Hierbas medicinales chinas para el cáncer esofágico
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004520.pub7Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 enero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud digestiva
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Xin Wei (XW), Zhiyu Chen (ZC) and Taixiang Wu (TW) were responsible for development of the protocol.
Xi Chen (XC), Linyu Deng (LD) and TW were responsible for searching for trials, quality assessment of the trials, data extraction, data analysis and review development. TW interviewed the original trial authors of self described "RCTs" included in the initial version of this review. XC and Xuehua Jiang (XJ) interviewed the trial authors of this version of the review.
Sources of support
Internal sources
-
Chinese Cochrane Centre, West China Hospital of Sichuan University, China.
External sources
-
Chinese Medical Board of New York (CMB), USA.
Declarations of interest
XC: none known.
LD: none known.
XJ: none known.
TW: none known.
Acknowledgements
The authors would like to thank Karin Dearness, Janet Lilleyman, Iris Gordon, Cathy Bennett and Racquel Simpson of the Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group, and peer referees Professors Sheila Greenfield, Liz Gardener, Edzard Ernst, David Kirby, Miguel Sanchez Gonzalez, Weidong Lu, Chun‐Tao Che, Sarah Rhodes and Jane Blazeby for advice on writing this review. We also thank authors of the included studies who patiently discussed the trials with XC and provided information of their studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jan 22 | Chinese herbal medicine for oesophageal cancer | Review | Xi Chen, Linyu Deng, Xuehua Jiang, Taixiang Wu | |
| 2009 Oct 07 | Chinese herbal medicines for esophageal cancer | Review | Xin Wei, Zhiyu Chen, Xiaoyan Yang, Taixiang Wu | |
| 2009 Jul 08 | Medicinal herbs for esophageal cancer | Review | Xin Wei, Zhiyu Chen, Xiaoyan Yang, Taixiang Wu | |
| 2007 Jul 18 | Medicinal herbs for esophageal cancer | Review | W ei Xin, C hen Zhiyu, W u Taixiang, Y ang Xiaoyan, L iu Guanjian, Taixiang Wu, Guanjian Liu, Xiaoyan Yang, Xin Wei, Zhiyu Chen | |
| 2007 Apr 18 | Medicinal herbs for esophageal cancer | Review | Xin Wei, Zhiyu Chen, Xiaoyan Yang, Taixiang Wu | |
| 2007 Jan 24 | Medicinal herbs for esophageal cancer | Review | WU Taixiang, X Wei, Xiaoyan Yang, Chen Zhiyu | |
| 2003 Oct 20 | Medicinal herbs for esophageal cancer | Protocol | Zhiyu Chen, T Wu, X Yang, J Wei, Q Wang, G Liu, Wu Taixiang, LIU Guanjian, WEI Jiafu, Wang Qin, YANG Xiaoyan | |
Differences between protocol and review
-
We changed the title of the review from 'Medicinal herbs for oesophageal cancer' to 'Chinese herbal medicine for oesophageal cancer.'
-
Types of studies: we had intended to include cross‐over trials and within‐patient studies, but limited our review to only RCTs comparing radiotherapy or chemotherapy with or without additional Chinese herbal medicine.
-
Types of interventions: we revised and limited control treatment 'other treatments that are widely used by the doctors for oesophageal cancer' to 'active cancer therapy such as radiotherapy or chemotherapy'. We changed 'Chinese medicinal herbs are used by oral intake' to 'Water extractions of TCM were administered either orally (in capsules or as powders) or by intravenous infusion'. These revised inclusion criteria are more common in clinical studies.
-
Types of outcome measures: we added 'Improvement, defined as complete response (CR) and partial response (PR) as clarified by Miller 1981 or short‐term therapeutic effects or TCM symptoms' to the 'Secondary outcomes'.
-
Search strategy: we changed from the Chinese Biomedical Database and CISCOM (the Research Council for Complementary Medicine) to the VIP and Wanfang databases.
-
We added effect of time‐to‐events data analysis to the 'Measures of treatment effect' section, and added 'GRADE and Summary of findings' tables.
-
We revised and rewrote the 'Assessment of risk of bias in included studies', 'Subgroup analysis and investigation of heterogeneity', and 'Sensitivity analysis' sections.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
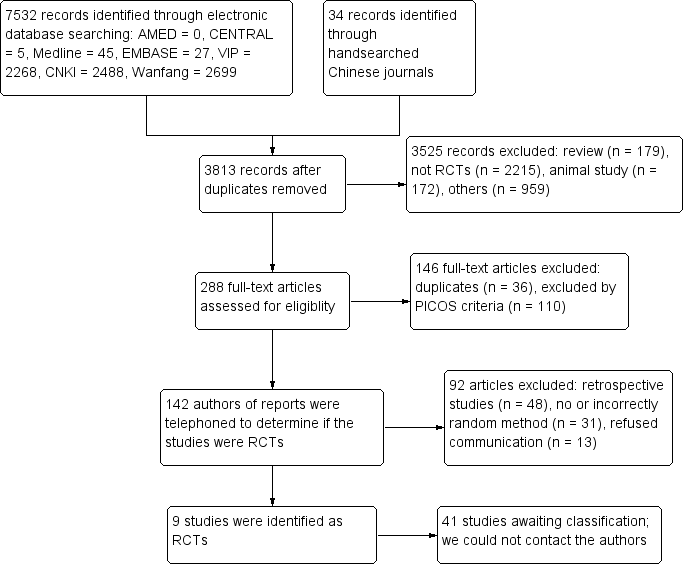
Study flow diagram.
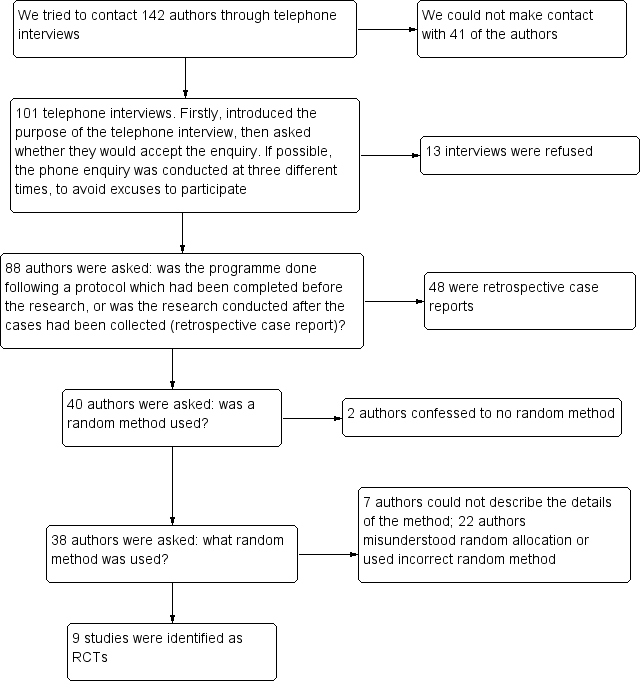
Flow diagram of telephone interviews.
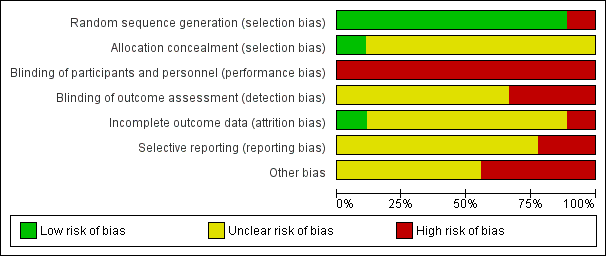
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
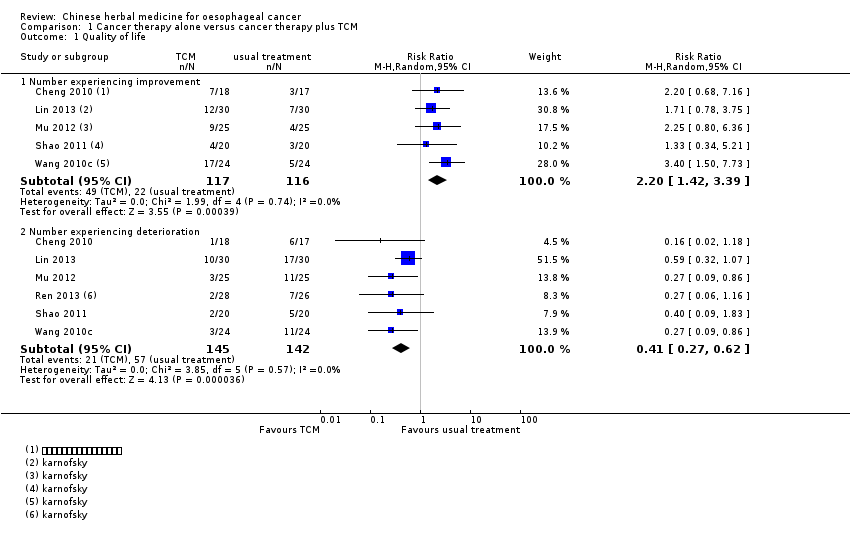
Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 1 Quality of life.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 2 Quality of life.
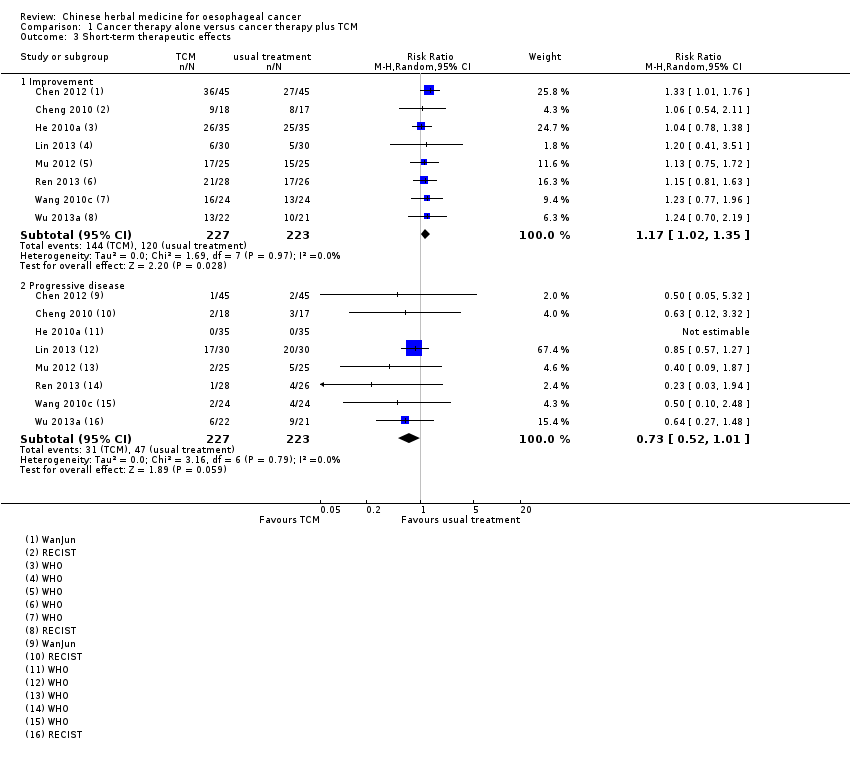
Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 3 Short‐term therapeutic effects.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 4 TCM symptoms.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 5 Adverse events.
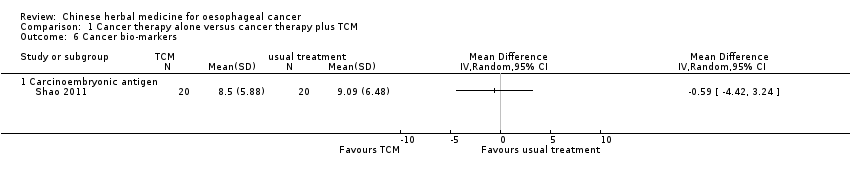
Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 6 Cancer bio‐markers.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 7 Weight.
| Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer | ||||||
| Patient or population: patients with oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual treatment | Usual treatment combined with TCM | |||||
| All‐cause mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Median survival times ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Time to progression ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Quality of life ‐ number experiencing improvement | Study population | RR 2.2 | 233 | ⊕⊕⊝⊝ | ||
| 190 per 1000 | 417 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 389 per 1000 | |||||
| Quality of life ‐ number experiencing deterioration | Study population | RR 0.41 | 287 | ⊕⊕⊝⊝ | ||
| 401 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 397 per 1000 | 163 per 1000 | |||||
| Quality of life ‐ Karnofsky performance status | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ | |
| Short‐term therapeutic effects ‐ improvement (complete response + partial response) | Study population | RR 1.17 | 450 | ⊕⊕⊕⊝ | ||
| 538 per 1000 | 630 per 1000 | |||||
| Moderate | ||||||
| 571 per 1000 | 668 per 1000 | |||||
| Short‐term therapeutic effects ‐ progressive disease | Study population | RR 0.73 | 450 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 154 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 126 per 1000 | |||||
| TCM symptoms ‐ total effectiveness | Study population | RR 1.84 | 88 | ⊕⊝⊝⊝ | ||
| 477 per 1000 | 878 per 1000 | |||||
| Moderate | ||||||
| 471 per 1000 | 867 per 1000 | |||||
| TCM symptoms ‐ ineffectiveness | Study population | RR 0.22 | 88 | ⊕⊝⊝⊝ | ||
| 523 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 529 per 1000 | 116 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The studies were parallel‐group RCTs, but not blinded. The outcome may be affected by the subjective effect of the researcher, thereby resulting in very serious limitations. | ||||||
| Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer | ||||||
| Patient or population: oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual treatment | Usual treatment combined with TCM | |||||
| Adverse events ‐ mucositis | Study population | RR 0.92 | 50 | ⊕⊕⊝⊝ | ||
| 1000 per 1000 | 920 per 1000 | |||||
| Moderate | ||||||
| 1000 per 1000 | 920 per 1000 | |||||
| Adverse events ‐ radiation oesophagitis | Study population | RR 0.66 | 160 | ⊕⊕⊝⊝ | ||
| 550 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 546 per 1000 | 360 per 1000 | |||||
| Adverse events ‐ arrest of bone marrow | Study population | RR 0.28 | 90 | ⊕⊕⊕⊝ | ||
| 556 per 1000 | 156 per 1000 | |||||
| Moderate | ||||||
| 556 per 1000 | 156 per 1000 | |||||
| Adverse events ‐ gastrointestinal reactions | Study population | RR 0.54 | 268 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 270 per 1000 | |||||
| Moderate | ||||||
| 558 per 1000 | 301 per 1000 | |||||
| Adverse events ‐ renal and hepatic impairment ‐ Fuzheng Guben granules | Study population | RR 0.33 | 90 | ⊕⊕⊝⊝ | ||
| 333 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 110 per 1000 | |||||
| Adverse events ‐ renal and hepatic impairment‐ Xiaoaiping | Study population | RR 0.90 | 60 | ⊕⊕⊝⊝ | ||
| 133 per 1000 | 120 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 210 per 1000 | |||||
| Adverse events ‐ white blood cell descent | Study population | RR 0.60 | 224 | ⊕⊝⊝⊝ | ||
| 486 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 446 per 1000 | 268 per 1000 | |||||
| Adverse events ‐ neurotoxicity | Study population | RR 0.73 | 60 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 367 per 1000 | 268 per 1000 | |||||
| Adverse events ‐ cardiac toxicity | Study population | RR 0.80 | 60 | ⊕⊕⊝⊝ | ||
| 167 per 1000 | 133 per 1000 | |||||
| Moderate | ||||||
| 167 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The study was a parallel‐group RCT, but not blinded. The outcome may be affected by the subjective effect of the researcher, thereby resulting in very serious limitations. | ||||||
| Study ID | TCM | Pinyin name | Latin name | English name | Preparation |
| Fuzheng Guben granules | Huangqi Dangshen Shanzha Chenpi Nuzhenzi Buguzhi Baizhu Gouqizi Fuling Shenqu Maiya Jixueteng Yinchenhao Tusizi | Radix Astragali Radix Codonopsis Fructus Crataegi Pericarpium Citri Reticulatae Fructus Ligustri Lucidi Fructus Psoraleae Rhizoma Atractylodis Macrocephalae Fructus Lycii Poria Massa Medicata Fermentata Fructus Hordei Germinatus Caulis Spatholobi Herba Artemisiae Scopariae Semen Cuscutae | Milkvetch Root Pilose Asiabell Root Hawthorn Fruit Tangerine Peel Glossy Privet Fruit Malaytea Scurfpea Fruit Largehead Atractylodes Rhizome Barbary Wolfberry Fruit Indian Buead Medicated Leaven Malt Suberect Spatholobus Virgate Wormwood Herb South Dodder Seed | Not described in detail Produced by Gansu Prorincial Wuwei Tumour Hospital | |
| Xihuang Pill | Niuhuang Ruxiang Moyao Shexiang | Calculus Bovis Olibanum Myrrha Moschus | Bezoar Frankincense Myrrh Musk | Frankincense and Myrrh were treated by vinegar‐processing method Produced by Beijing Tongrentang Pharmaceutical Factory | |
| Brucea Javanica oil emulsion | YadanzI | Fructus Bruceae | Java Brucea Fruit | Not described in detail | |
| Xiaoaiping injection | Wuguteng | Radix Fissistigmae Glaucescentis | Root of Glaucescent Fissistigma | Xiaoaiping injection was extracted from Glaucescent Fissistigma Produced by Nanjing Shenghe Pharmaceutical Co Ltd | |
| Kangai injection | Renshen Huangqi Kushen | Radix Ginseng Radix Astragali Radix Sophorae Flavescentis | Ginseng Milkvetch Root Lightyellow Sophora Root | Not described in detail | |
| Yiqiyangyin Huatanquyu decoction | Taizishen Nanshashen Beishashen Maidong Chenpi Banxia Ezhu Banzhilian Baihuasheshecao Zhigancao Jineijin Maiya Guya Baizhu Shanyao Tiandong Wuweizi Fuling Yiyiren Danggui | Radix Pseudostellariae Radix Adenophorae Radix Glehniae Radix Ophiopogonis Pericarpium Citri Reticulatae Rhizoma Pinelliae Rhizoma Curcumae Herba Scutellariae Barbatae Herba Hedyotidis Diffusae Glycyrrhizae Endothelium Corneum Gigeriae Galli Fructus Hordei Germinatus Fructus Oryzae Germinatus Rhizoma Atractylodis Macrocephala Rhizoma Diosscoreae Radix Asparagi Fructus Schisandrae Poria Semen Coicis Radix Angelicae Sinensis | Heterophylly Falsestarwort Root Ladybell Root Coastal Glehnia Root Dwarf Lilyturf Tuber Tangerine Peel Pinellia Tuber Zedoary Barbed Skullcap Herb Spreading Hedyotis Herb Radix glycyrrhizae preparata Chicken's Gizzard‐membrane Malt Rice‐grain Sprout Largehead Atractylodes Rhizome Common Yam Rhizome Cochinchinese Asparagus Root Chinese Magnoliavine Fruit Indian Buead Coix Seed Chinese Angelic | Modification according to symptoms: Largehead Atractylodes Rhizome Common Yam Rhizome Cochinchinese Asparagus Root Chinese Magnoliavine Fruit Indian Buead, Coix Seed and Chinese Angelic Yiqiyangyin Huatanquyu decoction was prepared by researchers of hospital | |
| Zhisheng capsule | Shexiang Ezhu Bingpian Renshen Niuhuang Dongcongxiacao Xiyangshen | Moschus Rhizoma Curcumae Borneolum Radix Ginseng Calculus Bovis Cordyceps Radix Panacis Quinquefolii | Musk Zedoary Borneol Ginseng Bezoar Chinese Caterpillar Fungus American Ginseng | The composition of Zhisheng capsule contained 16 traditional Chinese medicines, only 7 of them were given Produced by the first affiliated Hospital of Henan University of Traditional Chinese Medicine | |
| Aidi injection | Renshen, Ciwujia, Bianmao, Huangqi. | Radix Ginseng, Radix Acanthopanacis Senticosi, Mylabris, Radix Astragali. | Ginseng, Manyprickle Acanto‐Panax Root, Blister Beetle, Milkvetch Root. | Not described in detail |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Number experiencing improvement | 5 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.42, 3.39] |
| 1.2 Number experiencing deterioration | 6 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.27, 0.62] |
| 2 Quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Karnofsky performance status | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term therapeutic effects Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Improvement | 8 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.35] |
| 3.2 Progressive disease | 8 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.01] |
| 4 TCM symptoms Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Total effectiveness | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.20, 2.81] |
| 4.2 Ineffectiveness | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.93] |
| 5 Adverse events Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Mucositis | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 5.2 Radiation oesophagitis | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.94] |
| 5.3 Arrest of bone marrow | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.58] |
| 5.4 Gastrointestinal reactions | 4 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] |
| 5.5 Renal and hepatic impairment‐Fuzheng Guben granules | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.84] |
| 5.6 Renal and hepatic impairment‐Xiaoaiping | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.88, 7.10] |
| 5.7 White blood cell descent | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| 5.8 Neurotoxicity | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.34, 1.55] |
| 5.9 Cardiac toxicity | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.24, 2.69] |
| 5.10 Anaemia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.14] |
| 6 Cancer bio‐markers Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Carcinoembryonic antigen | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Weight Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Increased | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Decreased | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

