Tratamiento farmacológico para los síntomas asociados con la ansiedad en pacientes adultos bajo cuidados paliativos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004596.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 mayo 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
2012 update:
-
all authors revised the search strategy;
-
LJ and BC screened studies;
-
KCJ, AT, MK and LJ commented on the draft review;
-
all authors agreed the final document.
2017 update:
-
SS updated the drugs listed in the search strategy;
-
SS and CM searched trials registers and handsearched conference abstracts;
-
SS and CM undertook screening of papers with deferment to NP for points of disagreement;
-
all authors commented on the draft manuscript and agreed the final version.
Declarations of interest
SS: none known; SS is a specialist in palliative care and manages patients with anxiety.
CM: none known.
NP: none known.
Acknowledgements
Original review: the authors acknowledge the assistance of the Cochrane Pain, Palliative and Supportive Care Group, specifically Phil Wiffen and Frances Fairman. We also acknowledge the contribution of Arthur G Lipman as an author of the original review. Thank you to Nathaniel Gordon for translating from Polish two trials for potential inclusion.
2012 update: the authors acknowledge the assistance of the Cochrane Pain, Palliative and Supportive Care Group, specifically Jessica Thomas and Jane Haynes. The update was funded by Marie Curie Cancer Care.
2017 update: the authors acknowledge the assistance of the Cochrane Pain, Palliative and Supportive Care Group, and in particular Anne Erskine, Managing Editor, and Joanne Abbott, Information Specialist. We thank the authors of the original review and the 2012 update for allowing us the opportunity to update this review. We are very grateful to Kenny Jackson for his support and contributions to this update.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 May 18 | Drug therapy for symptoms associated with anxiety in adult palliative care patients | Review | Susan Salt, Caroline A Mulvaney, Nancy J Preston | |
| 2012 Oct 17 | Drug therapy for symptoms associated with anxiety in adult palliative care patients | Review | Bridget Candy, Kenneth C Jackson, Louise Jones, Adrian Tookman, Michael King | |
| 2004 Jan 26 | Drug therapy for anxiety in adult palliative care patients | Review | Kenneth C Jackson, Arthur G Lipman | |
Differences between protocol and review
2012 update
-
The Background, Methods, Results and Discussion were updated and revised to conform with current Cochrane style guidelines.
2017 update
-
In the first update and this second update we searched a range of trial registers that were not searched in the original Cochrane Review. However, while the ISRCTN Trials Register (www.controlled‐trials.com/isrctn) was searched in the first update, it was not searched in this latest update as it is covered by WHO Portal.
-
We did not search the Cochrane Pain, Palliative & Supportive Care Register as its contents are captured by CENTRAL.
-
We added an additional 25 drugs to our search and thus database searches for these were rerun from 2012 (see Appendix 9).
-
We updated the Background section to include references to more recently published work.
-
We updated the Methods section to take into account changes in the search strategy. We thought that we should be searching for studies currently in progress, in addition to published studies, and thus undertook searches of trial registers.
-
We added new outcomes, and specified primary and secondary outcomes. We specified that we will report outcomes assessed at one week.
-
We changed the description of 'Types of participants' and 'Types of interventions' to reflect current terminology. We also added comparators of interest.
-
We expanded our risk of bias descriptions.
-
We stated that we planned to construct a 'Summary of findings' table and to assess the quality of the evidence used the GRADE approach.
-
The Results and Discussion sections were updated to take into account the fact that no studies were found to include in the review and to include references to more recently published work.
-
All sections were updated and revised to conform with current Cochrane style guidelines.
Notes
A restricted search in March 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
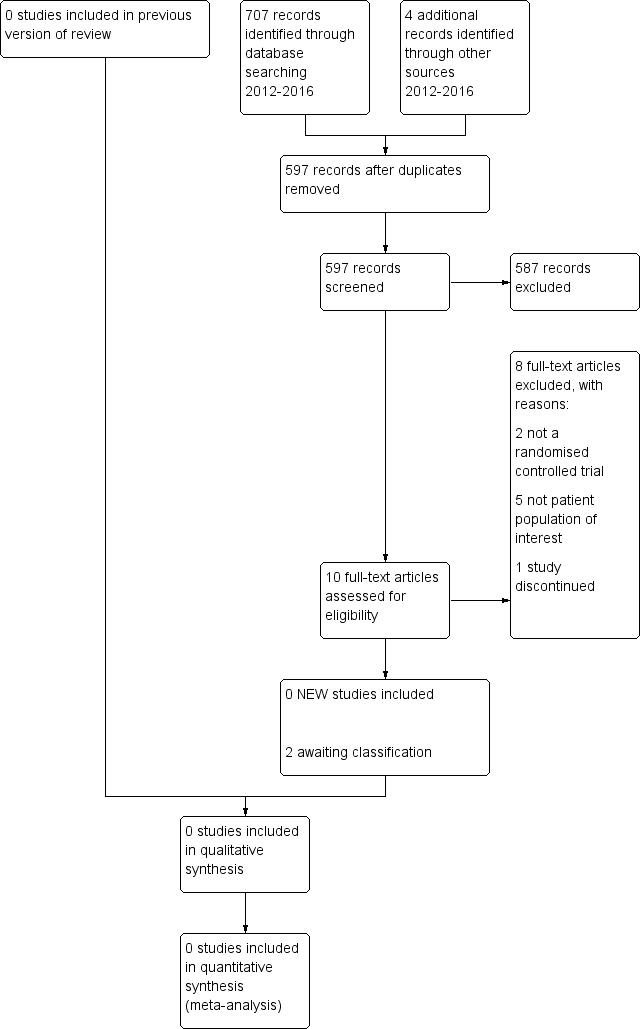
Study flow diagram.

