| | Analgesia | Rescue medication |
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: v good or excellent | Median time to use (hr) | % using |
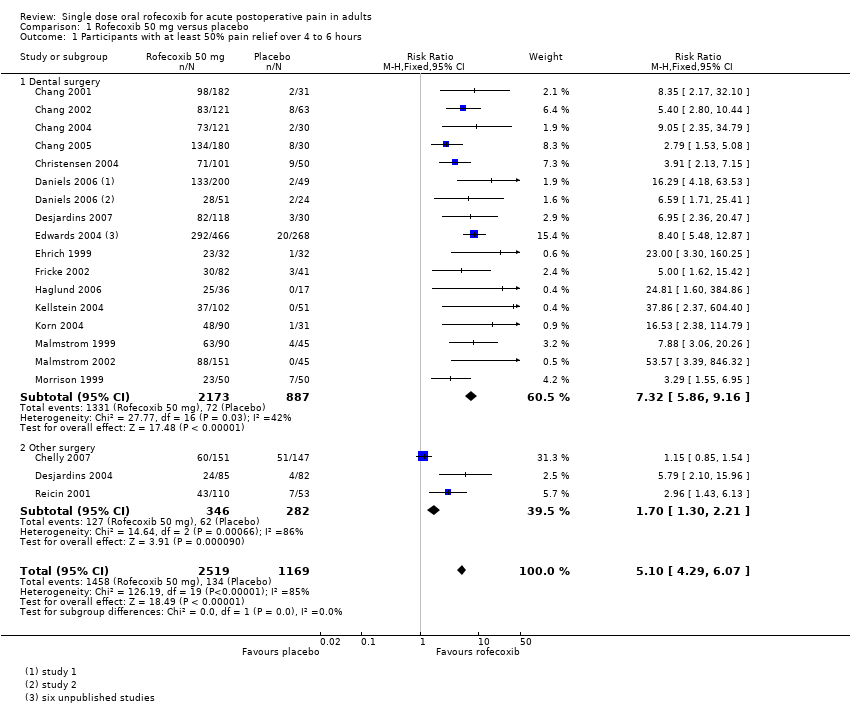
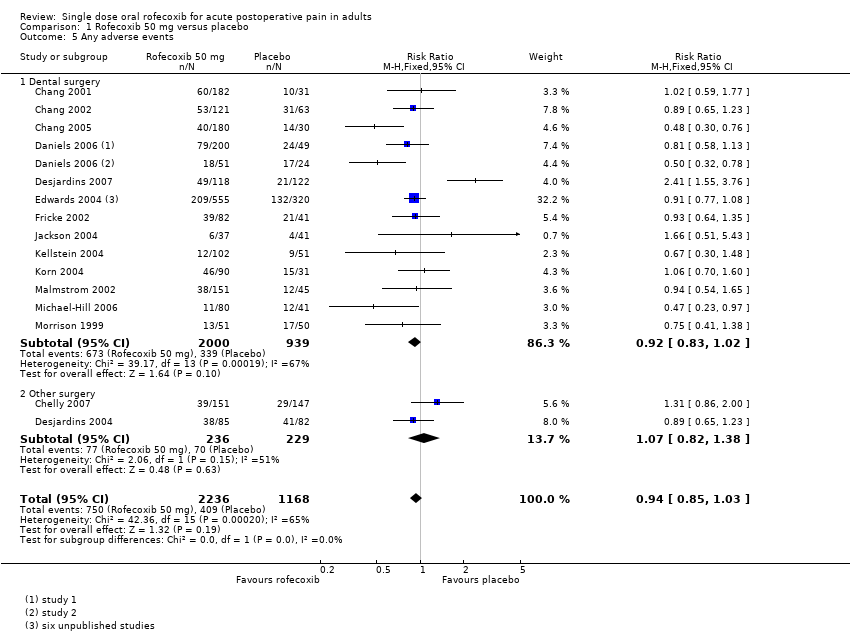
| Chang 2001 | (1) Rofecoxib 50 mg, n = 182 (2) Paracetamol 600 mg plus codeine 60 mg, n = 180 (3) Placebo, n = 31 | TOTPAR 6: (1) 12.4 (2) 7.0 (3) 3.4 | (1) 98/182 (2) 23/180 (3) 2/31 | At 6 h: (1) 87/178 (2) 26/176 (3) 0/29 | (1) 9.6 (2) 2.3 (3) 1.6 | No data |
| Chang 2002 | (1) Rofecoxib 50 mg, n = 121 (2) Diclofenac sodium 50 mg, n = 121 (3) Placebo, n = 63 | TOTPAR 6: (1) 14.5 (2) 6.9 (3) 4.5 | (1) 83/121 (2) 32/121 (3) 8/63 | At 8 h: (1) 79/121 (2) 21/121 (3) 6/63 | (1) >24:00 (2) 1.62 (3) 1.62 | At 8 h: (1) 19.8 (2) 66.9 (3) 76.2 |
| Chang 2004 | (1) Rofecoxib 50 mg, n = 121 (2) Oxycodone/acetaminophen 10/650 mg, n = 120 (3) Placebo, n = 30 | TOTPAR 6: (1) 12.9 (2) 11.3 (3) 3.1 | (1) 73/121 (2) 61/120 (3) 2/30 | At 6 h: (1) 59/120 (2) 56/116 (3) 0/30 | (1) >6 (2) 5:05 (3) 1:37 | At 6 h: (1) 33.9 (2) 53.3 (3) 90.0 |
| Chang 2005 | (1) Rofecoxib 50 mg, n = 180 (2) Codeine/acetaminophen 60/600 mg, n = 180 (3) Placebo, n = 30 | TOTPAR 6: (1) 15.5 (2) 10.7 (3) 6.7 | (1) 134/180 (2) 86/180 (3) 8/30 | At 6 h: (1) 124/180 (2) 68/180 (3) 3/30 | (1) >24 (2) 6.5 (3) 3.2 | At 24 h: (1) 43 (2) 86 (3) 73 |
| Chelly 2007 | (1) Rofecoxib 50 mg, n = 151 (2) Hydrocodone/acetaminophen 7.5/750 mg, n = 145 (3) Placebo, n = 147 | TOTPAR 6: (1) 9.3 (2) 10.7 (3) 8.4 | (1) 60/151 (2) 69/145 (3) 51/147 | At 6 h: (1) 91/151 (2) 99/147 (3) 76/147 | (1) 4.4 (2) 6.3 (3) 3.6 | At 6 h: (1) 58.3 (2) 47.9 (3) 67.1 |
| Christensen 2004 | (1) Rofecoxib 50 mg, n = 101 (2) Valdecoxib 40 mg, n = 99 (3) Placebo, n = 50 | TOTPAR 6: (1) 14.84 (2) 16.27 (3) 5.20 | (1) 71/101 (2) 78/99 (3) 9/50 | No usable data | (1) >24:00 (2) >24:00 (3) 2:10 | At 24 h: (1) 8.9 (2) 17.2 (3) 72.0 |
| Daniels 2006 study 1 | (1) Rofecoxib 50 mg, n = 200 (2) Valdecoxib 20 mg, n = 201 (3) Placebo, n = 49 | TOTPAR 4: (1) 9.4 (2) 9.0 (3) 1.8 | (1) 133/200 (2) 127/201 (3) 2/49 | No usable data | (1) >24:00 (2) 23:58 (3) 2:04 | At 6 h: (1) 12.3 (2) 15.8 (3) 82.5 At 12 h: (1) 31.6 (2) 38.6 (3) 87.7 |
| Daniels 2006 study 2 | (1) Rofecoxib 50 mg, n = 51 (2) Valdecoxib 40 mg, n = 50 (3) Placebo, n = 24 | TOTPAR 4: (1) 8.0 (2) 8.7 (3) 2.4 | (1) 28/51 (2) 30/50 (3) 2/24 | No usable data | (1) >24:00 (2) >24:00 (3) 2:15 | At 6 h: (1) 17.5 (2) 21.1 (3) 84.2 At 12 h: (1) 35.1 (2) 29.8 (3) 93.0 |
| Desjardins 2004 | (1) Rofecoxib 50 mg, n = 85 (2) Diclofenac 100 mg, n = 85 (3) Placebo, n = 82 | TOTPAR 6: (1) 7.28 (2) 4.81 (3) 2.87 | (1) 24/85 (2) 13/85 (3) 4/82 | no usable data | (1) 4:02 (2) 2:03 (3) 1:41 | No data |
| Desjardins 2007 | (1) Rofecoxib 50 mg, n = 118 (2) Oxycodone/acetaminophen 10/650 mg, n = 122 (3) Placebo, n = 30 | TOTPAR 6: (1) 14.6 (2) 12.8 (3) 3.9 | (1) 82/118 (2) 72/122 (3) 3/30 | No usable data | (1) >24:00 (2) >24:00 (3) 1:50 | At 6 h: (1) 20.3 (2) 37.7 (3) 73.3 |
| Edwards 2004 trial 111 dental | (1) Rofecoxib 50 mg, n = 159 (2) Ibuprofen 400 mg, n = 53 (3) Placebo, n = 52 | No data | (1) 109/159 (2) 38/53 (3) 1/45 | No data | No data | No data |
| Edwards 2004 trial 154 dental | (1) Rofecoxib 50 mg, n = 91 (2) Acetaminophen/codeine 325/5 mg, n = 89 (3) Placebo, n = 30 | No data | (1) 60/91 (2) 17/89 (3) 4/30 | No data | No data | No data |
| Edwards 2004 trial 27 dental | (1) Rofecoxib 50 mg, n = 38 (2) Naproxen 550 mg, n = 39 (3) Placebo, n = 39 | No data | (1) 26/38 (2) 28/39 (3) 6/39 | No data | No data | No data |
| Edwards 2004 trial 51 dental | (1) Rofecoxib 50 mg, n = 72 (2) Naproxen 550 mg, n = 49 (3) Placebo, n = 48 | No data | (1) 40/72 (2) 24/49 (3) 3/48 | No data | No data | No data |
| Edwards 2004 trial 71 dental | (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Placebo, n = 50 | No data | (1) 27/50 (2) 19/52 (3) 4/50 | No data | No data | No data |
| Edwards 2004 trial 84 dental | (1) Rofecoxib 50 mg, n = 56 (2) Ibuprofen 400 mg, n = 56 (3) Placebo, n = 56 | No data | (1) 30/56 (2) 30/56 (3) 2/56 | No data | No data | No data |
| Edwards 2004 all dental surgery | (1) Rofecoxib 50 mg (2) Placebo | | | | (1) 15.5 (2) 1.6 | |
| Edwards 2004 all other surgery | (1) Rofecoxib 50 mg (2) Placebo | | | | (1) 5.8 (2) 2.8 | |
| Ehrich 1999 | (1) Rofecoxib 50 mg, n = 32 (2) Rofecoxib 500 mg, n = 20 (3) Ibuprofen 400 mg, n = 20 (4) Placebo, n = 32 | TOTPAR 6: (1) 15.05 (2) 14.31 (3) 16.25 (4) 2.70 | (1) 23/32 (2) 14/20 (3) 16/20 (4) 1/32 | No usable data | (1) >6 (2) >6 (3) >6 (4) 1.6 | No usable data |
| Fricke 2002 | (1) Rofecoxib 50 mg, n = 82 (2) Valdecoxib 40 mg, n = 80 (3) Placebo, n = 41 | TOTPAR 6: (1) 8.6 (2) No usable data (3) 3.4 | (1) 30/82 (2) No usable data (3) 3/41 | No usable data | No data | At 24 h: (1) 58.5 (2) 40.0 (3) 85.4 |
| Haglund 2006 | (1) Rofecoxib 50 mg, n = 36 (2) Rofecoxib/acetaminophen 50 mg/1 g, n = 34 (3) Acetaminophen 1 g, n = 20 (4) Placebo, n = 17 | TOTPAR 6: (1) 14.5 (2) 16 (3) 11.5 (4) 0.25 | (1) 25/36 (2) 26/34 (3) 10/20 (4) 0/17 | No usable data | MEAN time to use of rescue medication (h) (1) 4.5 (2) 3.9 (3) 3.8 (4) 2.1 | At 8 h: (1) 16.7 (2) 8.8 (3) 40.0 (4) 70.6 |
| Jackson 2004 | (1) Rofecoxib 50 mg, n = 37 (2) Dexketoprofen trometamol 25 mg, n = 42 (3) Placebo, n = 41 | No usable data | No usable data | No usable data | (1) >24:00 (2) 6.6 (3) 2.5 | At 24 h: (1) 40.5 (2) 83.3 (3) 87.8 |
| Kellstein 2004 | (1) Rofecoxib 50 mg, n = 102 (2) Lumiracoxib 400 mg, n = 101 (3) Celecoxib 200 mg, n = 101 (4) Placebo, n = 51 | TOTPAR 6: (1) 8.64 (2) 10.68 (3) 6.13 (4) 1.4 | (1) 37/102 (2) 48/101 (3) 23/101 (4) 0/51 | Rated excellent at 24 h: (1) 26/102 (2) 32/101 (3) 14/101 (4) 1/51 | (1) 3.8 (2) 7.2 (3) 2.0 (4) 1.3 | No usable data |
| Korn 2004 | (1) Rofecoxib 50 mg, n = 90 (2) Oxycodone/acetaminophen 5/325 mg, n = 91 (3) Placebo, n = 31 | TOTPAR 4: (1) 7.2 (2) 4.0 (3) 1.2 TOTPAR 6: (1) 11.7 (2) 5.9 (3) 1.9 | (1) 48/90 (2) 13/91 (3) 1/31 | At 6 h: (1) 42/90 (2) 17/91 (3) 0/31 | (1) 8.3 (2) 3.3 (3) 1.7 | At 24 h: (1) 72.2 (2) 94.5 (3) 96.8 |
| Malmstrom 1999 | (1) Rofecoxib 50 mg, n = 90 (2) Celecoxib 200 mg, n = 91 (3) Ibuprofen 400 mg, n = 46 (4) Placebo, n = 45 | TOTPAR 6: (1) 13.17 (2) 9.55 (3) 12.85 (4) 3.07 | (1) 55/90 (2) 38/91 (3) 27/46 (4) 2/45 | At 8 h: (1) 50/90 (2) 26/91 (3) 23/46 (4) 3/45 | (1) >24 (2) 5.1 (3) 8.9 (4) 1.5 | At 24 h: (1) 78 (2) 49 (3) 76 (4) 91 |
| Malmstrom 2002 | (1) Rofecoxib 50 mg, n = 151 (2) Celecoxib 400 mg, n = 151 (3) Celecoxib 200 mg, n = 90 (4) Ibuprofen 400 mg, n = 45 (5) Placebo, n = 45 | TOTPAR 6: (1) 12.60 (2) 11.00 (3) 8.56 (4) 11.67 (5) 1.05 | (1) 88/151 (2) 75/151 (3) 32/90 (4) 24/45 (5) 0/45 | No usable data | (1) 15.9 (2) 10.6 (3) 6.8 (4) 10.0 (5) 1.6 | At 24 h: (1) 51.3 (2) 65.6 (3) 68.9 (4) 86.7 (5) 97.8 |
| Michael‐Hill 2006 | (1) Rofecoxib 50 mg, n = 80 (2) AZD3582 500 mg, n = 78 (3) AZD3582 750 mg, n = 83 (4) Placebo, n = 41 | No usable data | No usable data | No data | (1) 10.6 (2) 3.6 (3) 6.1 (4) 1.5 | No usable data |
| Morrison 1999 | (1) Rofecoxib 50 mg, n = 50 (2) Ibuprofen 400 mg, n = 51 (3) Placebo, n = 50 | TOTPAR 6: (1) 10.12 (2) 9.26 (3) 4.15 | (1) 23/50 (2) 22/51 (3) 7/50 | No usable data | (1) 9.5 (2) 6.1 (3) 2.4 | At 24 h: (1) 84 (2) 56 (3) 92 |
| Reicin 2001 | (1) Rofecoxib 50 mg, n = 110 (2) Naproxen sodium 550 mg, n = 55 (3) Placebo, n = 53 | TOTPAR 6: (1) 10.7 (2) 9.8 (3) 5.4 | (1) 43/110 (2) 20/55 (3) 7/53 | No usable data | No data | No data |