Artesunato más mefloquina versus mefloquina para el tratamiento del paludismo no complicado
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomized controlled trial Length of follow up: 42 d Generation of allocation sequence: no details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 9.1% (2/22) excluded after randomization due to vomiting of trial drugs | |
| Participants | Number enrolled: 22 participants aged 17 to 48 years Inclusion criteria: adult male patients; aged 17 to 48 years; 45 to 70 kg bodyweight; uncomplicated malaria; asexual parasites < 5% Exclusion criteria: history of liver or kidney disease | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 200 mg Mefloquine: 750 mg followed by 500 mg 6 h later | |
| Outcomes | 1. Mean fever clearance time | |
| Notes | Location: Bangkok, Thailand Date: not given Source of funding: UNDP/World Bank/WHO Special Programme for Training in Tropical Diseases, Atlantic Co. Ltd (Thailand), and Hoffmann‐La Roche (Thailand) | |
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: no details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 9.4% (8/85) excluded at day 28 from analysis due to severe vomiting of study drugs and concomitant illness but other losses to follow up were not accounted for | |
| Participants | Number enrolled: 127 participants between 16 and 56 years old Inclusion criteria: acute uncomplicated falciparum malaria; 100 to 200,000 parasites/µL blood; 16 to 60 years old; 45 to 60 kg bodyweight who agreed to stay in hospital for 28 days Exclusion criteria: pregnancy; severe malaria; and history of antimalarial drug treatment in preceding 1 week | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 100 mg followed by 50 mg/12 h for 5 d Mefloquine: 750 mg followed by 500 mg after 6 h Not included in this review: | |
| Outcomes | 1. Number cured at 28 d | |
| Notes | Location: Bangkok, Thailand Date: January to May 1991 Source of funding: Mahidol University research grant and Roche Research Foundation of Hong Kong; Atlantic Pharmaceutical Co. Ltd supplied artesunate tablets | |
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: table of random numbers Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 14.8% (17/115) were excluded from analysis at day 28 because of loss to follow up and taking antimalarial medication outside of the trial | |
| Participants | Number enrolled: 115 children and adults aged 5 to 50 years Inclusion criteria: falciparum mono infection with 500 to 30,000 parasites/µl of blood; axillary temperature at least 37.5 °C and/or history of fever in previous 48 h Exclusion criteria: severe malaria; presence of other causes of fever; pregnancy; and history of allergy to trial medication | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 4 mg/kg/d for 3 d, single dose | |
| Outcomes | 1. Fever on day 3 | |
| Notes | Location: Peruvian Amazon Date: June to September 2000 Source of funding: US Agency for International Development, the US Naval Medical Research Command, and the Government of Peru | |
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: randomization in pairs, no further details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 21.4% (65/304) excluded at day 28 due to vomiting trial medication and loss to follow up | |
| Participants | Number enrolled: 304 adults and children Inclusion criteria: slide‐confirmed malaria; weight > 5 kg; no use of other antimalarial in previous month; no signs of severe disease; and not pregnant | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 4 mg/kg, single dose | |
| Outcomes | 1. Fever clearance time | |
| Notes | Location: Thai‐Burmese boarder near Mae Sot Date: January to July 1992 Source of funding: not reported Nosten 1994a and Nosten 1994b are published in the same article | |
| Methods | Randomized controlled trial Length of follow up: 63 d Generation of allocation sequence: no details Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 11.8% excluded at day 28 and 31.9% excluded at day 63 due to vomiting trial medication and attrition | |
| Participants | Number enrolled: 348 adults and children Inclusion criteria: slide‐confirmed malaria; weight > 5 kg; no use of other antimalarial in previous month; no signs of severe disease; and not pregnant | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 4 mg/kg followed by 2 mg daily for 3 d Mefloquine: 25 mg/kg, single dose | |
| Outcomes | 1. Fever clearance time | |
| Notes | Location: Thai‐Burmese boarder near Mae Sot Date: October 1992 to June 1993 Source of funding: not reported Nosten 1994a and Nosten 1994b are published in the same article | |
| Methods | Randomized controlled trial Length of follow up: 63 d Generation of allocation sequence: randomized in blocks of three, no further details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 14.1% (46/362) lost to follow up at day 28, and 28.3% (98/362) lost to follow up at day 63; participants excluded because of vomiting and for reasons reportedly unrelated to the trial | |
| Participants | Number enrolled: 550 Inclusion criteria: weight at least 5 kg; slide‐confirmed falciparum malaria; and no antimalarial treatment in preceding 63 d Exclusion criteria: pregnancy; signs of severe malaria or concomitant illness requiring hospitalization; and history of neuropsychiatric illness | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 4 mg/kg/d for 3 d Mefloquine: 25 mg/kg, single dose on day 2 Not included in this review: | |
| Outcomes | 1. Parasite clearance time | |
| Notes | Location: Thai‐Myanmar border Date: June 1993 to May 1994 Source of funding: Wellcome Trust of Great Britain | |
| Methods | Randomized controlled trial Length of follow up: 42 d Generation of allocation sequence: not mentioned Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: not possible to calculate losses to follow up because numbers were not clear | |
| Participants | Number enrolled: 317, but only 156 given interventions relevant to this review Inclusion criteria: axillary temperature at least 37.5 °C or history of fever in previous 2 d; Plasmodium falciparum parasites of at least 1000/mm3 blood Exclusion criteria: body weight < 5 kg; pregnant women; signs and symptoms of complicated malaria or suggestive of another cause of fever; patients with a recent history of mefloquine use; patients with a parasite count > 250,000/mm3 and mixed infections | |
| Interventions | 1. Artesunate plus mefloquine Artesunate: 4 mg/kg, single dose Not included in this review: | |
| Outcomes | 1. Treatment failure at days 14, 28, and 42 | |
| Notes | Location: Kachin State, north Myanmar Date: July to August 2004 Source of funding: Médecins Sans Frontières (Holland) | |
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: no details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 3% (12/394) excluded post‐randomization for taking wrong regimen and vomiting of trial drugs but distribution across groups not given | |
| Participants | Number enrolled: 394 participants Inclusion criteria: symptomatic adults > 15 years old with malaria contracted within 50 km radius of the trial clinics; asexual parasitaemia 500 to 400,000 parasites/µL blood; no signs of complications; no history of antimalarial drug intake in previous 2 weeks; and consent to stay in malaria free area for the period of follow up Exclusion criteria: pregnancy | |
| Interventions | 1. Artesunate plus mefloquine Not included in this review: | |
| Outcomes | 1. Cure rate at day 28 Not included in this review: | |
| Notes | Location: Thai‐Cambodia border Date: July 1993 to December 1994 Source of funding: World Health Organization country budget for Thailand (THA/DPC/001) | |
Allocation concealment: B = unclear, see 'Methods of the review' for details and a summary of the quality assessment in Table 04
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Used different mefloquine doses for combination and mefloquine alone study arms | |
| Used different mefloquine doses for combination and mefloquine alone study arms | |
| Used different mefloquine doses for combination and mefloquine alone study arms | |
| Used different mefloquine doses for combination and mefloquine alone study arms | |
| Compared artesunate plus mefloquine with artesunate rather than mefloquine |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 1 Treatment failure. | ||||
| 1.1 Day 28 | 4 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.06, 0.47] |
| 1.2 Day 42 | 1 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.14, 0.39] |
| 1.3 Day 63 | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| 2 Parasitaemia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 2 Parasitaemia. | ||||
| 2.1 Day 3 | 2 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.11] |
| 2.2 Day 7 | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.11] |
| 2.3 Day 14 | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.28] |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 3 Fever. | ||||
| 3.1 Day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean fever clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 4 Mean fever clearance time (h). | ||||
| 5 Mean parasite clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 5 Mean parasite clearance time (h). | ||||
| 6 Adverse events Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 6 Adverse events. | ||||
| 6.1 Serious adverse events | 1 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.04] |
| 6.2 Adverse events requiring discontinuation of treatment | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.23] |
| 6.3 Abdominal pain | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.20] |
| 6.4 Cardiovascular abnormalities | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.08, 46.79] |
| 6.5 Central nervous system abnormalities | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.53, 1.36] |
| 6.6 Diarrhoea | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.48, 2.77] |
| 6.7 Dizziness | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.24] |
| 6.8 Headache | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.38, 0.69] |
| 6.9 Itching and rash | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [0.12, 67.83] |
| 6.10 Nausea | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.16] |
| 6.11 Palpitations/anxiety | 1 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 72.38] |
| 6.12 Psychosis | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.91] |
| 6.13 Vomiting | 4 | 1218 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.62] |
| 6.14 Other adverse events | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 8.43 [0.49, 146.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 1 Treatment failure. | ||||
| 1.1 Day 28 | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.38] |
| 1.2 Day 42 | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.36, 19.71] |
| 2 Parasitaemia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 2 Parasitaemia. | ||||
| 2.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 3 Fever. | ||||
| 3.1 Day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean fever clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 4 Mean fever clearance time (h). | ||||
| 5 Mean parasite clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 5 Mean parasite clearance time (h). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Parasitaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 1 Parasitaemia. | ||||
| 1.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 2 Fever. | ||||
| 2.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 3 Adverse event. | ||||
| 3.1 Insomnia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 1 Treatment failure. | ||||
| 1.1 Day 14 | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.35, 4.53] |
| 1.2 Day 28 | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.21] |
| 1.3 Day 42 | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.48, 1.65] |
| 2 Parasitaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 2 Parasitaemia. | ||||
| 2.1 Day 3 | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.29] |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 3 Fever. | ||||
| 3.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 1 Treatment failure.
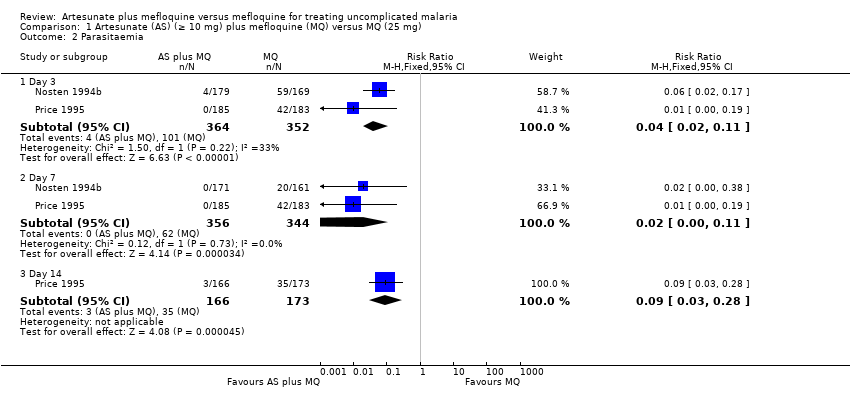
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 2 Parasitaemia.
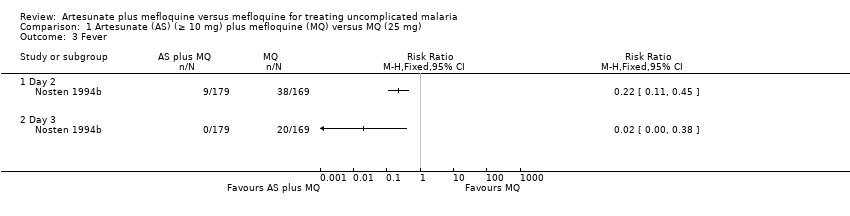
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 3 Fever.

Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 4 Mean fever clearance time (h).

Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 5 Mean parasite clearance time (h).
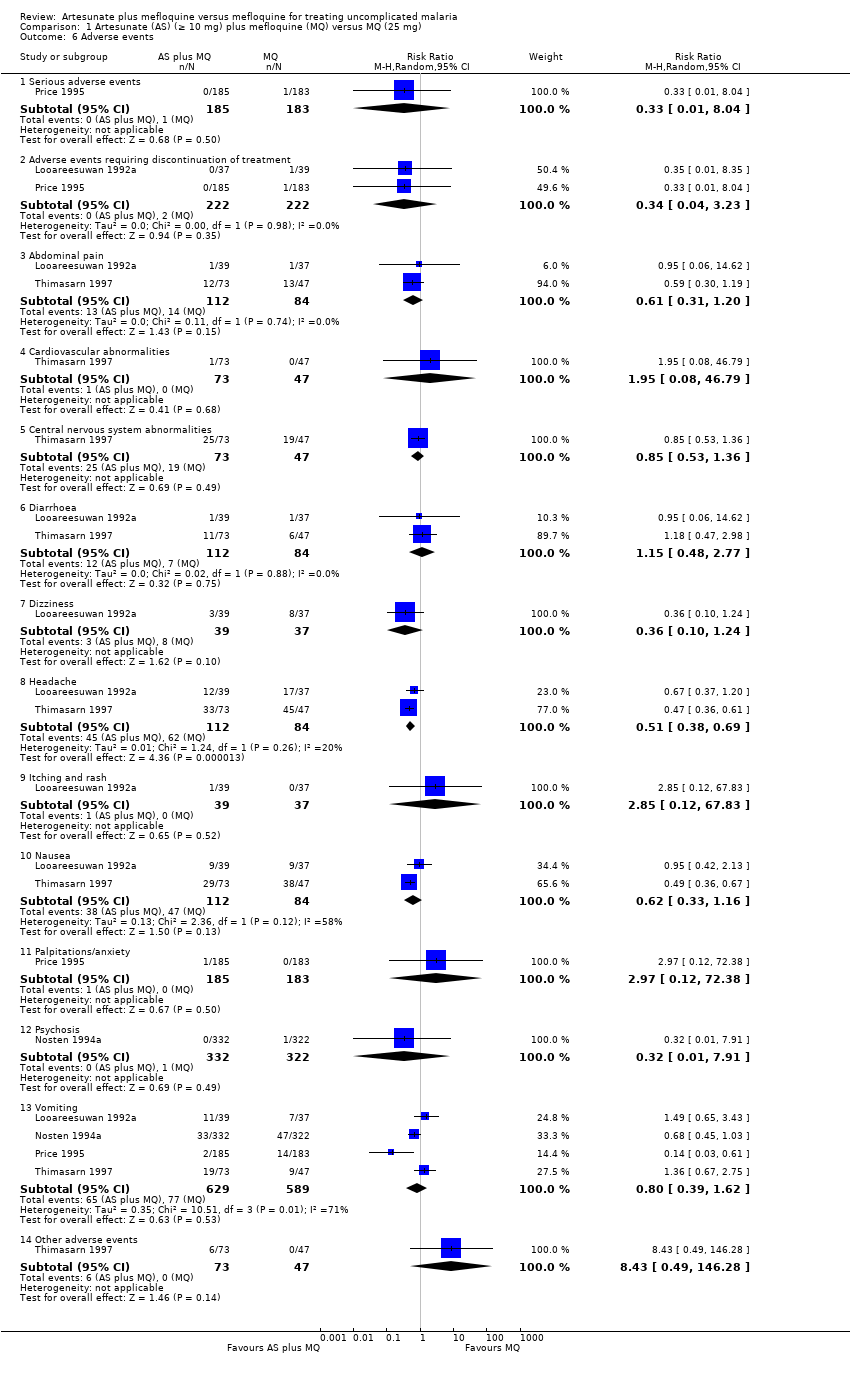
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 6 Adverse events.

Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 1 Treatment failure.

Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 2 Parasitaemia.
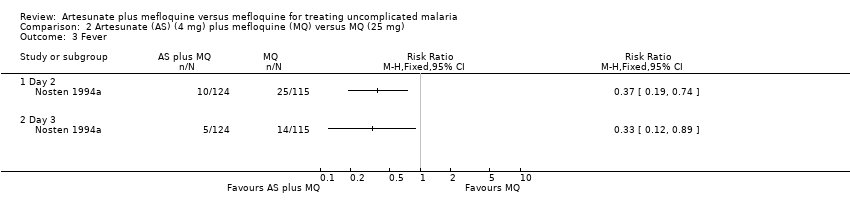
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 3 Fever.

Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 4 Mean fever clearance time (h).
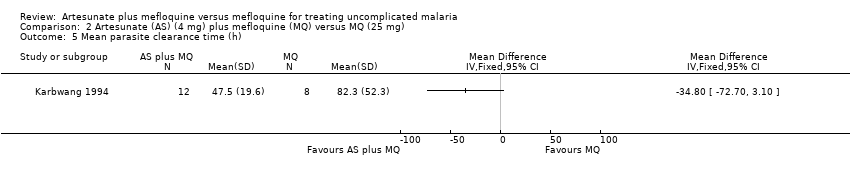
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 5 Mean parasite clearance time (h).
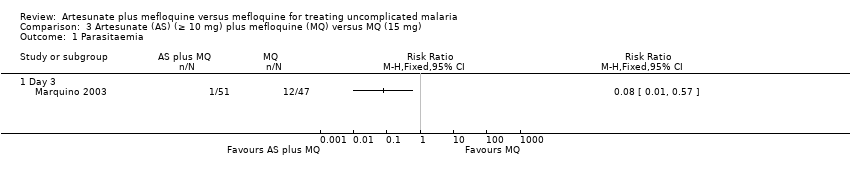
Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 1 Parasitaemia.

Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 2 Fever.

Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 3 Adverse event.

Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 1 Treatment failure.
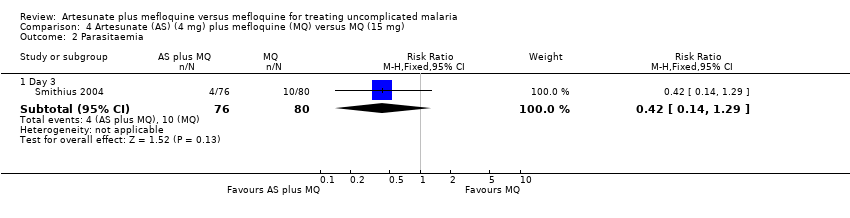
Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 2 Parasitaemia.

Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 3 Fever.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | BIOSIS |
| 1 | artesunate | artesunate | ARTESUNATE | ARTESUNATE | artesunate | artesunate |
| 2 | mefloquine | mefloquine | artesunate | artesunate | mefloquine | mefloquine |
| 3 | Lariam | Lariam | arsumax | arsumax | malaria | Lariam |
| 4 | — | 2 or 3 | 1 or 2 or 3 | 1 or 2 or 3 | 1 and 2 and 3 | — |
| 5 | — | 1 and 4 | MEFLOQUINE | mefloquine | — | — |
| 6 | — | — | mefloquine | MEFLOQUINE | — | — |
| 7 | — | — | Lariam | mephaquim | — | — |
| 8 | — | — | 5 or 6 or 7 | Lariam | — | — |
| 9 | — | — | 4 and 8 | 5 or 6 or 7 or 8 | — | — |
| 10 | — | — | exp MALARIA | 4 and 9 | — | — |
| 11 | — | — | malaria | malaria | — | — |
| 12 | — | — | exp PLASMODIUM | MALARIA | — | — |
| 13 | — | — | plasmodium | PLASMODIUM‐FALCIPARUM | — | — |
| 14 | — | — | 10 or 11 or 12 or 13 | 11 or 12 or 13 | — | — |
| 15 | — | — | 9 and 14 | 10 and 14 | — | — |
| aCochrane Infectious Diseases Group Specialized Register. | ||||||
| Method | Details |
| Recrudesced and new infections | In areas of intense malaria transmission, blood smears positive for malaria parasites after day 14 may be a result of new infections or a recrudescence of the original infection. Polymerase chain reaction (PCR) is a method that can be used to differentiate between new and old infections. We will use the results of PCR analyses, if they become available, to differentiate between recrudesced and new infections |
| Continuous data reported with geometric means | We will extract the standard deviations on the log scale, and extract minimum and maximum values for medians. We will combine the findings on a log scale and report on the original scale; we will report medians and ranges in tables |
| Exploring potential sources of heterogeneity using subgroup analyses | 1. Intervention: simultaneous versus sequential regimens; mefloquine dose; and artesunate dose |
| Sensitivity analyses | We will conduct sensitivity analyses for each of the components of methodological quality |
| Funnel plots | We will examine funnel plots for asymmetry, keeping in mind that the asymmetry could be caused by publication bias, differences in methodological quality, or heterogeneity |
| Trial | Artesunate | Mefloquine | ||
| Total dose (mg/kg) | Regimen | Total dose (mg/kg) | Regimen | |
| 3.33a | 1 dose | 21b | 2 doses over 1 day | |
| 10 | 11 doses over 5 days | 21b | 2 doses over 1 day | |
| 12 | 3 doses over 3 days | 15 | 1 dose | |
| 4 | 1 dose | 25 | 1 dose | |
| 10 | 4 doses over 3 days | 25 | 1 dose | |
| 12 | 3 doses over 3 days | 25 | 1 dose | |
| 4 | 1 dose | 15 | 1 dose | |
| 10a | 2 doses over 2 days | 21b | 2 doses over 3 days | |
| aAssuming 60 kg person (actual dose 200 mg). | ||||
| Trial | Generation of allocation sequencec | Allocation concealmentd | Blindinge | Inclusion of all randomized participants in final analysisb |
| Unclear | Unclear | Unclear | Adequate | |
| Unclear | Unclear | Unclear | Inadequate | |
| Adequate | Unclear | Unclear | Inadequate | |
| Unclear | Unclear | Unclear | Inadequate | |
| Unclear | Unclear | Unclear | Inadequate | |
| Unclear | Unclear | Unclear | Inadequate | |
| Unclear | Unclear | Unclear | Adequate | |
| Unclear | Unclear | Unclear | Inadequate | |
| aSee the 'Assessment of risk of bias in included studies' for the assessment methods, and the Characteristics of included studies' for the methods used in each trial. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 28 | 4 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.06, 0.47] |
| 1.2 Day 42 | 1 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.14, 0.39] |
| 1.3 Day 63 | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| 2 Parasitaemia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 3 | 2 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.11] |
| 2.2 Day 7 | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.11] |
| 2.3 Day 14 | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.28] |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean fever clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean parasite clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse events Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Serious adverse events | 1 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.04] |
| 6.2 Adverse events requiring discontinuation of treatment | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.23] |
| 6.3 Abdominal pain | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.20] |
| 6.4 Cardiovascular abnormalities | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.08, 46.79] |
| 6.5 Central nervous system abnormalities | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.53, 1.36] |
| 6.6 Diarrhoea | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.48, 2.77] |
| 6.7 Dizziness | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.24] |
| 6.8 Headache | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.38, 0.69] |
| 6.9 Itching and rash | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [0.12, 67.83] |
| 6.10 Nausea | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.16] |
| 6.11 Palpitations/anxiety | 1 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 72.38] |
| 6.12 Psychosis | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.91] |
| 6.13 Vomiting | 4 | 1218 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.62] |
| 6.14 Other adverse events | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 8.43 [0.49, 146.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Day 28 | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.38] |
| 1.2 Day 42 | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.36, 19.71] |
| 2 Parasitaemia Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean fever clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean parasite clearance time (h) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Parasitaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Insomnia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 14 | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.35, 4.53] |
| 1.2 Day 28 | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.21] |
| 1.3 Day 42 | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.48, 1.65] |
| 2 Parasitaemia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 3 | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.29] |
| 3 Fever Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

