Ibuprofeno y paracetamol (acetaminofeno) para el alivio del dolor posterior a la extracción quirúrgica de la muela de juicio inferior
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blinded, randomised, placebo‐controlled, active comparator, 2‐centre study. Parallel group. Dummy medications given. Single dose. Postoperative dosing. | |
| Participants | Males and females 16–40 years of age with at least 1 bony impacted third molar or 2 ipsilateral impacted third molars. An impaction score was assigned to the third molars to demonstrate that they were suitably impacted in bone for inclusion. The main exclusion criteria were history of significant disease, ongoing painful conditions (other than the third molar), migraine, malabsorption states, allergy/intolerance to the study medication, gastrointestinal complaints, psychotic illness or drug abuse, concomitant medication that would have interfered with the study drugs, pregnancy/lactation and taking NSAIDs from midnight the night before surgery. 614 patients screened, 322 randomised and 318 completed study. | |
| Interventions | Patients underwent surgical removal of 1 partially or full bone impacted mandibular third molar, or 2 ipsilateral third molars under local anaesthetic with nitrous oxide sedation. Following surgery, patients who fulfilled the inclusion criteria regarding baseline pain intensity were randomly allocated to 1 of 4 treatment groups in the ratio 1:1:1:1. These were.
Rescue medication was provided, if required within the first 4 hours following surgery, an intramuscular injection of ketorolac tromethamine (60 mg) was given. After 4 hours, acetaminophen 500 mg/hydrocodone Antibiotics were prescribed postoperatively. Caffeine‐containing foods and drinks were to be discontinued from midnight prior to surgery until the end of the 6‐hour postdose assessment period. Patients were randomised to treatment when they rated their baseline PI as moderate or severe, and the score on the VAS was ≥ 50 mm but ≤ 85 mm. | |
| Outcomes | Pain intensity at baseline (immediately following surgery), 5, 10, 15, 20, 25, 30, 35, 40, 45, 60, 90, 120, 180, 240, 300 and 360 minutes after dosing measured using VAS and categorical scale of 0 (none) to 3 (severe). Pain relief measured at the same time as PI with the exception of baseline. Scale of 0‐4 used (0 = none and 4 = complete). Also asked whether starting pain has at least half gone (no = 0, yes = 1). Stopwatches were started at the time of dosing, 1 was stopped when the patient felt any pain relief whatsoever and the second was stopped when the patient decided that the relief was meaningful to them. If the patient did not stop the watches within the first 4 hours or if rescue medication was used, the stopwatches were discontinued for that patient. Distractibility from pain was assessed at baseline and at 60% 360 minutes after dosing. VAS was used in response to the question 'How easy is it for you to distract yourself from your pain?.' The Rainier Scale was completed at baseline and at 60 and 360 minutes after dosing. This assessed perceived functional impairment of activities of daily living, measured on a 1‐10 scale (1 = wound not interfere at all, 10 = would completely interfere). Time of rescue medication was recorded, patients taking rescue medication completed all pain intensity and pain relief assessments immediately before medication was taken and continued to record their pain assessments throughout the 6‐hour assessment period. Global evaluation was scored at the end of the 6‐hour period or at the time of rescue medication. Patients were asked, 'How effective do you think the study medication is as a treatment for pain?.' Response choices were 1 = excellent, 2 = very good, 3 = good, 4 = fair or 5 = poor. A postoperative review was conducted 5‐12 days after surgery. | |
| Notes | Sodium ibuprofen 256 mg is equivalent to 200 mg ibuprofen acid. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients "were randomly allocated to one of four treatment groups... in a 1:1:1:1 ratio according to a computer‐generated randomisation schedule that stratified patients by sex and baseline pain intensity." |
| Allocation concealment (selection bias) | Unclear risk | This is unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Study claims to be double blinded, but no indication of how blinding of study participants, nurses or assessors was implemented. |
| Incomplete outcome data (attrition bias) | Low risk | All data reported on with intention‐to‐treat flow diagram presented in paper. All drop‐outs accounted for. |
| Selective reporting (reporting bias) | Low risk | All adverse events and outcomes reported as planned. |
| Other bias | Low risk | Drop‐outs, end points and adverse effects were all documented. |
| Methods | Double‐blinded, randomised, placebo‐controlled, single centre study (2 sites). Parallel group. Single dose. Postoperative dosing. Pennsylvania, USA and other collaborators. | |
| Participants | Private outpatients at least 15 years of age who had 1 or more impacted third molars surgically removed. Patients were excluded if they were pregnant or lactating; had any history of hypersensitivity or serious adverse reaction to any agent similar to the study medications; had any clinically significant condition that would affect the absorption, metabolism, or excretion of the study medications; or required concomitant medication that would make it difficult to quantify analgesia. Long‐term users of analgesics and tranquillisers were also excluded. 269 patients were randomised, 206 patients completed the study. | |
| Interventions | Treatments (1 or more third molar surgical extractions) were carried out under general anaesthetic with additional local anaesthetic (lignocaine). Patients were instructed to take the study medication when they had moderate or severe pain (not specified as VAS equivalent). Interventions.
The medications were issued as 2 tablets identical in appearance. Rescue medication was provided: combinations of acetaminophen with codeine and/or oxycodone. The patients returned to the surgeon's office 5 days postoperatively for a follow‐up visit. | |
| Outcomes | Following the first dose of study medication, subjects responded to the following statements at hourly intervals up to 6 hours.
Participants continued with the study drugs for 15 doses. Adverse effect data were also collected and summarised in the paper. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random numbers generator, a computer assigned patient numbers, in blocks of 12, to the six treatment groups." |
| Allocation concealment (selection bias) | High risk | No details given. |
| Blinding (performance bias and detection bias) | Low risk | The study personnel were not aware of the identity of the study medication at the time it was administered and evaluated. |
| Incomplete outcome data (attrition bias) | Low risk | All missing data accounted for, reasons for not completing study included in paper. |
| Selective reporting (reporting bias) | Low risk | All adverse events and outcomes reported as planned. |
| Other bias | Low risk | All outcomes well documented. |
| Methods | RCT, double‐blinded. Single dose. Postoperative dosing. Single centre: University Dental Hospital, Pennsylvania. | |
| Participants | 210 participants. Participants had to be at least 16 years of age, be in good general health, requiring removal of > 1 bony impacted wisdom teeth, and have no specific contraindications to the use of ibuprofen, aspirin, related NSAIDs, or acetaminophen. Women who were sexually active had to be using a medically approved method of contraception and had to have a negative urine pregnancy test on the day of surgery. Pregnant or lactating women and any patient who had received other analgesics, anti‐inflammatory drugs, sedatives (except for conscious sedation during the surgical procedure), or psychotropic agents within 12 hours of the study were excluded. | |
| Interventions | All surgery carried out under local anaesthetic with "most patients" also receiving intravenous conscious sedation. Treatment groups.
Administered by mouth with water when postsurgical pain became moderate or severe (> 50 mm on a 100 mm VAS severity scale). Patients who did not experience pain within 5 hours were not given medication. Rescue medication (500 mg acetaminophen plus hydrocodone bitartrate 5 mg) was given at any time after the 1 hour assessment period. | |
| Outcomes | Pain relief and pain intensity at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5 and 6 hours after initial dosing. Pain relief was assessed on a 5‐point scale on 0 (no relief) to 4 (complete relief). Pain intensity was assessed on a 3‐point scale 0 (none) to 3 (severe). Exact timings of onset of first perceptible relief and meaningful relief were both recorded using stopwatches. Derived data: Hourly categorical scores for PID. SPID was scored at 2 and 6 hours (SPID2 and SPID6). Time weighted pain relief scores summed to derive 2‐ and 6‐hour total pain relief (TOTPAR2 and TOTPAR6). At conclusion patients were asked to provide a global assessment of study medication and adverse reactions if and when occurred were recorded. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "..double blind, double dummy, placebo controlled, randomised, parallel group clinical trial in which patients were stratified for sex and baseline pain." However, no detail given as to how patients were selected for each group. |
| Allocation concealment (selection bias) | High risk | The drugs appear to have been similar in number and form but this was not clearly stated and no further details were given. |
| Blinding (performance bias and detection bias) | Unclear risk | The study was said to be "double blinded" but no other details were given. |
| Incomplete outcome data (attrition bias) | Low risk | 210 patients participated and were included in statistical analysis. There were no drop‐outs. |
| Selective reporting (reporting bias) | Low risk | All adverse effects and outcomes reported as planned. |
| Other bias | High risk | Different methods of conscious sedation were used in addition to local anaesthetic. Quote: "most patients also received conscious sedation", this is not quantified. |
| Methods | Double‐blinded, double‐dummy, parallel‐group RCT. Single dose. Postoperative dosing. | |
| Participants | Healthy male and female patients at least 15 years of age, who required surgical removal of 2 or more impacted (at least 1 partially embedded in bone) third molars. In order to be included, they had to experience moderate or severe pain associated with the surgical procedure (not specified as to how this was measured). Patients were excluded from the study if they received any analgesic within 4 hours or a long‐acting analgesic within 12 hours of the study medication; received anaesthesia other than mepiva‐Caine hydrochloride, fentanyl, or methohexital during the surgery; or were taking any concurrent medication that could confound the evaluation of analgesia or safety. | |
| Interventions | All patients had surgery performed under general anaesthetic with supplemental local anaesthetic. 3 treatment groups.
Patients received a single dose of the test medication when the pain was moderate or severe (not specified as to how this was measured). Rescue medication (backup) was provided but not stated as to what the drug was. Patients were asked not to re‐medicate during the first 1 hour period. If a patient did re‐medicate within the trial period, the time was noted and no further efficacy evaluations were taken. | |
| Outcomes | A stopwatch was started when the study drug was administered and patients were instructed to stop the watch when they experienced meaningful pain relief. If they did not experience meaningful relief within 2 hours after dosing, use of the stopwatch was discontinued. Response to treatment was evaluated by patient self rating of pain intensity (0 = none, 1 = slight, 2 = moderate, 3 = severe) and degree of pain relief (0 = none,1 = a little, 2 = some, 3 = a lot, 4 = complete) at 15, 30, 45, 60, and 90 minutes and 2, 3, 4, 5, and 6 hours postdose. At the last evaluation time, the patient provided a global evaluation of the study drug (0 = poor, 1 = fair, 2 = good, 3 = very good, 4 = excellent). Adverse clinical experiences were recorded by the study co‐ordinator. Data recorded for.
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to one of three treatment groups... according to an allocation schedule of random numbers." |
| Allocation concealment (selection bias) | High risk | No details given. |
| Blinding (performance bias and detection bias) | Unclear risk | The study was said to be a "double blind, double‐dummy", however, no further details were given. |
| Incomplete outcome data (attrition bias) | Unclear risk | "One of the patients in the ibuprofen lysine group had only one third molar removed and did not record efficacy evaluations; this patient was excluded from the efficacy analysis." Otherwise all participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All adverse effects and outcomes reported as planned. |
| Other bias | High risk | No detail of distribution of study medications or if they were identifiable to the participant or operator. |
| Methods | Randomised, double‐blinded, placebo‐controlled, parallel‐group, 2‐centre, modified factorial study. Single dose. Postoperative dosing. | |
| Participants | Healthy male or female outpatients were eligible for the study if they were aged 16 to 40 years, were scheduled to undergo surgical removal of 3 to 4 impacted molars 2 of which would be mandibular molars requiring bone removal. Impaction scoring (1‐4) was used to assess the molars. Exclusion criteria included a history of migraine headaches within the previous year; gastrointestinal disorders such as peptic or duodenal ulcer, dyspepsia, or heartburn; hypersensitivity to the study medications; and drug or alcohol abuse. Patients with a current history of significant disease, including psychotic illness or neurosis, were also excluded, as were those who had other painful conditions, were taking medications that might confound the assessment of pain relief, or were unable to refrain from smoking. Women who were pregnant or lactating were not eligible for enrolment. | |
| Interventions | Standard oral surgical procedures carried out under local anaesthetic and conscious sedation using nitrous oxide, diazepam, and methohexital (barbiturate drug). Following surgery, eligible patients were randomly assigned in a ratio of 2:1:2:1:1 to a single oral dose of the following.
Medication was given when postoperative pain intensity was rated at least moderate on the pain intensity categorical rating scale where 0 = none; 1 = mild; 2 = moderate; and 3 = severe and pain intensity was ≥ 50 mm on a 100‐mm VAS. Rescue medication was provided within the first 4 hours using tramadol 100 mg, and paracetamol 500 mg in combination with hydrocodone 5 mg or tramadol 100 mg after the first 4‐hour period. All assessments completed after the patient had taken rescue medication were considered missing. | |
| Outcomes | Pain was assessed immediately after surgery (before dosing) and at specified intervals for up to 8 hours after dosing (15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480 minutes). Primary efficacy end point was the sum of pain relief and pain intensity differences from baseline (0 hours) to 8 hours postdosing (SPRID8). This measure was defined as the area under the curve (AUC) for the sum of the 2 measures (pain relief and PID) at each time point from 0 to 8 hours. Secondary efficacy end points were: total pain relief from 0 to 8 hours (TOTPAR8), sum of pain intensity differences from 0 to 8 hours (SPID8), SPID on the VAS from 0 to 8 hours (SPID8 VAS), TOTPAR from 0 to 4 and from 0 to 6 hours (TOTPAR4, TOTPAR6), SPID4, SPID6, SPRID4, SPRID6, SPID4 VAS, SPID6 VAS, individual pain relief from 15 minutes to 8 hours, peak pain relief over 8 hours, individual PID from 15 minutes to 8 hours, PID VAS from 15 minutes to 8 hours, peak PID and peak PID VAS over 8 hours, time to PID ≥1, PRID, time to first perceptible pain relief, time to first confirmed perceptible pain relief, time to first meaningful pain relief, time to use of rescue medication, time to pain half gone, and patient's global assessment of pain relief on a 5‐point scale (1 = poor; 2 = fair; 3 = good; 4 = very good; 5 = excellent). The 2‐stopwatch method was also used to assess perceptible and meaningful pain relief (as in Daniels 2009). Patients were assessed 5‐7 days postoperatively in relation to their surgery and to assess tolerability of the study medications. | |
| Notes | No reason given for 2:1:2:1:1 ratio used for allocating participants into different study groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Upon study entry, patients at each site were stratified by sex and baseline pain intensity and given a unique number in sequence according to a predefined schedule. The block design for the randomisation schedule was in groups of 7. |
| Allocation concealment (selection bias) | Low risk | The randomisation code was supplied to the investigator in a sealed envelope and only broken if necessary due to safety concerns. |
| Blinding (performance bias and detection bias) | Low risk | Unblinded study personnel were not involved in any part of the study other than the preparation and verification of doses. Identical medication was dispensed by blindfolded staff to blindfolded patients. |
| Incomplete outcome data (attrition bias) | Low risk | All data reported on with intention‐to‐treat flow diagram presented in paper. All drop‐outs accounted for. |
| Selective reporting (reporting bias) | Low risk | All adverse effects and outcomes reported as planned. No obvious selective reporting of outcomes. |
| Other bias | Low risk | Drop‐outs, end points, adverse effects all documented. |
| Methods | Multicentre, 2‐stage, randomised, double‐blinded, parallel‐group, placebo‐controlled, factorial study. Related to Mehlisch 2010 but using an observation period of 72 hours and a 2‐stage study design. 3‐dose trial. Postoperative dosing. | |
| Participants | Male or female outpatients aged ≥ 16 years undergoing surgical removal of at least 3 impacted third molars (2 of which had to be mandibular impacted molars). Impaction scoring (1‐4) was used to assess the molars. Same exclusion criteria as Mehlisch 2010. | |
| Interventions | Stage 1: (first 8 hours). Patients randomly assigned to 1 of the following treatment groups.
Stage 2: (72 hours). Patients who had been taking the combination drugs or placebo stayed on these, but those on monotherapy received the combination drugs incorporating the same dose of active monotherapy from phase 1. Medication was administered when postoperative pain intensity was rated at least moderate on the pain intensity categorical rating scale where 0 = none; 1 = mild; 2 = moderate; and 3 = severe and pain intensity was ≥ 50 mm on a 100‐mm VAS. Medication had to be given within 6 hours of surgery but > 3 hours after fentanyl was last administered in order for the participant to be included. In stage 2, patients were instructed to take their assigned study medication when at least 8 hours had elapsed since their previous dose of study medication during stage 1, when their pain VAS score was ≥ 30 mm, and provided that they had not consumed > 2 doses of first‐line rescue medication in the previous 24 hours. As in stage 1, rescue medication was available as needed, but to ensure that the daily maximum dose of paracetamol was not exceeded, patients were allowed first‐line rescue medication only twice in any 24‐hour period. Patients who required > 2 doses of first‐line rescue medication in any 24‐hour period, in addition to the 3 doses of study medication, were considered treatment failures and were allowed to take tramadol 100 mg as second‐line rescue medication. Patients were given 6 tablets to take in stage 1 and 2 tablets in stage 2 to ensure adequate concealment. Rescue medication was provided (hydrocodone 7.5 mg and paracetamol 500 mg) at any time after dosing, but any patient in stage 1 who required rescue medication in the first 60 minutes was considered a "drop‐out" and any patient requiring > 2 doses in the first 24‐hour period of stage 2 were considered treatment failures. 735 patients initially randomised, 715 entered stage 2, 678 completed both stages. Follow‐up assessment was carried out 7‐10 days postoperatively to assess vital signs and perform a physical examination, adverse events were also recorded. | |
| Outcomes | Stage 1. Same primary efficacy end points as in Mehlisch 2010 (SPRID8) along with (PRID) scores at 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480 minutes. The key secondary end point was the patient's global assessment of the study medication, which was evaluated in response to the question 'How do you rate the study medication?'; response choices were 1 = poor; 2 = fair; 3 = good; 4 = very good; and 5 = excellent. This was assessed at the end of 8 hours, or at the time of rescue medication if earlier than 8 hours. Other secondary end points included, among others, TOTPAR from 0 to 8 hours, SPID from 0 to 8 hours, SPID on the VAS (SPID VAS) from 0 to 8 hours, time to pain half gone, and duration of effect (time to first administration of rescue medication). The 2‐stopwatch method was also used as described in the previous paper. Tolerability of the study medications was also assessed at 8 hours after dosing in stage 1, at 72 hours in stage 2 and also at the follow‐up visit. | |
| Notes | Does not state how the molars were removed and under what type of anaesthetic. Although fentanyl is mentioned in the patients and treatment section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation tables were used. The patients were stratified by sex and severity of pain, then assigned a unique number following a predefined schedule. |
| Allocation concealment (selection bias) | Low risk | The randomisation codes for each patient were supplied to the investigator in a sealed envelope. |
| Blinding (performance bias and detection bias) | Low risk | After preparation and checking, all tablets were deprinted and re‐packaged to match the placebos. |
| Incomplete outcome data (attrition bias) | Low risk | All data reported on with intention‐to‐treat flow diagram presented in paper. All drop‐outs accounted for. |
| Selective reporting (reporting bias) | Low risk | All adverse effects and outcomes reported as planned. No obvious selective reporting of outcomes. |
| Other bias | Low risk | Drop‐outs, end points, adverse effects all documented. |
| Methods | Randomised, double‐blinded, triple‐dummy, parallel‐group study. Single centre. Single dose. Postoperative dosing. | |
| Participants | Healthy ambulatory male or female subjects, ages 16 to 65 years, who experienced moderate or severe pain after undergoing the surgical removal of 1 or more impacted third molars, 1 of which must have been at least a partial bony mandibular impaction, were included in the study. Pregnant females, nursing mothers, and subjects with known sensitivity to acetaminophen, ketoprofen, ibuprofen, or other NSAIDs were excluded from participating in the study. Subjects with a recent history of serious medical condition or presence of bleeding disorders were excluded from the investigation. Subjects were also excluded if they had prior use of any analgesic, sedative, or psychotropic agent within 5 half‐lives for that drug before taking the study medication (except for local anaesthesia for the procedure). Prior use of any antihistamines within 48 hours of study entry was also prohibited. Subjects with a history of chronic abuse of analgesics or alcohol or substance abuse and subjects receiving other investigational drugs within 30 days of the study. | |
| Interventions | All surgery performed under local anaesthetic (lignocaine), patients fasted from midnight the previous night. The surgeon assessed the trauma rating of the procedure from mild to severe. Subjects remained at the study centre whilst medication was given and for 6 hours after receiving medication. Treatment groups.
Rescue medication provided but not specified as to what drug it was. Patients were medicated when they scored at least moderate pain on a categorical scale and a VAS of > 50. | |
| Outcomes | Pain severity was evaluated using the categorical pain scale at 15, 30, and 45 minutes and then at 1, 1.5, 2, 3, 4, 5, and 6 hours following study drug administration. At each assessment, patients rated their pain intensity and pain relief using the following categorical rating scale for pain intensity: none = 0, slight = 1, moderate = 2, or severe = 3. For pain relief, the following were used: none = 0, a little = 1, some = 2, a lot = 3, or complete relief = 4. A stopwatch was used to describe meaningful pain relief time. At the conclusion of the 6‐hour evaluation period or at the time of re‐medication (if it occurred before the 6th hour), each subject provided an overall evaluation of the study medication on a 5‐point categorical scale (from poor = 0 to excellent = 4). If rescue medication was taken, no further measures were made. Time to rescue medication was also recorded. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment assignments were determined by a randomisation schedule generated by the sponsor." This was independent of clinicians. |
| Allocation concealment (selection bias) | Low risk | Randomisation was controlled by the sponsor and all unit doses were identical in appearance. |
| Blinding (performance bias and detection bias) | Unclear risk | The study was said to be "double blinded", however, no details were given. |
| Incomplete outcome data (attrition bias) | Low risk | 239 patients included, all data appear to have been reported. |
| Selective reporting (reporting bias) | Low risk | All adverse effects and outcomes reported as planned. No obvious selective reporting of outcomes. |
| Other bias | Low risk | Drop‐outs, end points, adverse effects all documented. |
NSAIDs = non‐steroidal anti‐inflammatory drugs; PI = pain intensity; PID = pain intensity difference; PRID = pain relief and pain intensity difference; RCT = randomised controlled trial; SPID = summed pain intensity difference; TOTPAR = total pain relief; VAS = visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Multiple dose study, unable to extract reliable single dose data. | |
| Analgesic dosing continued for 7 days postoperatively and it was not possible to extract data from the study for the responses to the first dose. | |
| Multiple dose study, unable to extract reliable single dose data. Also included preoperative analgesic dosing. | |
| This was not available as a full paper, only an abstract from the 2002 International Association for Dental Research (IADR) conference. | |
| Multiple dose study, unable to extract reliable single dose data. Also included preoperative analgesic dosing. | |
| Multiple dose study, unable to extract reliable single dose data. Also included preoperative analgesic dosing. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours Show forest plot | 6 | 926 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.31, 1.61] |
| Analysis 1.1 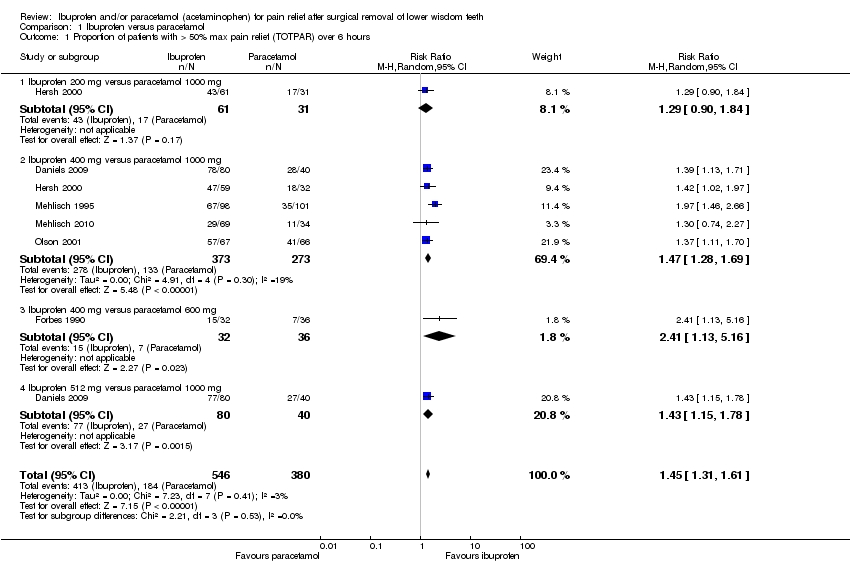 Comparison 1 Ibuprofen versus paracetamol, Outcome 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours. | ||||
| 1.1 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.90, 1.84] |
| 1.2 Ibuprofen 400 mg versus paracetamol 1000 mg | 5 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.28, 1.69] |
| 1.3 Ibuprofen 400 mg versus paracetamol 600 mg | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.13, 5.16] |
| 1.4 Ibuprofen 512 mg versus paracetamol 1000 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours Show forest plot | 6 | 926 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.13, 1.46] |
| Analysis 1.2  Comparison 1 Ibuprofen versus paracetamol, Outcome 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours. | ||||
| 2.1 Ibuprofen 512 mg versus paracetamol 1000 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.98, 1.67] |
| 2.2 Ibuprofen 400 mg versus paracetamol 1000 mg | 5 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.09, 1.55] |
| 2.3 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.85, 1.41] |
| 2.4 Ibuprofen 400 mg versus paracetamol 600 mg | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.96, 3.14] |
| 3 Number of patients using rescue medication at 6 hours Show forest plot | 5 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.26, 1.64] |
| Analysis 1.3  Comparison 1 Ibuprofen versus paracetamol, Outcome 3 Number of patients using rescue medication at 6 hours. | ||||
| 3.1 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.94, 2.02] |
| 3.2 Ibuprofen 400 mg versus paracetamol 1000 mg | 4 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.25, 1.79] |
| 3.3 Ibuprofen 512 mg versus paracetamol 1000 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.60] |
| 3.4 Ibuprofen 400 mg versus paracetamol 600 mg | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.87, 4.30] |
| 4 Number of patients using rescue medication at 8 hours Show forest plot | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.57, 2.60] |
| Analysis 1.4  Comparison 1 Ibuprofen versus paracetamol, Outcome 4 Number of patients using rescue medication at 8 hours. | ||||
| 4.1 Ibuprofen 200 mg versus paracetamol 500 mg | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.31, 4.25] |
| 4.2 Ibuprofen 400 mg versus paracetamol 500 mg | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.57, 4.89] |
| 4.3 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.10, 3.17] |
| 4.4 Ibuprofen 400 mg versus paracetamol 1000 mg | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.11, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Combined (ibuprofen and paracetamol) versus single drugs, Outcome 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours. | ||||
| 1.1 Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Combined (ibuprofen and paracetamol) versus single drugs, Outcome 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours. | ||||
| 2.1 Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of patients using rescue medication at 8 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Combined (ibuprofen and paracetamol) versus single drugs, Outcome 3 Number of patients using rescue medication at 8 hours. | ||||
| 3.1 Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.36, 1.88] |

Study flow diagram.
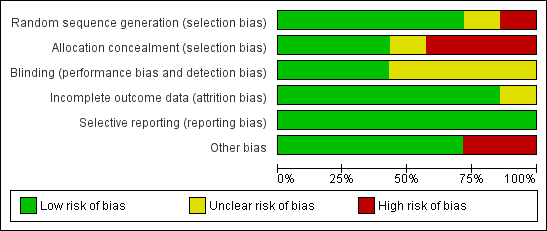
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
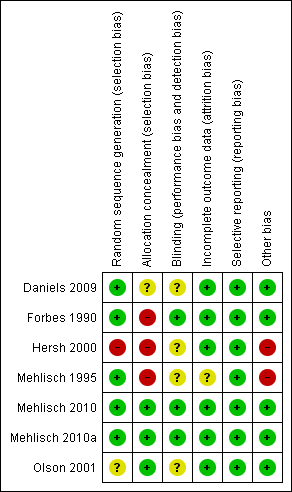
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
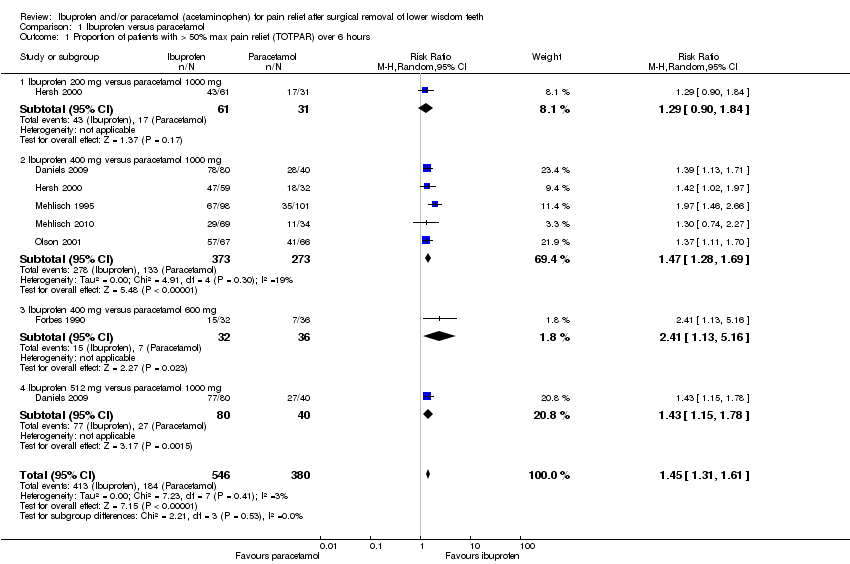
Comparison 1 Ibuprofen versus paracetamol, Outcome 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours.

Comparison 1 Ibuprofen versus paracetamol, Outcome 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours.
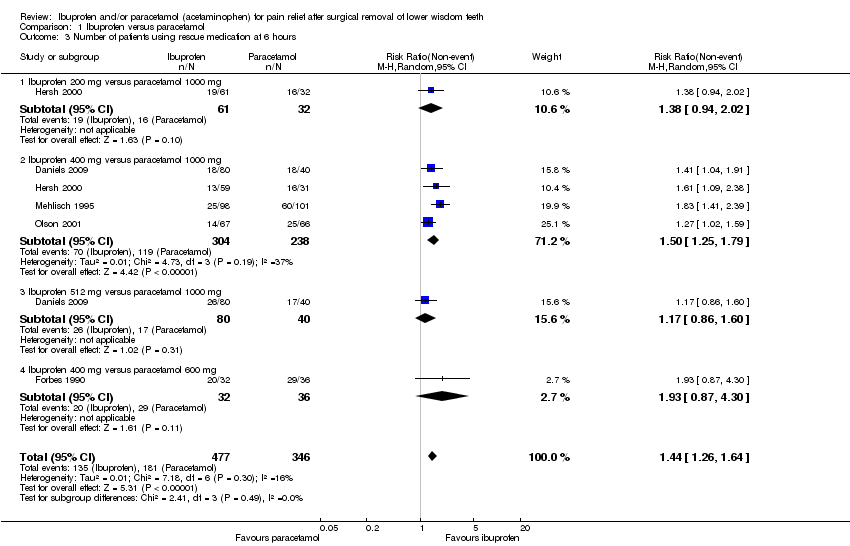
Comparison 1 Ibuprofen versus paracetamol, Outcome 3 Number of patients using rescue medication at 6 hours.
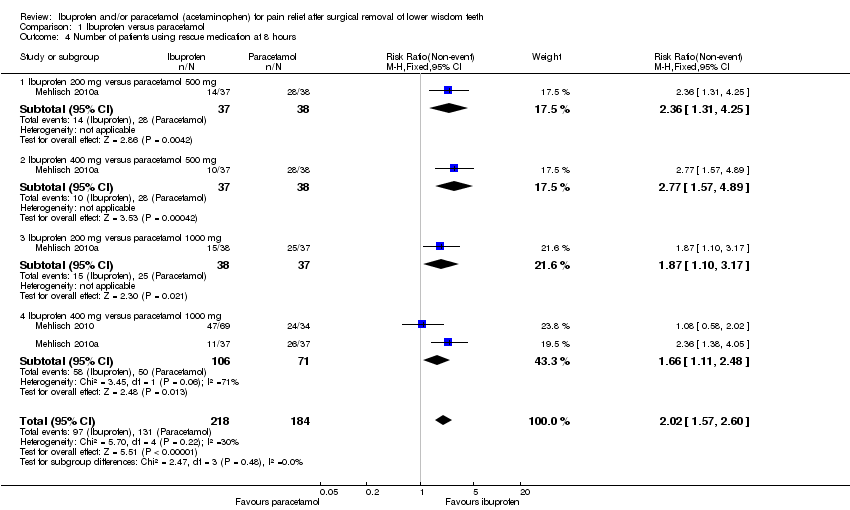
Comparison 1 Ibuprofen versus paracetamol, Outcome 4 Number of patients using rescue medication at 8 hours.

Comparison 2 Combined (ibuprofen and paracetamol) versus single drugs, Outcome 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours.

Comparison 2 Combined (ibuprofen and paracetamol) versus single drugs, Outcome 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours.
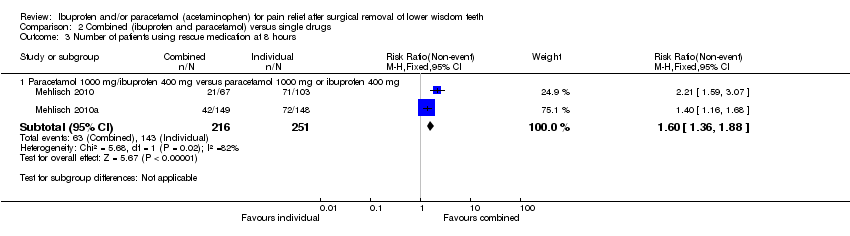
Comparison 2 Combined (ibuprofen and paracetamol) versus single drugs, Outcome 3 Number of patients using rescue medication at 8 hours.
| Ibuprofen versus paracetamol for pain relief following the surgical removal of lower wisdom teeth | ||||||
| Patient or population: Patients with pain after surgical removal of lower wisdom teeth Control: Paracetamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed1 risk | Corresponding risk | |||||
| Control | Ibuprofen | |||||
| Proportion of patients with > 50% maximum pain relief (TOTPAR) over 6 hours ‐ Ibuprofen 400 mg versus paracetamol 1000 mg | Study population | RR 1.47 | 646 | ⊕⊕⊕⊕ | When all doses considered RR = 1.45 (95% CI 1.31 to 1.61) (6 studies, 926 participants; high quality evidence) | |
| 56 per 100 | 83 per 100 | |||||
| Proportion of patients with > 50% maximum pain relief (TOTPAR) over 2 hours ‐ Ibuprofen 400 mg versus paracetamol 1000 mg | Study population | RR 1.30 | 645 | ⊕⊕⊕⊕ | When all doses considered RR = 1.29 (95% CI 1.13 to 1.46) (6 studies, 926 participants; high quality evidence) | |
| 62 per 100 | 81 per 100 | |||||
| Number of patients not using rescue medication at 6 hours (non‐event) Follow‐up: 6 hours | Study population | RR 1.50 | 542 | ⊕⊕⊕⊕ | When all doses considered RR = 1.44 (95% CI 1.13 to 1.64) (5 studies, 823 participants; high quality evidence) | |
| 50 per 100 | 75 per 100 | |||||
| Adverse events | The majority of adverse events were minor in nature and usually included nausea, vomiting, headaches and dizziness. Side effect profiles have not been included in a meta‐analysis as multiple adverse events were recorded in single patients. However, the differences in the observed adverse events for ibuprofen and paracetamol were small | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 This is the median control group risk based on paracetamol being the control group. | ||||||
| Combined (ibuprofen and paracetamol) versus single drugs for pain relief after surgical removal of lower wisdom teeth | ||||||
| Patient or population: Patients with pain after surgical removal of lower wisdom teeth Control: Single drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed1 risk | Corresponding risk | |||||
| Control | Combined (ibuprofen and paracetamol) | |||||
| Proportion of patients with > 50% maximum pain relief (TOTPAR) over 6 hours ‐ Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | Study population | RR 1.77 | 170 | ⊕⊕⊕⊝ | ||
| 38 per 100 | 67 per 100 | |||||
| Proportion of patients with > 50% maximum pain relief (TOTPAR) over 2 hours ‐ Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | Study population | RR 1.29 | 170 | ⊕⊕⊕⊝ | ||
| 37 per 100 | 48 per 100 | |||||
| Number of patients not using rescue medication at 8 hours ‐ Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | Study population | RR 1.60 | 467 | ⊕⊕⊕⊕ | ||
| 50 per 100 | 80 per 100 | |||||
| Adverse events | The majority of adverse events were minor in nature and usually included nausea, vomiting, headaches and dizziness. Side effect profiles have not been included in a meta‐analysis as multiple adverse events were recorded in single patients. However, the differences in the observed adverse events for ibuprofen and paracetamol were small | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 This is the median control group risk based on paracetamol/ibuprofen as single drugs being the control group. 2 Quality of evidence downgraded due to single study and serious imprecision. | ||||||
|
| Daniels 2009 | Forbes 1990 | Hersch 2000* | Mehlisch 1995 | Mehlisch 2010 | Mehlisch 2010a | Olson 2001* |
| Paracetamol 500 mg
|
|
|
|
|
| √ |
|
| Paracetamol 600 mg
|
| √ |
|
|
|
|
|
| Paracetamol 1000 mg
| √ |
| √ | √ | √ | √ | √ |
| Ibuprofen 200 mg
|
|
| √ |
|
| √ |
|
| Ibuprofen 400 mg
| √ | √ | √ | √ | √ | √ | √ |
| Ibuprofen 512 mg
| √ |
|
|
|
|
|
|
| Paracetamol 250 mg/ibuprofen 100 mg
|
|
|
|
|
| √ |
|
| Paracetamol 500 mg/ibuprofen 200 mg
|
|
|
|
| √ | √ |
|
| Paracetamol 1000 mg/ibuprofen 400 mg
|
|
|
|
| √ | √ |
|
| *Liquigel formula. | |||||||
| Study | Use of rescue medication (RM) | ||||
| Mean time to RM | Use of RM (%) | Use of RM (n) | Total | Observation period | |
| Daniels 2009 | 6 hours | ||||
| Paracetamol 1000 | 44 | 35 | 80 | ||
| Ibuprofen 400 | 23 | 18 | 80 | ||
| Ibuprofen 512 | 33 | 26 | 80 | ||
| Forbes 1990 | 6 hours | ||||
| Paracetamol 600 | 3.89 hours | 81 | 29 | 36 | |
| Ibuprofen 400 | 4.63 hours | 63 | 20 | 32 | |
| Hersch 2000 | 6 hours | ||||
| Paracetamol 1000 | 51 | 32 | 63 | ||
| Ibuprofen 200 | 31 | 19 | 61 | ||
| Ibuprofen 400 | 22 | 13 | 59 | ||
| Mehlisch 1995 | 6 hours | ||||
| Paracetamol 1000 | 60 | 60 | 101 | ||
| Ibuprofen 400 | 26 | 25 | 98 | ||
| Mehlisch 2010 | 8 hours | ||||
| Paracetamol 1000 | 261 minutes | 71 | 24 | 34 | |
| Ibuprofen 400 | 296 minutes | 68 | 47 | 69 | |
| Paracetamol 1000/ibuprofen 400 | 376 minutes | 31 | 21 | 67 | |
| Paracetamol 500/ibuprofen 200 | 329 minutes | 61 | 20 | 33 | |
| Mehlisch 2010a | 8 hours in phase 1 | ||||
| Paracetamol 500 | 74 | 56 | 76 | ||
| Paracetamol 1000 | 69 | 51 | 74 | ||
| Ibuprofen 200 | 39 | 29 | 75 | ||
| Ibuprofen 400 | 28 | 21 | 74 | ||
| Paracetamol 250/ibuprofen 100 | 38 | 27 | 71 | ||
| Paracetamol 1000/ibuprofen 400 | 28 | 42 | 149 | ||
| Paracetamol 500/ibuprofen 200 | 22 | 31 | 143 | ||
| Olson 2001 | 6 hours | ||||
| Paracetamol 1000 | 38 | 25 | 66 | ||
| Ibuprofen 400 | 21 | 14 | 67 | ||
| Study | Adverse events (AE) | |||
| Total AE (n) | Total AE % | Serious/Severe AE | Serious/Severe AE % | |
| Daniels 2009 | ||||
| Paracetamol 1000 | 25 (81) | 31 | 3 | 12 |
| Ibuprofen 400 | 19 (80) | 24 | 1 | 3 |
| Ibuprofen 512 | 24 (80) | 30 | 2 | 6 |
| Forbes 1990 | ||||
| Paracetamol 600 | 5 (41) | 12 | Not specified | Not specified |
| Ibuprofen 400 | 8 (43) | 19 | Not specified | Not specified |
| Hersch 2000 | ||||
| Paracetamol 1000 | 12 (63) | 19 | 0 | 0 |
| Ibuprofen 200 | 7 (61) | 11 | 0 | 0 |
| Ibuprofen 400 | 4 (59) | 7 | 0 | 0 |
| Mehlisch 1995 | ||||
| Paracetamol 1000 | 17 (101) | 17 | 0 | 0 |
| Ibuprofen 400 | 12 (98) | 12 | 0 | 0 |
| Mehlisch 2010 | ||||
| Paracetamol 1000 | 24 (34) | 71 | 11 | 46 |
| Ibuprofen 400 | 39 (69) | 57 | 14 | 36 |
| Paracetamol 1000/ibuprofen 400 | 38 (67) | 57 | 11 | 29 |
| Paracetamol 500/ibuprofen 200 | 14 (33) | 42 | 6 | 43 |
| Mehlisch 2010a | ||||
| Paracetamol 500 | 38 (76) | 50 | 7 | 18 |
| Paracetamol 1000 | 30 (74) | 40 | 5 | 17 |
| Ibuprofen 200 | 31 (75) | 41 | 7 | 23 |
| Ibuprofen 400 | 33 (74) | 45 | 5 | 15 |
| Paracetamol 250/ibuprofen 100 | 22 (71) | 31 | 8 | 36 |
| Paracetamol 1000/ibuprofen 400 | 44 (149) | 30 | 15 | 34 |
| Paracetamol 500/ibuprofen 200 | 51 (143) | 36 | 7 | 14 |
| Olson 2001 | ||||
| Paracetamol 1000 | 10 (66) | 15 | 1 | 10 |
| Ibuprofen 400 | 7 (67) | 11 | 2 | 29 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours Show forest plot | 6 | 926 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.31, 1.61] |
| 1.1 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.90, 1.84] |
| 1.2 Ibuprofen 400 mg versus paracetamol 1000 mg | 5 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.28, 1.69] |
| 1.3 Ibuprofen 400 mg versus paracetamol 600 mg | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.13, 5.16] |
| 1.4 Ibuprofen 512 mg versus paracetamol 1000 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours Show forest plot | 6 | 926 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.13, 1.46] |
| 2.1 Ibuprofen 512 mg versus paracetamol 1000 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.98, 1.67] |
| 2.2 Ibuprofen 400 mg versus paracetamol 1000 mg | 5 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.09, 1.55] |
| 2.3 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.85, 1.41] |
| 2.4 Ibuprofen 400 mg versus paracetamol 600 mg | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.96, 3.14] |
| 3 Number of patients using rescue medication at 6 hours Show forest plot | 5 | 823 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.26, 1.64] |
| 3.1 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.94, 2.02] |
| 3.2 Ibuprofen 400 mg versus paracetamol 1000 mg | 4 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.25, 1.79] |
| 3.3 Ibuprofen 512 mg versus paracetamol 1000 mg | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.60] |
| 3.4 Ibuprofen 400 mg versus paracetamol 600 mg | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.87, 4.30] |
| 4 Number of patients using rescue medication at 8 hours Show forest plot | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.57, 2.60] |
| 4.1 Ibuprofen 200 mg versus paracetamol 500 mg | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.31, 4.25] |
| 4.2 Ibuprofen 400 mg versus paracetamol 500 mg | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.57, 4.89] |
| 4.3 Ibuprofen 200 mg versus paracetamol 1000 mg | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.10, 3.17] |
| 4.4 Ibuprofen 400 mg versus paracetamol 1000 mg | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.11, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of patients with > 50% max pain relief (TOTPAR) over 6 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of patients with > 50% max pain relief (TOTPAR) over 2 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of patients using rescue medication at 8 hours Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Paracetamol 1000 mg/ibuprofen 400 mg versus paracetamol 1000 mg or ibuprofen 400 mg | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.36, 1.88] |

