Hierbas medicinales chinas para el cáncer esofágico
Resumen
Antecedentes
El cáncer esofágico es la séptima causa de muerte por cáncer en el mundo. Las hierbas medicinales chinas tradicionales se utilizan en ocasiones como complemento de la radioterapia o de la quimioterapia en este tipo de cáncer. Esta revisión fue publicada por primera vez en 2007 y se actualizó en 2009; esta actualización de 2016 es la última versión de la revisión.
Objetivos
Evaluar la eficacia y los posibles efectos adversos del agregado de hierbas medicinales chinas al tratamiento con radioterapia o quimioterapia del cáncer esofágico.
Métodos de búsqueda
Se hicieron búsquedas en el el registro de ensayos del Grupo Cochrane de Enfermedades Esófago‐gástricas del Intestino Delgado y Pancreáticas (Cochrane Upper Gastrointestinal and Pancreatic Diseases Group), the Cochrane Library, MEDLINE, EMBASE, Allied and Complementary Medicine Database (AMED), China National Knowledge Infrastructure (CNKI), VIP database, Wanfang database y en el Chinese Cochrane Centre Controlled Trials Register hasta el 1 octubre 2015. También se realizaron búsquedas en las bases de datos de ensayos en curso, en Internet y en listas de referencias.
Criterios de selección
Ensayos controlados aleatorios (ECA) que compararon el uso de la radioterapia o la quimioterapia con y sin el agregado de hierbas medicinales chinas.
Obtención y análisis de los datos
Al menos dos autores de la revisión extrajeron los datos de forma independiente y evaluaron la calidad de los ensayos.
Resultados principales
Se trató de contactar con los autores de los 142 estudios por teléfono, y finalmente se incluyeron nueve estudios con 490 participantes. Todos los estudios incluidos se realizaron en China, y los pacientes con cáncer esofágico avanzado se asignaron a los grupos de radioterapia o quimioterapia, con y sin hierbas medicinales chinas adicionales. En estos estudios se informaron la calidad de vida, los efectos terapéuticos a corto plazo, los síntomas de la MCT y los eventos adversos causados por la radioterapia o la quimioterapia. En general, se consideró que los ensayos tuvieron riesgo de sesgo incierto o alto.
La calidad de vida se midió antes y después de la intervención; los análisis de esta revisión mostraron un efecto beneficioso en el número de participantes que experimentaron una mejoría (cociente de riesgos [CR] 2,20; intervalo de confianza [IC] del 95%: 1,42 a 3,39; cinco ECA, 233 participantes, cambio en la puntuación del estado funcional ≥ 10) y en el número de participantes que experimentaron un deterioro (CR 0,41; IC del 95%: 0,27 a 0,62; seis ECA, 287 participantes, cambio en la puntuación del estado funcional ≤ 10). Estas pruebas se consideraron de calidad baja, se disminuyó la calidad de las pruebas por el riesgo de sesgo y la imprecisión y se mejoró la calidad de las pruebas por el efecto grande.
Para los efectos terapéuticos a corto plazo, los resultados indican que la medicina china tradicional (MCT) tiene una repercusión positiva sobre la mejoría (respuesta completa + respuesta parcial) (CR 1,17; IC del 95%: 1,02 a 1,35; ocho ECA, 450 participantes), pruebas de calidad moderada, que se disminuyó por el riesgo de sesgo. No hubo diferencias significativas para la enfermedad progresiva (CR 0,73; IC del 95%: 0,52 a 1,01; ocho ECA, 450 participantes), pruebas de calidad baja, que se disminuyó por el riesgo de sesgo y la imprecisión. Tres estudios evaluaron este resultado después de cuatro semanas o tres meses de seguimiento, los estudios restantes no proporcionaron información detallada para este resultado. Los síntomas de la MCT, que fueron similares a los efectos terapéuticos a corto plazo evaluados con los criterios clínicos de la MCT, se diagnosticaron en dos estudios con 88 pacientes al final de la intervención. Los resultados indican que la MCT tiene una repercusión positiva sobre la efectividad total (CR 1,84; IC del 95%: 1,20 a 2,81) y sobre la ineficacia (CR 0,22; IC del 95%: 0,05 a 0,93); se consideró que los estudios proporcionaron pruebas de calidad muy baja, que se disminuyó por el riesgo de sesgo y la imprecisión.
Nueve estudios informaron una serie de eventos adversos causados por la radioterapia o la quimioterapia al final de la intervención, que incluyeron mucositis, esofagitis por radiación, inhibición de la médula ósea, reacciones gastrointestinales, deficiencia renal y hepática, disminución de los leucocitos, neurotoxicidad, toxicidad cardíaca y anemia. Para los que contenían estudios múltiples, se realizó un análisis agrupado. Como resultado, la MCT mostró un efecto significativo sobre la esofagitis por radiación (CR 0,66; IC del 95%: 0,47 a 0,94; dos ECA, 90 participantes), las reacciones gastrointestinales (CR 0,54; IC del 95%: 0,36 a 0,81; cuatro ECA, 268 participantes) y la disminución de los leucocitos (CR 0,60; IC del 95%: 0,44 a 0,83; cuatro ECA, 224 participantes). La calidad de las pruebas fue baja o muy baja y se disminuyó por el riesgo de sesgo y la imprecisión.
Conclusiones de los autores
Hasta el momento no se han encontrado pruebas para determinar si la MCT es un tratamiento eficaz para el cáncer esofágico. El efecto de la MCT sobre los efectos terapéuticos a corto plazo no está claro. La MCT probablemente tiene efectos positivos sobre la calidad de vida y sobre algunos eventos adversos causados por la radioterapia o la quimioterapia en los pacientes con cáncer esofágico avanzado que reciben radioterapia o quimioterapia. Es necesario interpretar con precaución los resultados de la revisión debido a que en general las pruebas son de calidad baja. Los ensayos futuros deben ser grandes y diseñarse correctamente para detectar efectos clínicos importantes y reducir al mínimo el riesgo de sesgo.
PICO
Resumen en términos sencillos
Hierbas medicinales chinas para el cáncer esofágico
Pregunta de la revisión
Las hierbas medicinales chinas se utilizan ampliamente como tratamiento complementario de la quimioterapia o la radioterapia en pacientes tratados por cáncer esofágico. Hasta el momento no existen pruebas claras de que las hierbas medicinales chinas resulten efectivas para esta función.
Antecedentes
Se realizó una revisión sistemática de los posibles efectos beneficiosos de las hierbas medicinales chinas en comparación con la quimioterapia o la radioterapia para el cáncer esofágico con y sin hierbas medicinales concurrentes. Se hicieron búsquedas en el el registro de ensayos del Grupo Cochrane de Enfermedades Esófago‐gástricas del Intestino Delgado y Pancreáticas (Cochrane Upper Gastrointestinal and Pancreatic Diseases Group), the Cochrane Library, MEDLINE, EMBASE, Allied and Complementary Medicine Database (AMED), China National Knowledge Infrastructure (CNKI), VIP database, Wanfang database y en el Chinese Cochrane Centre Controlled Trials Register hasta el 1 octubre 2015. También se realizaron búsquedas en las bases de datos de ensayos en curso, en Internet y en listas de referencias.
Características de los estudios
Se identificaron nueve ensayos controlados aleatorios (ECA). Todos los ensayos se realizaron en China.
Resultados clave
Esta revisión actualizada incluyó nueve ensayos con 490 participantes. Cuando las hierbas medicinales chinas se utilizaron como tratamiento complementario a la quimioterapia o la radioterapia para el cáncer esofágico, los resultados mostraron un efecto positivo sobre la calidad de vida y una mayor tolerancia de los pacientes a los efectos adversos causados por la radioterapia o la quimioterapia (p.ej. esofagitis por radiación, reacciones gastrointestinales y disminución de los glóbulos), pero no mostraron efectos sobre la mortalidad por todas las causas, la mediana del tiempo de supervivencia o el tiempo hasta la progresión.
Calidad de la evidencia
Se consideró que la calidad de las pruebas fue baja a moderada debido al riesgo de sesgo; y a que el escaso número de pacientes reclutados en estos estudios provocó imprecisión y resultados inconsecuentes. Los nueve ensayos incluidos no permitieron realizar un análisis de subgrupos de diferentes clases de hierbas medicinales chinas, y los objetivos terapéuticos de cada una de ellas son diferentes entre sí, lo que pudiera ser otra razón para los resultados imprecisos. En el futuro, se requieren ensayos de alta calidad.
Authors' conclusions
Summary of findings
| Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer | ||||||
| Patient or population: patients with oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual treatment | Usual treatment combined with TCM | |||||
| All‐cause mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Median survival times ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Time to progression ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Quality of life ‐ number experiencing improvement | Study population | RR 2.2 | 233 | ⊕⊕⊝⊝ | ||
| 190 per 1000 | 417 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 389 per 1000 | |||||
| Quality of life ‐ number experiencing deterioration | Study population | RR 0.41 | 287 | ⊕⊕⊝⊝ | ||
| 401 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 397 per 1000 | 163 per 1000 | |||||
| Quality of life ‐ Karnofsky performance status | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ | |
| Short‐term therapeutic effects ‐ improvement (complete response + partial response) | Study population | RR 1.17 | 450 | ⊕⊕⊕⊝ | ||
| 538 per 1000 | 630 per 1000 | |||||
| Moderate | ||||||
| 571 per 1000 | 668 per 1000 | |||||
| Short‐term therapeutic effects ‐ progressive disease | Study population | RR 0.73 | 450 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 154 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 126 per 1000 | |||||
| TCM symptoms ‐ total effectiveness | Study population | RR 1.84 | 88 | ⊕⊝⊝⊝ | ||
| 477 per 1000 | 878 per 1000 | |||||
| Moderate | ||||||
| 471 per 1000 | 867 per 1000 | |||||
| TCM symptoms ‐ ineffectiveness | Study population | RR 0.22 | 88 | ⊕⊝⊝⊝ | ||
| 523 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 529 per 1000 | 116 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The studies were parallel‐group RCTs, but not blinded. The outcome may be affected by the subjective effect of the researcher, thereby resulting in very serious limitations. | ||||||
| Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer | ||||||
| Patient or population: oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual treatment | Usual treatment combined with TCM | |||||
| Adverse events ‐ mucositis | Study population | RR 0.92 | 50 | ⊕⊕⊝⊝ | ||
| 1000 per 1000 | 920 per 1000 | |||||
| Moderate | ||||||
| 1000 per 1000 | 920 per 1000 | |||||
| Adverse events ‐ radiation oesophagitis | Study population | RR 0.66 | 160 | ⊕⊕⊝⊝ | ||
| 550 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 546 per 1000 | 360 per 1000 | |||||
| Adverse events ‐ arrest of bone marrow | Study population | RR 0.28 | 90 | ⊕⊕⊕⊝ | ||
| 556 per 1000 | 156 per 1000 | |||||
| Moderate | ||||||
| 556 per 1000 | 156 per 1000 | |||||
| Adverse events ‐ gastrointestinal reactions | Study population | RR 0.54 | 268 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 270 per 1000 | |||||
| Moderate | ||||||
| 558 per 1000 | 301 per 1000 | |||||
| Adverse events ‐ renal and hepatic impairment ‐ Fuzheng Guben granules | Study population | RR 0.33 | 90 | ⊕⊕⊝⊝ | ||
| 333 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 110 per 1000 | |||||
| Adverse events ‐ renal and hepatic impairment‐ Xiaoaiping | Study population | RR 0.90 | 60 | ⊕⊕⊝⊝ | ||
| 133 per 1000 | 120 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 210 per 1000 | |||||
| Adverse events ‐ white blood cell descent | Study population | RR 0.60 | 224 | ⊕⊝⊝⊝ | ||
| 486 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 446 per 1000 | 268 per 1000 | |||||
| Adverse events ‐ neurotoxicity | Study population | RR 0.73 | 60 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 367 per 1000 | 268 per 1000 | |||||
| Adverse events ‐ cardiac toxicity | Study population | RR 0.80 | 60 | ⊕⊕⊝⊝ | ||
| 167 per 1000 | 133 per 1000 | |||||
| Moderate | ||||||
| 167 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The study was a parallel‐group RCT, but not blinded. The outcome may be affected by the subjective effect of the researcher, thereby resulting in very serious limitations. | ||||||
Background
Description of the condition
Oesophageal cancer is a global health problem that has two subtypes: squamous carcinoma and adenocarcinoma. As the seventh leading cause of cancer death worldwide, the occurrence of oesophageal cancer varies by geographic area, ethnic group and gender. The incidence of oesophageal carcinoma can be as high as 30 to 800 cases per 100,000 persons in particular areas of northern Iran, some areas of southern Russia, and in northern China; the incidence in the US is approximately three to six cases per 100,000 persons (Nishihira 1993; Pera 2001). Oesophageal cancer is generally more common in men than in women, with a male‐to‐female ratio of 7:1, and occurs most commonly during the sixth and seventh decades of life (Blot 1993; Kirby 1994).
Oesophageal carcinoma arises in the mucosa. Subsequently it tends to invade the submucosa and the muscular layer of the oesophagus. Eventually contiguous structures such as the tracheobronchial tree, the aorta, or the recurrent laryngeal nerve become involved. Though firstly metastasising to the perioesophageal lymph nodes, oesophageal cancer can also spread to almost any other part of the body, including the liver, lungs, brain and bones (Nishihira 1993).
The etiology of oesophageal carcinoma is thought to be related to exposure of the oesophageal mucosa to noxious or toxic stimuli, resulting in a sequence of dysplasia, carcinoma in situ and carcinoma (Lam 2000).
Some factors believed to trigger oesophageal carcinoma are: cigarette smoking (Broitman 1983); alcohol consumption (Tuyns 1979); drinking exceptionally hot beverages such as tea (Victora 1987); diets low in beta‐carotene, vitamins A, C and B, magnesium and zinc; poor oral hygiene; protease inhibitors (Sammon 1998); high‐level exposure to asbestos (O'Byrne 2001); tylosis palmaris et plantaris (Mandard 2000); swallowing lye or other caustic substances (Messmann 2001); and Barrett's oesophagus (Williamsom 1991). A recent investigation reported that the high incidence of oesophageal carcinoma in Taiwan and India may be associated with betel chewing as a major independent risk factor (Wu 2001a).
Description of the intervention
The management of oesophageal cancer presents great challenges in the current practice of gastroenterology; it is highly treatable in its earliest stages but is usually fatal when more advanced (Clark 1994; Steup 1996).
Non‐operative therapy is usually reserved for patients who are not candidates for surgery. The goal of therapy for these patients is palliation of dysphagia, to allow them to eat. Chemotherapy has limited use as a single modality (Cooper 1999; Entwistle 2002), but radiation therapy is successful in relieving dysphagia in approximately 50% of patients. In patients with advanced oesophageal cancer, the preoperative combination of chemotherapy and radiotherapy has shown good results (Cooper 1999; Herskovic 1992). Intracavitary radiotherapy, with or without surgery, is safe for many patients (Homs 2003).
Laser therapy can destroy cancerous tissue and relieve a blockage in the oesophagus when the cancer cannot be removed by surgery. The relief of a blockage can help to reduce symptoms, especially swallowing problems (Carter 1992). When a tracheoesophageal fistula is present, intubating with expandable metallic stents is particularly useful (Knyrim 1993; Sarper 2003).
Oesophageal resection (oesophagectomy) is used in patients who are suitable for surgery. If an oesophagectomy is indicated there are three main procedures that range from minimally invasive to highly invasive (Wu 2003). A transhiatal oesophagectomy without a thoracotomy, a 'standard' (transthoracic) oesophagectomy, or an en bloc oesophagectomy are current methods used. Many studies have been conducted using the three procedures but a consensus has not been formed as to the preferred technique (Goldminc 1993; Swanstrom 2002; Teng 1999). The application of these treatments is dependent on the stage of the disease at diagnosis.
Chinese herbal medicine for oesophageal cancer has commonly been used as adjunct treatment for alleviating the side effects of chemotherapy or radiotherapy, and for improving the quality of life of cancer patients. An investigation conducted in New Zealand found that 49% of cancer patients used complementary and alternative medicine (CAM), including vitamins, antioxidants, alternative diets and herbal therapies. Usage was more common in younger patients. CAM was used by 47% of people to improve quality of life and by 30% in the hope of a cancer cure (Chrystal 2003). In one study, all participants used three or more types of CAM, most commonly herbal or nutritional supplements (Shumay 2001); evidence suggests that at least one cancer patient in three uses some form of CAM (Jacobson 1999). The most commonly‐cited reasons for seeking complementary medicine were to reduce side effects of treatment, and because of the lack of effectiveness of standard therapies (Hilsden 1999).
How the intervention might work
Possible benefits of herbal medicine includes increasing appetite (Jin 2003), boosting the immune system (Chen 2002a), facilitating the recovery of the body and preventing tumour regeneration or development of metastases (Wang 1999). Some herbs may be effective against cancer (Ye 2002). For example, artemisinin‐type compounds inhibit tumour cell growth (Chen 2003), and membranous milkvetch root, Barbary wolfberry fruit, and heterophylly falsestarwort root may enhance immunity (Du 1998). Common yam rhizome and Indian bread could decrease the side effects of radiotherapy and give a better outcome (Jia 2001). The Chinese herbal medicine spreading hedyotis can repress the proliferation of cancer cells and block the progression of oesophageal cancer (Zhou 1996a).
Traditional Chinese medicine (TCM) is used in the treatment of cancer by following a particular theoretical and methodological pathway that involves assessing the cause, diagnosis and treatment. Drug treatment within TCM typically consists of complex prescriptions of a combination of components.
Why it is important to do this review
The evidence to support the use of herbal medicine for anticancer effects, reducing chemotherapy side effects and improving quality of life is mainly theoretical and experiential. Furthermore, there is evidence to indicate that not all CAM is risk‐free, with concerns regarding adverse event issues such as allergic reactions and Chinese herbal nephropathy (Lampert 2002; Lord 2001; Nortier 2000). Despite these concerns, herbal medicine is widely used by cancer patients (Dooley 2004; Jacobson 1999).
Objectives
We aim to update our previous review that was published in 2009 to assess the efficacy and possible adverse effects of combining Chinese herbal medicine with radiotherapy or chemotherapy for the treatment of oesophageal cancer (Wei 2007).
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) comparing radiotherapy or chemotherapy with or without additional Chinese herbal medicine.
Types of participants
Patients with oesophageal cancer of different subtypes.
Types of interventions
All traditional Chinese medicine (TCM) used in the treatment of oesophageal cancer as an adjunct to active cancer therapy such as radiotherapy or chemotherapy. Water extractions of TCM were administered either orally (in capsules or as powders) or by intravenous infusion.
Types of outcome measures
Primary outcomes
-
All‐cause mortality.
-
Median survival times.
-
Time to progression.
-
Quality of life (using validated measures).
Secondary outcomes
-
Improvement, defined as complete response and partial response as clarified by Miller 1981, or short‐term therapeutic effects.
-
Any adverse event that caused: (1) death; (2) life‐threatening illness; or (3) significant toxicity.
Search methods for identification of studies
We conducted searches to identify all published and unpublished RCTs of traditional Chinese herbal therapy for oesophageal cancer. The search strategy identified studies in all languages. We translated non‐English language papers so that we could fully assess them for inclusion in the review, as necessary.
Electronic searches
We searched the following databases.
-
CENTRAL (the Cochrane Library, September 2015; Appendix 1).
-
MEDLINE (1950 to September 2015; Appendix 2).
-
EMBASE (1980 to September 2015; Appendix 3).
-
AMED (1985 to September 2015; Appendix 4).
-
CNKI (1979 to September 2015; Appendix 5).
-
Chinese journals full‐text database (1979 to September 2015; Appendix 5).
-
Chinese journals full‐text database century journals (1979 to September 2015; Appendix 5).
-
Chinese Doctoral degree thesis full‐text database (1979 to September 2015; Appendix 5).
-
Chinese outstanding master degree thesis full‐text database (1979 to September 2015; Appendix 5).
-
VIP Database (1989 to September 2015; Appendix 6).
-
Wanfang Database (1993 to September 2015; Appendix 7).
We constructed the search strategy for Chinese databases by using a combination of MeSH subject headings and text words relating to the use of Chinese medicinal herbs in the treatment of oesophageal cancer (Appendix 8).
Searching other resources
We also handsearched the following Chinese journals.
-
Acta Medicinae Sinica.
-
Cancer Research on Prevention and Treatment.
-
China Journal of Chinese Materia Medica.
-
China Oncology.
-
Chinese Journal of Cancer Research.
-
Chinese Journal of Clinical Oncology and Rehabilitation.
-
Chinese Journal of Integrated Traditional and Western Medicine on Digestion.
-
Chinese Journal of Oncology.
-
Chinese Journal of Radiation Oncology.
-
Henan Journal of Traditional Chinese Medicine.
-
Jiangshu Journal of Traditional Chinese Medicine.
-
Journal of Beijing of Traditional Chinese Medicine.
-
Journal of Fujian of Traditional Chinese Medicine.
-
Journal of Jilin of Traditional Chinese Medicine.
-
Journal of Practical Oncology.
-
Journal of Nanjing University of Traditional Chinese Medicine.
-
Journal of Sichuang of Traditional Chinese Medicine.
-
Journal of Traditional Chinese Medicine.
-
Traditional Chinese Medicinal Research.
Data collection and analysis
Three review authors (XC, LD, TW) undertook data collection and analysis.
Selection of studies
To determine the studies to be assessed further we scanned the titles, abstracts and keywords of every record retrieved. We retrieved full articles for further assessment if the information given suggested that the studies:
-
included patients with oesophageal cancer of different subtypes;
-
compared the combination of conventional treatment and Chinese medicinal herbs with conventional therapies without Chinese medicinal herbs;
-
assessed one or more relevant clinical outcome measures;
-
used random allocation to the comparison groups.
We then telephoned the authors of potential included studies to determine if the studies were RCTs.
There were no recorded disagreements between the review authors. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (XC, TW) independently extracted data concerning details of study population, intervention used and outcomes by using a data extraction form. We designed the form specifically for this review and it included the following items.
-
General information: published or unpublished, title, authors, reference or source, contact address, country, urban or rural etc., language of publication, year of publication, duplicate publication, sponsor, setting.
-
Trial characteristics: design, duration of follow‐up, method of randomisation, allocation concealment, blinding (patients, people administering treatment, outcome assessors).
-
Intervention(s): intervention(s) (dose, route, timing), comparison intervention(s) (dose, route, timing), comedication(s) (dose, route, timing).
-
Patients: exclusion criteria, total number and number in comparison groups, age (adults), baseline characteristics, diagnostic criteria, similarity of groups at baseline (including any comorbidity), assessment of compliance, withdrawals or losses to follow‐up (reasons or description), subgroups.
-
Outcomes: outcomes specified in this review, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes.
-
Results: for outcomes (including a measure of variation) and times of assessment. If necessary, we converted these to measures of effect as specified below, and using an intention‐to‐treat analysis.
-
Other: the Pinyin, Latin and English names of the TCM preparations of included studies are in Table 1.
| Study ID | TCM | Pinyin name | Latin name | English name | Preparation |
| Fuzheng Guben granules | Huangqi Dangshen Shanzha Chenpi Nuzhenzi Buguzhi Baizhu Gouqizi Fuling Shenqu Maiya Jixueteng Yinchenhao Tusizi | Radix Astragali Radix Codonopsis Fructus Crataegi Pericarpium Citri Reticulatae Fructus Ligustri Lucidi Fructus Psoraleae Rhizoma Atractylodis Macrocephalae Fructus Lycii Poria Massa Medicata Fermentata Fructus Hordei Germinatus Caulis Spatholobi Herba Artemisiae Scopariae Semen Cuscutae | Milkvetch Root Pilose Asiabell Root Hawthorn Fruit Tangerine Peel Glossy Privet Fruit Malaytea Scurfpea Fruit Largehead Atractylodes Rhizome Barbary Wolfberry Fruit Indian Buead Medicated Leaven Malt Suberect Spatholobus Virgate Wormwood Herb South Dodder Seed | Not described in detail Produced by Gansu Prorincial Wuwei Tumour Hospital | |
| Xihuang Pill | Niuhuang Ruxiang Moyao Shexiang | Calculus Bovis Olibanum Myrrha Moschus | Bezoar Frankincense Myrrh Musk | Frankincense and Myrrh were treated by vinegar‐processing method Produced by Beijing Tongrentang Pharmaceutical Factory | |
| Brucea Javanica oil emulsion | YadanzI | Fructus Bruceae | Java Brucea Fruit | Not described in detail | |
| Xiaoaiping injection | Wuguteng | Radix Fissistigmae Glaucescentis | Root of Glaucescent Fissistigma | Xiaoaiping injection was extracted from Glaucescent Fissistigma Produced by Nanjing Shenghe Pharmaceutical Co Ltd | |
| Kangai injection | Renshen Huangqi Kushen | Radix Ginseng Radix Astragali Radix Sophorae Flavescentis | Ginseng Milkvetch Root Lightyellow Sophora Root | Not described in detail | |
| Yiqiyangyin Huatanquyu decoction | Taizishen Nanshashen Beishashen Maidong Chenpi Banxia Ezhu Banzhilian Baihuasheshecao Zhigancao Jineijin Maiya Guya Baizhu Shanyao Tiandong Wuweizi Fuling Yiyiren Danggui | Radix Pseudostellariae Radix Adenophorae Radix Glehniae Radix Ophiopogonis Pericarpium Citri Reticulatae Rhizoma Pinelliae Rhizoma Curcumae Herba Scutellariae Barbatae Herba Hedyotidis Diffusae Glycyrrhizae Endothelium Corneum Gigeriae Galli Fructus Hordei Germinatus Fructus Oryzae Germinatus Rhizoma Atractylodis Macrocephala Rhizoma Diosscoreae Radix Asparagi Fructus Schisandrae Poria Semen Coicis Radix Angelicae Sinensis | Heterophylly Falsestarwort Root Ladybell Root Coastal Glehnia Root Dwarf Lilyturf Tuber Tangerine Peel Pinellia Tuber Zedoary Barbed Skullcap Herb Spreading Hedyotis Herb Radix glycyrrhizae preparata Chicken's Gizzard‐membrane Malt Rice‐grain Sprout Largehead Atractylodes Rhizome Common Yam Rhizome Cochinchinese Asparagus Root Chinese Magnoliavine Fruit Indian Buead Coix Seed Chinese Angelic | Modification according to symptoms: Largehead Atractylodes Rhizome Common Yam Rhizome Cochinchinese Asparagus Root Chinese Magnoliavine Fruit Indian Buead, Coix Seed and Chinese Angelic Yiqiyangyin Huatanquyu decoction was prepared by researchers of hospital | |
| Zhisheng capsule | Shexiang Ezhu Bingpian Renshen Niuhuang Dongcongxiacao Xiyangshen | Moschus Rhizoma Curcumae Borneolum Radix Ginseng Calculus Bovis Cordyceps Radix Panacis Quinquefolii | Musk Zedoary Borneol Ginseng Bezoar Chinese Caterpillar Fungus American Ginseng | The composition of Zhisheng capsule contained 16 traditional Chinese medicines, only 7 of them were given Produced by the first affiliated Hospital of Henan University of Traditional Chinese Medicine | |
| Aidi injection | Renshen, Ciwujia, Bianmao, Huangqi. | Radix Ginseng, Radix Acanthopanacis Senticosi, Mylabris, Radix Astragali. | Ginseng, Manyprickle Acanto‐Panax Root, Blister Beetle, Milkvetch Root. | Not described in detail |
Original reports of trial results were independently abstracted by XC and TW. Differences in data extraction were resolved by discussion and by referring back to the original article. When necessary, information was sought from the authors of the primary studies. We consulted a third author (LD) to resolve any disagreement.
For binary outcomes, we extracted number of events and total number in each group. For continuous outcomes, we extracted the mean, standard deviation and sample size of each group.
Assessment of risk of bias in included studies
At least two review authors (XC, TW) independently assessed risk of bias by using the following criteria described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011) and (Wu 2007).
We assessed the following characteristics.
Random sequence generation (selection bias)
We classified a study as a RCT if it was described as randomised (this includes the use of words such as randomly, random, and randomisation, etc.). We judged the study as low risk, high risk, or unclear risk according to the following.
-
Low risk, if the allocation sequence was generated by computer‐generated random numbers, published random number table, coin tossing, shuffling cards or envelopes, or throwing dice.
-
Unclear risk, if the trial was described as randomised but the method used for generation of the allocation sequence was not described.
-
High risk, if selection was based on patient numbers, birth dates, visit dates, or alternative allocation.
-
We excluded studies that described selection based on patient or clinical preference, or any selection mechanism that cannot be described as random. We also excluded studies that did not state whether the treatment was randomly allocated.
Allocation concealment (selection bias)
-
Low risk, if investigators were unaware of the allocation of each participant before they were entered in the trial. Acceptable methods included: central telephone randomisation schemes, pharmacy‐based schemes, sequentially numbered, opaque, sealed envelopes, or sequentially numbered drug containers of identical appearance.
-
Unclear risk, if the authors did not report or provide a description of an allocation concealment approach that allowed for classification as concealed or not concealed.
-
High risk, when investigators may have been aware of the allocation of each participant before they entered the trial, e.g. when allocation was based on patient data such as date of birth, hospital case note number, or visit dates, sealed envelopes that were not opaque, or a random number table that was not concealed from an investigator.
Blinding of participants and personnel (performance bias)
-
Low risk, if both participants and physicians were blinded to the treatment allocation, and it was unlikely that the blinding could have been broken.
-
Unclear risk, if no blinding information was available or there was insufficient information to permit a judgement of low risk or high risk.
-
High risk, if the authors defined the study as an open study, or no party was blinded. Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Blinding of outcome assessment (detection bias)
-
Low risk, if outcome assessors were blinded to the assigned treatment arm.
-
Unclear risk, if no information was provided for blinding of outcome assessment.
-
High risk, if outcome assessors were not blinded to the assigned treatment arm. Lack of blinding is likely to influence adverse events as an outcome.
Incomplete outcome data (attrition bias)
We assessed attrition bias for Helicobacter pylori (H. pylori) eradication and adverse events.
-
Low risk, if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to true outcome; missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups; the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; missing data were imputed using appropriate methods.
-
Unclear risk, if insufficient reporting of attrition or exclusions to permit judgement of low risk or high risk (e.g. no reasons for missing data provided).
-
High risk, if reasons for missing outcome data were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; the proportion of missing outcomes compared with observed event risk were enough to induce clinically relevant bias in intervention effect estimate; per protocol analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting (reporting bias)
-
Low risk, if the published reports included all expected outcomes, including those that were prespecified.
-
Unclear risk, if insufficient information to permit judgement of low risk or high risk.
-
High risk, if not all of the study’s prespecified primary outcomes were reported; the primary outcome was reported using measurements, analysis methods, or subsets of the data that were not prespecified; the primary outcome was not prespecified or was reported incompletely; or the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Other bias
-
Low risk, if the study appears to be free of other sources of bias.
-
Unclear risk, if there may be a risk of bias but there is either: insufficient information to assess whether an important risk of bias exists (e.g. limited information from a conference proceeding); or insufficient rationale or evidence that an identified problem introduces bias.
-
High risk, if there is at least one important risk of bias; a potential source of bias related to the specific study design used; stopped early due to a data‐dependent process (including a formal stopping rule); extreme baseline imbalance; has been claimed to have been fraudulent; or any other problem.
Measures of treatment effect
If data were sufficiently similar, we carried out meta‐analysis using the Review Manager software (RevMan 2014). We expressed results as risk ratios (RRs) for dichotomous outcomes; we used mean difference (MD) or the standardised mean difference (SMD) for continuous outcomes, both with 95% confidence intervals (CIs). We estimated time‐to‐event (survival) data by O ‐ E (observed value minus expected value) with variance.
Unit of analysis issues
For trials with non‐standard designs such as cluster‐randomised trials and trials with multiple intervention groups, unit of analysis issues were taken into account. The analysis was based on the individual participant rather than group of individuals. For a study with more than two treatment arms, we extracted only the relevant data.
Dealing with missing data
Where required, we requested information about missing data from original trial authors by telephone interview. We conducted an intention‐to‐treat analysis for missing participants due to drop‐out. If the missing data were unobtainable, we assumed that the unreported outcomes would provide dissatisfactory results. For example, the missing data from treatment groups would be added to the number experiencing deterioration in quality of life, progressive disease in short‐term therapeutic effects and suffering adverse events caused by radiotherapy or chemotherapy. The missing data from control groups would be added to the number experiencing improvement in quality of life and improvement in short‐term therapeutic effects.
Assessment of heterogeneity
We carried out the test for heterogeneity using the Chi2 test with significance set at P < 0.10. We used I2 to estimate the total variation across studies. We considered I2 < 25% as low level heterogeneity, 25% to 50% as a moderate level, and higher than 50% as high level heterogeneity (Higgins 2011). If there was evidence of heterogeneity, we performed subgroup analyses to find possible explanations.
Assessment of reporting biases
We considered reporting biases, which include publication bias and selective outcome reporting; they are listed in the 'Risk of bias' table of included studies. We would have assessed the potential publication bias using funnel plots when at least nine trials for one outcome were present. The result of asymmetric funnel plots should be interpreted carefully, because they may not necessarily be caused by publication bias. In this updated review, we could not perform a funnel plots analysis because of the small number of included studies.
Data synthesis
We did not intend to combine results of trials with different comparator drugs, and so we only included trials comparing radiotherapy or chemotherapy with or without additional Chinese herbal medicine. We used a random‐effects statistical model to conduct pooled analysis throughout. If the number of included studies was too small or heterogeneity was too apparent to conduct a meta‐analysis, we performed only a descriptive analysis.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes, all‐cause mortality, median survival times, time to progression, quality of life, short‐term therapeutic effects or TCM symptoms and adverse events. We used the five GRADE (Atkins 2004) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We explored reasons for heterogeneity between studies, and through sensitivity analyses examined the effects of excluding study subgroups, for example, those studies with lower methodological quality. We used a funnel plot to explore publication bias.
We intended to explore the following potential sources of heterogeneity using subgroup analyses or meta‐regression.
-
Stage of disease.
-
Different TCM formulations.
-
Duration of treatment.
Sensitivity analysis
We checked if the results were affected by the inclusion of results from certain trials by using a sensitivity analysis, and by comparing a random‐effects model to a fixed‐effect model.
Results
Description of studies
Results of the search
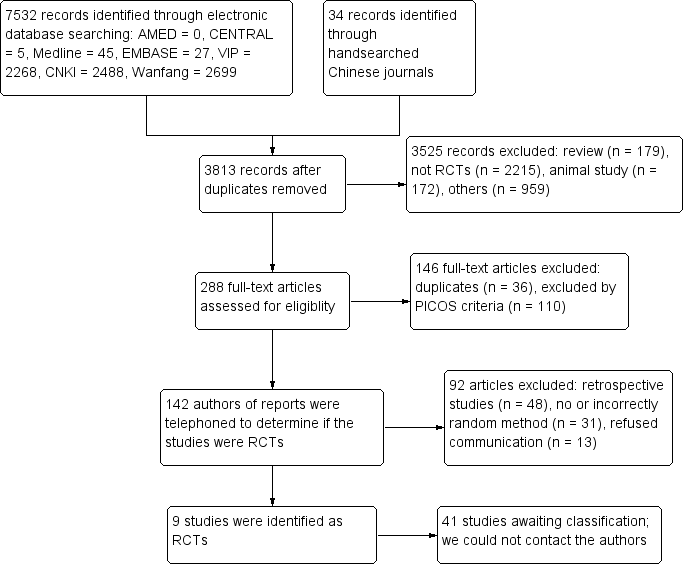
In this updated review, the initial search of the electronic databases and handsearching yielded 3813 records after we removed duplicates. After scrutinising these records, we assessed 288 full‐text records for eligibility (see Figure 1 for details of the screening process of the search results). We excluded 146 full‐text records and retained 142 full‐text records which claimed "randomly allocated patients" in their text. Then we telephoned authors of 142 reports to determine if the trials were authentic RCTs or not (see Figure 2 for details of the telephone interview process and the results). Finally, we judged 92 studies as not true RCTs (see Characteristics of excluded studies). We identified nine studies to be true RCTs which met the inclusion criteria (see Characteristics of included studies). However, up to October 30, 2015, we have been unable to contact 41 authors in this update, and we have provided details of these studies in Studies awaiting classification.

Study flow diagram.

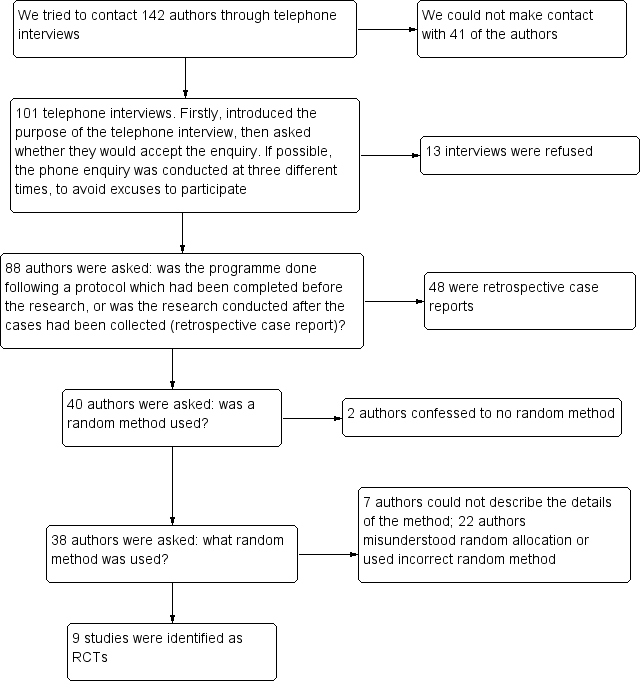
Flow diagram of telephone interviews.
Included studies
In the last version of this review, no study was eligible for inclusion. We included nine studies that met our inclusion criteria in this updated review (Chen 2012; Cheng 2010; He 2010a; Lin 2013; Mu 2012; Ren 2013; Shao 2011; Wang 2010c; Wu 2013a). All included trials were conducted in China, and were single‐centre two‐arm studies, except Wang 2010c which involved patients from two hospitals. The details are presented in the Characteristics of included studies tables.
Trial characteristics
All studies used a parallel‐group design. No details were given about the duration of follow‐up, except Wu 2013a, in which the median time of follow‐up was 5.3 months. No traditional Chinese medicine (TCM) placebo was used in any of the control groups, and so they could not be blinded.
Interventions
All studies compared radiotherapy or chemotherapy with and without additional Chinese herbal medicine. Chemoradiotherapy was given in Chen 2012, five trials used chemotherapy (Cheng 2010; Lin 2013; Shao 2011; Wang 2010c; Wu 2013a), and the remaining trials used radiotherapy (He 2010a; Mu 2012; Ren 2013).
-
Chen 2012 compared Fuzheng Guben granules combined with chemoradiotherapy versus chemoradiotherapy. The duration of chemoradiotherapy lasted six weeks; Fuzheng Guben granules lasted six to seven weeks.
-
Cheng 2010 compared Xihuang Pill combined with chemotherapy versus chemotherapy. The duration of treatment continued for six weeks.
-
He 2010a compared Brucea javanica oil emulsion plus radiotherapy versus radiotherapy. The duration of radiotherapy treatment continued for six to seven weeks; Brucea javanica oil emulsion was given for 18 to 27 days.
-
Lin 2013 compared Xiaoaiping injection combined with chemotherapy versus chemotherapy. The duration of treatment continued for eight weeks.
-
Mu 2012 and Ren 2013 compared Kangai injection plus radiotherapy versus radiotherapy. The duration of treatment continued for six weeks.
-
Shao 2011 compared Yiqi yang yin Huatan quyu decoction combined with chemotherapy versus chemotherapy. The duration of treatment continued for two months.
-
Wang 2010c compared Zhisheng capsule combined with chemotherapy versus chemotherapy. The duration of treatment continued for nine weeks.
-
In Wu 2013a, all participants received chemotherapy for a maximum of six weeks; an additional Aidi injection was given to participants in the treatment group for 14 days.
The Pinyin, Latin and English names of the TCM preparations of the included studies are in Table 1.
Patients
A total of 490 participants (355 males, 135 females) were included in these nine studies, with 247 participants in the treatment group and 243 participants in the control group. All participants were recruited from Chinese hospitals and had oesophageal carcinoma confirmed by pathology or imaging (computed tomography (CT), magnetic resonance imaging (MRI), X‐ray). All reports described no significant clinically statistical difference between treatment and control groups.
-
In Chen 2012, patients were diagnosed to be stage Ⅱb, Ⅲ, Ⅳ.
-
In Cheng 2010, the trial author diagnosed the patients as having advanced or metastatic oesophageal cancer by CT and X‐ray.
-
In He 2010a, the trial author diagnosed the disease to be stage Ⅱ, Ⅲ using the International Union for Cancer Control staging system.
-
In Lin 2013, the trial author diagnosed the disease to be stage Ⅲ, Ⅳ.
-
In Mu 2012, the trial author described the cancer as having developed to an advanced stage.
-
In Ren 2013, the stage of disease was not mentioned.
-
In Shao 2011, the trial author identified the oesophageal cancer to be stage Ⅲ, Ⅳ.
-
In Wang 2010c, the cancer was diagnosed to be stage Ⅱ, Ⅲ using the International Union for Cancer Control staging system.
-
In Wu 2013a, the author diagnosed the cancer to be stage Ⅲ, Ⅳ using the American Joint Committee on Cancer staging system.
None of the trials described withdrawals or losses to follow‐up.
Outcomes
Primary outcomes
No study reported all‐cause mortality, median survival times or time to progression.
Eight studies assessed quality of life, however, two studies did not use a validated measure, and could not be adopted (He 2010a; Wu 2013a). The remaining six studies, Lin 2013, Mu 2012, Ren 2013, Wang 2010c, Shao 2011 used the Karnofsky scoring scale for quality of life, while Cheng 2010 used the Revision of Quality of Life Index Scale of Chinese cancer patients. The measurement for quality of life was conducted before and after the intervention.
Secondary outcomes
The included studies reported two different therapeutic evaluation methods; short‐term therapeutic effects and TCM symptoms. Short‐term therapeutic effects referred to improvement (complete response + partial response) and progressive disease. There were three evaluation criteria, five studies used the World Health Organization Response Evaluation Criteria in Solid Tumours (He 2010a; Lin 2013; Mu 2012; Ren 2013; Wang 2010c), two studies used the New Response Evaluation Criteria in Solid Tumours (RECIST) (Cheng 2010; Wu 2013a), and one study used the WanJun evaluation criteria (Chen 2012). One study measured this outcome after four weeks' follow‐up (Chen 2012), two studies followed up for three months after the intervention (He 2010a; Ren 2013), and the remaining gave no detailed information. TCM symptoms were diagnosed in two studies (Shao 2011; Wang 2010c) at the end of the intervention. The studies classified participants as to total effectiveness and ineffectiveness according to Zheng 2002. The total effectiveness meant the symptom scores decreased ≥30% after intervention, the ineffectiveness represented the symptom scores decreased ≤30% after intervention.
Adverse events
We focused on any adverse event that caused death, life‐threatening illness or significant toxicity. In this updated review, the nine included studies reported many adverse events, such as, mucositis, radiation oesophagitis, arrest of bone marrow, gastrointestinal reactions (vomiting, dyspepsia, nausea), renal and hepatic impairment, white blood cell descent, neurotoxicity, cardiac toxicity, anaemia and low fever. All these adverse events were observed at the end of the intervention.
In addition, we were also concerned with outcomes which could reflect the adjunct treatment characteristics of Chinese herbal medicine, such as bodyweight (reflecting increased appetite) and T‐lymphocyte and cancer bio‐markers (reflecting boost the immune system).
Excluded studies
In the last version of this review (Wei 2007), we included Chen 2002a and Liu 2005 as real RCTs based on the initial interview with the original trial authors. On 31 May, 2007, TW interviewed Dr. Degui Liu and Prof. Zhijian Chen, the original trial authors, to discuss the design and process of the study in detail. The two trial authors very kindly explained that they had not used any randomisation method to generate an allocation sequence; Liu 2005 was actually a retrospective case report. We therefore identified them as non‐RCTs and excluded them from the review.
According to this situation, it was necessary to critically identify whether the studies which claimed they had "randomly allocated patients" were true RCTs or not. For this purpose, we telephoned the authors of these studies. In conclusion, 13 trial authors refused an interview, 48 studies were retrospective case reports, two trial authors confessed to using no random method, seven trial authors could not describe the details of the random method, and 22 trial authors misunderstood the random allocation method or used an incorrect random method. We recorded the process of telephone interviews, and two review authors (XC and TW) were responsible for judgements (see Figure 2).
Summary details of all the trials are given in the Characteristics of excluded studies.
Risk of bias in included studies
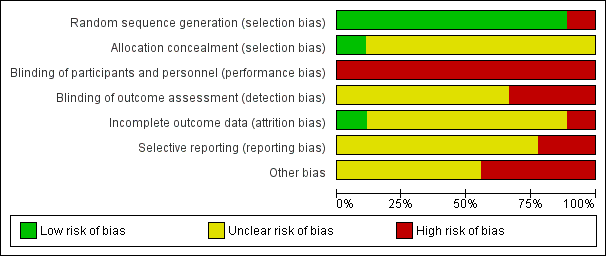
The risk of bias is described below and summarised in Figure 3 and Figure 4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Four included studies mentioned ''randomly allocated patients'' (Chen 2012; He 2010a; Mu 2012; Wang 2010c), and five studies stated using random number tables in the articles (Cheng 2010; Lin 2013; Ren 2013; Shao 2011; Wu 2013a), but all studies were short of details of randomisation.
After telephone interviews with the trial authors who kindly gave descriptions of random sequence generation, we graded the included studies at low risk of bias, apart from Cheng 2010, which we judged to be at high risk of bias because the descriptions were inconsistent between the telephone interview and reports.
Allocation concealment
None of the studies reported on the use of allocation concealment. However, in a telephone interview, one author explained that ''computer‐generated random numbers were sealed in envelopes, which were used to allocate patients'' (Wu 2013a). We judged this study at low risk bias for allocation concealment.
Blinding
None of the nine studies employed a placebo mimicking TCM in the control group, so it was not possible to mask participants and personnel. We therefore judged the risk of performance bias to be high.
In detail, blinding was not performed in three studies, one mentioned "no blinding method" in the text (Cheng 2010), two studies transmitted "no blinding" through interviews (Shao 2011; Wu 2013a), and so we judged the detection bias to be high. The remaining six studies did not provide any information about masking, and so we judged the detection bias to be unclear (Chen 2012; He 2010a; Lin 2013; Mu 2012; Ren 2013; Wang 2010c).
Incomplete outcome data
No study had a statement on drop‐outs or withdrawals. In telephone interviews, one author described " no drop‐outs from the TCM group and control group" (Shao 2011), and so we judged the risk to be low.
However, in Wang 2010c, there were only 22 patients represented in the outcome of short‐term therapeutic effects and 23 patients represented in the TCM clinical symptoms outcome, which were not matched to the total 24 patients allocated in the control group. No intention‐to‐treat (ITT) analysis was performed either. We considered this study to be at high risk for attrition bias. In addition, three trials showed unequal participant numbers in the two arms (Cheng 2010; Ren 2013; Wu 2013a), especially in Ren 2013 where there were 28 participants in the TCM group and 26 in the control group. The reports did not interpret whether the imbalance of participant numbers in the two arms was produced by withdrawals during follow‐up or inadequate randomisation, or other reasons.
Selective reporting
The protocols of the included studies were unavailable. Two studies did not report one outcome in the results, which had been presented in the methods section of the study (Lin 2013; Ren 2013). We considered these two studies at high risk of reporting bias.
Other potential sources of bias
Other bias refers to factors such as conflict of interest, baseline imbalances, the standard deviation and other obvious potential sources that have a disadvantageous impact on risk. Ultimately, we judged three studies to have conflicts of interest (Chen 2012; Shao 2011; Wang 2010c), and He 2010a contained an incorrect description of significant difference; we judged these four studies to be at high risk of bias for this domain.
Effects of interventions
See: Summary of findings for the main comparison Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer; Summary of findings 2 Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer
See: summary of findings Table for the main comparison and summary of findings Table 2.
Primary outcomes
All‐cause mortality, median survival times and time to progression were not reported in any of the included studies.
Quality of life
We reported quality of life in terms of number of participants experiencing an improvement and number experiencing a deterioration in quality of life. More specifically, we assessed quality of life pre‐ and post‐intervention with the Karnofsky performance status scale or other validated scales. Participants experiencing an improvement meant that the participants performance score would improve ≥ 10 after the intervention, while experiencing a deterioration meant a change score ≤ 10.
Five studies reported improvements in quality of life with a total of 233 participants undergoing radiotherapy or chemotherapy, with or without additional Chinese herbal medicine (Cheng 2010; Lin 2013; Mu 2012; Shao 2011; Wang 2010c). Six studies reported a deterioration in quality of life with 287 participants (Cheng 2010; Lin 2013; Mu 2012; Ren 2013; Shao 2011; Wang 2010c). TCM in these studies probably had a positive effect on quality of life, both in number experiencing improvement (risk ratio (RR) 2.20, 95% confidence interval (CI) 1.42 to 3.39; I² = 0%) and number experiencing deterioration (RR 0.41, 95% CI 0.27 to 0.62; I² = 0%) (Analysis 1.1). All studies in this outcome were parallel‐group RCTs, but not blinded; the evaluation may have been affected by the subjective effect of the researcher, resulting in very serious risk of bias. Further, these studies included relatively few patients and few events and have wide confidence intervals around the estimate of the effect that could lead to serious imprecision. Due to the very serious risk of bias and serious imprecision, we downgraded the quality of evidence by three levels. With a RR of number experiencing improvement > 2.0 and RR of number experiencing deterioration < 0.5, the effect was large and so we upgraded the quality of evidence by one level. Overall, we judged this outcome to have low quality evidence.
In addition, one study provided the Karnofsky score for quality of life in 60 participants (Lin 2013). Results of this study suggest that Xiaoaiping injection may improve the Karnofsky score (mean difference (MD) 6.80, 95% CI 3.86 to 9.74) (Analysis 1.2). We judged this to have low quality evidence, downgrading for very serious risk of bias (summary of findings Table for the main comparison).
Secondary outcomes
Short‐term therapeutic effects
For short‐term therapeutic effects, participants were divided into four different categories ‐ complete response, partial response, stable disease and progressive disease, which should have clear judgement criteria in individual trials. Improvement was defined as complete response and partial response, and it represented the patients being in a more beneficial state after therapy. In contrast to that, progressive disease represented a disadvantageous state after therapy.
In detail, eight studies reported short‐term therapeutic effects in 450 participants experiencing radiotherapy or chemotherapy, with or without additional Chinese herbal medicine (Chen 2012; Cheng 2010; He 2010a; Lin 2013; Mu 2012; Ren 2013; Wang 2010c; Wu 2013a). The data suggest that TCM probably leads to little or no difference in improvement (RR 1.17, 95% CI 1.02 to 1.35; I² = 0%, P = 0.03) and progressive disease (RR 0.73, 95% CI 0.52 to 1.01; I² = 0%, P = 0.06) (Analysis 1.3). If Chen 2012 is removed from the analysis, the difference for improvement is no longer statistically significant (RR 1.12, 95% CI 0.95 to 1.32; 7 studies, 360 participants; I² = 0%, P = 0.17). This may be caused by different TCM formulations. However, the data were not sufficient to conduct a subgroup analysis, and we could not explore the potential diverse effects of TCM on short‐term therapeutic effects. All studies were parallel‐group RCTs but not blinded, the outcomes were measured by imaging such as CT, MRI or X‐ray, and judged using the WanJun, WHO or RECIST Evaluation Criteria in Solid Tumours. Due to the lack of blinding, the observations would have experienced the Hawthorne effect. Therefore, the results might have serious limitations. In addition, the effect of progressive disease had wide confidence intervals, resulting in serious imprecision. Overall, we judged improvement to have moderate quality evidence, downgrading for serious risk of bias; and progressive disease to have low quality evidence due to serious risk of bias and serious imprecision (summary of findings Table for the main comparison).
TCM symptoms
Similar to short‐term therapeutic effects, TCM symptoms divided participants into total effectiveness and ineffectiveness. The TCM symptoms scores were measured before and after intervention according to Zheng 2002. The participants whose symptom scores decreased ≥30% were assigned to the total effectiveness group, the participants whose symptom scores decreased ≤30% were assigned to the ineffectiveness group. This outcome only included two studies with 88 patients (Shao 2011; Wang 2010c). As a result, TCM showed a positive impact on both total effectiveness (RR 1.84, 95% CI 1.20 to 2.81; I² = 37%) and ineffectiveness (RR 0.22, 95% CI 0.05 to 0.93; I² = 49%) (Analysis 1.4). The two studies were not blinded and did not conceal allocation. TCM clinical criteria was ambiguous, the outcome may be more affected by the researcher, who prepared the TCM, so the risk of bias was very serious. Total effectiveness had serious limitations with only two included studies with few patients and few events, while the result for ineffectiveness had very serious limitations due to few patients, few events and wide confidence intervals. With a RR for ineffectiveness of < 0.5, the effect was large and so we upgraded the quality of evidence by one level. Overall, we judged total effectiveness to have very low quality evidence due to very serious risk of bias and serious imprecision; we judged ineffectiveness to also have very low quality evidence, downgrading for very serious risk of bias and very serious imprecision, and upgrading for the large effect.
Adverse events
Nine studies reported ten kinds of adverse events, including mucositis, radiation oesophagitis, arrest of bone marrow, gastrointestinal reactions, renal and hepatic impairment, white blood cell descent, neurotoxicity, cardiac toxicity, anaemia and low fever (see Analysis 1.5 and summary of findings Table 2).
-
One study reported mucositis caused by radiotherapy (Mu 2012). Twenty‐three out of 25 patients in the TCM group and 25/25 patients in the control group suffered from different degrees of mucositis; there was no significant difference between the two groups (RR 0.92, 95% CI 0.80 to 1.06; P = 0.24). We judged the quality of evidence to be low; downgrading for very serious risk of bias.
-
Two studies reported radiation oesophagitis (Chen 2012; He 2010a). TCM exhibited a potential ability to reduce radiation oesophagitis (RR 0.66, 95% CI 0.47 to 0.94; I² = 0%). We judged this to have low quality evidence; downgrading for serious risk of bias and serious imprecision.
-
One study reported arrest of bone marrow (Chen 2012). The result showed that TCM probably had an effect on arrest of bone marrow caused by chemoradiotherapy (RR 0.28, 95% CI 0.14 to 0.58). We judged this to be of moderate quality evidence; downgrading for very serious risk of bias, and upgrading for the large effect.
-
Four studies reported gastrointestinal reactions (Chen 2012; He 2010a; Lin 2013; Wang 2010c). The data showed that TCM probably had a potential effect on gastrointestinal reactions caused by chemotherapy or radiotherapy (RR 0.54, 95% CI 0.36 to 0.81; I² = 30%). We judged this to be of very low quality evidence; downgrading for very serious risk of bias and serious imprecision.
-
Two studies reported renal and hepatic impairment (Chen 2012; Lin 2013). However, pooled analysis showed significant heterogeneity with I² = 88%, (50% is regarded as high level heterogeneity). Because only two trials were included in this adverse event, we could not explore potential sources of heterogeneity.
-
Four studies reported white blood cell descent (He 2010a; Lin 2013; Ren 2013; Shao 2011). The data showed that oesophageal cancer patients may benefit from TCM after chemotherapy or radiotherapy (RR 0.60, 95% CI 0.44 to 0.83; I² = 0%) in blood cell descent. We judged this to be of very low quality evidence; downgrading for very serious risk of bias and serious imprecision.
-
One study reported neurotoxicity (Lin 2013), there was no significant difference between the groups (RR 0.73, 95% CI 0.34 to 1.55; P = 0.41). We judged this to be of low quality evidence; downgrading for very serious risk of bias.
-
One study reported cardiac toxicity (Lin 2013), there was no significant difference between the groups (RR 0.80, 95% CI 0.24 to 2.69; P = 0.72). We judged this adverse outcome to be of low quality evidence; downgrading for very serious risk of bias.
-
One trial reported anaemia (Shao 2011); there was no significant difference between the groups (RR 1.33, 95% CI 0.57 to 3.14; P = 0.51). We judged this adverse outcome to be of low quality evidence; downgrading for very serious risk of bias.
-
In addition, He 2010a reported two cases of low fever in the treatment group; the symptoms disappeared after two to three days. The trial author suggested that low fever was related to the usage of Brucea javanica oil emulsion.
Discussion
Summary of main results
In this systematic review, we intended to evaluate the efficacy and possible adverse effects of traditional Chinese medicine (TCM) when used as an adjunct to treatment with radiotherapy or chemotherapy for oesophageal cancer. The overall treatment concept for TCM is different from that used in Western medicine, and we hoped to assess if TCM could be used in the effective treatment of oesophageal cancer. TCM is well established and widely used, and based on the principles that the root cause of illness, not the symptoms, must be treated. TCM can be described as being holistic in its approach; it views every aspect of the balance between mind, body, spirit and emotions. In using TCM to treat oesophageal cancer, physicians hope to strengthen and support the body's systems, using preparations that are formulated to act specifically on imbalances in the way the body functions.
In the last version of this review, we excluded all 43 studies which were self described by trial authors as being RCTs, as we later discovered this not to be the case. This may have been due to some Chinese trial authors in the field having misunderstood what the term means, underestimating the importance of randomly allocating participants with oesophageal cancer and knowing how to minimise risk of bias in a RCT. In this updated review, we tried to contact authors of 142 studies to critically identify whether the studies which claimed to be RCTs, were in fact true RCTs. Ultimately, we included nine of the studies (all conducted in China).
It is regrettable that none of the studies reported all‐cause mortality, median survival times or time to progression, and so we are uncertain as to whether TCM has an effect on oesophageal cancer for these outcomes or how effective it may be. Most studies reported quality of life and short‐term therapeutic effects. Both of these outcomes are measured by clinicians according to certain criteria, however, the process was not blinded, thereby increasing the risk of bias. The results of the meta‐analyses show that TCM probably had an effect on quality of life. While, the results are complicated for short‐term therapeutic effects, TCM showed a statistically significant effect on improvement (complete response + partial response), but no statistically significant effect on progressive disease. These inconsistent results may be caused by the small number of participants and few studies, which leads the result to be more sensitive and volatile. TCM symptoms, which were similar to short‐term therapeutic effects evaluated with TCM clinical criteria, were diagnosed in two studies and showed a positive effect.
Nine studies reported a series of adverse events, such as mucositis, radiation oesophagitis, arrest of bone marrow, gastrointestinal reactions, renal and hepatic impairment, white blood cell descent, neurotoxicity, cardiac toxicity, anaemia and low fever. All of these adverse effects were caused by radiotherapy or chemotherapy, except for low fever, which was considered to be associated with TCM. After analyses, TCM showed a positive impact on radiation oesophagitis, arrest of bone marrow, gastrointestinal reactions and white blood cell descent. The effects of TCM on the other adverse effects are uncertain.
In this updated review, we were also concerned with the details of other outcomes not specified in the protocol, such as T‐lymphocyte, cancer bio‐markers and bodyweight. To some extent, these three outcomes would reflect the TCM effect, and as auxiliary outcomes, they have attracted the attention of researchers. If necessary, we will consider the analysis of these outcomes in a future review update.
Overall completeness and applicability of evidence
It is difficult to reach definitive conclusions due to variation between TCM formulations in the included studies. Variation in the components of herbal medical preparations is not unique to Chinese traditional herbal medicine; the variation between formulations and batches of treatments being an inevitable consequence of the nature of TCM. In China, the government specifies the limits of variation that are acceptable. This variation should be considered and may be a factor that contributes to any heterogeneity between different study results. Therefore, much importance should be attached to a high quality study when a study uses a self prepared herbal formulation and this study should provide evidence that is strong enough to encourage further studies in different locations to strengthen its validity. Studies treating oesophageal cancer with the same main components, as contained within an efficacious self prepared herbal formulation, are also encouraged. Chinese patented drugs have priority over the self prepared herbal formulations in order to achieve consistency.
In this review, we attempted to contact 142 authors, and completed 101 telephone interviews. Eventually we identified nine true RCTs. These RCTs allocated 490 participants in total to either usual treatment or usual treatment combined with TCM. Interventions, either usual treatment or TCM, were complicated. The usual treatment contained chemotherapy, radiotherapy and chemoradiotherapy, while TCM contained eight different types. The included studies have addressed partial objectives assessing quality of life, short‐term therapeutic effects and possible adverse effects. However, the question about other outcomes including all‐cause mortality, median survival times and time to progression have not been answered.
In terms of applicability of evidence, all trials were conducted in China, this characteristic would limit the usage to a broader population. Further, the majority of trials gave no details about the duration of follow‐up; the effects on quality of life and adverse events were measured at the end of the intervention. Thus, it was uncertain how long the effects of the intervention would last.
Quality of the evidence
Overall, we rated the quality of evidence of the included studies between very low and moderate due to methodological limitations and imprecision.
Methodological quality
From the last published version of the review, we checked 142 studies labelled as "randomised controlled trial" by telephone interviews with authors, and finally obtained nine authentic RCTs. Although identified to be RCTs by telephone, all nine studies had problems with methodological quality. None of these studies provided information about allocation concealment, no TCM placebo was used in the control group (making it impossible to mask participants and physicians), there was no information about blinding of outcome assessors, no study had a statement on drop‐outs or withdrawals, few studies reported the results of outcomes completely according to the methods section of the study, and self prepared TCM brought about conflicts of interest. All the factors mentioned above could lead to selection bias, performance bias, detection bias, attrition bias, reporting bias or other bias ‐ resulting in false positive findings.
Outcomes
The cumulative sample size and the total number of events were rather low, and 95% confidence intervals around relative effects were wide. We downgraded the quality of evidence for this imprecision of outcomes. In addition, we did not observe dose‐response information in any outcomes of TCM to treatment with radiotherapy or chemotherapy for oesophageal cancer.
Other
The quality of trial reporting, particularly the descriptions of methodology, study processes and the results, were very poor, and we found it difficult to ascertain from the report how the studies were performed. In the future we would like to include high quality studies to determine the effect of Chinese herbal medicine on oesophageal cancer.
Potential biases in the review process
Publication bias was also a possibility but we were not able to assess this using a funnel plot due to having less than ten trials included in the review. Anther common problem was that the assessment of quality of life and TCM symptoms response of treatment were performed by authors who were not blinded in the trial conditions. This may have led to potential risk of detection bias and cause false positive results. Valuable assessment about quality of life and TCM symptoms response of treatment in a RCT should be participant‐based, include use of a validated multidimensional questionnaire completed by the participant and use the internationally evaluated 'minimum standard checklist' (Efficace 2003; Efficace 2006; Efficace 2007).
Agreements and disagreements with other studies or reviews
There is no other known systematic review of Chinese herbal medicine for oesophageal cancer. Well designed RCTs with large sample sizes in the future may allow us to draw reliable conclusions.
Study flow diagram.
Flow diagram of telephone interviews.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
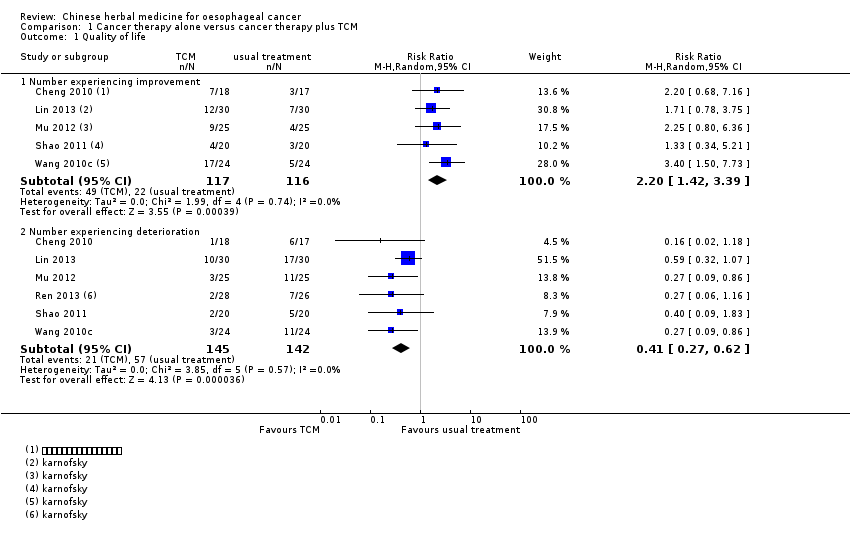
Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 1 Quality of life.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 2 Quality of life.
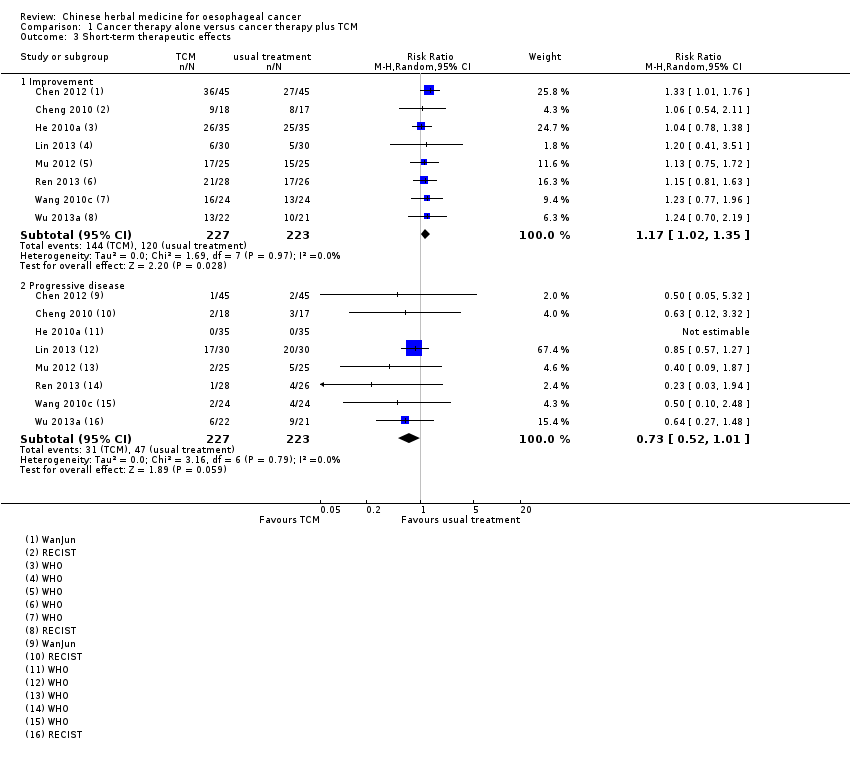
Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 3 Short‐term therapeutic effects.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 4 TCM symptoms.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 5 Adverse events.
Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 6 Cancer bio‐markers.

Comparison 1 Cancer therapy alone versus cancer therapy plus TCM, Outcome 7 Weight.
| Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer | ||||||
| Patient or population: patients with oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual treatment | Usual treatment combined with TCM | |||||
| All‐cause mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Median survival times ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Time to progression ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not investigated |
| Quality of life ‐ number experiencing improvement | Study population | RR 2.2 | 233 | ⊕⊕⊝⊝ | ||
| 190 per 1000 | 417 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 389 per 1000 | |||||
| Quality of life ‐ number experiencing deterioration | Study population | RR 0.41 | 287 | ⊕⊕⊝⊝ | ||
| 401 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 397 per 1000 | 163 per 1000 | |||||
| Quality of life ‐ Karnofsky performance status | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ | |
| Short‐term therapeutic effects ‐ improvement (complete response + partial response) | Study population | RR 1.17 | 450 | ⊕⊕⊕⊝ | ||
| 538 per 1000 | 630 per 1000 | |||||
| Moderate | ||||||
| 571 per 1000 | 668 per 1000 | |||||
| Short‐term therapeutic effects ‐ progressive disease | Study population | RR 0.73 | 450 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 154 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 126 per 1000 | |||||
| TCM symptoms ‐ total effectiveness | Study population | RR 1.84 | 88 | ⊕⊝⊝⊝ | ||
| 477 per 1000 | 878 per 1000 | |||||
| Moderate | ||||||
| 471 per 1000 | 867 per 1000 | |||||
| TCM symptoms ‐ ineffectiveness | Study population | RR 0.22 | 88 | ⊕⊝⊝⊝ | ||
| 523 per 1000 | 115 per 1000 | |||||
| Moderate | ||||||
| 529 per 1000 | 116 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The studies were parallel‐group RCTs, but not blinded. The outcome may be affected by the subjective effect of the researcher, thereby resulting in very serious limitations. | ||||||
| Active cancer therapy combined with TCM compared to active cancer therapy for oesophageal cancer | ||||||
| Patient or population: oesophageal cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual treatment | Usual treatment combined with TCM | |||||
| Adverse events ‐ mucositis | Study population | RR 0.92 | 50 | ⊕⊕⊝⊝ | ||
| 1000 per 1000 | 920 per 1000 | |||||
| Moderate | ||||||
| 1000 per 1000 | 920 per 1000 | |||||
| Adverse events ‐ radiation oesophagitis | Study population | RR 0.66 | 160 | ⊕⊕⊝⊝ | ||
| 550 per 1000 | 363 per 1000 | |||||
| Moderate | ||||||
| 546 per 1000 | 360 per 1000 | |||||
| Adverse events ‐ arrest of bone marrow | Study population | RR 0.28 | 90 | ⊕⊕⊕⊝ | ||
| 556 per 1000 | 156 per 1000 | |||||
| Moderate | ||||||
| 556 per 1000 | 156 per 1000 | |||||
| Adverse events ‐ gastrointestinal reactions | Study population | RR 0.54 | 268 | ⊕⊝⊝⊝ | ||
| 500 per 1000 | 270 per 1000 | |||||
| Moderate | ||||||
| 558 per 1000 | 301 per 1000 | |||||
| Adverse events ‐ renal and hepatic impairment ‐ Fuzheng Guben granules | Study population | RR 0.33 | 90 | ⊕⊕⊝⊝ | ||
| 333 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 110 per 1000 | |||||
| Adverse events ‐ renal and hepatic impairment‐ Xiaoaiping | Study population | RR 0.90 | 60 | ⊕⊕⊝⊝ | ||
| 133 per 1000 | 120 per 1000 | |||||
| Moderate | ||||||
| 233 per 1000 | 210 per 1000 | |||||
| Adverse events ‐ white blood cell descent | Study population | RR 0.60 | 224 | ⊕⊝⊝⊝ | ||
| 486 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 446 per 1000 | 268 per 1000 | |||||
| Adverse events ‐ neurotoxicity | Study population | RR 0.73 | 60 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 367 per 1000 | 268 per 1000 | |||||
| Adverse events ‐ cardiac toxicity | Study population | RR 0.80 | 60 | ⊕⊕⊝⊝ | ||
| 167 per 1000 | 133 per 1000 | |||||
| Moderate | ||||||
| 167 per 1000 | 134 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The study was a parallel‐group RCT, but not blinded. The outcome may be affected by the subjective effect of the researcher, thereby resulting in very serious limitations. | ||||||
| Study ID | TCM | Pinyin name | Latin name | English name | Preparation |
| Fuzheng Guben granules | Huangqi Dangshen Shanzha Chenpi Nuzhenzi Buguzhi Baizhu Gouqizi Fuling Shenqu Maiya Jixueteng Yinchenhao Tusizi | Radix Astragali Radix Codonopsis Fructus Crataegi Pericarpium Citri Reticulatae Fructus Ligustri Lucidi Fructus Psoraleae Rhizoma Atractylodis Macrocephalae Fructus Lycii Poria Massa Medicata Fermentata Fructus Hordei Germinatus Caulis Spatholobi Herba Artemisiae Scopariae Semen Cuscutae | Milkvetch Root Pilose Asiabell Root Hawthorn Fruit Tangerine Peel Glossy Privet Fruit Malaytea Scurfpea Fruit Largehead Atractylodes Rhizome Barbary Wolfberry Fruit Indian Buead Medicated Leaven Malt Suberect Spatholobus Virgate Wormwood Herb South Dodder Seed | Not described in detail Produced by Gansu Prorincial Wuwei Tumour Hospital | |
| Xihuang Pill | Niuhuang Ruxiang Moyao Shexiang | Calculus Bovis Olibanum Myrrha Moschus | Bezoar Frankincense Myrrh Musk | Frankincense and Myrrh were treated by vinegar‐processing method Produced by Beijing Tongrentang Pharmaceutical Factory | |
| Brucea Javanica oil emulsion | YadanzI | Fructus Bruceae | Java Brucea Fruit | Not described in detail | |
| Xiaoaiping injection | Wuguteng | Radix Fissistigmae Glaucescentis | Root of Glaucescent Fissistigma | Xiaoaiping injection was extracted from Glaucescent Fissistigma Produced by Nanjing Shenghe Pharmaceutical Co Ltd | |
| Kangai injection | Renshen Huangqi Kushen | Radix Ginseng Radix Astragali Radix Sophorae Flavescentis | Ginseng Milkvetch Root Lightyellow Sophora Root | Not described in detail | |
| Yiqiyangyin Huatanquyu decoction | Taizishen Nanshashen Beishashen Maidong Chenpi Banxia Ezhu Banzhilian Baihuasheshecao Zhigancao Jineijin Maiya Guya Baizhu Shanyao Tiandong Wuweizi Fuling Yiyiren Danggui | Radix Pseudostellariae Radix Adenophorae Radix Glehniae Radix Ophiopogonis Pericarpium Citri Reticulatae Rhizoma Pinelliae Rhizoma Curcumae Herba Scutellariae Barbatae Herba Hedyotidis Diffusae Glycyrrhizae Endothelium Corneum Gigeriae Galli Fructus Hordei Germinatus Fructus Oryzae Germinatus Rhizoma Atractylodis Macrocephala Rhizoma Diosscoreae Radix Asparagi Fructus Schisandrae Poria Semen Coicis Radix Angelicae Sinensis | Heterophylly Falsestarwort Root Ladybell Root Coastal Glehnia Root Dwarf Lilyturf Tuber Tangerine Peel Pinellia Tuber Zedoary Barbed Skullcap Herb Spreading Hedyotis Herb Radix glycyrrhizae preparata Chicken's Gizzard‐membrane Malt Rice‐grain Sprout Largehead Atractylodes Rhizome Common Yam Rhizome Cochinchinese Asparagus Root Chinese Magnoliavine Fruit Indian Buead Coix Seed Chinese Angelic | Modification according to symptoms: Largehead Atractylodes Rhizome Common Yam Rhizome Cochinchinese Asparagus Root Chinese Magnoliavine Fruit Indian Buead, Coix Seed and Chinese Angelic Yiqiyangyin Huatanquyu decoction was prepared by researchers of hospital | |
| Zhisheng capsule | Shexiang Ezhu Bingpian Renshen Niuhuang Dongcongxiacao Xiyangshen | Moschus Rhizoma Curcumae Borneolum Radix Ginseng Calculus Bovis Cordyceps Radix Panacis Quinquefolii | Musk Zedoary Borneol Ginseng Bezoar Chinese Caterpillar Fungus American Ginseng | The composition of Zhisheng capsule contained 16 traditional Chinese medicines, only 7 of them were given Produced by the first affiliated Hospital of Henan University of Traditional Chinese Medicine | |
| Aidi injection | Renshen, Ciwujia, Bianmao, Huangqi. | Radix Ginseng, Radix Acanthopanacis Senticosi, Mylabris, Radix Astragali. | Ginseng, Manyprickle Acanto‐Panax Root, Blister Beetle, Milkvetch Root. | Not described in detail |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Number experiencing improvement | 5 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.42, 3.39] |
| 1.2 Number experiencing deterioration | 6 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.27, 0.62] |
| 2 Quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Karnofsky performance status | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term therapeutic effects Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Improvement | 8 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.35] |
| 3.2 Progressive disease | 8 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.01] |
| 4 TCM symptoms Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Total effectiveness | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.20, 2.81] |
| 4.2 Ineffectiveness | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.93] |
| 5 Adverse events Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Mucositis | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 5.2 Radiation oesophagitis | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.94] |
| 5.3 Arrest of bone marrow | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.58] |
| 5.4 Gastrointestinal reactions | 4 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.36, 0.81] |
| 5.5 Renal and hepatic impairment‐Fuzheng Guben granules | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.84] |
| 5.6 Renal and hepatic impairment‐Xiaoaiping | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.88, 7.10] |
| 5.7 White blood cell descent | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| 5.8 Neurotoxicity | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.34, 1.55] |
| 5.9 Cardiac toxicity | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.24, 2.69] |
| 5.10 Anaemia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.14] |
| 6 Cancer bio‐markers Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Carcinoembryonic antigen | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Weight Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Increased | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Decreased | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

