Tratamiento antimicótico sistémico para la tiña capitis en niños
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
| |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: There was only one drop‐out (2%, 1/50). Although which group this drop‐out belonged to was unclear and ITT analysis was not performed, the proportion of missing outcomes compared with the observed event risk seemed to be not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Single‐blind, parallel group RCT for 8 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Participants were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Investigators were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 5 participants (12.5%, 5/40) were lost to follow‐up. It was unclear which group these drop‐outs belonged to. ITT analysis was not performed. The reason for drop‐outs was not clear. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | 3‐arm, parallel group RCT for 1 year | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
One of the primary outcomes of interest in this review (complete cure rate) was not reported in this study. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Whether the participants and personnel were blinded was not stated. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Investigators were not blinded. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 12.5% (11/88) participants lost to follow‐up. It was unclear which group these drop‐outs belonged to. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Multicentre, single‐blind, parallel group RCT for 10 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
| |
| Notes | This article included 2 RCTs, but the results of the 2 RCTs were reported together. Funding: Novartis Pharmaceuticals Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 43): "Eligible patients were randomized in a 2:1 ratio to terbinafine and griseofulvin treatment arms, respectively (Fig 1). Patients were randomized to the lowest available randomization number at each site based on treatment allocation cards received by a pharmacist or designee at the site after they had fulfilled the inclusion/exclusion criteria". Comment: Standared randomisation method was applied |
| Allocation concealment (selection bias) | Low risk | Quote (page 43): "Randomization data were accessible only to the dispenser of medication and were kept confidential until database lock." Comment: The method for allocation concealment seemed to be adequate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 45): "Investigators and others performing assessments, recording data, or analysing data were blinded to treatment identity from the time of randomisation until database lock." Comment: The method of blinding was not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 45): "Investigators and others performing assessments, recording data, or analysing data were blinded to treatment identity from the time of randomisation until database lock." Comment: The method of blinding was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 3.6% (56/1549) participants lost to follow‐up. Among them, 40 participants were in the terbinafine group and 16 participants in the griseofulvin group. ITT analyses were performed. The proportion of missing outcomes compared with the observed event risk didn't seem to be enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Multicentre, single‐blind, parallel group RCT for 10 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
One of the primary outcomes of interest in this review (complete cure rate) was not reported in this study. | |
| Notes | Main data of this article were from 2 identical studies. The safety data from this article were from 3 studies. Overall, 90% of participants were from USA. Funding: Prifzer, Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Quote (page 799): "Sealed envelopes containing randomly assigned treatments" were applied. Comment: A standard method of location concealment was done. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Whether the participants and personnel were blinded was not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Investigators were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 9.3% (82/880) participants lost to follow‐up. Among them, 37 participants were in the fluconazole 3 weeks group; 24 participants were in the fluconazole 6 weeks group; and 21 participants were in the griseofulvin group. ITT analyses were performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
Followed by placebo to complete 4 weeks when needed Co‐treatment: non‐medicated shampoo twice weekly | |
| Outcomes |
| |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 23.2% (41/177) participants lost to follow‐up. Among them, 14 patents were in the terbinafine for 1 week group; 15 participants were in the terbinafine for 2 weeks group; 12 participants were in the terbinafine for 4 weeks group. ITT analyses were performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Open‐label, parallel group RCT for 24 weeks | |
| Participants |
| |
| Interventions |
Co‐treatment: selenium sulphide shampoo, twice weekly for the first 2 weeks of treatment | |
| Outcomes |
| |
| Notes | Funding: Novartis Pharmaceuticals UK Ltd (Terbinafine). The report of the study stated that "T. tonsurans accounted for 77% of the terbinafine group and 88% of the griseofulvin group. Microsporum species accounted for 14% of both groups" (i.e. 88% plus 14% = 102%). We used the data from this paper's tables (which seemed to be reliable). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 323): "Computer generated", "participants randomised in blocks of four" Comment: standard methods of randomisation performed |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) | High risk | Comment: 54.8% (115/210) participants lost to follow‐up. Among them, 62 participants were in the terbinafine group; 53 participants were in the griseofulvin group. ITT analyses were performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Open‐label, parallel group RCT for 26 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
One of the primary outcomes of interest in the review (adverse events) was not reported in this study. | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 47): "Table of random numbers" was used. Comment: A standard method of randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) | High risk | Comment: 21.3% (17/80) participants lost to follow‐up. Among them, 11 participants were in the griseofulvin group, whereas 6 were in the ketoconazole group. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: publication reported findings on all outcomes listed in the Methods section |
| Other bias | Unclear risk |
|
| Methods | Multicentre, double‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 7% (14/200) participants lost to follow‐up. Among them, 4 were in the griseofulvin group, 2 were in the terbinafine group, 4 were in the itraconazole group, 4 were in the fluconazole group. ITT analysis was not performed. The proportion of missing outcomes compared with the observed event risk seemed to be not enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
| |
| Notes | Participants were observed for 12 weeks. After 4 weeks, non‐responders were offered an additional 4 weeks of treatment followed by a second observation period Funding: Novartis (terbinafine) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding: Sandoz | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: insufficient information although stated to be a "double‐blind comparative study of terbinafine and griseofulvin in tinea capitis" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: insufficient information although stated to be a "double‐blind comparative study of terbinafine and griseofulvin in tinea capitis" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
| |
| Notes | Funding: Sandoz (terbinafine) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
| |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 6 weeks (although the author stated this was a "third party blind" study in the abstract, it was unclear whether they applied blinding method or not). | |
| Participants |
| |
| Interventions |
Co‐treatment: not mentioned | |
| Outcomes |
| |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: unclear, although the author stated this was a "third party blind" study in the abstract |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: unclear, although the author stated this was a "third party blind" study in the abstract |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Single‐blind, parallel group RCT for 20 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
One of the primary outcomes of interest in this review (adverse events) was not reported in this study. | |
| Notes | The proportions and percentages were done including the adults, because we are unaware which group they belonged to. Funding: Sandoz (terbinafine) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: study was described as "single blind and open trial study" but no mention of which one (participant or observers), and the method of blinding was not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: study was described as "single blind and open trial study" but no mention of which one (participant or observers), and the method of blinding was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 8.5% (7/82) participants lost to follow‐up. It was unclear which group these lost participants belonged to. ITT analysis was not performed. The proportion of missing outcomes compared with the observed event risk seemed insufficient to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Multicentre, triple‐blind, parallel group RCT for 16 weeks | |
| Participants |
| |
| Interventions |
Participants were provided with baby‐shampoo to clean the scalp. | |
| Outcomes |
| |
| Notes | Funding: Novartis Pharma AG | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Participants, clinicians and outcome assessors blinded, except for the arm taking griseofulvin, where both participants and investigators were informed from day 1" Comment: The method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Participants, clinicians and outcome assessors blinded, except for the arm taking griseofulvin, where both participants and investigators were informed from day 1" Comment: The method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 21.8% (36/165) participants lost to follow‐up. Among them, 7 were in the terbinafine for 6 weeks group, 4 were in the terbinafine for 8 weeks group, 6 were in the terbinafine for 10 weeks group, 12 were in the terbinafine for 12 weeks group, 7 were in the griseofulvin group. ITT analyses were performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding: Janssen (itraconazole) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 8.6% (3/35) participants lost to follow‐up. Among them, 1 was in the itraconazole group, 2 were in the griseofulvin group. ITT analysis was not performed. The proportion of missing outcomes compared with the observed event risk seemed to be not enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 6 weeks | |
| Participants |
| |
| Interventions |
Co‐treatment: manual depilation in cases of inflammatory tinea capitis. | |
| Outcomes |
One of the primary outcomes of interest in this review (complete cure rate) was not reported in this study. | |
| Notes | Tinea corporis and tinea capitis study, information poorly stated Funding: Laboratories Dr Esteve (ketoconazole) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 38): "Computer generated random number table" Comment: A standard method of randomisation was done. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | At the beginning there were 39 participants in each group, after the drop‐outs there were 32 and 35 left, and so the percentages do not match:
These fungi percentages are the total over 67, not over 78 participants. Funding: not mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 14.1% (11/78) participants lost to follow‐up. Among them, 7 were in the griseofulvin group, whereas 4 were in the terbinafine group. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Open‐label, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
Co‐treatment: ketoconazole shampoo twice a week and econazole cream nightly was recommended | |
| Outcomes |
One of the primary outcomes of interest in this review (complete cure rate) was not reported in this study. | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding |
| Blinding of outcome assessment (detection bias) | High risk | Comment: no blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: not mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Double‐blind, parallel group RCT for 16 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
One of the primary outcomes of interest in this review (adverse events) was not reported in this study | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants were not blinded, but the outcomes were unlikely to be influenced; clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 51.8% (14/27) participants lost to follow‐up. It was unclear which group these lost participants belonged to. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Single‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
No co‐treatment | |
| Outcomes |
| |
| Notes | Funding: Sandoz (terbinafine) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Only participants blinded, but the method of blinding was not stated. The outcomes were likely to be influenced if the clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Comment: Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 18.2% (24/132) participants lost to follow‐up. Among them, 6 were in the terbinafine for 1 week group, 6 were in the terbinafine for 2 weeks group, and 12 were in the terbinafine for 4 weeks group. ITT analyses were performed. The reasons for drop‐outs were not clear. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 6 weeks | |
| Participants |
| |
| Interventions |
Co‐treatment: antiseborrhoeic shampoo | |
| Outcomes |
One of the primary outcomes of interest in this review (complete cure rate) was not reported in this study | |
| Notes | Funding: Janssen (ketoconazole) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 36.4% (8/22) participants lost to follow‐up. Among them, 3 were in the griseofulvin group, whereas 5 were in the ketoconazole group. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
| Methods | Triple‐blind, parallel group RCT for 12 weeks | |
| Participants |
| |
| Interventions |
Co‐treatment: antiseborrhoeic shampoos | |
| Outcomes |
| |
| Notes | Not much information given apart from the total cured results Funding: Janssen (ketoconazole) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | High risk | Comment: 39.2% (31/79) participants lost to follow‐up. Among them, 20 were in the griseofulvin group, whereas 11 were in the ketoconazole group. ITT analysis was not performed. |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
There were no statistically significant differences between the groups in terms of age, sex, weight or duration of infection |
| Methods | Triple‐blind, parallel group RCT for 20 weeks | |
| Participants |
| |
| Interventions |
| |
| Outcomes |
One of the primary outcomes of interest in this review (adverse events) was not reported in this study | |
| Notes | Funding: Institute of Dermatology Research Funds and Novartis (Thailand) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: There was no information on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: There was no information on the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Participants and clinicians were blinded, but the method of blinding was not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Outcome assessors were blinded, but the method of blinding was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no drop‐outs |
| Selective reporting (reporting bias) | Low risk | Comment: The publication reported findings on all outcomes listed in the Methods section. |
| Other bias | Unclear risk |
|
ITT: intention‐to‐treat; KOH: potassium hydroxide; RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Excluded because this study evaluated the therapy for inflammatory lesions caused by tinea capitis.The main aim of this study was to analyse the treatment of kerions in tinea capitis, combining the tinea capitis treatment of griseofulvin plus intralesional corticosteroid to try to reduce the inflammation. | |
| Excluded because this study evaluated the therapy for inflammatory lesions caused by tinea capitis. It combined griseofulvin for the tinea capitis with steroids to modulate the immune‐mediated inflammation, hasten resolution of kerions and minimise scar formation. | |
| Excluded because this study evaluated the therapy for inflammatory lesions caused by tinea capitis. It combined griseofulvin treatment and griseofulvin treatment plus prednisolone, a glucocorticoid. | |
| Excluded because this study was not a RCT | |
| Excluded because this study was not a RCT |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Single‐blinded, randomised controlled trial |
| Participants | 64 children with tinea capitis aged 4‐12 years randomised to 3 treatment groups. 5 participants lost to follow‐up. |
| Interventions |
|
| Outcomes | Primary outcome: mycological cure rate at week 6 Secondary outcomes: clinical improvement according to clinical symptom score; mycological cure rate at month 6; the type and frequency of adverse events |
| Notes | — |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 5 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| Analysis 1.1  Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 Trichophyton infections | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.2 Microsporum infections | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.15, 1.35] |
| 1.3 Mixed Trichophyton/Microsporum infections | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.64, 2.42] |
| 2 Primary outcome: adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 2 Primary outcome: adverse events. | ||||
| 2.1 Drug‐related adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 3 Secondary outcome: proportion of participants with clinical cure only. | ||||
| 3.1 2‐week terbinafine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 4‐week terbinafine | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 4 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.74, 1.88] |
| Analysis 2.1  Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 T. tonsurans infections | 1 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.22, 1.77] |
| 1.2 T. violaceum infections | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.24] |
| 2 Primary outcome: drug‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 2 Primary outcome: drug‐related adverse events. | ||||
| 3 Primary outcome: severe adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 3 Primary outcome: severe adverse events. | ||||
| 4 Secondary outcomes: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 4 Secondary outcomes: proportion of participants with clinical cure only. | ||||
| 4.1 T. tonsurans infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 T. violaceum infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Terbinafine, medium‐ (6‐8 weeks) and long‐term (10‐12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 Medium terbinafine treatment duration (6‐8 weeks) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.86] |
| 1.2 Long terbinafine treatment duration (10‐12 weeks) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 2 Secondary outcome: clinical cure only Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Terbinafine, medium‐ (6‐8 weeks) and long‐term (10‐12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up, Outcome 2 Secondary outcome: clinical cure only. | ||||
| 2.1 Medium terbinafine treatment duration (6‐8 weeks) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |
| 2.2 Long terbinafine treatment duration (10‐12 weeks) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.52, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Terbinafine, short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12‐20 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 1‐2 weeks versus 4 weeks | 4 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.86] |
| 1.2 Medium term (6‐8 weeks) versus long term (10‐12 weeks) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.97, 2.17] |
| 2 Secondary outcome: clinical cure only Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Terbinafine, short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12‐20 weeks follow‐up, Outcome 2 Secondary outcome: clinical cure only. | ||||
| 2.1 1‐2 weeks versus 4 weeks | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.06] |
| 2.2 Medium term (6‐8 weeks) versus long term (10‐12 weeks) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.90, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Terbinafine standard dose versus double dose in Microsporum infections; 20 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| Analysis 6.1  Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 2 Secondary outcome: proportion of participants with clinical cure only. | ||||
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.19] |
| Analysis 7.1  Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 2 Secondary outcome: clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 2 Secondary outcome: clinical cure only. | ||||
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 Ketoconazole (12 weeks) versus griseofulvin (12 weeks) assessed at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ketoconazole (up to 26 weeks) versus griseofulvin (up to 26 weeks) assessed at 26 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Ketoconazole (12 weeks) versus griseofulvin (12 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only. | ||||
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 Short‐term (2‐4 weeks) fluconazole | 3 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 1.2 Medium‐term (6 weeks) fluconazole | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.46] |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only. | ||||
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.3  Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Fluconazole (2‐3 weeks) versus terbinafine (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 11.2  Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only. | ||||
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 11.3  Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 Fluconazole low dose versus higher dose (1.5, 3.0 and 6.0 mg/kg/d) in Trichophyton infections; 4 months follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||
| 1.1 1.5 mg versus 3.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1.5 mg versus 6.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 3.0 mg versus 6.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 13.1  Comparison 13 Fluconazole 3 weeks versus 6 weeks; 10 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
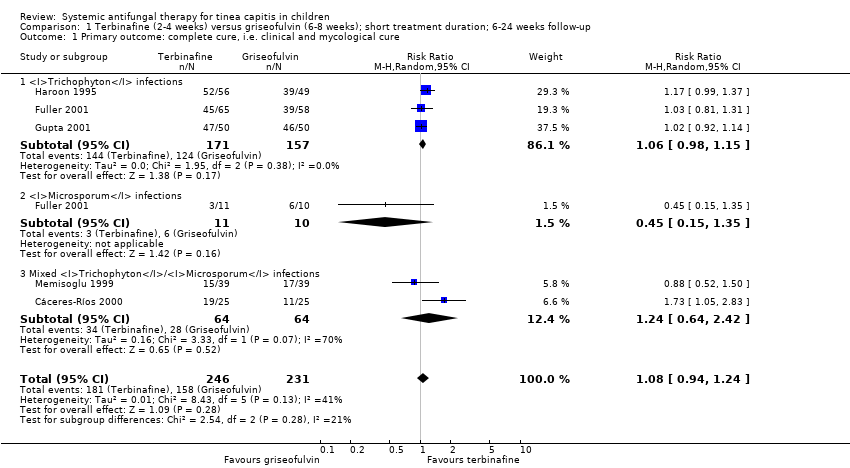
Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 2 Primary outcome: adverse events.

Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 3 Secondary outcome: proportion of participants with clinical cure only.

Comparison 1 Terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration; 6‐24 weeks follow‐up, Outcome 4 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 2 Primary outcome: drug‐related adverse events.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 3 Primary outcome: severe adverse events.

Comparison 2 Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration; 10 weeks follow‐up, Outcome 4 Secondary outcomes: proportion of participants with clinical cure only.
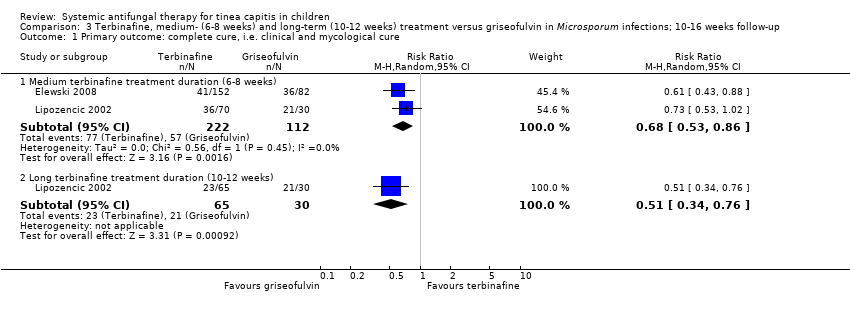
Comparison 3 Terbinafine, medium‐ (6‐8 weeks) and long‐term (10‐12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
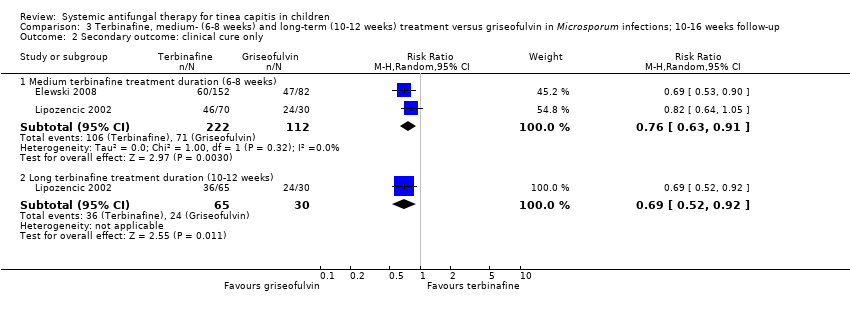
Comparison 3 Terbinafine, medium‐ (6‐8 weeks) and long‐term (10‐12 weeks) treatment versus griseofulvin in Microsporum infections; 10‐16 weeks follow‐up, Outcome 2 Secondary outcome: clinical cure only.

Comparison 4 Terbinafine, short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12‐20 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 4 Terbinafine, short‐term versus long‐term for treating Trichophyton and Microsporum infections; 12‐20 weeks follow‐up, Outcome 2 Secondary outcome: clinical cure only.

Comparison 5 Terbinafine standard dose versus double dose in Microsporum infections; 20 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
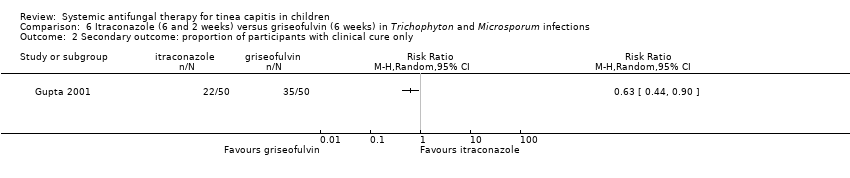
Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 6 Itraconazole (6 and 2 weeks) versus griseofulvin (6 weeks) in Trichophyton and Microsporum infections, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 2 Secondary outcome: clinical cure only.

Comparison 7 Itraconazole versus terbinafine (both 2 weeks) in Trichophyton infections, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.
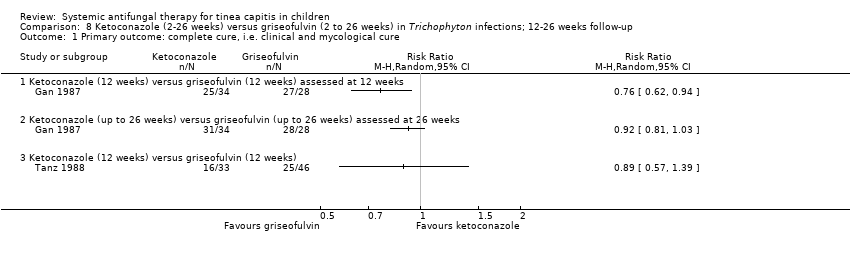
Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
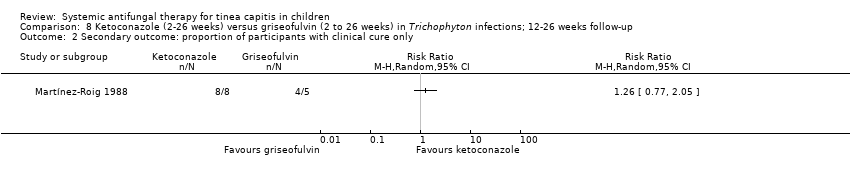
Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 8 Ketoconazole (2‐26 weeks) versus griseofulvin (2 to 26 weeks) in Trichophyton infections; 12‐26 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 9 Fluconazole (2‐6 weeks) versus griseofulvin (6 weeks); 8‐12 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 10 Fluconazole (2‐3 weeks) versus terbinafine (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 2 Secondary outcome: proportion of participants with clinical cure only.

Comparison 11 Fluconazole (2‐3 weeks) versus itraconazole (2‐3 weeks) in Trichophyton infections; 12 weeks follow‐up, Outcome 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence.

Comparison 12 Fluconazole low dose versus higher dose (1.5, 3.0 and 6.0 mg/kg/d) in Trichophyton infections; 4 months follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.

Comparison 13 Fluconazole 3 weeks versus 6 weeks; 10 weeks follow‐up, Outcome 1 Primary outcome: complete cure, i.e. clinical and mycological cure.
| Terbinafine versus griseofulvin for children with tinea capitis | ||||||
| Patient or population: children with tinea capitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Griseofulvin | Terbinafine | |||||
| Proportion of participants with complete cure | 790 per 1000 | 837 per 1000 | RR 1.06 | 328 | ⊕⊕⊝⊝ | This outcome was for children infected with Trichophyton, terbinafine (2‐4 weeks) versus griseofulvin (6‐8 weeks); short treatment duration |
| Proportion of participants with complete cure | 378 per 1000 | 446 per 1000 | RR 1.18 | 1006 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton (T. tonsurans and T. violaceum) Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 354 per 1000 | 521 per 1000 | RR 1.47 | 764 | ⊕⊕⊕⊝ | This outcome was for children infected with T. tonsurans Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 451 per 1000 | 411 per 1000 | RR 0.91 | 242 | ⊕⊕⊝⊝ | This outcome was for children infected with T. violaceum Terbinafine (6 weeks) versus griseofulvin (6 weeks) in Trichophyton infections; medium treatment duration |
| Proportion of participants with complete cure | 509 per 1000 | 346 per 1000 | RR 0.68 | 334 | ⊕⊕⊕⊝ | This outcome was for children infected with Microsporum. Terbinafine medium‐ (6 to 8 weeks) and long‐term (10 to 12 weeks) treatment versus griseofulvin |
| Proportion of participants with complete cure | 600 per 1000 | 270 per 1000 (90 to 810) | RR 0.45 (0.15 to 1.35) | 21 (1 study) | ⊕⊝⊝⊝ | This outcome was for children infected with Microsporum. Terbinafine short‐term (4 weeks) versus griseofulvin |
| Adverse events attributed to the study drugs | 83 per 1000 | 92 per 1000 | RR 1.11 | 1549 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton and Microsporum Terbinafine (6 weeks) versus griseofulvin (6 weeks), medium treatment duration |
| Severe adverse events | 6 per 1000 | 6 per 1000 | RR 0.97 | 1549 | ⊕⊕⊕⊝ | This outcome was for children infected with Trichophyton and Microsporum Terbinafine (6 weeks) versus griseofulvin (6 weeks), medium treatment duration |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because 1 of the 3 studies was at high risk of bias, the other two studies were at unclear risk of bias. | ||||||
| Itraconazole versus griseofulvin for children infected with Trichophyton and Microsporum | |||||
| Patient or population: children infected with Trichophyton and Microsporum | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Griseofulvin | Itraconazole | ||||
| Proportion of participants with complete cure | 910 per 1000 | 838 per 1000 | RR 0.92 | 134 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because both studies were at unclear risk of bias. | |||||
| Itraconazole versus terbinafine in children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Terbinafine | Itraconazole | ||||
| Proportion of participants with complete cure | 788 per 1000 | 732 per 1000 | RR 0.93 | 160 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because both studies were at unclear risk to bias. | |||||
| Ketoconazole versus griseofulvin in children infected with Trichophyton | ||||||
| Patient or population: children infected with Trichophyton | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Griseofulvin | Ketoconazole | |||||
| Proportion of participants with complete cure Follow‐up: 12 weeks | 964 per 1000 | 733 per 1000 (598 to 906) | RR 0.76 (0.62 to 0.94) | 62 | ⊕⊕⊝⊝ | Ketoconazole (12 weeks) versus griseofulvin (12 weeks) |
| Proportion of participants with complete cure Follow‐up: 26 weeks | 1000 per 1000 | 920 per 1000 (810 to 1000) | RR 0.92 (0.81 to 1.03) | 62 (1 study) | ⊕⊕⊝⊝ | Ketoconazole (up to 26 weeks) versus griseofulvin (up to 26 weeks) |
| Proportion of participants with complete cure Follow‐up: 12 weeks | 543 per 1000 | 484 per 1000 (310 to 755) | RR 0.89 (0.57 to 1.39) | 79 (1 study) | ⊕⊕⊝⊝ | Ketoconazole (12 weeks) versus griseofulvin (12 weeks) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because the study was at high risk of bias. | ||||||
| Fluconazole versus griseofulvin in children with tinea capitis | |||||
| Patient or population: children with tinea capitis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Griseofulvin | Fluconazole | ||||
| Proportion of participants with complete cure | 449 per 1000 | 413 per 1000 | RR 0.92 | 615 | ⊕⊕⊕⊝ |
| Proportion of participants with complete cure | 322 per 1000 | 341 per 1000 | RR 1.06 | 361 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aDowngraded one level because one of the three studies was at high risk of bias, the other two were at unclear risk of bias. | |||||
| Fluconazole versus terbinafine for children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Terbinafine | Fluconazole | ||||
| The proportion of participants with complete cure | 940 per 1000 | 818 per 1000 | RR 0.87 | 100 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Fluconazole versus itraconazole in children infected with Trichophyton | |||||
| Patient or population: children infected with Trichophyton | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Itraconazole | Fluconazole | ||||
| Proportion of participants with complete cure Follow‐up:12 weeks | 820 per 1000 | 820 per 1000 | RR 1.00 | 100 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Different durations of fluconazole in children infected with T. tonsurans and M. canis | |||||
| Patient or population: children infected with T. tonsurans and M. canis Comparison: fluconazole (6 weeks duration) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Fluconazole ( 6 weeks duration) | Fluconazole ( 3 weeks duration) | ||||
| Proportion of participants with complete cure Follow‐up: 8‐12 weeks | 341 per 1000 | 300 per 1000 | RR 0.88 | 491 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Downgraded one level because the study was at unclear risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 5 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 1.1 Trichophyton infections | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 1.2 Microsporum infections | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.15, 1.35] |
| 1.3 Mixed Trichophyton/Microsporum infections | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.64, 2.42] |
| 2 Primary outcome: adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Drug‐related adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 2‐week terbinafine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 4‐week terbinafine | 3 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | 1006 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.74, 1.88] |
| 1.1 T. tonsurans infections | 1 | 764 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.22, 1.77] |
| 1.2 T. violaceum infections | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.24] |
| 2 Primary outcome: drug‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Primary outcome: severe adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Secondary outcomes: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 T. tonsurans infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 T. violaceum infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Medium terbinafine treatment duration (6‐8 weeks) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.86] |
| 1.2 Long terbinafine treatment duration (10‐12 weeks) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 2 Secondary outcome: clinical cure only Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Medium terbinafine treatment duration (6‐8 weeks) | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.63, 0.91] |
| 2.2 Long terbinafine treatment duration (10‐12 weeks) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.52, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 1‐2 weeks versus 4 weeks | 4 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.86] |
| 1.2 Medium term (6‐8 weeks) versus long term (10‐12 weeks) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.97, 2.17] |
| 2 Secondary outcome: clinical cure only Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 1‐2 weeks versus 4 weeks | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.06] |
| 2.2 Medium term (6‐8 weeks) versus long term (10‐12 weeks) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.90, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.19] |
| 2 Secondary outcome: clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Ketoconazole (12 weeks) versus griseofulvin (12 weeks) assessed at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ketoconazole (up to 26 weeks) versus griseofulvin (up to 26 weeks) assessed at 26 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Ketoconazole (12 weeks) versus griseofulvin (12 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term (2‐4 weeks) fluconazole | 3 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 1.2 Medium‐term (6 weeks) fluconazole | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.46] |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: proportion of participants with clinical cure only Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Secondary outcome: percentage of drop‐outs as a surrogate for participant adherence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 1.5 mg versus 3.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1.5 mg versus 6.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 3.0 mg versus 6.0 mg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: complete cure, i.e. clinical and mycological cure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

