugar a alopecia cicatrizal (pérdida del pelo) o calvicie permanente (Elewski 2000).
Los médicos deben confirmar el diagnóstico clínico al identificar la presencia de los hongos dentro del eje del pelo en las muestras de cabello visualizadas bajo el microscopio, por el cultivo del hongo de dichas muestras en condiciones de laboratorio (diagnóstico micológico) o ambos (Higgins 2000). Los métodos principales de obtención de las muestras para el diagnóstico microbiológico incluyen el raspado o cepillado el cuero cabelludo y la extracción de los cabellos afectados (Fuller 2003). Analizar la muestra bajo un microscopio es la manera más rápida de diagnosticar la infección y, si el resultado es positivo, el tratamiento puede comenzar de inmediato (Fuller 2003). Sin embargo, a veces este método indica que el paciente no presenta la afección aunque en realidad la tenga. El cultivo de los raspados es más sensible y permite la identificación exacta del microorganismo involucrado; sin embargo, este método puede tomar hasta cuatro semanas para proporcionar un resultado (Fuller 2003; Gupta 1999). Se puede utilizar la luz de Wood (luz ultravioleta con filtro) para detectar infecciones que despiden luz fluorescente bajo este tipo de luz como M. canis y M. audouinii, pero no es útil para diagnosticar la tiña capitis por T. tonsurans (Elewski 2000).
Descripción de la intervención
El objetivo primario del tratamiento para la tiña capitis es lograr la curación clínica (signos y síntomas) y micológica (cultivar negativo) completa lo más rápido posible, con eventos adversos mínimos. La mayoría de las micosis superficiales pueden ser tratadas tópicamente (tratamiento aplicado directamente a la piel), pero la tiña capitis siempre requiere medicación sistémica (que se propaga a todo el cuerpo) porque la micosis se encuentra en la raíz de los folículos del pelo, que puede no ser alcanzada por los agentes tópicos (Higgins 2000). Los fármacos tópicos sólo se utilizan como tratamientos coadyuvantes junto con los tratamientos sistémicos (Higgins 2000).
La tiña capitis ocurre principalmente en niños y puede haber problemas para persuadirles para que tomen la medicina (Hay 2006). Los factores que mejoran la adherencia incluyen un sabor aceptable y un ciclo terapéutico corto. Este último factor puede ser importante para reducir el riesgo de eventos adversos.
La griseofulvina ha sido tradicionalmente el tratamiento prescrito con más frecuencia y el antimicótico habitual administrado para la tiña capitis en la práctica clínica (Bennett 2000; Friedlander 2000). La dosis pediátrica de griseofulvina es de 10 a 25 mg/kg/día durante seis a ocho semanas (Blumer 1999). Todavía es un fármaco con un costo relativamente bajo y se ha utilizado como la norma para evaluar muchos agentes más nuevos (Blumer 1999). Sin embargo, tiene un sabor amargo desagradable y se debe tomar con las comidas durante uno a dos meses, lo que puede afectar la adherencia en los niños (Bennett 2000). La presentación líquida no siempre está disponible.
Los médicos consideran cada vez más la posibilidad de tratar la tiña capitis con agentes antimicóticos más nuevos como el ketoconazol, el itraconazol, la terbinafina o el fluconazol (Friedlander 2000; Gonzalez 2007), pero hay inquietudes con respecto al uso de estos fármacos en los niños debido a la posibilidad de efectos secundarios poco frecuentes pero potencialmente graves como la toxicidad hepática o la interacción medicamentosa (Blumer 1999). Estos agentes más nuevos también son costosos, lo que es una consideración importante debido a que la tiña capitis es endémica en algunas de las comunidades más pobres del mundo.
De qué manera podría funcionar la intervención
El mecanismo principal de acción de la griseofulvina es unirse y desactivar las proteínas microtubulares que son fundamentales para la mitosis (división celular), por lo que detienen la división celular de las células micóticas. La griseofulvina también inhibe la síntesis del ácido nucleico y daña la síntesis de la pared de las células micóticas (Fuller 2014).
El mecanismo de acción de la terbinafina incluye la inhibición de la escualeno epoxidasa, que es una enzima clave en la biosíntesis de esterol en la célula micótica. Lo anterior causa una deficiencia de ergosterol dentro de la membrana de las células micóticas que daña la membrana de las células micóticas (Abdel‐Rahman 2005).
El mecanismo de acción de los antimicóticos azoles (p.ej. itraconazol y fluconazol) es la inhibición de la enzima lanosterol 14‐alfa‐demetilasa dependiente del citocromo P450, que es esencial para la conversión de lanosterol a ergosterol. Las interrupciones en la síntesis de ergosterol provocan daño en la membrana de las células de los hongos y daño a la célula micótica (Zonios 2008).
Por qué es importante realizar esta revisión
Es posible elegir entre varios fármacos antimicóticos sistémicos para tratar a los niños con tiña capitis.
Se deseaba determinar la eficacia comparativa y los perfiles de seguridad de estos fármacos. Además, debido a la distribución global de esta infección y a la respuesta de las especies micóticas a diferentes fármacos, las implicaciones de costo también pueden ser muy importantes. También existía interés en comparar diferentes duraciones del tratamiento porque los tratamientos más cortos, si son efectivos, serían preferibles al tratamiento prolongado, que puede aumentar el riesgo de eventos adversos y la probabilidad de no adherencia.
Objetivos
Evaluar los efectos de los fármacos antimicóticos sistémicos para la tiña capitis en niños.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos controlados con asignación aleatoria (ECA).
Tipos de participantes
Niños menores de 18 años de edad con inmunidad normal con tiña capitis confirmada por microscopía, crecimiento de dermatófitos en el cultivo, o ambos.
Tipos de intervenciones
Se consideraron los estudios de todos los regímenes de cualquier intervención de fármacos antimicóticos sistémicos para la tiña capitis a través de las siguientes comparaciones.
-
Cualquier tratamiento sistémico versus ningún tratamiento o placebo.
-
Comparación de dos o más tratamientos sistémicos.
-
Comparación de diferentes dosis y regímenes del mismo tratamiento sistémico.
-
Comparación de tratamientos sistémicos versus tópicos.
Se previó que los estudios no se centrarían en algunos agentes antimicóticos sistémicos como anfotericina B, flucitosina, caspofungina o miconazol, debido a una falta de espectro de actividad antimicótica o a una falta de toxicidad aceptable en esta población.
Tipos de medida de resultado
Resultados primarios
-
Proporción de participantes con curación completa, es decir, curación clínica y micológica. La curación clínica completa se definió como la resolución del prurito y los signos clínicos como el enrojecimiento, las escamas y el edema. La curación micológica completa se definió como resultados negativos en la microscopía, ningún crecimiento en el cultivo, o ambos.
-
La frecuencia y el tipo de eventos adversos.
Resultados secundarios
-
Proporción de participantes con curación clínica solamente.
-
Medición de la recurrencia de la afección después del final del período de intervención.
-
Porcentaje de abandonos como resultado substituto para la adherencia de los participantes.
-
Tiempo transcurrido hasta la curación.
Results
Description of studies
Results of the search
The electronic database searches for this update yielded 85 studies after duplicates were removed. We discarded 79 studies after screening titles and abstract and examined the full text of the remaining 6 records. We excluded two further studies (Koumantaki‐Mathioudaki 2005; Shemer 2013; see 'Characteristics of excluded studies'), and we identified four new studies for inclusion in this update (Deng 2011; Elewski 2008; Foster 2005; Khan 2011; see 'Characteristics of included studies'). We did not identify any other studies in our searches of ongoing trial registers or from other resources.
The original review identified 21 trials of systemic treatments for tinea capitis (Gonzalez 2007).
Please see Figure 1 for the study flow diagram.
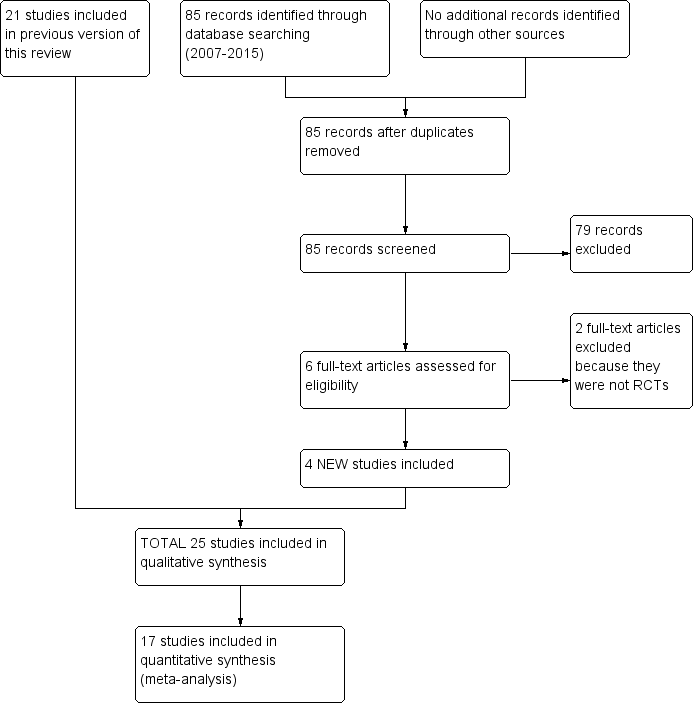
Study flow diagram.
Included studies
In total, we included 25 studies that randomised 4449 participants (Characteristics of included studies). We found no other trials that compared an active treatment to placebo. The trials compared different active treatments: either different drugs or different regimens of the same drug.
Design
All included studies were parallel group RCTs. Ten of them had a multi‐arm design (Deng 2011; Foster 2005; Friedlander 2002; Gupta 2001; Haroon 1996; Kullavanijaya 1997; Lipozencic 2002; Memisoglu 1999; Solomon 1997; Talarico Filho 1998).
Sample size
Only six studies reported a sample size calculation (Elewski 2008; Foster 2005; Fuller 2001, Khan 2011; Lipozencic 2002; Ungpakorn 2004).
Regarding the sample size, we organised the studies into three distinct groups.
-
Nine studies were small (N < 50) (Cáceres‐Ríos 2000; Dastghaib 2005; Hamm 1999; López‐Gómez 1994; Martínez‐Roig 1988; Rademaker 1998; Solomon 1997; Tanz 1985; Ungpakorn 2004).
-
Eight studies were medium (N = 51 to 150) (Deng 2011; Gan 1987; Jahangir 1998; Khan 2011; Kullavanijaya 1997; Memisoglu 1999; Talarico Filho 1998; Tanz 1988).
-
Eight studies were large (N > 150) (Elewski 2008; Foster 2005; Friedlander 2002; Fuller 2001; Gupta 2001; Haroon 1995; Haroon 1996; Lipozencic 2002).
Settings
The studies included in this review took place in many parts of the world.
Eight took place in Asia: four in Pakistan (Haroon 1995; Haroon 1996; Jahangir 1998; Khan 2011); two in Thailand (Kullavanijaya 1997; Ungpakorn 2004); one in China (Deng 2011); and one in Iran (Dastghaib 2005).
Five studies were carried out in Europe: one in Germany (Hamm 1999); one in Turkey (Memisoglu 1999); two in Spain (López‐Gómez 1994; Martínez‐Roig 1988); and one in the UK (Fuller 2001).
Two studies were from South America (Cáceres‐Ríos 2000; Talarico Filho 1998), five were completed in North America (Friedlander 2002; Gan 1987; Solomon 1997; Tanz 1985; Tanz 1988), and one study took place in New Zealand (Rademaker 1998).
Four of the studies were conducted in several locations: Gupta 2001 took place in the United States and South Africa; Lipozencic 2002, in Europe and South America; Elewski 2008 in the United States, Peru, the United Kingdom, Egypt, Russia and South Africa; and Foster 2005, in the United States, Guatemala, Chile, Costa Rica and India.
Participants
With regard to age, most of the participants of included studies were older than two years. Cáceres‐Ríos 2000 and Talarico Filho 1998 included participants as young as one year. Another study involved participants as young as six months of age (Gupta 2001). The upper age limit in the majority of the trials was 16 years, although Haroon 1995 included participants ranging in age from 2 to 65 years. In that trial, 94 of the 105 participants were under 12 years of age, so we assumed all the participants were under 16 years of age and therefore analysed the whole population. Three other studies reported a total of four adults in their samples (Kullavanijaya 1997; Lipozencic 2002; López‐Gómez 1994).
Fungal type
Each of the 25 studies reported the types of fungi cultured; some provided general percentages and reported exact proportions of the types of fungi within each arm. The Trichophyton species predominated over Microsporum species. T. tonsurans and M. canis were responsible for causing infection in the highest proportion of participants. T. tonsurans was the most commonly identified fungus in 11 studies (Cáceres‐Ríos 2000; Elewski 2008; Foster 2005; Friedlander 2002; Fuller 2001; Gan 1987; Ginsburg 1987; Khan 2011; Solomon 1997; Tanz 1985; Tanz 1988); T. violaceum was the predominant fungus in four studies (Deng 2011; Haroon 1995; Haroon 1996; Jahangir 1998); in one study, the proportions of M. canis and T. tonsurans were the same (Hamm 1999); and M. canis was the main fungus in five trials (Lipozencic 2002; López‐Gómez 1994; Memisoglu 1999; Rademaker 1998; Talarico Filho 1998). Three trials identified the causative fungi, but their relative frequencies were not provided so it was impossible to determine the frequencies: T. tonsurans and M. ferrugineum were identified in one study (Kullavanijaya 1997), T. tonsurans and T. violaceum in another (Gupta 2001), and T. mentagrophytes and M. canis in a third study (Martínez‐Roig 1988). In addition, one study failed to classify the causative species of fungi (Memisoglu 1999).
Interventions
The standard dose for griseofulvin used in trials is generally 10 to 20 mg/kg/d or 125 mg/d in participants weighing 10 to 20 kg; 250 mg/d in those weighing from 20 to 40 kg and 500 mg/d for those over 40 kg. Unless otherwise stated, the standard dosing for terbinafine studies was 62.5 mg/d in participants weighing from 10 to 20 kg; 125 mg/d from 20 to 40 kg, and 250 mg/d over 40 kg.
In total, we studied five different antifungal agents: griseofulvin, terbinafine, itraconazole, fluconazole and ketoconazole.
Comparisons
We evaluated a variety of regimens, including between‐drug and within‐drug comparisons. We considered griseofulvin to be the standard because it is the oldest agent, and 17 studies used it as a control. Of the 25 included studies, 17 compared griseofulvin as standard therapy with terbinafine (Cáceres‐Ríos 2000; Deng 2011; Elewski 2008; Fuller 2001; Gupta 2001; Haroon 1995; Khan 2011; Lipozencic 2002; Memisoglu 1999; Rademaker 1998), itraconazole (Gupta 2001; López‐Gómez 1994; Memisoglu 1999), ketoconazole (Gan 1987; Martínez‐Roig 1988; Tanz 1985; Tanz 1988), or fluconazole (Dastghaib 2005; Foster 2005; Gupta 2001; Memisoglu 1999). Gupta 2001 and Memisoglu 1999 compared griseofulvin, itraconazole and fluconazole.
Ten studies compared terbinafine versus griseofulvin (Cáceres‐Ríos 2000; Deng 2011; Elewski 2008; Fuller 2001; Gupta 2001;Haroon 1995; Khan 2011; Lipozencic 2002; Memisoglu 1999; Rademaker 1998), two versus itraconazole (Gupta 2001; Memisoglu 1999) and two versus fluconazole (Gupta 2001; Memisoglu 1999). Seven studies compared different treatment duration regimens for terbinafine (Deng 2011; Friedlander 2002; Hamm 1999; Haroon 1996; Kullavanijaya 1997; Lipozencic 2002; Talarico Filho 1998), and one compared different doses (Ungpakorn 2004).
Three studies (Gupta 2001; López‐Gómez 1994; Memisoglu 1999) compared itraconazole with other antifungals: three with griseofulvin (Gupta 2001; López‐Gómez 1994; Memisoglu 1999), two with terbinafine (Gupta 2001; Memisoglu 1999) and two with fluconazole (Gupta 2001; Memisoglu 1999). Ketoconazole was compared with griseofulvin in four trials (Gan 1987; Martínez‐Roig 1988; Tanz 1985; Tanz 1988).
Five trials studied fluconazole (Dastghaib 2005; Foster 2005; Gupta 2001; Memisoglu 1999; Solomon 1997); four with griseofulvin (Dastghaib 2005; Foster 2005; Gupta 2001; Memisoglu 1999), two with terbinafine (Gupta 2001; Memisoglu 1999), two with itraconazole (Gupta 2001; Memisoglu 1999), one by itself with varying doses (Solomon 1997), and one by itself with different durations of treatment (Foster 2005).
Outcomes
Primary outcomes
All but three studies reported the proportion of participants with complete cure, which was our pre‐specified primary outcome (Martínez‐Roig 1988; Rademaker 1998; Tanz 1985). Most of the studies reported complete cure at 12 to 16 weeks but three reported at 8 weeks (Dastghaib 2005; Deng 2011; Gan 1987), one at 10 weeks (Elewski 2008), one at 2 weeks, 4 weeks, 8 weeks and one year (Deng 2011), one at 3, 6 and 10 weeks (Foster 2005), one at 2, 4 and 6 weeks (Khan 2011), and two at 20 to 24 weeks (Fuller 2001; Ungpakorn 2004).
Three studies failed to report our other primary outcome: adverse events (Gan 1987; Kullavanijaya 1997; Solomon 1997).
Secondary outcomes
Fourteen studies reported the proportion of participants with clinical cure only, which was our first pre‐specified secondary outcome (Cáceres‐Ríos 2000; Elewski 2008; Friedlander 2002; Gupta 2001; Hamm 1999; Haroon 1996; Lipozencic 2002; López‐Gómez 1994; Martínez‐Roig 1988; Memisoglu 1999; Rademaker 1998; Solomon 1997; Talarico Filho 1998; Tanz 1988).
Only three studies reported recurrence of the condition after the end of the intervention period, which was our second pre‐specified secondary outcome (Martínez‐Roig 1988; Rademaker 1998; Solomon 1997).
Twelve studies reported the percentage of drop‐outs as a surrogate for participant adherence, our third pre‐specified secondary outcome (Deng 2011; Friedlander 2002; Fuller 2001; Gan 1987; Gupta 2001; Hamm 1999; Lipozencic 2002; López‐Gómez 1994; Memisoglu 1999; Talarico Filho 1998; Tanz 1985; Tanz 1988).
Four studies reported the time taken to cure, our fourth pre‐specified secondary outcome (Friedlander 2002; Gan 1987; Lipozencic 2002; Martínez‐Roig 1988).
Follow‐up
The follow‐up period ranged from six weeks in Martínez‐Roig 1988, Khan 2011 and Tanz 1985 to one year in Deng 2011. Although most studies had a 12‐week follow‐up period, five trials had longer follow‐up periods ranging from 16 to 24 weeks (Fuller 2001; Kullavanijaya 1997; Lipozencic 2002; Solomon 1997; Ungpakorn 2004). In addition, two trials had a 10‐week follow‐up period (Elewski 2008; Foster 2005).
Other
Some of the studies did not provide detailed information on the clinical setting or baseline characteristics of sex, age and infection severity, or they did not report the comparability between arms or the duration of symptoms or signs. Rademaker 1998 did not compare the baseline characteristics at all, and two trials did not report the information on comparability (Martínez‐Roig 1988; Solomon 1997). Finally, only five trials reported information about the severity of the infection (Cáceres‐Ríos 2000; Deng 2011; Elewski 2008; Gupta 2001; Tanz 1985). For the 25 studies, the most common reason for excluding a participant from the trial was treatment with any antifungal agent within one month prior to entering the trial.
Excluded studies
We provide details of the excluded studies in the 'Characteristics of excluded studies' table.
In this update, we excluded Koumantaki‐Mathioudaki 2005 and Shemer 2013 because we found that neither of them were RCTs after reading the full texts.
The original review excluded 3 of the initial 24 trials of systemic treatments for tinea capitis because they evaluated the therapy for the inflammatory component (kerion) caused by tinea capitis infection (Ginsburg 1987; Honig 1994; Hussain 1999).
Studies awaiting classification
One trial reported in a conference paper appeared to meet the inclusion criteria, but as we could not obtain further information, we could neither include or exclude it (Pather 2006). See Characteristics of studies awaiting classification.
Risk of bias in included studies
Please see Figure 2 for our judgements about each 'Risk of bias' item presented as percentages across all included studies and Figure 3 for the judgements about each domain for all the included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation
All included studies stated or implied that treatment allocation was randomised; however, there were only four studies that reported an adequate generation method of randomisation: two used a computer‐generated random number table (Fuller 2001; Martínez‐Roig 1988), and two used a table of random numbers (Elewski 2008; Gan 1987).
Allocation concealment
Only two studies reported adequate allocation concealment (Elewski 2008; Foster 2005).
Blinding
Performance bias
Five included studies reported that participants or personnel were not blinded; therefore, we judged these studies to be at high risk of performance bias (Dastghaib 2005; Fuller 2001; Gan 1987; Rademaker 1998; Talarico Filho 1998).
We judged the other 20 studies as being at unclear risk of performance bias, either because they did not report blinding of participants or personnel (Hamm 1999; Haroon 1995; Jahangir 1998; Khan 2011), or because they did not describe the method of blinding (Deng 2011; Elewski 2008; Foster 2005; Friedlander 2002; Gupta 2001; Haroon 1995; Haroon 1996; Kullavanijaya 1997; Lipozencic 2002; López‐Gómez 1994; Martínez‐Roig 1988; Memisoglu 1999; Solomon 1997; Tanz 1985; Tanz 1988; Ungpakorn 2004).
Detection bias
Five included studies reported that outcome assessors were not blinded; therefore, we judged these studies to be at high risk of detection bias (Deng 2011; Fuller 2001; Gan 1987; Rademaker 1998; Talarico Filho 1998).
We deemed the other 20 studies to be at unclear risk of detection bias, either because they did not report blinding of outcome assessors (Cáceres‐Ríos 2000; Hamm 1999; Jahangir 1998; Khan 2011), or because they did not describe the method of blinding (Dastghaib 2005; Elewski 2008; Foster 2005; Friedlander 2002; Gupta 2001; Haroon 1995; Haroon 1996; Kullavanijaya 1997; Lipozencic 2002; López‐Gómez 1994; Martínez‐Roig 1988; Memisoglu 1999; Solomon 1997; Tanz 1985; Tanz 1988; Ungpakorn 2004).
Incomplete outcome data
Overall, after randomising 4449 people, 471 participants (10.6%) were lost. Only six studies performed intention‐to‐treat (ITT) analyses (Elewski 2008; Foster 2005; Friedlander 2002; Fuller 2001; Lipozencic 2002; Talarico Filho 1998).
We considered 14 studies to be at low risk of attrition bias because either less than 10% of participants dropped out, or because the drop‐outs were between 10% and 20% but balanced in numbers across intervention groups (Cáceres‐Ríos 2000; Elewski 2008; Foster 2005; Gupta 2001; Hamm 1999; Haroon 1995; Haroon 1996; Jahangir 1998; Khan 2011; Kullavanijaya 1997; López‐Gómez 1994; Martínez‐Roig 1988; Talarico Filho 1998; Ungpakorn 2004).
We judged 10 studies to be at high risk of attrition bias because either more than 20% of participants dropped out, whether ITT analysis was performed or not (Friedlander 2002; Fuller 2001; Gan 1987; Lipozencic 2002; Solomon 1997; Tanz 1985; Tanz 1988), or the drop‐outs were between 10% and 20% but ITT analysis was not performed (Dastghaib 2005; Deng 2011; Memisoglu 1999).
Rademaker 1998 was at unclear risk of attrition bias, as it did not provide sufficient information on drop‐outs to make a judgement.
Selective reporting
All included studies reported findings on all outcomes listed in the 'Methods' section; therefore, we judged all included studies as being at low risk of reporting bias. However, we did not have access to the original study protocols in any of the included studies.
Other potential sources of bias
Ninteen of the 25 included studies did not report the method of sample size calculation (Cáceres‐Ríos 2000; Dastghaib 2005; Deng 2011; Friedlander 2002; Gan 1987; Gupta 2001; Hamm 1999; Haroon 1995; Haroon 1996; Jahangir 1998; Kullavanijaya 1997; López‐Gómez 1994; Martínez‐Roig 1988; Memisoglu 1999; Rademaker 1998; Solomon 1997; Talarico Filho 1998; Tanz 1985; Tanz 1988).
Nine studies did not report the funding sources (Dastghaib 2005; Friedlander 2002; Gan 1987; Gupta 2001; Jahangir 1998; Khan 2011; Memisoglu 1999; Rademaker 1998; Solomon 1997).
Three studies did not report their inclusion or exclusion criteria (Hamm 1999; Kullavanijaya 1997; Rademaker 1998); two other studies did not report baseline comparability (Khan 2011; Tanz 1988). However, whether these factors introduced bias to the results remained unclear. We therefore judged these studies as being at unclear risk of other bias.
In addition, all but two of the included studies reported the proportion of different types of fungi (Kullavanijaya 1997; Martínez‐Roig 1988). Most included studies recruited children infected with both Trichophyton and Microsporum.Lipozencic 2002 and Ungpakorn 2004 only recruited children infected with Microsporum, while Friedlander 2002, Gupta 2001, Jahangir 1998 and Solomon 1997 only recruited children infected with Trichophyton.Tanz 1985 recruited children infected with Trichophyton,Scopulariopsis,Penicillium and unidentified fungus. We list the details of type of fungi in each study in the 'Characteristics of included studies' tables.
Effects of interventions
See: Summary of findings for the main comparison Complete cure and adverse events for terbinafine versus griseofulvin in children with tinea capitis; Summary of findings 2 Complete cure for itraconazole versus griseofulvin in children infected with Trichophyton and Microsporum ; Summary of findings 3 Complete cure for itraconazole versus terbinafine in children infected with Trichophyton ; Summary of findings 4 Complete cure for ketoconazole versus griseofulvin in children infected with Trichophyton ; Summary of findings 5 Complete cure for fluconazole versus griseofulvin in children with tinea capitis; Summary of findings 6 Complete cure for fluconazole versus terbinafine in children infected with Trichophyton ; Summary of findings 7 Complete cure for fluconazole versus itraconazole in children infected with Trichophyton ; Summary of findings 8 Complete cure for different durations of fluconazole in children infected with T. tonsurans and M. canis
Numbers given show the total numbers of participants included in the analysis. When it was possible to calculate an effect size, we reported this with the 95% confidence interval (CI). We used the P value of 0.05 as the cutoff value to determine statistical significance; when P values were below this threshold, we stated whether the result favoured the intervention group or the control condition. In the text below, we report an I² statistical value for heterogeneity as moderate or high if it exceeds 50%.
We have presented the results for our pre‐specified outcomes below under the following 13 comparisons.
-
Terbinafine versus griseofulvin (short treatment duration).
-
Terbinafine versus griseofulvin in Trichophyton infections (medium treatment duration).
-
Terbinafine (medium‐ and long‐term treatment) versus griseofulvin in Microsporum infections.
-
Terbinafine short‐ versus long‐term.
-
Terbinafine standard dose compared to terbinafine double dose.
-
Itraconazole versus griseofulvin.
-
Itraconazole versus terbinafine.
-
Ketoconazole versus griseofulvin.
-
Fluconazole versus griseofulvin.
-
Fluconazole versus terbinafine.
-
Fluconazole versus itraconazole.
-
Fluconazole dosages (1.5, 3.0 and 6.0 mg/kg/d).
-
Treatment durations of fluconazole (short‐term versus medium‐term).
We have summarised the results of included studies that we could not combine in meta‐analyses because of differences between studies in terms of design. We present the results of studies that could not be pooled in meta‐analyses using data and information derived from the reports of individual studies.
We produced eight 'Summary of findings' tables for the first primary outcome of complete clinical cure (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6; summary of findings Table 7; summary of findings Table 8). In summary of findings Table for the main comparison we also reported our primary outcome of adverse events.
1. Terbinafine (2 to 4 weeks) versus griseofulvin (6 to 8 weeks); short treatment duration; 6 to 24 weeks follow‐up
Eight studies assessed the efficacy of terbinafine used in the short‐term for 2 to 4 weeks as compared to griseofulvin (used for 8 weeks) (Cáceres‐Ríos 2000; Deng 2011; Fuller 2001; Gupta 2001; Haroon 1995; Khan 2011; Memisoglu 1999; Rademaker 1998).
Primary outcomes
Complete cure, i.e. clinical and mycological cure, at 12 to 24 weeks follow‐up
Five studies reported on complete cure (Cáceres‐Ríos 2000; Fuller 2001; Gupta 2001; Haroon 1995; Memisoglu 1999). This update did not identify any new studies addressing this outcome.
A pooled analysis of the five studies found that the difference in the proportion of participants with complete cure between four weeks of terbinafine and eight weeks of griseofulvin was not statistically significant (73.6% versus 68.4%; RR 1.08, 95% CI 0.94 to 1.24; Analysis 1.1).
Trichophyton infections
Three studies included participants with Trichophyton infections (Fuller 2001; Gupta 2001; Haroon 1995). Haroon 1995 compared terbinafine for 4 weeks with 10 mg/kg/d of griseofulvin for 8 weeks in 105 participants, of whom 87.6% had T. violaceum tinea capitis. The proportion of participants with complete cure at week 12 was 93% (52/56) in the terbinafine group and 80% (39/49) in the griseofulvin group (RR 1.17, 95% CI 0.99 to 1.37; Analysis 1.1).
Fuller 2001 recruited 210 participants and included 147 in the ITT analyses. Trichophyton infection accounted for 84.4% of the terbinafine group (N = 65) and 82.9% of the griseofulvin group (N = 58). At 24 weeks, the proportion of participants with complete cure was 69.2% (45/65) in the terbinafine group and 67.2% (39/58) in the griseofulvin group (RR 1.03, 95% 0.81 to 1.31; Analysis 1.1)
Gupta 2001 compared 50 participants in each treatment group with infections caused by T. tonsurans and T. violaceum. In this trial, administration of terbinafine for 2 to 3 weeks was compared with microsize griseofulvin 20 mg/kg for 6 weeks. The proportion of participants with complete cure at week 12 was 94% (47/50) for the terbinafine group and 92% (46/50) for the griseofulvin treated group (RR 1.02, 95% CI 0.92 to 1.14; Analysis 1.1).
In the pooled analysis of 328 participants with a confirmed Trichophyton infection, terbinafine for four weeks and griseofulvin for 8 weeks showed similar efficacy in three studies (84.2% versus 79.0%; RR 1.06 95% CI 0.98 to 1.15; low quality evidence; Analysis 1.1; Fuller 2001; Gupta 2001; Haroon 1995; summary of findings Table for the main comparison).
Microsporum infections
In Fuller 2001, the proportion of the 21 children with Microsporum infections who achieved complete cure in the terbinafine (for four weeks) group and the griseofulvin (for eight weeks) group were 27.2% (3/11) and 60.0% (6/10), respectively (RR 0.45, 95% CI 0.15 to 1.35; N = 21; low quality evidence; Analysis 1.1; summary of findings Table for the main comparison).
Mixed Trichophyton and Microsporum infections
Cáceres‐Ríos 2000 and Memisoglu 1999 included participants with Trichophyton and Microsporum infections but did not report results separately. In Memisoglu 1999, complete cure at the final follow‐up visit (week 12) was 39% (15/39) for the group treated with four weeks of terbinafine compared with 44% (17/39) in the group treated with eight weeks of ultra microsized griseofulvin (RR 0.88, 95% CI 0.52 to 1.50; Analysis 1.1). There was a similar proportion of participants with complete cure within the subgroups infected with M. canis (48% of participants) and Trichophyton species according to the principal investigators of that study. The other study, Cáceres‐Ríos 2000, evaluated terbinafine for four weeks versus microsized griseofulvin for 8 weeks in 50 participants from Peru and found a significant increase in complete cure with terbinafine (76%; 19/25) compared to griseofulvin (44%; 11/25) measured at 12 weeks (RR 1.73, 95% CI 1.05 to 2.83; Analysis 1.1). The causative organisms were T. tonsurans and M. canis, at 74% and 26%, respectively.
A pooled analysis of the two studies showed no significant difference between the groups (53.1% versus 43.8%; RR 1.24, 95% CI 0.64 to 2.42; Analysis 1.1).
Adverse events
Seven studies reported this outcome (Cáceres‐Ríos 2000; Deng 2011; Fuller 2001; Gupta 2001; Haroon 1995; Khan 2011; Memisoglu 1999), of which two were new studies added in this update (Deng 2011; Khan 2011).
Khan 2011 reported that the incidence of adverse events was comparable between the two groups, with none of participants showing serious side effects, except for nausea and mild abdominal discomfort. Deng 2011 reported one case of vomiting in the griseofulvin group and no side effects in the terbinafine group.
Drug‐related adverse events
In the open study (Fuller 2001), 36 participants in the terbinafine group reported 57 adverse events (pruritus, urticaria, skin scaling), and 4 participants withdrew from the study due to adverse events (vomiting, dizziness, urticaria and weight loss). A total of 52 adverse events, predominantly abdominal discomfort and vomiting, were detected in 27 participants in the griseofulvin group, and 1 participant withdrew from the study due to abdominal pain, headache and vomiting. There was no significant difference regarding adverse events that might be attributed to either of the study drugs in the terbinafine (26/77) or griseofulvin (17/70) group (33.8% versus 24.3%, RR 1.39, 95% CI 0.83 to 2.34; Analysis 1.2).
Some studies reported good tolerability for terbinafine because there were no or few adverse events, and these had either an uncertain or no relationship to the treatment (Cáceres‐Ríos 2000; Haroon 1995). Haroon 1995 reported tonsillitis, cutaneous infestations, raised hepatic enzymes, raised triglycerides and eosinophilia, and Memisoglu 1999 reported mild elevated triglycerides, with an uncertain relationship to the drug. The following adverse events were reported less commonly and may not have been caused by griseofulvin: skin infections, skin infestations, elevated hepatic enzymes, elevated serum triglycerides, elevated serum uric acid, anaemia, eosinophilia, leucocytosis and granulocytopenia (Haroon 1995; Memisoglu 1999). Gupta 2001 reported three gastric problems and three cases of nausea in the griseofulvin group. Griseofulvin was associated with a small number of adverse events in other trials.
Secondary outcomes
None of the included studies reported measurement of recurrence of the condition after the end of the intervention period or the time taken to cure.
Proportion of participants with clinical cure only
Three studies reported the proportion of patients achieving only a clinical cure (Deng 2011; Gupta 2001; Khan 2011); two were new studies added in this update (Deng 2011; Khan 2011). We did not pool the data from these studies because of significant clinical heterogeneity, especially due to the various fungal types in different studies.
Deng 2011 compared the effects of terbinafine for two weeks, terbinafine for four weeks, and griseofulvin for treating participants infected with T. violaceum (55.1%), A. vanbreuseghemi (30.6%) and T. tonsurans (14.3%). Investigators found that the clinical cure in week 8 was 85.2% (23/27) in the 2‐week terbinafine group and 84.2% (16/19) in the griseofulvin group (RR 1.01, 95% CI 0.79 to 1.30; Analysis 1.3). The corresponding rate was 78.3% (18/23) in the 4‐week terbinafine group and 84.2% (16/19) in the griseofulvin group (RR 0.93, 95% CI 0.70 to 1.24; Analysis 1.3). When the follow‐up was extended to one year, all participants in the three groups achieved clinical cure.
Gupta 2001 reported that the proportion of participants with clinical cure was determined at the end of treatment (week four for terbinafine and week six for griseofulvin) showing better results in the griseofulvin group (70%, 35/50) than in the terbinafine group (40%, 20/50) (RR 0.57, 95% CI 0.39 to 0.84; Analysis 1.3).
Khan 2011 compared the effects of terbinafine for four weeks and griseofulvin for treating patients infected with T. tonsurans (75%) and M. canis (22%). The proportion of participants with clinical cure only at week six seemed to be higher in the terbinafine group than in the griseofulvin group, but the difference was not statistically significant (70% versus 55%; RR 1.27, 95% CI 0.96 to 1.69; Analysis 1.3).
Percentage of drop‐outs as a surrogate for participant adherence
Four studies reported on the percentage of drop‐outs as a surrogate for participant adherence (Deng 2011; Fuller 2001; Gupta 2001; Memisoglu 1999), including one new study added to this update (Deng 2011). Deng 2011 reported no drop‐outs in the terbinafine group and one in the griseofulvin group (5.3%, 1/19) (RR 0.13, 95% CI 0.01 to 3.08; Analysis 1.4).
The percentage of drop‐outs was 35.9% (37/103) versus 24.2% (26/107) (RR 1.48, 95% CI 0.97 to 2.26 Fuller 2001); 4.0% (2/50) versus 8.0% (4/50) (RR 0.50, 95% CI 0.10 to 2.61 Gupta 2001); 10.2% (4/39) versus 17.9% (7/39) (RR 0.57, 95% CI 0.18 to 1.80; Memisoglu 1999) in the terbinafine and griseofulvin groups, respectively (see Analysis 1.4). Only one study reported no drop‐outs from either treatment arm (Haroon 1995).
2. Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up
Primary outcomes
Complete cure, i.e. clinical and mycological cure
Two studies reported on complete cure (Elewski 2008; Lipozencic 2002), including one new study added to this update (Elewski 2008). This study included 1549 participants and compared terbinafine (5 to 8 mg/kg for 6 weeks) with griseofulvin (10 to 20 mg/kg for 6 weeks) in children with tinea capitis. In this study, 49.3% of the participants were infected with T. tonsurans, 15.6% were infected with T. violaceum, and 15.1% were infected with M. canis.
Trichopyton tonsurans infections
In those infected with T. tonsurans, 52.1% (264/507) of participants in the terbinafine group versus 35.4% (91/257) in the griseofulvin group achieved a complete cure (RR 1.47, 95% CI 1.22 to 1.77; N = 764; moderate quality evidence; Analysis 2.1; summary of findings Table for the main comparison).
Trichopyton violaceum infections
In those infected with T. violaceum, 41.3% (66/160) of participants in the terbinafine group versus 45.1% (37/82) in the griseofulvin group achieved a complete cure (RR 0.91, 95% CI 0.68 to 1.24; N = 242; low quality evidence; Analysis 2.1; summary of findings Table for the main comparison).
The pooled data of participants infected with Trichophyton indicated that there was no significant difference between the terbinafine group and the griseofulvin group (49.5% versus 37.8%; RR 1.18, 95% CI 0.74 to 1.88; N = 1006; low quality evidence; Analysis 2.1; summary of findings Table for the main comparison).
Adverse events
Drug‐related adverse events
Both Elewski 2008 and Lipozencic 2002 reported this outcome. New evidence from Elewski 2008 indicated that 51.9% (541/1042) of participants in the terbinafine group and 49.1% (249/507) in the griseofulvin group reported an adverse effect during the study (RR 1.06, 95% CI 0.95 to 1.18). A total of 9.2% (96/1042) of participants in the terbinafine group and 8.3% (42/507) in the griseofulvin group had adverse events attributed to the study drugs (RR 1.11, 95% CI 0.79 to 1.57; N = 1549; moderate quality evidence; Analysis 2.2; summary of findings Table for the main comparison). The most frequent adverse events, accounting for more than 5% in any group, were nasopharyngitis, headache, pyrexia, cough, and vomiting. These individual adverse events were also similar between the two groups.
In addition, Lipozencic 2002 reported "adverse events from 18.4% to 42.4% for the terbinafine treatment groups and 16.7% for the griseofulvin group". The most common adverse events, accounting for approximately 5% in any group, were fever, pharyngitis, infections (parasitic, viral and upper respiratory tract) and influenza‐like symptoms. Terbinafine was well tolerated in all treatment groups, although two participants prematurely discontinued treatment. One suffering from urticaria was in the terbinafine 6‐week group; and the other, suffering from asymptomatic and reversible neutropaenia, was in the terbinafine 10‐week group. Both events resolved without sequelae. Somnolence and gastrointestinal disorders were also reported.
Severe adverse events
Severe adverse events were rare (0.6% in both groups; RR 0.97, 95% CI 0.24 to 3.88; N = 1549; moderate quality evidence; Analysis 2.3; summary of findings Table for the main comparison).
Secondary outcomes
None of the studies comparing terbinafine (six weeks) to griseofulvin (six weeks) for Trichophyton infection reported recurrence of the condition after the end of the intervention period, percentage of drop‐outs as a surrogate for participant adherence or the time taken to cure.
Proportion of participants with clinical cure only
One new study reported the proportion of patients with a clinical cure only (Elewski 2008). In participants infected with T. tonsurans, the proportion of participants with clinical cure only at week 10 was 70% (355/507) in the terbinafine group and 57.2% (147/257) in the griseofulvin group (RR 1.22, 95% CI 1.09 to 1.38; Analysis 2.4). In participants infected with T. violaceum, the corresponding proportion was 65% (104/160) in the terbinafine group and 64.6% (53/82) in the griseofulvin group (RR 1.01, 95% CI 0.83 to 1.22; Analysis 2.4).
3. Terbinafine (medium (6 to 8 weeks) and long term (10 to 12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up
Primary outcome
Complete cure, i.e. clinical and mycological cure
Microsporum infections
We included two studies that reported data for participants infected with Microsporum (Elewski 2008; Lipozencic 2002). In Elewski 2008, a new study added in this update that included 1549 participants,15.1% were infected with M. canis. Of these, 27% (41/152) of participants in the medium‐term terbinafine group versus 43.9% (36/82) in the griseofulvin group achieved complete cure (Elewski 2008) (RR 0.61, 95% CI 0.43 to 0.88; Analysis 3.1).
Another open study, Lipozencic 2002, assessed medium‐ to long‐term treatment regimens of terbinafine versus griseofulvin. In this study, 98.5% of the 165 included participants were infected with M. canis, and the remainder were infected with M. audouini. A lower proportion of participants treated with medium‐term terbinafine achieved complete cure (51.4%, 36/70) compared to those treated with griseofulvin (70%, 21/30), but the difference was not statistically significant (RR 0.73, 95% CI 0.53 to 1.02; Analysis 3.1). Long‐term duration of treatment (10 or 12 weeks) resulted in the complete cure being higher in those in the griseofulvin group (70%, 21/30) compared to those in the terbinafine group (35%, 23/65) at 4 weeks after the end of treatment (RR 0.51, 95% CI 0.34 to 0.76; Analysis 3.1).
In participants infected with Microsporum, pooling the data from two studies for medium‐term treatment (six or eight weeks) resulted in an increase in complete cure for those in the griseofulvin group compared to those in the terbinafine group at four weeks after the end of treatment (34.7% versus 50.9%; RR 0.68, 95% CI 0.53 to 0.86; N = 334; moderate quality evidence; Analysis 3.1; Elewski 2008; Lipozencic 2002; summary of findings Table for the main comparison).
Adverse events
None of the studies (Elewski 2008; Lipozencic 2002) reported this outcome.
Secondary outcomes
Neither of the included studies comparing short‐ or long‐term treatment with terbinafine versus griseofulvin for Microsporum reported on recurrence of the condition after the end of the intervention period or the time taken to cure.
Proportion of participants with clinical cure only
Two studies reported the proportion of participants with a clinical cure only (Elewski 2008; Lipozencic 2002), including one new study added to this update (Elewski 2008). According to Elewski 2008, in participants infected with M. canis, the proportion of participants with clinical cure only at week 10 was 39.5% (60/152) in the terbinafine group and 57.3% (47/82) in the griseofulvin group (RR 0.69, 95% CI 0.53 to 0.90; Analysis 3.2). In Lipozencic 2002 at 16 weeks, the proportion of participants infected with Microsporum with clinical cure only was 61.1% (22/36) and 70.5% (24/34) in the groups treated with terbinafine for 6 and 8 weeks, respectively, and 60.6% (20/33) and 50% (16/32) in the groups treated with terbinafine for 10 and 12 weeks, respectively, compared to 80% (24/30) in the griseofulvin group. The control treatment (griseofulvin for 12 weeks) resulted in more cures compared with medium‐term terbinafine treatment duration (6 to 8 weeks) (RR 0.82, 95% CI 0.64 to 1.05; Analysis 3.2) and long‐term terbinafine treatment duration (10 to 12 weeks) (RR 0.69, 95% CI 0.52 to 0.92; Analysis 3.2), which was statistically significant in favour of griseofulvin. We pooled data from two studies (Elewski 2008; Lipozencic 2002) in a meta‐analysis. In participants infected with Microsporum, the proportion of participants with clinical cure only was significantly lower in the medium‐term terbinafine treatment group than in the griseofulvin group (RR 0.76, 95% CI 0.63 to 0.91; N = 334; Analysis 3.2).
Percentage of drop‐outs as a surrogate for participant adherence
Lipozencic 2002 reported on drop‐outs as a surrogate to measure adherence. The percentage of drop‐outs was 22.2% (8/36), 14.7% (5/34), 18.18% (6/33), 34.2% (12/32), and 23.3% (7/30), in the groups treated with terbinafine for 6, 8, 10, or 12 weeks and griseofulvin, respectively.
4. Terbinafine short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12 to 20 weeks follow‐up
Primary outcomes
Complete cure, i.e. clinical and mycological cure
One to two weeks versus four weeks
Four studies reported on complete cure (Friedlander 2002; Haroon 1996; Kullavanijaya 1997; Talarico Filho 1998). Meta‐analysis showed that a four‐week treatment duration of terbinafine was significantly better than one to two weeks (65.1% versus 48.6%; RR 0.73, 95% CI 0.62 to 0.86; Analysis 4.1).
Medium term (6 to 8 weeks) versus long‐term (10 to 12 weeks)
Medium‐term (six to eight weeks) treatment duration of terbinafine appeared to be better than long‐term (10 to 12 weeks) treatment duration, but the difference was not statistically significant (51.4% versus 35.3%; RR 1.45, 95% CI 0.97 to 2.17; Lipozencic 2002; Analysis 4.1).
Adverse events
Five studies reported on adverse events (Deng 2011; Friedlander 2002; Hamm 1999; Haroon 1996; Talarico Filho 1998), including one study added to this update (Deng 2011).
Deng 2011 reported that none of the terbinafine treated patients experienced adverse events.
Talarico Filho 1998 reported the following adverse events: mild itching and mild constipation in the one‐week arm; mild headache and nausea in the two‐week arm; mild urticaria, swelling of the lips (labial oedema), mild constipation, moderate loss of appetite, mild diarrhoea, mild nausea and moderate or partial loss of taste (recovered within eight weeks) in the four‐week arm.
Hamm 1999 reported: abdominal pain (mild to moderate), epistaxis (nose bleed), lack of appetite, headache, severe facial swelling, coughing and fever (mild to moderate) in the one‐week arm; abdominal pain, fatigue, nausea, dyspepsia, headache and fever in the two‐week arm. One additional participant had lack of appetite and gastroenteritis only during the additional four‐week treatment period.
In Friedlander 2002, around 44% of the participants experienced mild to moderate adverse events, which were probably not related to treatment. The most frequent adverse events were "upper respiratory tract infections, gastrointestinal upsets and other events common in this patient population". Authors did not report relevant data but stated that the frequency of adverse events was similar between groups.
Haroon 1996 compared three different regimens (at one, two and four weeks), reporting a few adverse events: headache, raised hepatic enzymes, raised triglycerides, eosinophilia and leucocytosis in the one‐week arm; raised hepatic enzymes and eosinophilia in the two‐week arm and raised hepatic enzymes, raised triglycerides, eosinophilia and leucocytosis in the four‐week arm.
Secondary outcomes
None of the studies reported recurrence of the condition after the end of the intervention period.
Proportion of participants with clinical cure only
Four studies reported on the proportion of participants achieving a clinical cure only, all of which were also included in the original review (Friedlander 2002; Haroon 1996; Lipozencic 2002; Talarico Filho 1998).
One to two weeks versus four weeks
Meta‐analysis of three included studies showed that a four‐week treatment duration of terbinafine seemed to be better than one‐ to two‐week treatment duration, but the difference was not statistically significant (75.1% versus 63.9%; RR 0.84, 95% 0.67 to 1.06; Friedlander 2002; Haroon 1996; Talarico Filho 1998; Analysis 4.2).
Medium term (6 to 8 weeks) versus long‐term (10 to 12 weeks)
Data from Lipozencic 2002 showed that medium‐term (6 to 8 weeks) treatment duration of terbinafine appeared to be better than long‐term (10 to 12 weeks), but the difference was also not statistically significant (65.7% versus 55.4%; RR 1.19, 95% CI 0.90 to 1.56; Analysis 4.2).
Percentage of drop‐outs as a surrogate for participant adherence
Two studies reported drop‐outs as a surrogate for adherence (Deng 2011; Friedlander 2002), including one new study added to this update (Deng 2011).
Deng 2011 reported there were no drop‐outs in the two‐week or four‐week terbinafine groups.
In Friedlander 2002, the percentage of drop‐outs in the one‐, two‐ and four‐week arms were reported as 25% (14/56), 25.4%(15/59) and 19.3% (12/62), respectively.
Time taken to cure
One study, also included in the original review, reported on time taken to cure (Hamm 1999). The time taken to cure was about two weeks if the causative organism was a Trichophyton. Participants infected with Microsporum only responded to an additional four‐week treatment course of terbinafine, i.e. two to three weeks after an initial course of one or two weeks.
5. Terbinafine standard dose versus double dose in Microsporum infections; 20 weeks follow‐up
This update did not identify any new studies addressing the outcomes for this comparison. Likewise, we did not find any studies reporting on our primary outcome of adverse events or the secondary outcomes of proportion of participants with clinical cure only; measurement of recurrence of the condition after the end of the intervention period; or percentage of drop‐outs as a surrogate for participant adherence.
Primary outcomes
Complete cure, i.e. clinical and mycological cure
Ungpakorn 2004 reported on complete cure and assessed the efficacy of the standard dose of terbinafine compared to double doses of terbinafine after 20 weeks of follow‐up. Both treatments were given in a pulsed protocol (one week on, three weeks off) for the treatment of tinea capitis caused by Microsporum species. The proportion with complete cure for the standard dose group reached 60.8% (14/23) and was similar to 68.4% (13/19) in the double dose group (RR 1.12, 95% CI 0.72 to 1.76; Analysis 5.1).
Secondary outcomes
Time taken to cure
Ungpakorn 2004 also reported time taken to cure: at week 20 all participants were cured with the exception of one who at the beginning had moderately severe tinea capitis.
6. Itraconazole (six and two weeks) versus griseofulvin (six weeks) in Trichophyton and Microsporum infections
This update did not identify any new studies addressing the outcomes for this comparison.
Primary outcomes
Complete cure, i.e. clinical and mycological cure
Two studies reported complete cure (Gupta 2001; López‐Gómez 1994).
Gupta 2001 compared six weeks of griseofulvin versus two to three weeks of itraconazole in 100 people, with the dose given according to the participant's weight. This study showed complete cure at 82% (41/50) for the itraconazole group and 92% (46/50) for the griseofulvin group (RR 0.89, 95% CI 0.76 to 1.04; Analysis 6.1). The main causative fungi were T. tonsurans and T. violaceum, although investigators did not report the exact percentages. According to this trial, when Trichophyton species are the infecting fungi, both griseofulvin and itraconazole reach high complete cure percentages, although griseofulvin tends to be more effective. However the disadvantage was that griseofulvin was administered for six weeks treatment, while itraconazole was administered only for two to three weeks.
In another study involving 34 participants, in whom M. canis was the most common fungi, complete cure was the same for both drugs at 88% (15/17 and 15/17) (López‐Gómez 1994). This study compared six weeks of treatment with ultra microsize griseofulvin 500 mg/d or itraconazole 100 mg/d with a follow‐up of 14 weeks. According to this trial, in tinea capitis involving Microsporum species, both itraconazole and griseofulvin reached high complete cure percentages within a treatment period of six weeks (RR 1.00, 95% CI 0.78 to 1.28; Analysis 6.1).
We did not see a significant statistical difference between the different doses of itraconazole employed in the two studies and griseofulvin in the pooled analysis (83.6% versus 91.0%; RR 0.92, 95% CI 0.81 to 1.05; N = 134; very low quality evidence; Analysis 6.1; summary of findings Table 2).
Adverse events
Two studies reported adverse events (Gupta 2001; López‐Gómez 1994).
Authors did not report adverse events in the itraconazole group of either of the trials. In those treated with griseofulvin, two participants experienced nausea and intense stomach ache with severe vomiting at weeks two and four of treatment, requiring discontinuation of therapy (López‐Gómez 1994). Gupta 2001 reported three gastric problems and three cases of nausea in the griseofulvin group. One of the participants who experienced nausea dropped out of the study.
Secondary outcomes
None of the studies under this comparison reported recurrence of the condition after the end of the intervention period or the time taken to cure.
Proportion of participants with clinical cure only
Only one study reported this outcome (Gupta 2001). The proportion of participants with clinical cure only at the end of treatment reported in the study were 44% (22/50) and 70% (35/50) in the itraconazole and in the griseofulvin groups, respectively (RR 0.63, 95% CI 0.44 t0 0.90; Analysis 6.2).
Percentage of drop‐outs as a surrogate for participant adherence
Two studies reported on drop‐outs (Gupta 2001; López‐Gómez 1994).
In Gupta 2001, the percentage of drop‐outs was the same for both treatment groups (8% versus 8%; RR 1.00, 95% CI 0.26 to 3.78; Analysis 6.3). In López‐Gómez 1994 the percentage of drop‐outs was 5.5% (1/18) in the itraconazole group and 11.7% (2/17) in the griseofulvin group (RR 0.47, 95% CI 0.05 to 4.74; Analysis 6.3).
7. Itraconazole versus terbinafine (both two weeks) in Trichophyton infections
This update did not identify any new studies addressing the outcomes for this comparison.
Primary outcomes
Complete cure, i.e. clinical and mycological cure
Gupta 2001 and Jahangir 1998 reported on complete cure.
Jahangir 1998 had 60 participants and compared a two‐week course of itraconazole (50 to 200 mg/d based on weight) with two weeks of terbinafine. T. violaceum was the causative fungus in 82% to 89% of the participants. Twelve weeks after the start of treatment, 53% (16/30) and 60% (18/30) of participants were completely cured in the terbinafine and itraconazole groups, respectively (RR 1.13, 95% CI 0.72 to 1.75; Analysis 7.1). Gupta 2001, where Trichophyton was the species of fungus, compared itraconazole and terbinafine with a two‐ to three‐week course of therapy. At 12 weeks, 94% (47/50) in the terbinafine group had a complete cure, compared to 82% (41/50) in the itraconazole group (RR 0.87, 95% CI 0.75 to 1.01; Analysis 7.1).
In the pooled analyses, there was very little difference in the proportion of participants achieving complete cure with itraconazole and terbinafine (as treatment of Trichophyton species) when used for periods of two to three weeks (73.8% versus 78.8%; RR 0.93, 95% CI 0.72 to 1.19; N = 160; low quality evidence; Analysis 7.1; summary of findings Table 3).
Adverse events
Only Jahangir 1998 reported adverse events. Two participants reported urticaria in the itraconazole group. In the terbinafine group, one participant experienced fever, body aches and vertigo, but no participant showed any significant haematological or biochemical change.
Secondary outcomes
No studies for this comparison reported recurrence of the condition after the end of the intervention period or the time taken to cure.
Proportion of participants with clinical cure only
Only Gupta 2001 reported on the proportion of participants achieving clinical cure at the end of the four‐week treatment, with similar results in both terbinafine and itraconazole treatment groups: 40% (20/50) and 44% (22/50), respectively (RR 1.10, 95% CI 0.69 to 1.75; Analysis 7.2).
Percentage of drop‐outs as a surrogate for participant adherence
Gupta 2001 reported that the percentage of drop‐outs was 8% (4/50) for the itraconazole group and 4% (2/50) for the terbinafine group (RR 2.00, 95% CI 0.38 to 10.43, Analysis 7.3).
8. Ketoconazole versus griseofulvin (12 to 26 weeks) in Trichophyton infections; 12 to 26 weeks follow‐up
This update did not identify any new studies addressing the outcomes for this comparison.
Primary outcomes
Complete cure, i.e. clinical and mycological cure
Two studies reported complete cure, but because of the differences in treatment durations between them, we did not pool the results (Gan 1987; Tanz 1988).
Gan 1987 was an open study in 80 participants where Trichophyton species predominated; investigators compared once‐daily ketoconazole 5 mg/kg/d with once‐daily griseofulvin 15 mg/kg/d. The children were examined every two weeks while they were receiving therapy (and at least one follow‐up after the end of therapy). Treatment was stopped when there was either complete cure or after six months had passed. At the end of 12 weeks of therapy, 73.5% (25/34) of participants treated with ketoconazole had complete cure of their infection, compared with 96.4% (27/28) of the participants given griseofulvin (RR 0.76, 95% CI 0.62 to 0.94; N = 62; low quality evidence; Analysis 8.1; summary of findings Table 4 ).
Those who did not achieve a complete clinical cure within 12 weeks continued to take therapy and were assessed between 12 and 26 weeks of therapy, until they exhibited complete resolution of clinical disease and negative hair sample cultures. During this period, the one remaining griseofulvin participant and six of the ketoconazole participants with continuing disease had complete clearance of clinical and mycological disease.
Thus, by the end of 26 weeks, all of the participants in the griseofulvin group were completely cured, and only three ketoconazole‐treated patients remained with persistent clinical disease and positive mycological cultures (i.e. 91.2% (31/34) participants receiving ketoconazole compared with 100% (28/28) in the griseofulvin group (RR 0.95, 95% CI 0.83 to 1.07; N = 62; low quality evidence; Analysis 8.1; summary of findings Table 4).
In Tanz 1988, participants randomly received ketoconazole or griseofulvin for 12 weeks. The proportion of participants with complete cure was similar at 48% (16/33) in the ketoconazole group and 54% (25/46) in the griseofulvin group (RR 0.89, 95% CI 0.57 to 1.39; N = 79; low quality evidence; Analysis 8.1; summary of findings Table 4).
Adverse events
Four studies reported adverse events (Gan 1987; Martínez‐Roig 1988; Tanz 1985; Tanz 1988). Despite reports of liver disease (Lewis 1984), we did not find any studies reporting this adverse effect. Ketoconazole use was associated with two cases of abdominal pain and one case of urticaria (Tanz 1985). Only one participant from the ketoconazole group withdrew from the study and reported to have nausea. Other than this, there were no serious adverse reactions in any of the two groups (Tanz 1988). No adverse events were reported in the ketoconazole group (Gan 1987; Martínez‐Roig 1988). One griseofulvin‐treated participant showed a two‐fold increase in serum alanine aminotransferase and aspartate aminotransferase after three weeks of treatment, but values returned to normal at the following weekly clinic visits (Martínez‐Roig 1988).
Secondary outcomes
Proportion of participants with clinical cure only
Only one study reported this outcome (Martínez‐Roig 1988). The proportion of participants with clinical cure evaluated at the end of treatment were 100% (8/8) and 80% (4/5) in the ketoconazole and griseofulvin groups, respectively (RR 1.26, 95% CI 0.77 to 2.05; Analysis 8.2).
Proportion of participants with recurrence of the condition after the end of the intervention period
Gan 1987 reported (page 48) that "three patients (two treated with ketoconazole and one treated with griseofulvin) had a recurrence of tinea capitis at four weeks (two patients) and at four months (one patient) following discontinuation of therapy".
Percentage of drop‐outs as a surrogate for participant adherence
Two studies reported this outcome (Tanz 1985; Tanz 1988).
In Tanz 1985, the percentage of drop‐outs was 30% (3/10) and 41.6% (5/12) in the ketoconazole and griseofulvin groups, respectively (RR 0.72, 95% CI 0.23 to 2.30, Analysis 8.3).
In Tanz 1988, the percentage of drop‐outs was 66.6% (22/33) and 56.5% (26/46) in the ketoconazole and griseofulvin groups, respectively (RR 1.18, 95% CI 0.83 to 1.67; Analysis 8.3).
Time taken to cure
Two studies reported this outcome (Gan 1987; Martínez‐Roig 1988).
In Gan 1987, the time needed to improve was 60 days and 108 days in the ketoconazole and griseofulvin groups, respectively. Hair sample cultures took significantly longer to become negative (sterile) in the ketoconazole group (median eight weeks) than in the griseofulvin group (four weeks).
Martínez‐Roig 1988 reported the mean time to clinical cure in weeks: 4.2 weeks in the griseofulvin group and 5.0 weeks in the ketoconazole group. They also reported the mean time needed to achieve negative cultures in weeks: 3.6 and 4.7 weeks in the griseofulvin and ketoconazole groups, respectively.
9. Fluconazole (2 to 6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up
The study by Foster 2005 was new to this update.
Primary outcomes
Complete cure, i.e. clinical and mycological cure
Three studies reported this outcome (Dastghaib 2005; Foster 2005; Gupta 2001).
Dastghaib 2005 assessed 40 participants: 16 were infected with T. violaceum, 16 with T. verrucosum and 8 with M. canis. The children were treated with 5 mg/kg/d of fluconazole or 15 mg/kg/d griseofulvin for four and six weeks, respectively. Complete cure was reported for 79% (15/19) of the fluconazole arm and 76% (16/21) of the griseofulvin arm (RR 1.04, 95% CI 0.74 to 1.45; Analysis 9.1).
Foster 2005 included 880 participants: 86% were infected with T. tonsurans and 11% with M. canis; 721 of them were included in the analyses: 245 participants on short‐term fluconazole, 246 participants on the medium‐term fluconazole and 230 participants in the griseofulvin group. As we stated in the 'Methods' section, for RCTs with multiple intervention groups, we split the shared griseofulvin group (N = 230) in two (N = 115 each) to avoid double‐counting.
The children were randomly assigned to three groups and treated with a short‐term course of fluconazole (6 mg/kg/d for three weeks; N = 245); medium‐term use of fluconazole (6 mg/kg/d for six weeks; N = 246); or griseofulvin (11 mg/kg/d for six weeks; 230), respectively. Complete cure was not significantly different between the short‐term fluconazole group and the griseofulvin group (30.2% versus 31.3%; RR 0.96, 95% CI 0.69 to 1.34; Analysis 9.1).
Gupta 2001 assessed 100 participants who were infected with T. tonsurans and/or T. violaceum (the exact percentages were not reported). They were treated with either fluconazole 6 mg/kg/d for two to three weeks or with microsize griseofulvin 20 mg/kg/d for six weeks. The proportion of participants with complete cure were 82% (41/50) and 92% (46/50), respectively (RR 0.89, 95% CI 0.76 to 1.04; Analysis 9.1).
Short‐term (2‐4 weeks) fluconazole
Meta‐analysis of the three studies failed to show any significant difference between short‐term use of fluconazole and griseofulvin for proportions of participants with complete cure i.e. clinical and mycological cure (41.4% versus 52.7%; RR 0.92, 95% CI 0.81 to 1.05; N = 615; moderate quality evidence; Analysis 9.1; Dastghaib 2005; Foster 2005; Gupta 2001; summary of findings Table 5).
Medium‐term (6 weeks) fluconazole
There is a single trial in this subgroup (N = 361; Foster 2005). There was no clear difference between between the medium‐term fluconazole group and the griseofulvin group (34.1% versus 32.1%; RR 1.06, 95% CI 0.77 to 1.46; N = 361; low quality evidence; Analysis 9.1; summary of findings Table 5).
Adverse events
Three studies reported this outcome (Dastghaib 2005; Foster 2005; Gupta 2001).
Foster 2005 included 1063 patients for safety evaluation and reported that the most frequent treatment‐related adverse events were abdominal pain (1.3%) and diarrhoea (0.7%) in the short‐term fluconazole group; headache (0.9%) and rash (0.6%) in the medium‐term fluconazole group; and headache (1.7%), abdominal pain (1.4%) and dyspepsia (1.0%) in the griseofulvin group. There were no significant differences between the three groups with regard to all causality and treatment‐related adverse events.
Two studies reported nausea as an adverse effect in the griseofulvin group (Dastghaib 2005; Gupta 2001).
Secondary outcomes
None of the studies in this comparison reported on recurrence of the condition after the end of the intervention period or the time taken to cure.
Proportion of participants with clinical cure only
Two studies reported this outcome (Foster 2005; Gupta 2001).
Foster 2005 reported that the proportions of participants with clinical cure only at week 10 were 40% (98/245), 46% (112/246), and 40% (92/230) in the short‐term fluconazole, medium‐term fluconazole, and griseofulvin groups, respectively.
Gupta 2001 reported the proportions of participants with clinical cure only at the end of treatment (week 4 for fluconazole and week 6 for griseofulvin) were 26% (13/50) and 70% (35/50) in the fluconazole and griseofulvin groups, respectively (RR 0.37, 95% CI 0.22 to 0.61; Analysis 9.2).
Percentage of drop‐outs as a surrogate for participant adherence
Two studies reported this outcome (Foster 2005; Gupta 2001).
Foster 2005 reported that the percentages of drop‐outs were 12% (37/302), 7% (21/286), and 8% (24/292) in the short‐term fluconazole, medium‐term fluconazole and griseofulvin groups, respectively.
The same percentage of drop‐outs was reported for both groups in Gupta 2001 (8% versus 8%, RR 1.00, 95% CI 0.26 to 3.78; Analysis 9.3).
10. Fluconazole (2 to 3 weeks) versus terbinafine (2 to 3 weeks) in Trichophyton infections; 12 weeks follow‐up
This update did not identify any new studies addressing the outcomes for this comparison. Moreover, none of the trials evaluating this comparison reported on adverse events, recurrence of the condition after the end of the intervention period or the time taken to cure.
Primary outcome
Complete cure, i.e. clinical and mycological cure
Only one study reported this outcome (Gupta 2001). The efficacy of fluconazole for two to three weeks was compared with terbinafine, dosed according to weight for two to three weeks in Trichophyton infections. The proportions of participants with complete cure were 82% (41/50) for the fluconazole arm and 94% (47/50) for the terbinafine arm (RR 0.87, 95% CI 0.75 to 1.01; N = 100; low quality evidence; Analysis 10.1; summary of findings Table 6).
Secondary outcomes
Proportion of participants with clinical cure only
Only one study reported this outcome (Gupta 2001). The proportion of participants with clinical cure only at the end of treatment (week 4 for terbinafine and fluconazole) was not significantly different in the terbinafine group compared with the fluconazole group ((40% versus 26%; RR 1.54, 95% CI 0.86 to 2.74).
Percentage of drop‐outs as a surrogate for participant adherence
Only one study reported this outcome (Gupta 2001). The percentage of drop‐outs was 4% (2/50) and 8% (4/50) in the terbinafine and fluconazole groups, respectively (RR 0.50, 95% CI 0.10 to 2.61).
11. Fluconazole (2 to 3 weeks) versus itraconazole (2 to 3 weeks) in Trichophyton infections; 12 weeks follow‐up
This update did not identify any new studies addressing the outcomes for this comparison. Moreover, none of the trials evaluating this comparison reported on adverse events, recurrence of the condition after the end of the intervention period or the time taken to cure.
Primary outcome
Complete cure, i.e. clinical and mycological cure
Only one study reported this outcome (Gupta 2001). When fluconazole treatment was compared with itraconazole in participants with Trichophyton tinea capitis, in doses of 5 mg/kg/d daily for two to three weeks, the proportion of participants with complete cure was 82% (41/50 and 41/50) for both groups (RR 1.00, 95% CI 0.83 to 1.20; N = 100; low quality evidence; Analysis 11.1; summary of findings Table 7).
Secondary outcome:
Proportion of participants with clinical cure only
Only one study reported this outcome (Gupta 2001). The proportion of participants with clinical cure only at the end of treatment (week four in itraconazole and fluconazole groups) was nearly double in the itraconazole group: 44% (22/50) compared with 26% (13/50) in the fluconazole group, but the difference was not statistically significant (RR 1.69, 95% CI 0.96 to 2.97; Analysis 11.2).
Percentage of drop‐outs as a surrogate for participant adherence
Only one study reported this outcome (Gupta 2001). The same percentage of drop‐outs as a surrogate for participant adherence was reported in both treatment groups (8%, 4/50; RR 1.00, 95% CI 0.26 to 3.78; Analysis 11.3).
12. Fluconazole low dose versus higher dose (1.5, 3.0 and 6.0 mg/kg/d) in Trichophyton infections; 4 months follow‐up
This update did not identify any new studies addressing the outcomes for this comparison. In fact, we found a single study for this comparison, and it only reported on one primary outcome (complete cure) and not on any of our secondary outcomes (Solomon 1997). We categorised data into three subgroups comprising 1.5 mg versus 3.0 mg; 1.5 mg versus 6.0 mg; and 3.0 mg versus 6.0 mg.
Primary outcome
Complete cure, i.e. clinical and mycological cure
Solomon 1997 compared different doses of fluconazole: 1.5 mg/kg/d, 3.0 mg/kg/d, and 6.0 mg/kg/d, for 20 days in a group of 41 participants with tinea capitis caused by the Trichophyton species. Authors reported efficacy in only 27 participants and did not provide details on drop‐outs by group. However, they reported the total missing participants (34%) in the study. Thus, we assumed that the same percentage was applied for the missing participants in each group, being originally 12, 15 and 14 participants randomly assigned in the 1.5 mg/kg/d, 3.0 mg/kg/d and 6.0 mg/kg/d groups, respectively. Intention‐to‐treat efficacy rates in the 1.5 mg/kg/d, 3.0 mg/kg/d and 6.0 mg/kg/d groups were 17% (2/12), 40% (6/15) and 57% (8/14), respectively.
Although higher doses resulted in more cures than lower doses, none of the comparisons reached statistical significance (3.0 mg versus 1.5 mg: RR 2.40, 95% CI 0.59 to 9.82; Analysis 12.1; 6.0 mg versus 1.5 mg: RR 3.43, 95% CI 0.89 to 13.15; Analysis 12.1; 6.0 mg versus 3.0 mg: RR 1.43, 95% CI 0.66 to 3.08; Analysis 12.1).
13. Treatment durations of fluconazole (3 weeks versus 6 weeks); 10 weeks follow‐up
One new study added to this update evaluated this comparison, but it did not report on adverse events, recurrence of the condition after the end of the intervention period or the time taken to cure (Foster 2005).
Primary outcome
Complete cure, i.e. clinical and mycological cure
Foster 2005 compared different treatment duration of fluconazole: short‐term use of fluconazole (6 mg/kg/d for three weeks) and medium‐term use of fluconazole (6 mg/kg/d for six weeks). At week 10, there was no significant difference with regard to the proportion of participants with complete cure for the short‐term and medium‐term use of fluconazole in children infected with T. tonsurans and M. canis (30.2%, 74/245 versus 34.1%, 84/246; RR 0.88, 95% CI 0.68 to 1.14; N = 491; low quality evidence; Analysis 13.1; summary of findings Table 8).
Secondary outcomes
Proportion of participants with clinical cure only
Foster 2005 reported that short‐term use of fluconazole and medium‐term use of fluconazole had similar effects on clinical cure (RR 0.88, 95% CI 0.72 to 1.08).
Percentage of drop‐outs as a surrogate for participant adherence
Foster 2005 reported the percentage of drop‐outs was 12% (37/302) and 7% (21/286) in the short‐term and medium‐term fluconazole groups, respectively (RR 1.67, 95% CI 1.00 to 2.78).
Discusión
Resumen de los resultados principales
En esta revisión actualizada se incluyeron 25 estudios (con 4449 participantes), entre ellos cuatro estudios nuevos (2637 participantes) que se agregaron a esta actualización. Los datos de los estudios incluidos produjeron 13 comparaciones principales de intervenciones.
Terbinafina versus griseofulvina
En los metanálisis de terbinafina durante cuatro semanas versus griseofulvina durante ocho semanas, evaluadas a las 12 a 24 semanas de seguimiento, el resultado no favoreció a la terbinafina ni a la griseofulvina para las infecciones por Trichophyton (CR 1,06; IC del 95%: 0,98 a 1,15; N = 328, tres ECA; pruebas de baja calidad; Resumen de los hallazgos para la comparación principal) para el resultado primario de curación. Lo anterior también fue el caso para las infecciones mixtas por Trichophyton y Microsporum en dos estudios y en el análisis de un estudio único de las infecciones por Microsporum.
Nuevas pruebas de Elewski 2008 indicaron que la terbinafina (a dosis estándar según el peso del participante durante seis semanas) logró una proporción de participantes con curación completa similar a la de la griseofulvina (a dosis estándar según el peso del participante durante seis semanas) en niños infectados por Trichophyton, cuando se siguieron durante diez semanas (CR 1,18; IC del 95%: 0,74 a 1,88; N = 1006; un ECA; pruebas de baja calidad, Resumen de los hallazgos para la comparación principal). El análisis de subgrupos que evaluó la respuesta al tratamiento en niños infectados por T. tonsurans mostró que la terbinafina es mejor que la griseofulvina. Estas nuevas pruebas dieron lugar a un cambio en las conclusiones de esta revisión. Sin embargo, ambos regímenes tuvieron efectos similares en los niños con T. violaceum.
Hay pruebas de calidad moderada de que la griseofulvina (seis a 12 semanas) es mejor que el uso a plazo medio (seis a ocho semanas) de terbinafina con respecto a la proporción de participantes que lograron la curación completa (CR 0,68; IC del 95%: 0,53 a 0,86; N = 334; dos ECA; Resumen de los hallazgos para las comparación principal). Esta prueba a favor de la eficacia de la griseofulvina en la infección por Microsporum se agregó recientemente a esta actualización (Elewski 2008).
Pruebas de calidad moderada también confirmaron que los eventos adversos y los eventos adversos graves son comparables entre la terbinafina y la griseofulvina y los eventos adversos son leves y reversibles en la mayoría de los casos (CR 1,11; IC del 95%: 0,79 a 1,57; N = 1549; un ECA; Resumen de los hallazgos para la comparación principal).
Diferentes regímenes de dosis de terbinafina
Se investigó una posible relación entre la dosis, la duración y la respuesta con la terbinafina. Un metanálisis de cuatro estudios indicó que una duración del tratamiento de cuatro semanas con terbinafina fue significativamente mejor que una o dos semanas de tratamiento con respecto a la curación completa de las infecciones por Trichophyton y Microsporum (CR 0,73; IC del 95%: 0,62 a 0,86; N = 552; cuatro ECA). No hubo diferencias en la proporción de participantes con curación completa, o clínicamente curados, al comparar la administración a plazo medio (seis a ocho semanas) versus a largo plazo (diez a 12 semanas) de terbinafina en un análisis de un estudio único. Las comparaciones de los eventos adversos entre los diferentes plazos del tratamiento indicaron que los eventos adversos fueron leves y comparables entre los grupos. Una comparación de un estudio único de la dosis estándar de terbinafina en comparación con una dosis doble en un estudio de infecciones por Microsporum indicó que la efectividad para la curación completa de la infección fue comparable entre los regímenes cuando se evaluaron a las 20 semanas de seguimiento.
Itraconazol versus griseofulvina
Un metanálisis de dos estudios no encontró diferencias significativas entre el itraconazol y la griseofulvina para lograr una curación completa en los niños con infección por Trichophyton y Microsporum (CR 0,92; IC 0,81 a 1,05; N = 134; dos ECA; pruebas de muy baja calidad; Resumen de los hallazgos 2).
Itraconazol versus terbinafina
Un metanálisis de dos estudios indicó que no hubo diferencias significativas entre el itraconazol y la griseofulvina para lograr una curación completa en los niños infectados por Trichophyton (CR 0,93; IC 0,72 a 1,19; N = 160; dos ECA; pruebas de baja calidad; Resumen de los hallazgos 3).
Ketoconazol versus griseofulvina
Las pruebas actuales con respecto al ketoconazol versus la griseofulvina fueron limitadas. Un estudio favoreció a la griseofulvina porque el ketoconazol (por 12 semanas) pareció ser menos efectivo en cuanto a la curación completa que la griseofulvina durante el mismo período (CR 0,76; IC del 95%: 0,62 a 0,94; Gan 1987). Sin embargo, al final del estudio, cuando la duración del tratamiento se prolongó hasta un máximo de 26 semanas en los que no lograron una curación a las 12 semanas, los efectos parecieron ser similares (CR 0,95; IC del 95%: 0,83 a 1,07). Otro estudio indicó que la proporción de pacientes con una curación completa fue similar en los grupos de ketoconazol (por 12 semanas) y griseofulvina (por 12 semanas) (CR 0,89; IC del 95%: 0,57 a 1,39; Resumen de los hallazgos 4).
Fluconazol versus otras terapias (griseofulvina o terbinafina o fluconazol)
Un metanálisis de tres estudios indicó que el fluconazol y la griseofulvina tuvieron efectos similares para lograr la curación completa a corto plazo de dos a cuatro semanas (CR 0,92; IC del 95%: 0,81 a 1,05; N = 500; pruebas de calidad moderada de tres ECA) o a plazo medio de seis semanas (CR 1,06; IC del 95%: 0,77 a 1,46; N = 361; un ECA pruebas de baja calidad, Resumen de los hallazgos 5).
Un análisis de estudio único de un ensayo pequeño no mostró diferencias entre las dos intervenciones para el resultado curación completa cuando el fluconazol se comparó con la terbinafina, ambos administrados durante dos a tres semanas (CR 0,87; IC 0,75 a 1,01; N = 100; un ECA; pruebas de baja calidad; Resumen de los hallazgos 6), o cuando el fluconazol se comparó con el itraconazol, cada uno administrado durante dos a tres semanas, para las infecciones por Trichophyton (CR IC del 1,00 0,83 a 1,20; N = 100, un ECA pruebas de baja calidad, Resumen de los hallazgos 7).
Diferentes dosis de fluconazol
La dosis de fluconazol se comparó en un análisis pequeño de estudio único, o sea, 1,5; 3,0 y 6,0 mg/k/día en las infecciones por Trichophyton, sin diferencias significativas entre los grupos, aunque las dosis mayores dieron lugar a más curaciones en cada comparación cuando se realizó un seguimiento a los cuatro meses.
Diferente duración del tratamiento con fluconazol
Un análisis de estudio único adicional de la duración del tratamiento con fluconazol que comparó el tratamiento a corto plazo (tres semanas) versus a plazo medio (seis semanas) a las diez semanas de seguimiento no mostró diferencias significativas entre los grupos en los niños infectados por T. tonsurans y M. canis.
Compleción y aplicabilidad general de las pruebas
Esta revisión puede ayudar a los médicos en todo el mundo a que sopesen las ventajas y las desventajas de los agentes antimicóticos sistémicos y las opciones cuando tratan a los niños con tiña capitis. Se basa en la información detallada obtenida de ensayos clínicos que evalúan la eficacia de los tratamientos para la tiña capitis. Sin embargo, se recomienda precaución al interpretar los datos porque los 25 estudios incluidos publicados entre 1987 a 2013 se realizaron en diferentes países e incluyeron pacientes de diferentes grupos étnicos. Además, los tipos de hongos variaron de un ensayo a otro. Lo anterior significa que los resultados pueden no ser aplicables directamente desde el punto de vista clínico a participantes individuales y a situaciones específicas.
Calidad de la evidencia
La calidad de las pruebas de esta revisión para cada resultado, como se presenta en las tablas "Resumen de los hallazgos", fue muy baja a moderada. Las razones principales para la disminución de la calidad de las pruebas de estos resultados fueron la imprecisión debido a los tamaños de la muestra y el riesgo bajo de sesgo en los estudios incluidos.
Limitaciones en el diseño, la realización y el informe de los estudios
Los estudios incluidos en esta revisión tienen varias limitaciones metodológicas: sólo cuatro estudios describieron adecuadamente el método de asignación al azar y sólo dos describieron de forma adecuada la asignación de la ocultación. El cegamiento de los participantes y el personal y el cegamiento de los evaluadores de resultado no fueron adecuados en los estudios. Diez estudios tuvieron alto riesgo de sesgo de informe incompleto de los datos de resultado (sesgo de desgaste) y, aunque se consideró que ninguno tuvo alto riesgo de sesgo de informe selectivo, en realidad no se tuvo acceso a los protocolos de estudio para realizar la comparación.
Se detectaron otras fuentes de sesgo que incluyen el fracaso para informar la gravedad de la enfermedad o la comparabilidad inicial, pero no estuvo claro el grado en el que estos factores pueden haber introducido sesgo. Por lo tanto, es importante recalcar que cualquier conclusión establecida se basó en estudios primarios con grados variables de sesgo. Los lectores deben considerar el riesgo de sesgo cuando se interpretan estos resultados (Figura 3) y tener cuidado con los resultados derivados de estudios con riesgo alto o incierto de sesgo.
Inconsistencia de los resultados
La inconsistencia se refiere a una heterogeneidad no explicada de los resultados entre los estudios.
En esta revisión, la mayoría de las comparaciones se evaluaron en estudios únicos, de manera que no fue necesaria la evaluación de la consistencia de los resultados entre los estudios. En los resultados en los que se agruparon los datos solamente se identificó una inconsistencia de los efectos en un resultado (curación completa, terbinafina versus griseofulvina, Resumen de los hallazgos para la comparación principal). En este resultado la calidad se disminuyó un nivel porque la I2 del resultado agrupado fue del 85%, que indica heterogeneidad significativa. Una fuente de heterogeneidad en este análisis de las infecciones por Trichophyton puede ser que el análisis incluyera infecciones por T. tonsurans y T. violaceum; sin embargo, no está claro el grado en el que esta heterogeneidad clínica contribuyó a la heterogeneidad estadística observada.
Imprecisión de los resultados
Los resultados se consideran imprecisos cuando los estudios incluyen relativamente pocos pacientes y pocos efectos. En esta revisión se identificó imprecisión en algunas comparaciones, y la razón de la imprecisión fue que el número total de efectos fue menor de 300, como recomienda el manual GRADE (Schünemann 2013).
Dificultad para generalizar las pruebas
La mayoría de los 4449 participantes con micosis en los 25 estudios incluidos tuvieron edades de dos a 16 años, de manera que las pruebas encontradas se relacionan directamente con la población con la enfermedad de interés. En esta revisión, los resultados informados por los pacientes (RIP), como la calidad de vida, se informaron pocas veces en los estudios incluidos. Los RIP son importantes para tomar decisiones clínicas basadas en pruebas y se deben analizar en los estudios futuros.
Sesgo de publicación
Fue imposible realizar un análisis de los gráficos en embudo para evaluar el posible sesgo de publicación de cualquiera de los resultados debido al número limitado de ensayos; sólo hubo uno a tres estudios para cada comparación.
Sesgos potenciales en el proceso de revisión
La evaluación de los estudios incluidos se basó en los textos publicados; por lo tanto, los resultados estuvieron influenciados inevitablemente por la calidad del informe de estos estudios. No hay dudas de que la calidad deficiente del informe influye en la exactitud de las evaluaciones, y no fue posible obtener información adicional de los investigadores de los ensayos con los que se estableció contacto. Pather 2006 fue un artículo de un congreso que pareció cumplir los criterios de inclusión, pero como no fue posible obtener información adicional, no se pudo incluir o excluir. Esta puede ser una fuente de sesgo potencial, ya que las conclusiones de la revisión podrían cambiar una vez que se haya evaluado el estudio. Se intentó realizar una búsqueda exhaustiva de los estudios, pero teóricamente, quizás haya algunos estudios faltantes porque no se efectuaron búsquedas en todas las bases de datos médicas locales en todo el mundo.
Además, la heterogeneidad clínica entre los estudios incluidos es inevitable porque cada estudio incluyó participantes de diferentes grupos étnicos, tipos de micosis, gravedad de la enfermedad, dosis de los fármacos y duraciones del tratamiento y del seguimiento. La heterogeneidad clínica podría provocar sesgo cuando se combinan los resultados de diferentes estudios en un metanálisis.
Acuerdos y desacuerdos con otros estudios o revisiones
Griseofulvina
En esta revisión, la griseofulvina se empleó como un tratamiento estándar para la evaluación de cualquier tratamiento más nuevo para la tiña capitis.
Un metanálisis anterior encontró que la griseofulvina fue un tratamiento efectivo para la tiña capitis (Gupta 2008). Esta conclusión está apoyada por los resultados de la presente revisión: en los metanálisis de griseofulvina versus terbinafina (dos a cuatro semanas), la griseofulvina (seis a ocho semanas) fue al menos tan efectiva como la terbinafina para las infecciones por Trichophyton, Microsporum y mixtas por Trichophyton y Microsporum para el resultado primario curación completa.
Tey 2011, otro metanálisis, concluyó que la "griseofulvina es más eficaz que la terbinafina para tratar la tiña capitis causada por las especies de Microsporum". Además, Gupta 2013 realizó un metanálisis adicional de ensayos controlados aleatorios y no encontró diferencias en la eficacia general de los dos fármacos a las dosis especificadas pero se observaron diferencias según las especies, infecciosas, es decir la griseofulvina fue superior para Microsporum spp., mientras que la terbinafina fue superior para Trichophyton spp. La guía reciente de la British Association of Dermatologists (BAD) también recomendó la griseofulvina como el tratamiento de primera línea para la tiña capitis en los niños, especialmente en los que presentan infección por Microsporum spp. (Fuller 2014). Esta actualización de la revisión también proporciona pruebas nuevas de calidad moderada de que para las infecciones por Microsporum, la griseofulvina es mejor que terbinafina con respecto a la proporción de participantes que logran la curación completa (Resumen de los hallazgos para la comparación principal), lo que coincide con revisiones y guías anteriores.
En las otras comparaciones de la griseofulvina con agentes antimicóticos triazoles (itraconazol, fluconazol y ketoconazol) no se encontraron pruebas de que la griseofulvina tuviera una eficacia superior para el resultado de curación completa, pero estos fueron ensayos pequeños y la calidad de las pruebas fue moderada en el mejor de los casos.
Solamente se encontraron pruebas limitadas acerca de las dosis, la duración y las formulaciones más apropiadas de la griseofulvina. Las recomendaciones de dosis para la griseofulvina varían debido a las diferentes formas farmacéuticas (Higgins 2000), y tomar la griseofulvina con alimentos grasos mejora su absorción y biodisponibilidad. Algunos ensayos recomendaron dosis mayores para la griseofulvina micronizada pero no para la griseofulvina ultramicronizada, aunque pueden ser necesarios hasta 25 mg/kg (Higgins 2000). No se encontraron pruebas que compararan directamente diferentes formulaciones de griseofulvina.
La desventaja principal de la griseofulvina es la necesidad de una duración larga del tratamiento, lo que puede reducir la adherencia. La European Society for Pediatric Dermatology (ESPD) recomendó, "La decisión de tratamiento entre la griseofulvina y los agentes antimicóticos más nuevos para los niños con tiña capitis por Trichophyton spp. se puede basar en un paciente individual en el equilibrio entre la duración del tratamiento �?� la adherencia y las consideraciones con económicas" (Kakourou 2010). Todavía no está claro si hay una diferencia en cuanto a la adherencia entre cuatro semanas de terbinafina versus ocho semanas de griseofulvina, ya que en esta revisión no se encontraron pruebas que apoyaran la hipótesis de que la adherencia mejora con ciclos de tratamientos más cortos. No obstante, se podría argumentar que los ciclos largos de griseofulvina versus los ciclos más cortos de terbinafina, por ejemplo, ya tienen incorporada alguna reducción en la adherencia sencillamente debido a la duración más larga del primero.
Se incluyeron dos estudios que informaron datos sobre la duración del tratamiento en los participantes infectados por Microsporum ( Elewski 2008; Lipozencic 2002). Una proporción inferior de participantes tratados durante seis a ocho semanas con terbinafina para las infecciones por M. canis y M. audouini lograron la curación completa, en comparación con los tratados con griseofulvina (no fue estadísticamente significativo). La duración a largo plazo del tratamiento (diez o 12 semanas) dio lugar a que una proporción mayor de participantes lograra la curación completa en el grupo de griseofulvina en comparación con el grupo de terbinafina (Resumen de los hallazgos para la comparación principal). Sin embargo, a falta de comparaciones directas adicionales de la duración o las formulaciones, no es posible hacer observaciones adicionales sobre los regímenes óptimos para tratar la tiña capitis con griseofulvina en los niños.
Se ha informado que la griseofulvina es un fármaco seguro, aunque habitualmente provoca efectos secundarios como cefaleas y molestias gastrointestinales (Bennassar 2010). A pesar de la suposición de que los trastornos gastrointestinales son un inconveniente importante en los fármacos más antiguos como la griseofulvina, no se encontraron informes de trastornos gastrointestinales asociados con la administración de griseofulvina en los estudios incluidos. Se reconoce que, aunque los ECA no siempre pueden proporcionar una plataforma para informar los efectos secundarios poco frecuentes, son un método razonable para detectar los efectos habituales. Los eventos adversos habituales se incluyeron como un resultado en esta revisión; sin embargo, los estudios incluidos registraron algunos eventos adversos poco frecuentes que pueden o no estar relacionados con el tratamiento con griseofulvina. Se encontraron pruebas de que, cuando ocurren, los eventos adversos graves son comparables entre la terbinafina y la griseofulvina. No obstante, en la mayoría de los casos los eventos adversos fueron leves y reversibles. Dichos eventos incluyeron enzimas hepáticas séricas, triglicéridos y ácido úrico elevados; anemia, eosinofilia; leucocitosis y granulocitopenia. Aunque pocos eventos adversos observados se consideraron graves, es aconsejable la monitorización periódica todos los meses de las funciones de los sistema de órganos que incluyen los sistemas hepático, renal y hematopoyético (sangre) en los pacientes que reciben griseofulvina durante más de ocho semanas (Möhrenschlager 2005).
La griseofulvina ha sido tradicionalmente el tratamiento sistémico utilizado con más frecuencia para la tiña capitis y sus ventajas son que es de bajo costo, hay vasta experiencia en su uso, la formulación en suspensión permite la dosis exacta en los niños y está autorizada en la mayoría de los países. Sin embargo, la griseofulvina ya no está disponible en Nueva Zelanda y otros países, incluido Canadá (Bortolussi 2016), donde ha sido reemplazado por fármacos antimicóticos más nuevos.
Terbinafina
La versión original de esta revisión encontró que la terbinafina fue al menos tan efectiva como la griseofulvina para el tratamiento de las infecciones del cuero cabelludo por Trichophyton. En esta actualización se agregaron algunas pruebas nuevas que apoyan este resultado. Se encontró que para T. tonsurans, la terbinafina es mejor que la griseofulvina, mientras que los dos regímenes tuvieron efectos similares en los niños con T. violaceum. Este resultado también coincide con el de un metanálisis anterior que compara la terbinafina con la griseofulvina (Fleece 2004).
La eficacia del tratamiento con terbinafina puede variar según las especies micóticas aisladas. Nuevas pruebas en esta actualización indican que la terbinafina tiene un efecto más deficiente sobre la curación completa en los participantes con infecciones por Microsporum. Este resultado guarda relación con la guía BAD de 2014 (Fuller 2014), que concluyó que la terbinafina fue más efectiva contra especies de Trichophyton; mientras que la griseofulvina fue más efectiva contra especies de Microsporum.
No se encontraron pruebas acerca de las formulaciones de terbinafina. Aunque los comprimidos pueden ser preferidos por algunos niños (quizás con cinco años de edad y más), pueden no tener en cuenta la individualización de la dosis (generalmente calculada por el peso corporal, por lo que se pueden necesitar dosis más pequeñas que las proporcionadas por estos comprimidos). Las gotas o jarabes son preferibles a los comprimidos para individualizar las dosis en los niños y hay una forma granulada de terbinafina para espolvorear en los alimentos (Fuller 2014).
La duración del tratamiento con terbinafina puede ser importante. Pruebas limitadas de estudios observacionales indican que los regímenes terapéuticos más largos de terbinafina pueden mejorar la curación completa en los pacientes con infección por especies de Microsporum (Aste 2004; Commens 2003; Devliotou 2004). En el metanálisis de cuatro estudios que compararon directamente dos semanas versus cuatro semanas de duración del tratamiento con terbinafina, una duración más larga fue significativamente mejor que una duración corta para una curación completa.
Itraconazol
El itraconazol es actualmente el agente preferido en la mayoría de los países europeos, aunque no se ha autorizado en algunos países (p.ej. el Reino Unido) para el tratamiento de la tiña capitis en los niños de 12 años de edad o más jóvenes (Fuller 2014). Sin embargo, en esta revisión solamente se identificaron pruebas limitadas basadas en ensayos pequeños que indicaron que el itraconazol oral a dosis ajustadas por el peso fue efectivo y seguro para la tiña capitis causada por T. violaceum (dos semanas de tratamiento) y M. canis (seis semanas de tratamiento). Se necesitan más ECA bien diseñados con tamaños de la muestra grandes para confirmar la seguridad, la eficacia y los regímenes de tratamiento óptimos de itraconazol en pacientes pediátricos con tiña capitis.
Fluconazol
En esta actualización se incluyó un ECA grande que comparó la administración a corto plazo (6 mg/kg/día por tres semanas) con la administración a plazo medio de fluconazol (6 mg/kg/día por seis semanas) (Foster 2005). Según este estudio, la administración a corto plazo y a plazo medio de fluconazol tuvo un efecto similar sobre la curación completa. En la guía BAD 2014 (Fuller 2014) se recomendó el fluconazol como una opción a la terbinafina para la tiña capitis en los niños. Sin embargo, todavía no está claro el régimen óptimo de fluconazol para tratar la tiña capitis pediátrica. Según Fuller 2014 y los hallazgos de esta revisión, la duración a corto plazo del fluconazol puede ser un régimen de tratamiento útil.
Ketoconazol
Esta revisión identificó pruebas de baja calidad que indicaron que el ketoconazol pareció tener una eficacia similar a la griseofulvina para la tiña capitis en los niños, aunque parece ser menos efectivo que la griseofulvina en los niños con tiña capitis causada por especies de Trichophyton (Gan 1987). Sin embargo, no se recomienda el uso del ketoconazol en niños debido a los eventos adversos potenciales, especialmente la hepatotoxicidad (Elewski 2000), y el ketoconazol oral se retiró en el Reino Unido y Europa en 2013 (Fuller 2014).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
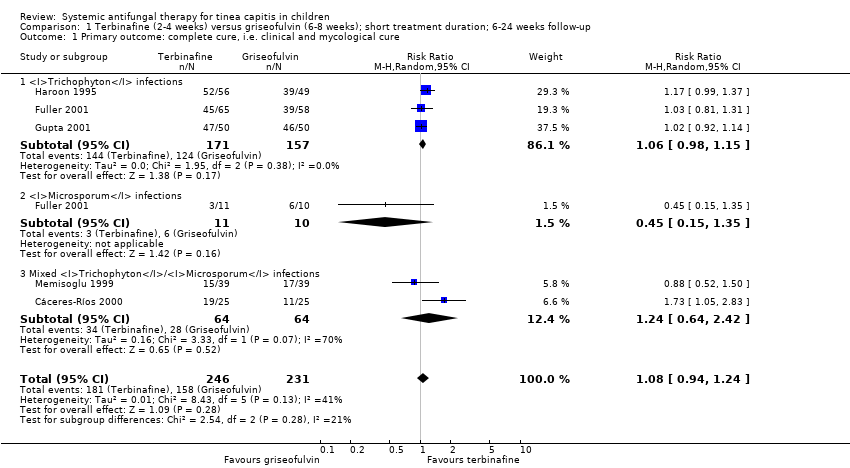
Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 2 Primary outcome: adverse events.

Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 3 Secondary outcome: proportion of participants with clinical cure only.

Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 4 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 2 Primary outcome: drug‐related adverse events.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 3 Primary outcome: severe adverse events.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 4 Secondary outcomes: proportion of participants with clinical cure only.
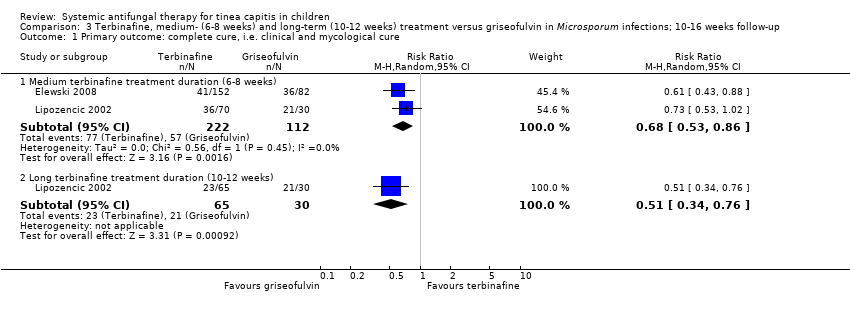
Comparison 3 Terbinafine, medium‐ (6‐8 weeks) and long‐term (10‐12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
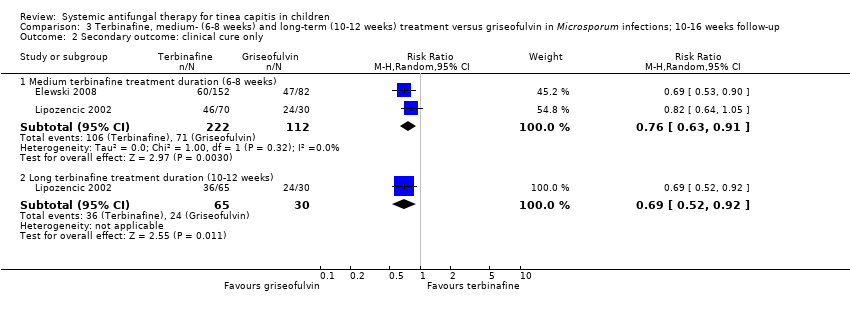
Comparison 3 Terbinafine, medium‐ (6‐8 weeks) and long‐term (10‐12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up, Outcome 2 Secondary outcome: clinical cure only.

Comparison 4 Terbinafine, short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12‐20 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 4 Terbinafine, short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12‐20 weeks follow‐up, Outcome 2 Secondary outcome: clinical cure only.

Comparison 5 Terbinafine standard dose versus double dose in Microsporum infections; 20 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
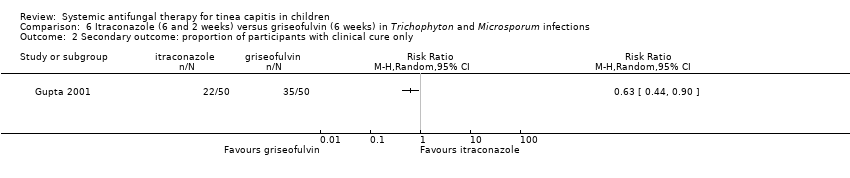
Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 2 Secondary outcome: clinical cure only.

Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.
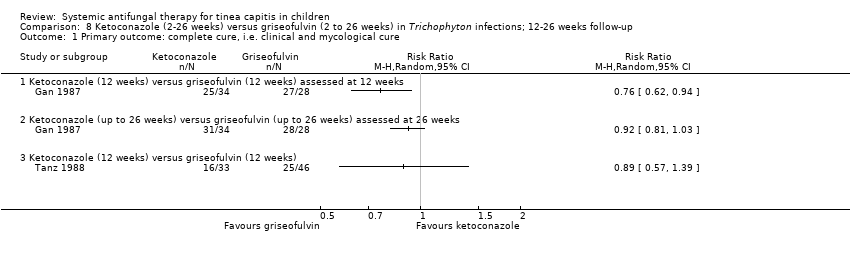
Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
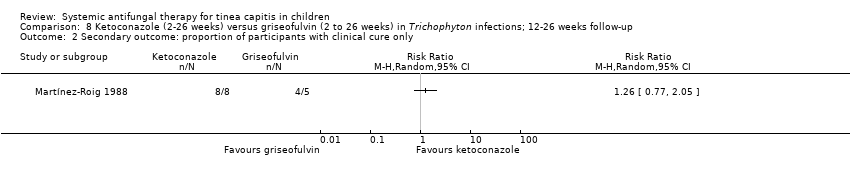
Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 10 Fluconazole (2‐3 weeks) versus terbinafine (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 12 Fluconazole low dose versus higher dose (1.5, 3.0 and 6.0 mg/kg/d) in Trichophyton infections; 4 months follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 13 Fluconazole 3 weeks versus 6 weeks; 10 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
| Terbinafine versus griseofulvin for children with tinea capitis | ||||||
| Patient or population: children with tinea capitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Griseofulvin | Terbinafine | |||||
| Proportion of participants with complete cure | 790 per 1000 | 837 per 1000 | RR 1.06 | 328 | ⊕⊕⊝⊝ | This outcome was for children infected with Trichophyton, terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration |
| Proportion of participants with complete cure | 378 per 1000 | 446 per 1000 | RR 1.18 | 1006 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton (T. tonsurans and T. violaceum) Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 354 per 1000 | 521 per 1000 | RR 1.47 | 764 | ⊕⊕⊕⊝ | This outcome was for children infected with T. tonsurans Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 451 per 1000 | 411 per 1000 | RR 0.91 | 242 | ⊕⊕⊝⊝ | This outcome was for children infected with T. violaceum Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 509 per 1000 | 346 per 1000 | RR 0.68 | 334 | ⊕⊕⊕⊝ | This outcome was for children infected with Microsporum. Terbinafine medium‐ (6 to 8 weeks) and long‐term (10 to 12 weeks) treatment versus griseofulvin |
| Proportion of participants with complete cure | 600 per 1000 | 270 per 1000 (90 to 810) | RR 0.45 (0.15 to 1.35) | 21 (1 study) | ⊕⊝⊝⊝ | This outcome was for children infected with Microsporum. Terbinafine short‐term (4 weeks) versus griseofulvin |
| Adverse events attributed to the study drugs | 83 per 1000 | 92 per 1000 | RR 1.11 | 1549 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton and Microsporum Terbinafine (6 weeks) versus griseofulvin (6 weeks), medium treatment duration |
| Severe adverse events | 6 per 1000 | 6 per 1000 | RR 0.97 | 1549 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton and Microsporum Terbinafine (6 weeks) versus griseofulvin (6 weeks), medium treatment duration |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because 1 of the 3 studies was at high risk of bias, the other two studies were at unclear risk of bias. | ||||||
| Itraconazole versus griseofulvin for children infected with Trichophyton and Microsporum | |||||
| Patient or population: children infected with Trichophyton and Microsporum | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Griseofulvin | Itraconazole | ||||
| Proportion of participants with complete cure | 910 per 1000 | 838 per 1000 | RR 0.92 | 134 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because both studies were at unclear risk of bias. | |||||
| Itraconazole versus terbinafine in children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Terbinafine | Itraconazole | ||||
| Proportion of participants with complete cure | 788 per 1000 | 732 per 1000 | RR 0.93 | 160 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because both studies were at unclear risk to bias. | |||||
| Ketoconazole versus griseofulvin in children infected with Trichophyton | ||||||
| Patient or population: children infected with Trichophyton | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Griseofulvin | Ketoconazole | |||||
| Proportion of participants with complete cure Follow‐up: 12 weeks | 964 per 1000 | 733 per 1000 (598 to 906) | RR 0.76 (0.62 to 0.94) | 62 | ⊕⊕⊝⊝ | Ketoconazole (12 weeks) versus griseofulvin (12 weeks) |
| Proportion of participants with complete cure Follow‐up: 26 weeks | 1000 per 1000 | 920 per 1000 (810 to 1000) | RR 0.92 (0.81 to 1.03) | 62 (1 study) | ⊕⊕⊝⊝ | Ketoconazole (up to 26 weeks) versus griseofulvin (up to 26 weeks) |
| Proportion of participants with complete cure Follow‐up: 12 weeks | 543 per 1000 | 484 per 1000 (310 to 755) | RR 0.89 (0.57 to 1.39) | 79 (1 study) | ⊕⊕⊝⊝ | Ketoconazole (12 weeks) versus griseofulvin (12 weeks) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because the study was at high risk of bias. | ||||||
| Fluconazole versus griseofulvin in children with tinea capitis | |||||
| Patient or population: children with tinea capitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Griseofulvin | Fluconazole | ||||
| Proportion of participants with complete cure | 449 per 1000 | 413 per 1000 | RR 0.92 | 615 | ⊕⊕⊕⊝ |
| Proportion of participants with complete cure | 322 per 1000 | 341 per 1000 | RR 1.06 | 361 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because one of the three studies was at high risk of bias, the other two were at unclear risk of bias. | |||||
| Fluconazole versus terbinafine for children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Terbinafine | Fluconazole | ||||
| The proportion of participants with complete cure | 940 per 1000 | 818 per 1000 | RR 0.87 | 100 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Fluconazole versus itraconazole in children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Itraconazole | Fluconazole | ||||
| Proportion of participants with complete cure Follow‐up:12 weeks | 820 per 1000 | 820 per 1000 | RR 1.00 | 100 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Different durations of fluconazole in children infected with T. tonsurans and M. canis | |||||
| Patient or population: children infected with T. tonsurans and M. canis Comparison: fluconazole (6 weeks duration) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Fluconazole ( 6 weeks duration) | Fluconazole ( 3 weeks duration) | ||||
| Proportion of participants with complete cure Follow‐up: 8‐12 weeks | 341 per 1000 | 300 per 1000 | RR 0.88 | 491 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 5 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 1.1 Trichophyton infections | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.2 Microsporum infections | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.15, 1.35] |
| 1.3 Mixed Trichophyton/Microsporum infections | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.64, 2.42] |
| 2 Primary outcome: adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Drug‐related adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 2‐week terbinafine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 4‐week terbinafine | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.74, 1.88] |
| 1.1 T. tonsurans infections | 1 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.22, 1.77] |
| 1.2 T. violaceum infections | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.24] |
| 2 Primary outcome: drug‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Primary outcome: severe adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Secondary outcomes: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 T. tonsurans infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 T. violaceum infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Medium terbinafine treatment duration (6‐8 weeks) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.86] |
| 1.2 Long terbinafine treatment duration (10‐12 weeks) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 2 Secondary outcome: clinical cure only Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Medium terbinafine treatment duration (6‐8 weeks) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |
| 2.2 Long terbinafine treatment duration (10‐12 weeks) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.52, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 1‐2 weeks versus 4 weeks | 4 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.86] |
| 1.2 Medium term (6‐8 weeks) versus long term (10‐12 weeks) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.97, 2.17] |
| 2 Secondary outcome: clinical cure only Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 1‐2 weeks versus 4 weeks | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.06] |
| 2.2 Medium term (6‐8 weeks) versus long term (10‐12 weeks) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.90, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.19] |
| 2 Secondary outcome: clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Ketoconazole (12 weeks) versus griseofulvin (12 weeks) assessed at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ketoconazole (up to 26 weeks) versus griseofulvin (up to 26 weeks) assessed at 26 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Ketoconazole (12 weeks) versus griseofulvin (12 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (2‐4 weeks) fluconazole | 3 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 1.2 Medium‐term (6 weeks) fluconazole | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.46] |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 1.5 mg versus 3.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1.5 mg versus 6.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 3.0 mg versus 6.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

