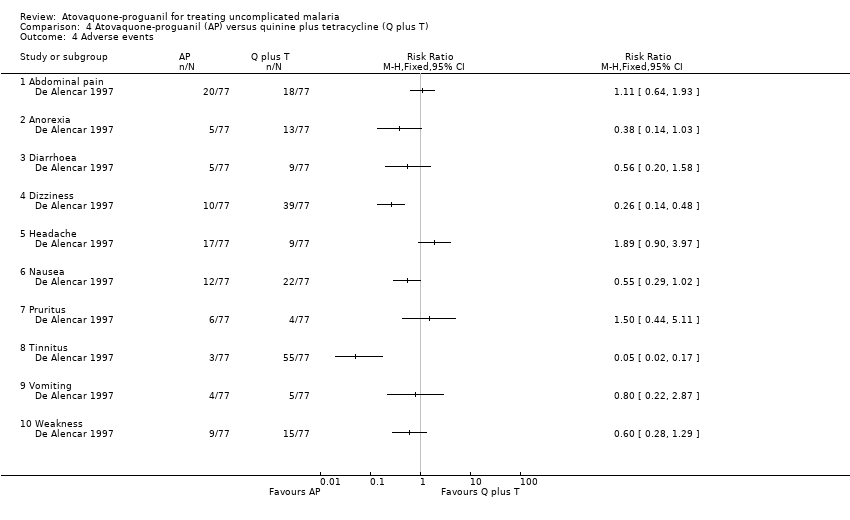
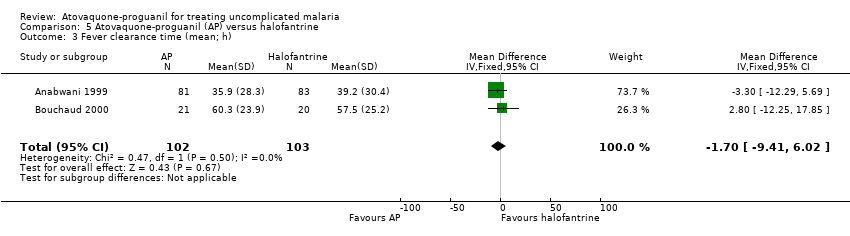
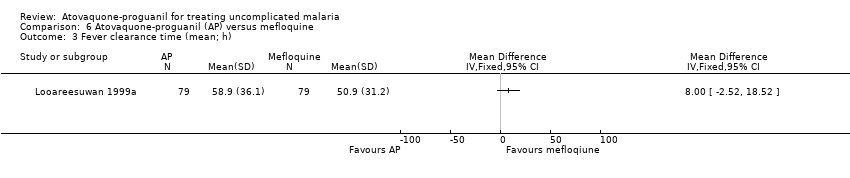
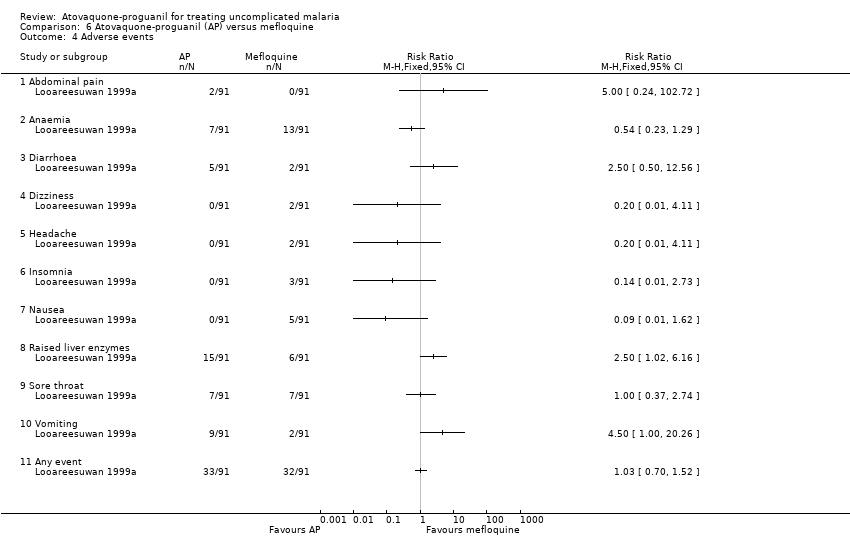
Contenido relacionado
Revisiones y protocolos relacionados
Andrew Blanshard, Paul Hine | 18 enero 2021
Anna M van Eijk, Dianne J Terlouw | 16 febrero 2011
Babalwa Zani, Michael Gathu, Sarah Donegan, Piero L Olliaro, David Sinclair | 20 enero 2014
Aika AA Omari, Carrol L Gamble, Paul Garner | 19 abril 2006
Rachel Isba, Babalwa Zani, Michael Gathu, David Sinclair | 23 febrero 2015
Maya Tickell‐Painter, Nicola Maayan, Rachel Saunders, Cheryl Pace, David Sinclair | 30 octubre 2017
David Sinclair, Babalwa Zani, Sarah Donegan, Piero Olliaro, Paul Garner | 8 julio 2009
Sabine Bélard, Michael Ramharter, Florian Kurth | 8 diciembre 2020
Nithya Gogtay, Sridharan Kannan, Urmila M Thatte, Piero L Olliaro, David Sinclair | 25 octubre 2013
Aika AA Omari, Carrol L Gamble, Paul Garner | 19 octubre 2005