Seguimiento telefónico iniciado por un profesional de asistencia sanitaria del hospital para los problemas posteriores al alta de pacientes que dejan el hospital hacia el domicilio
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: whether post‐discharge care counseling was a factor in improving patients' overall compliance with treatment in a hospital setting. AIM OF STUDY: To test whether counseling of surgical and cardiac patients by a pharmacist would improve patients' drug compliance. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: unclear. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: at least 3 medications and having a scheduled appointment at 8 weeks in hospital. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: mental illness. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: number assigned: odd number in control, even numbers in intervention. METHOD OF CONCEALMENT OF ALLOCATION: as above, no further information given. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION : cardiac patients / surgery patients. GEOGRAPHIC LOCATION: Saudi Arabia. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 18‐70. GENDER (% MALE): 50. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: unclear. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: not given. | |
| Interventions | DETAILS OF INTERVENTION: A pharmacist counseled patients on day of discharge about indication of drug use, expected therapeutic outcome, dosage and method of administration, storage conditions, duration of therapy and what to do when a dose is missed. The pharmacist gave TFU every 3 days for up to eight weeks; also patients could call pharmacist when they needed further counseling. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: access to the pharmacist by phone (in intervention group only) and counseling by pharmacist on discharge day (both groups). DELIVERY OF INTERVENTION: PROVIDERS: pharmacist. INTERVENTION QUALITY: unclear how much intervention was given in each patient. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 3. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT: | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to enhance knowledge. AIM OF STUDY: To examine the effectiveness of three 'survival skill' based teaching strategies for cardiac surgical patient education. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: unclear. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: admitted for elective coronary artery bypass surgery; not further specified. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: anova/t‐test/chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION : cardiac patients / surgery patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 63.1 (9.8). GENDER (% MALE): 81. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: coronary artery disease. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: coronary bypass surgery. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 90% married. Average hospital stay of 6.2 days. | |
| Interventions | DETAILS OF INTERVENTION: A teaching protocol was implemented during postdischarge week two by the cardiac rehabilitation nurse via telephone. The telephone contact was a method to reinforce the teaching content which had been previously given in the hospital. There were also opportunities for the patient to clarify any additional topics with the cardiac rehabilitation nurse. DETAILS OF CONTROL Two control groups: one received inhospital teaching only, the other inhospital teaching plus post discharge group teaching. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: All groups received inhospital teaching by the cardiac rehabilitation nurse on postoperative day 5 or 6; the content of this inpatient teaching protocol consisted on wound healing, signs and symptoms of angina/MI, guidelines for activity progression, incisional care, risk factor modification, dietary modifications and method for enrolling in cardiac rehabilitation. Patients also received two teaching booklets. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): knowledge / self‐developed: heart disease management questionnaire / partly; Kuder‐Richardson coefficient 0.36 / unclear / unclear, but in first month after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reinforce cognitive and affective information that was given to patients during hospitalisation and to supplement information about specific concerns/ AIM OF STUDY: To investigate the impact of supportive‐educative telephone program on the levels of knowledge and anxiety of patients undergoing coronary artery bypass graft surgery during the first 6 weeks after hospital discharge. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: the first 74 patients scheduled. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: scheduled for first‐time non emergency CABG/ oriented/ able to read, write and speak english/ access to telephone/ no major cardiac complications/ intent to return to cardiac surgeon within 6 weeks. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: pyschiatric problems or history. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT:unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION :cardiac patients / surgery patients. GEOGRAPHIC LOCATION: Canada. SETTING:discharged home from an acute care setting. NUMBER OF PARTTICIPANTS: AGE: RANGE OR MEAN (SD): 50 to 70. GENDER (% MALE): 86. ETHNICITY: white. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: coronary artery disease. OTHER HEALTH PROBLEM/S: none. TREATMENT RECEIVED/RECEIVING: coronary bypass surgery. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 81% married/ 58% employed/ 57% rural homes/ 80% more than 10 years education | |
| Interventions | DETAILS OF INTERVENTION: The supportive‐educative telephone program was an interactive program involving information exchange between patient and the cardiac rehabilitation NURSE specialist through a series of 4 to 6 telephone calls initiated by the nurse during the first 6 weeks home convalescent period. The program was designed to assist patients to gain knowledge and improve decision making and coping skills, thereby decreasing their anxiety. A first call was made early in the first week after discharge in which the time and number of subsequent calls was made. DETAILS OF CONTROL usual care: group and individual teaching during hospital stay, visit of self help group member, information about cardiac rehabilitation program. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: good. FIDELITY/INTEGRITY: yes. | |
| Outcomes | NUMBER OF OUTCOMES: 4 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): knowledge / self‐developed / partly, based on instrument of Horn and Swain (1977) / unclear / 6 weeks after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): readmission / hospital record / unclear / status analysis / 6 weeks after discharge | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: To improve patients' symptoms and/or side effects, such as delayed nausea and vomiting, as well as to detect and correct new symptoms that develop after discharge. AIM OF STUDY: To evaluate the effect of telephone follow‐up on the physical well‐being dimension of health‐related quality of life in patients with cancer. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: all patients admitted within a 1 year period; informed consent obtained during hospital stay. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: patients with hematologic or solid tumor malignancies/ chemotherapy/ speak English/ access to telephone. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: speech or hearing impairment/ mental or cognitive disorder/ live outside USA/ receiving weekly chemotherapy/ having allogeneic bone marrow transplant. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY EPOC‐ QUALITY CRITERIA 2002: B.moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: spreadsheet with a block size of 4. METHOD OF CONCEALMENT OF ALLOCATION: each patient received a number and study assignment from the investigational pharmacist BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: anova/t‐test/chi‐square/wilcoxon. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: oncology patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 53 (14). GENDER (% MALE): 73. ETHNICITY: 87% Caucasian, PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: hematologic or solid tumor malignancy, OTHER HEALTH PROBLEM/S: none. TREATMENT RECEIVED/RECEIVING: chemotherapy. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 44% inpatient/mean Karnofsky score 83 | |
| Interventions | DETAILS OF INTERVENTION: TFU 48‐72 hours after discharge by PHARMACIST. During the call patients were asked if they had experienced any problems since discharge. Information was solicited on both drug‐related and non‐drug related problems. When appropriate, patients were given advice, support and reinforcement of education provided at the time of discharge, and appropriate follow‐up was recommended. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: pharmacist. INTERVENTION QUALITY: call‐back duration was 7.4 minutes (range 0‐30). FIDELITY/INTEGRITY: intervention was given in 80% by pharmacist as intended; in 20% by student. | |
| Outcomes | NUMBER OF OUTCOMES: 5 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): functional well‐being / Fact‐G scale (Cella 1993) / yes / questionnaire / 3 weeks after discharge. Physical well‐being / Fact‐G scale (Cella 1993) / yes / questionnaire / 3 weeks after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): symptom distress / Memorial Symptom Assessment Scale (Portenoy 1994) / yes / questionnaire / 3 weeks after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: The telephone nursing care link project was designed to provide discharged patients with a means for continuing the health education that was begun by nursing staff during their hospitalisation. AIM OF STUDY: To assess differences in patient satisfaction with the meeting of their healthcare education needs among the patients who received a telephone call after discharge, those who are given the opportunity to call and those who receive no additional telephone follow‐up and to assess differences in readmissions within 30 days of discharge between the three groups. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: Patients from 5 units were eligible during a 3 month period; using a counterbalanced method patients were allocated to one of three groups. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: being discharged home from one of five participating units (general surgery, neurosurgery, orthopedic, general medicine, urology)/ English speaking. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? unclear. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: alternating scheme by time period and nursing unit. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: no. STATISTICAL METHODS AND THEIR APPROPRIATENESS: anova/chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: mixed specialties. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): GENDER (% MALE): ETHNICITY: PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: unclear. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: not given. | |
| Interventions | DETAILS OF INTERVENTION: The nurse contacted the patient 2 to 3 days after discharge. Additional calls were made as needed. DETAILS OF CONTROL: Two control groups: one received usual care and the other were given a brochure at discharge that described the project and contained information on how to contact the nurse specialist, the hours of operation and a description of the types of questions that were appropriate for this service. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear how much additional phone calls were made. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): readmission / hospital record / unclear / status analysis / 4 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to decrease postdischarge problems. AIM OF STUDY: To investigate the effect of a nurse‐initiated telephone reassurance program on postdischarge problems reported by recently discharged ophthalmic patients. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: patients were informed about the research project during their hospital stay and informed consent was obtained. Immediately after discharge patients were randomized by an independent researcher and using opaque envelopes (not published information). INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: adult ophthalmic patients/ at least 2 days in hospital/ dutch speaking. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: admitted from another ward or care institute to the pthalic unit/ discharged to institutional care setting/not able to answer the telephone/not having a telephone. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY EPOC‐ QUALITY CRITERIA 2002: C. high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified in article; additional information from authors obtained says opaque envelopes were used for randomization by an independent researcher. METHOD OF CONCEALMENT OF ALLOCATION: randomization after discharge by independent researcher. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test/u‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION : ophthalmology patients / surgery patients. GEOGRAPHIC LOCATION: The Netherlands. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 66.6 (16.1). GENDER (% MALE): 43. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: ophthalmic disease (cataract 43%, glaucoma 15%, retina disorder 14%, cornea disorder 13%). OTHER HEALTH PROBLEM/S: 93% self‐supporting in ADL/IADL. TREATMENT RECEIVED/RECEIVING: ophthalmic surgery. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 38% living alone. | |
| Interventions | DETAILS OF INTERVENTION: Patients were phoned 3‐6 days after discharge by an experienced nurse. Before calling the nurse went through a structured form containing relevant information about the patient's admission and discharge conditions. During the call, the nurse used a structured interview schedule, covering 10 aspects. all aspects were discussed with the patient. Six nurses participated in the project. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: good. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 4. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Functional limitations / Problems after Discharge Questionnaire (Mistiaen, 1997) / yes / questionnaire / 1 and 4 weeks after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to improve parental compliance with primary care follow‐up. AIM OF STUDY: It was hypothesized that physician initiated follow‐up phone calls to parents of moderately ill children seen in the pediatric emergency department would improve parental compliance with primary care follow‐up. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: unclear. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: patients between 6 months and 8 years of age treated as outpatients in the pediatric emergency department for pneumonia or croup or asthma or bronchiolitis or vomiting or fever eci or fever > 39.5 or seizure with fever/ having telephone, EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified, INFORMED CONSENT OBTAINED? unclear. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION : ED patients / pediatric patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 3.2 (2.3). GENDER (% MALE): 63. ETHNICITY: 83% afro‐american. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS:pneumonia or croup or asthma or bronchiolitis or vomiting or fever eci or fever > 39.5 or seizure with fever OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 70.5% on medical assistance insurance; 2.3% had no primary care physician. | |
| Interventions | DETAILS OF INTERVENTION: families in the intervention group were called by a physician within 12‐30 hours after discharge. At that time they were reminded to fill their prescriptions, to call their regular doctors, and to follow‐up any other specific instructions that had been documented on the discharge sheet. Parents were also given the opportunity to ask questions about other issues related to their child's health. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: physician. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): compliance / self‐developed / no / telephone interview / 10‐20 days after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: A mechanism that may improve patient satisfaction and clinical outcomes at the time of discharge is the use of follow‐up telephone calls. AIM OF STUDY: whether pharmacist involved in discharge planning can improve patient satisfaction and outcomes by providing telephone follow‐up after hospital discharge. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: all patients admitted within a 1 year period, who received a pharmacy‐facilitated discharge (= provision of patient counseling on all discharge medications, assistance in obtaining medications and completing insurance forms) and are discharged home. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: Patients from a general medical service discharged home with a pharmacy‐facilitated discharge EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: discharged to nursing home or other care facility/ homeless/ non‐English speaker/unable to participate in a telephone conversation or complete a written survey INFORMED CONSENT OBTAINED? yes ETHICAL APPROVAL? yes FUNDING: yes ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/t‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: general medical patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): GENDER (% MALE) ETHNICITY PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: OTHER HEALTH PROBLEM/S: TREATMENT RECEIVED/RECEIVING: OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: within 2 days after discharge patients received a telephone call from a member of the pharmacy service. The content of the call followed a script to ensure consistency. During the call patients were asked how they had been feeling since returning home, if they had any questions regarding follow‐up appointments or the in‐hospital care, if they were able to obtain their medication, if they had experienced any medication related side‐effects, and if they had any other question or concern. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: pharmacist. INTERVENTION QUALITY: good. FIDELITY/INTEGRITY: yes. | |
| Outcomes | NUMBER OF OUTCOMES: 3 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / hospital record / unclear / status analysis / 4 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. Yes (see reference Dudas 2001). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: provider initiated follow‐up calls to a patient may provide the opportunity to decrease the frequency of unnecessary return office visits; this could save money and time for both provider and patient. AIM OF STUDY: to determine the effects of follow‐up telephone calls on the number of return office visits of vasectomy patients. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: in the first 3 months all patients were considered intervention patients and received TFU; the next 3 months all patients were considered control patients and received only written postoperative instructions and no TFU. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: vasectomy patients from an urology group. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? no, ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: inadequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: none. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: surgery patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): GENDER (% MALE) ETHNICITY PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: OTHER HEALTH PROBLEM/S: TREATMENT RECEIVED/RECEIVING: OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: A telephone call that is specific to the needs/concerns of a vasectomy patient made within 24 to 48 hours of the procedure by a nurse regarding pain, swelling, redness and fever. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Unnecessary return office visits / hospital record / unclear / status analysis / 4 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: To assess the patient's level of recuperation, evaluate the care received and identify inadequacies of the process; furthermore it demonstrates a sense of caring about patients and assists in marketing an ambulatory surgery program. AIM OF STUDY: To investigate the post‐discharge follow‐up required for patients who have undergone laparoscopic cholecystectomy on an outpatient basis and to determine if there was a significant difference in mean concern scores and satisfaction level of patients followed up by a home visit versus a telephone call. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: a convenience sample of patients scheduled for elective or urgent laparoscopic cholecystectomy. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not requiring postoperative admission; willing to be discharged on the day of operation, have a responsible caregiver and have a telephone. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: open cholecystectomy. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: adequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: predetermined schedule; patients allocated by an operating room slating clerk. METHOD OF CONCEALMENT OF ALLOCATION: patients allocated by an operating room slating clerk. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/t‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: surgery patients. GEOGRAPHIC LOCATION: Canada. SETTING: discharged home from an acute care setting. NUMBER OF PARTTICIPANTS: AGE: RANGE OR MEAN (SD): 42 (13). GENDER (% MALE): 20. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: unclear. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: cholecystectomy. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 25% smoker. | |
| Interventions | DETAILS OF INTERVENTION: not specified. DETAILS OF CONTROL: home visit by a nurse. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: usual care. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 4 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / hospital record / unclear / status analysis / 4 weeks after discharge. ED‐visits / hospital record / unclear / status analysis / 4 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: To enhance compliance and improve outcomes. AIM OF STUDY: To evaluate the impact of personalized telephone follow‐up on compliance rates in high‐risk hypercholesteraemic patients receiving combination drug therapy. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: During a 7 month period patients who had undergone CABG surgery or percutaneous transluminal coronary angioplasty were eligible; patients were recruited from the coronary care unit. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: CABG or PTCA patients/ baseline fasting LDL above 130 mg/dl/ able to read, understand and speak English/ have telephone at home EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: serum transaminase level twice above normal/concomitant therapy with cyclosporine, warfarin or erythromycin/history of gastrointestinal disease. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C. high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: a computer generated list of random numbers. METHOD OF CONCEALMENT OF ALLOCATION: unclear. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/t‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION : surgery patients / cardiac patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 62.5 (12). GENDER (% MALE): 57. ETHNICITY: 70% Caucasian. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: cardiovascular disease. OTHER HEALTH PROBLEM/S: hypercholesterolaemia. TREATMENT RECEIVED/RECEIVING: CABG (20x)/PTCA(10). OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: A pharmacist telephoned patients at their home every week for 12 weeks. To ensure consistency, the same pharmacist was involved in each patient contact and a standard set of questions was asked. Emphasis was placed on the importance of therapy in reducing the risk of recurrent cardiac events. Patients were questioned about when and where prescriptions were filled, how they paid their prescriptions, potential side‐effects, overall well‐being, and specific reasons for non‐compliance when applicable. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: in hospital all patients were extensively counseled on the appropriate use of the drugs and all patients received dietary instructions. DELIVERY OF INTERVENTION PROVIDERS: pharmacist. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (e.g. treatment adherence, knowledge, adverse events, ..): Compliance / pill count / no / pill count at the clinic visit / 6 and 12 weeks after discharge and at 1 and 2 years. Lipid‐profiles / blood sample / unclear / blood analysis / 6 and 12 weeks after discharge. D. Health service delivery oriented outcomes (e.g. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN English. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to increase patient knowledge in six content areas. AIM OF STUDY: to investigate the effect of a planned telephone follow‐up program to provide information and support to post myocardial infarction patients at home in the 6 to 8 week period after hospital discharge. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: all patients entering the coronary care unit with a diagnosis of MI were eligible; further procedure unclear. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: Myocardial infarction/ able to communicate in english/ have a telephone. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: disoriented to time place or person/ history of previous MI/ psychiatric history/ too ill/ not able to return at the clinic at 2 months afterwards. INFORMED CONSENT OBTAINED? unclear. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: B.moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients. GEOGRAPHIC LOCATION: Canada. SETTING: discharged home from an acute care setting. NUMBER OF PARTTICIPANTS: AGE: RANGE OR MEAN (SD): 54. GENDER (% MALE): 86. ETHNICITY: not clear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: myocardial infarction. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: the cardiac rehabilitation research nurse made the follow‐up phone calls; they assessed understanding of teaching done before discharge. Information that was unclear or confusing was clarified and new information introduced. To promote retention of information, topic areas addressed during each call included each of the six teaching areas of the study. Time was dependent on the patient's difficulty or ease in remembering or understanding the information provided. Approximately 3 calls were made to each subject; additional follow‐up was based on the nurse assessment of the subject's knowledge. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: inhospital teaching. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Knowledge / self‐developed / partly, based on instrument of Horn and Swain (1977) and interrater reliability testing / telephone interview / unclear, probably at 8 weeks post discharge. D. Health service delivery oriented outcomes (e.g. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to resolve past and current problems, to provide more personalized care, to increase patient satisfaction and thereby increasing the likelihood they will return and recommend the institution to family and friends and so establishing a competitive advantage over local health providers who do not have such a program. AIM OF STUDY: it was hypothesized that calling discharged patients 3 weeks after leaving the hospital would provide an opportunity to correct any problems, offer additional service, and reinforce the hospital's concern for the patient's medical recovery. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: all patients discharged from a surgical unit were identified through the hospital's database shortly after they left the hospital. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: discharged from surgery department (general surgery or otorhinolaryngology)/ had at least one overnight stay in the hospital/not being readmitted before scheduled telephone follow‐up. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: international patients/ being discharged to a nursing home. INFORMED CONSENT OBTAINED? no. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test (probably). CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: surgery patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): ? GENDER (% MALE): ? ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: unclear. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: surgery. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: Three weeks after discharge, the patient was called using an interview guide; the 3 week period was selected because it gives the surgical patient sufficient time to overcome most of the normal problems associated with any surgery; yet if problems persisted, they could be identified and addressed before the routine 6 weeks follow‐up. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? unclear. DETAILS OF CO‐INTERVENTIONS: unclear. DELIVERY OF INTERVENTION: PROVIDERS: someone who was not a nurse, but had worked in a number of hospital units and was familiar with hospital patient's concerns. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: The TFU was aimed to monitor recovery, to reinforce risk‐factor reduction, coach toward activity and to provide reassurance. AIM OF STUDY: whether in‐patient education and telephone monitoring during convalescence enhanced perceptions of cardiac efficacy and reported activity. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: patient and their family members were approached the day before surgery and invited to participate in the study. Randomization occurred following transfer from the ICU and was carried out according to cluster randomized design. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: adequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: "Randomization occured following transfer from the intensive care unit and was carried out according to a cluster randomization design (Donner et al. 1981). In this procedure, a a group or cluster of subjects is formed and then is assigned as a group, using computer‐generated random numbers, to experimental or control conditions. Cluster size was randomly determined, and usually was eight to ten patients...Random assignments were made in accordance with plans drawn by W. Hauck, the consulting statistician; the sequence of randomization was not revealed to research assistants." (p. 1134). METHOD OF CONCEALMENT OF ALLOCATION: as above. BLINDING: INTENTION TO TREAT ANALYSIS: not stated BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: STATISTICAL METHODS AND THEIR APPROPRIATENESS: CONSUMER INVOLVEMENT: | |
| Participants | DESCRIPTION : cardiac patients / surgery patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 58. GENDER (% MALE): 80. ETHNICITY: 42% caucasian, PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: cardiovascular disease, OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: CABG and/or valve replacement. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: Both groups were provided with routine information on recovery. The experimental group additionally received a slide programme and brief counseling session before discharge. After discharge the experimental group was followed by weekly telephones from a NURSE for 4 weeks and biweekly telephone between 4th and 8th week after discharge. DETAILS OF CONTROL: usual care, CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: routine information on recovery, consisting of a booklet and slide program. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: poor since outcome assessment coincides with intervention. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 3 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Activity level / self‐developed / no / patient's self‐report during telephone interview / 4, 8, 12 and 24 weeks. Self‐efficacy / Jenkins Self‐Efficacy Scale (Jenkins 1988) / yes / telephone interview / at discharge, 4, 8, 12 and 24 weeks after discharge. C.Other consumer oriented outcomes (e.g. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (e.g. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR: yes. ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: it was expected that patients who received a weekly telephone call would have less anxiety, less severe side effects, use more helpful self‐care strategies and cope better than patients who did not receive TFU. AIM OF STUDY: to investigate the effects that a weekly telephone call intervention had on patients' well‐being. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: potential subjects from the physician's practice and meeting the study criteria were identified by the physician nurse team. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: undergoing radiation therapy for cure /able to communicate by telephone. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? unclear. ETHICAL APPROVAL? unclear. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test/anova. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: oncology patients GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 58. GENDER (% MALE): 40. ETHNICITY: PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: cancer patients receiving radiotherapy (34% breast cancer; plus 7 other types of cancer). OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: radiotherapy. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: Experimental patients received usual care ; in addition they received a weekly telephone call by NURSE to further assess problems and reinforce teaching. The weekly telephone calls continued until the first follow‐up visit, which usually occurred 1 month after treatment was completed/ DETAILS OF CONTROL: usual care consisting of weekly on‐treatment visits with both the physician and nurse during the course of the treatment, usually lasting 6 weeks. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: usual care consisting of weekly on‐treatment visits with both the physician and nurse during the course of the treatment, usually lasting 6 weeks. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: coincides with weekly treatment and counseling by nurse and physician. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 4 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Selfcare / self‐developed / unclear / telephone interview / 1, 2, 3, 4 and 6 weeks after discharge C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Side‐effects / self‐developed: side‐effects profile / unclear / telephone interview / 1, 2, 3, 4 and 6 weeks after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce anxiety in patients and partners. AIM OF STUDY: to determine the effectiveness of an information and support telephone intervention for reducing anxiety in patients who have undergone CABG surgery and their partners STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: convenience sample. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: first elective CABG without valve replacement/had a partner at home involved in their care/older than 18 years/able to understand and speak english/have a telephone/ able to hear telephone conversations. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: major comorbidity/ psychiatric diagnosis/ generalized anxiety or panic disorder. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: adequate. EPOC‐ QUALITY CRITERIA 2002: B.moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: random number assignment and using opaque envelopes. METHOD OF CONCEALMENT OF ALLOCATION: opaque envelopes. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/anova/repeated measures analysis. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients / surgery patients. GEOGRAPHIC LOCATION: Canada. SETTING: discharged home from an acute care setting. NUMBER OF PARTTICIPANTS: AGE: RANGE OR MEAN (SD): 63 (8). GENDER (% MALE): 86. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: cardiovascular disease. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: CABG. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 98% married, 50% high school, 58% retired. | |
| Interventions | DETAILS OF INTERVENTION: The intervention consisted of information and support to assist patients (and partners) in meeting their needs. Standardized protocols for predefined problems and concerns identified in the literature were developed. The intervention began on the day of discharge; this was followed by 6 telephone calls by NURSE on days 1, 2, 4, 7, 14 and 21 after discharge. The nurse was also on call 24 hours a day. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D.Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR: yes. ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce readmissions. AIM OF STUDY: To compare the effectiveness of 3 hospital discharge care models for reduction congestive heart failure related readmission charges: 1/ home telecare delivered via a 2‐way video‐conference device with an integrated electronic stethoscope; 2/ nurse telephone calls; and 3/ usual outpatient care. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: During a one year period all patients with a primary admission diagnosis of chronic heart failure were screened on the inclusion criteria. Patients who agreed to participate were randomized before discharge, to one of 3 models, using sealed envelopes. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: CHF/ aged 40 and older/ telephone at home/ English speaking/ area university of California/ adequate vision and hearing. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: to much comorbidity (Charlson score >6), Geriatric depression score >7, Mini mental state<20, symbol digit modalities test low. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: adequate. EPOC‐ QUALITY CRITERIA 2002: B. moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE:random number assignment and using opaque envelopes. METHOD OF CONCEALMENT OF ALLOCATION: opaque envelopes. BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/anova. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): GENDER (% MALE) ETHNICITY PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: OTHER HEALTH PROBLEM/S: TREATMENT RECEIVED/RECEIVING: OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: All 3 groups received home visit of a nurse shortly after discharge and at day 60. Patient in telephone group received scheduled phone calls from nurse, whereas patients of the telecare group received scheduled telecare visits. Both groups also had possibility to contact study nurse. Difference between the intervention groups is that the telecare group could also see and not only hear study nurse, and vice versa. During contacts several health status measures were filled out. DETAILS OF CONTROL: The telecare was instructed in the use of the equipment and received home telecare visits/ Patients in the usual care group received 2 home visits. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: all patients received an in‐person home nurse visit shortly after discharge and second in‐person home nurse visit 60 days later. During both visits completed some questionnaires. DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: good. FIDELITY/INTEGRITY: good. | |
| Outcomes | NUMBER OF OUTCOMES: 3 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Physical status / SF‐36 (Ware, 1992), Minnesota Living wit Heart Failure Questionnaire (Rector, 1992) / yes / interview / 60 days after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL: readmissions/ed‐visits and charges were also measured but only at 6 months, which is outside the scope of this review. ·CONTACT WITH AUTHOR: yes. ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. Yes, see Jerant 2003. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: To enhance compliance (based on health belief model)/ AIM OF STUDY: the purpose of the study was to test the effect of clinical and telephone intervention on compliance for ED‐patients. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: consecutive patients who met the sampling criteria were randomly assigned to one of 4 groups. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: Patients presenting at the ED with one of following problems (chest pain, hypertension, asthma, otitis media, diabetes, urinary tract infection, headache, urethritis, vaginitis, low back pain, rash)/ signed release of information/ able to respond to HBM intervention/ did not require hospital admission/ had a referral follow‐up recommendation/ telephone at home. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: 2X2X11 factorial design/ blocked randomization within each presenting problem. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/logistic regression. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: ED patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 0 to 60+ GENDER (% MALE): unclear. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: mixed (largest groups: chest pain, urinary tract infection and low back pain). OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 61% single. | |
| Interventions | DETAILS OF INTERVENTION: Telephone call, which was a modified and shortened application of the Health Belief Model (HBM) clinical intervention, by a nurse 1 or 2 days after the ED‐visit/ DETAILS OF CONTROL: There are 3 control groups: group 1 received usual care, group 2 received a HBM clinical intervention during their ED‐visit, and group 3 received a HBM clinical intervention during their ED‐visit and a telephone HBM follow‐up. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Compliance / self‐developed / unclear / telephone interview to health agency where patient had referral / different across patients. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. yes, Jones et al. published 5 manuscripts, in which they present results for several subgroups (eg. hypertension, low back pain, otitis media, urinary tract infection). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to enhance compliance with the use of the home apnea monitor for infants discharged on an apnea monitor. AIM OF STUDY: this study was designed to test whether weekly telephone contact with a health professional would improve the use of the home apnea monitor. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: all infants discharged on apnea monitor during a 1.5 year period, were eligible for this study. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: preterm infants with abnormal pneumocardiogram, patients with bronchopulmonary disease requiring oxygen support/ siblings of a sudden infant death syndrome victim, others infants with various pulmonary, cardiac or neurologic problems. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C. high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: stratified balanced block technique. METHOD OF CONCEALMENT OF ALLOCATION: unclear. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test/Mann‐Whitney U‐test/chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: pediatric patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 0.5 in infants/ 25 years in mothers. GENDER (% MALE): not stated. ETHNICITY: not stated. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: preterm infants with abnormal pneumocardiogram, patients with bronchopulmonary disease requiring oxygen support/ siblings of a sudden infant death syndrome victim, others infants with various pulmonary, cardiac or neurologic problems. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: OTHER SOCIAL/DEMOGRAPHIC DETAILS: 50% of mothers married. | |
| Interventions | DETAILS OF INTERVENTION: above the care comparable to the control group, patients in the experimental group received an additional phone call consisting of a structured interview every week for a total of 8 weeks. The questionnaire addressed the use of monitors and the well‐being of the baby, including any need for an office visit or hospitalisation. DETAILS OF CONTROL: care for all groups: access to a physician in the neonatal intensive care unit at all times, initial instruction as well as support 24 hours per day from the monitor vending company, follow‐up visits with a neonatologist or pediatrician within 2 weeks of discharge and about every month for the next 3 months and most infants had 1‐3 visits by a home nurse in the first 2 weeks following discharge. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: see control intervention. DELIVERY OF INTERVENTION: PROVIDERS: unclear, probably physician. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Knowledge / self‐developed: heart disease management questionnaire / partly; Kuder‐Richardson coefficient 0.36 / unclear / unclear, but in first month after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to enhance support. AIM OF STUDY: the hypothesis tested was that routine contact by telephone might significantly improve the adequacy of support for patients during the potentially stressful period between completing radiotherapy and the first follow‐up visit. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: consecutive unselected outpatients attending for radiotherapy. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: radiotherapy patients. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: did not understand English/ not having a telephone/ HIV‐related malignancy/ less than 5 dose of radiotherapy/ inhospital patients. INFORMED CONSENT OBTAINED? unclear. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: unclear. METHOD OF CONCEALMENT OF ALLOCATION: unclear. BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: oncology patients. GEOGRAPHIC LOCATION: UK. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 30 to 88. GENDER (% MALE): 42. ETHNICITY: not stated. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: radiotherapy for cancer (breast 43%, lung, 31%,..). OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: Telephone contact comprised telephone calls to the patient on day 4, 8, 14 and 18 after completing radiotherapy; the telephone calls were made by a member of staff (radiographer, nurse, or doctor who was known to the patient). The calls were semistructured, the questions to be asked being: 'how are you feeling?', 'are you having any problems?', 'have you any further side‐effects from treatment?', 'do you need to make an appointment ..?'. patients were asked if they had any additional worries or concerns. wherever possible action was taken. DETAILS OF CONTROL: all patients were seen once a week in the clinic by a doctor during the radiotherapy treatment. Additional advice and support was given, where necessary, by radiographers and nurses. In the usual care group no attempt was made to contact the patients between completing treatment and the first follow‐up visit. If patients telephoned the department for advice or support this was provided. CO‐INTERVENTION? yes. DETAILS OF CO‐INTERVENTIONS: see control intervention. DELIVERY OF INTERVENTION PROVIDERS: mixed (nurse, radiographer, doctor). INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: improve the appropriateness of the parents use of follow‐up care. AIM OF STUDY: To test the hypothesis that the appropriateness of parents' use of early follow‐up care after ED visits can be improved by post visit support from a nurse practitioner. We hypothesized that telephone support, given by pediatric nurse practitioners to parents within 1 day after their ED‐visit for their children's acute illness could improve the appropriateness of the parents use of follow‐up care. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: potential subjects were identified on arrival at the ED. Patients were told that they were conducting a survey to try to learn ways of improving pediatric ED care. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: child younger than 8 years accompanied by parent or usual caretaker, free of active chronic illness, presenting with a chief complaint suggesting an acute infectious or allergic condition/ parents speaking English, acces to telephone, primary care source is hospital's primary care center. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: requiring hospital admission. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: adequate. EPOC‐ QUALITY CRITERIA 2002: B. moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: use of a random number table and a balanced block‐randomization METHOD OF CONCEALMENT OF ALLOCATION: sealed envelopes given to the parents on leaving the ED. BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: ED patients / pediatric patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): GENDER (% MALE) ETHNICITY PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: OTHER HEALTH PROBLEM/S: TREATMENT RECEIVED/RECEIVING: OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: The intervention consisted of only a single telephone call; mostly the call required less than 5 minutes. The NP called each parent in 6 to 18 hours after discharge from the ED. She offered further explanation about the child's diagnosis and treatment, reinforced follow‐up instructions and offered around the clock access to herself or another NP by telephone if needed; the protocol allowed her to answer questions or offer clinical assistance over the phone if it seemed warranted. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Compliance (appropriate use of follow‐up care) / self‐developed / yes / hospital record/ telephone interview / 1 week after discharge D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to help ease surgical orthopaedic patients' transition from hospital to home and to identify problems associated with this transition. AIM OF STUDY: this was a pilot study designed to explore the effectiveness of a post‐discharge telephone call for surgical orthopaedic patients; the focus of the study was to identify and resolve problems associated with the study protocol and the data collection tools. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: the sample was drawn from a pool of adult patients admitted to the orthopaedic unit for either elective or emergency orthopaedic surgery during a 3 month period. Prospective participants were identified through the use of posters placed in patients' rooms. Patients who expressed an interest in the study were approached prior to their discharge by a research assistant who explained the purpose of the study. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: English speaking/ 17 years and older/ discharged to a private residence with phone. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C. high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: unclear. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: not stated. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: surgery patients. GEOGRAPHIC LOCATION: Canada. SETTING: discharged home from an acute care setting. NUMBER OF PARTTICIPANTS: AGE: RANGE OR MEAN (SD): 56.8 (17.6). GENDER (% MALE): 55. ETHNICITY: not stated. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: orthopedic problem. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: orthopedic surgery (68% elective, 32% emergency). OTHER SOCIAL/DEMOGRAPHIC DETAILS: 69% high school or more. | |
| Interventions | DETAILS OF INTERVENTION: The intervention consisted of a follow‐up call made by the unit manager (=nurse), or her designate, 24 to 72 hours post discharge. Information obtained was recorded on a form which consisted of a checklist of specific concerns/problems often encountered by post‐op patients and a list of relevant nursing interventions in addition to information about the call. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION: PROVIDERS: nurse. INTERVENTION QUALITY: length of intervention between 1‐25 minutes. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 3 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..):Symptoms / self‐developed / yes / telephone interview / 4 weeks after discharge. Recovery / self‐developed / yes / telephone interview / 4 weeks after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / self‐developed / yes / telephone interview / 4 weeks after discharge. ED‐visits. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL: no comparison results given. ·CONTACT WITH AUTHOR: yes. ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: telehealth interventions offer a potentially promising way to improve care and continue patient education once patients have returned to the community/ AIM OF STUDY: to determine which of 3 approaches to care produces the lowest incidence or pressure ulcers, promotes the most effective care of sores that develop and leads to the fewest hospitalizations in newly injured patients with spinal cord injury after discharge. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: During a 6 month period, patients with newly injured spinal cords were recruited. Patients for the video group were first recruited; those qualifying were informed about the study and asked to volunteer; following recruitment of the video group, matched controls (age, race, severity of injury) were recruited for the telephone group and the standard care group. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: scheduled for discharge to the community/ have telephone at home and for the video group living in Georgia. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: inadequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE:not done. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: kruskall‐wallis test/ chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: neurological patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 33 (12). GENDER (% MALE): 74. ETHNICITY: 31% African American. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: new spinal cord injury. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 46% married; 91% employed; 48% post high school education; slight differences in video group. | |
| Interventions | DETAILS OF INTERVENTION: both the video and the telephone group received weekly interventions for 10‐12 weeks. The telephone group participated in telephone only counseling sessions for approximately 10 weeks after discharge. During the telephone sessions patients were guided through skin check‐ups and were also assisted in problem solving related to bowel, diet or any matter of concern. DETAILS OF CONTROL: The videogroup received weekly interventions for approximately. 10‐12 weeks. In the video group, patients participated in weekly counseling sessions using the video‐unit for the first 6‐8 weeks following discharge; through the images generated the nurse was able to check visually the condition of the patients skin and to monitor him for ulcers; through visual contact the nurse could also help resolve problems related to wheelchairs, mattresses and mobility about the house. Following the video‐sessions, patients then received weekly telephone counseling for approximately. 4‐6 weeks. The standard care group was given instruction on using the centers' help line; this line provides information and counseling free of charge to patients who call the center on their own initiative. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Tracking/diagnosing pressure ulcer / self‐developed / no / unclear / every 8‐12 weeks but only reported for a 1 year period (therefore not included in the analysis for this review). C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / unclear / unclear / unclear / at 1 year (not included therefore in review data‐analysis) | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce the incidence of secondary conditions among people with mobility impairment resulting from spinal cord injury. AIM OF STUDY: the results are presented on health related outcomes of a randomized trial of telehealth interventions to reduce the incidence of secondary conditions among people with mobility impairment resulting from spinal cord injury. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: patients with spinal cord injury were recruited during their initial stay. Any patient from 18 to 60 years of age with a newly acquired spinal cord injury was eligible. All participants were research volunteers; once they agreed to participate they were randomly assigned to 1 of 3 groups. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: new spinal cord injury/age 18‐60/ have telephone/ discharged to the community. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: concomitant diagnosis of brain injury, known active substance abuse, mobility impairment level mild. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C. high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: unclear. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: kruskall‐wallis test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: neurological patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 37 (12). GENDER (% MALE): 77. ETHNICITY: 17% African American. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS:new spinal cord injury. OTHER HEALTH PROBLEM/S: unclear. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 55% married. | |
| Interventions | DETAILS OF INTERVENTION: 3 groups: telephone, videophone and standard care. Video and phone groups took part in individual educational rehabilitation sessions with a nurse once a week for 5 weeks, then once every 2 weeks for one month. These sessions were in addition to any other regularly scheduled care, such as the two month post discharge care. The content and structure of the education sessions for the phone and video group were similar, except that the video group also saw real time images of the nurse. The intervention sessions lasted a total of nine weeks. DETAILS OF CONTROL: 2 groups: video group and standard care. The standard care group were offered the routine care, which requires patients to call the hospital help line if and when they need assistance prior to the regular 2 months post discharge visit. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION: PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 4 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / unclear / unclear / unclear / at 1 year (not included therefore in review data‐analysis). | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce resource use (readmissions, hospital days, costs). AIM OF STUDY: to assess the effectiveness of a standardized telephonic case‐management intervention in decreasing resource use in patients with chronic heart failure. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: physicians known to admit patients with heart failure were matched by specialty (cardiology, internal medicine), practice size and number of HF admissions in the prior year. After matching, physicians were randomized to the intervention or usual care group. Although it was the physicians who were randomized, patients were the unit of analysis for this study. It was not feasible to randomize patients in the same physician practice to different groups because of the possibility that the physicians would modify care in the control group to mimic aspects of the intervention. Physicians were not informed of the group to which they were assigned. A total of 281 physicians were randomized. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: confirmed diagnosis of heart failure as the primary reason for their hospital visit/ speak English or Spanish. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: cognitive impairment or psychiatric illness/ severe renal failure requiring dialysis/ terminal disease// discharge to a long term care setting/ previous enrolment in a HF disease program. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: unclear. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: no. STATISTICAL METHODS AND THEIR APPROPRIATENESS: anova/logistic regression/linear regression. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients. GEOGRAPHIC LOCATION: USA. SETTING:discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 72 (12). GENDER (% MALE): 49. ETHNICITY: 25% Spanish speaking. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: heart failure (49% ischemic, 20% hypertensive). OTHER HEALTH PROBLEM/S: 41% low comorbidity, 20% high comorbidity (68% hypertension, 35% COPD, 42% diabetes, 28% renal disease). TREATMENT RECEIVED/RECEIVING: medication (62% digoxin, 54% ACE‐inhibitor, 86% diuretic,…). OTHER SOCIAL/DEMOGRAPHIC DETAILS: 56% single. | |
| Interventions | DETAILS OF INTERVENTION: Telephonic case management by a registered nurse was provided using a decision support software program; the software program uses automated tool for setting priorities for patient education, data collection and documentation; best practices are supported by the program. The intervention group was phoned within 5 days after discharge and thereafter at a frequency guided by the software and case manager judgment based on patient symptoms, knowledge and needs. Patients received an average of 17 calls at decreasing levels of intensity, length and frequency over the 6 month follow‐up period; printed educational material was mailed every month to the patients. DETAILS OF CONTROL: usual care, not standardized. CO‐INTERVENTION? unclear. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: large range. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / self‐developed / unclear / hospital record / 3 and 6 months. Costs / self‐developed / no / hospital record / 3 and 6 months. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL: Riegel 2002 (in journal Disease Management & Health Outcomes) is subanalysis for Spanish speaking patients. ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to improve patient attendance at outpatients clinic after ED visit. AIM OF STUDY: to determine whether the intervention of a telephone call within 3 days of ED attendance would improve the proportion of patients making recommended appointments and the proportion of patients attending scheduled appointments. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: consecutive patients who were advised by ED doctors to make outpatient appointments were identified for inclusion using the ED computer system over a 4 week period. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: do not speak english/ confused or demented with no carer/ had no telephone/ age under 16 years/haven been included previously in the study/ are referred to a private specialist or another hospital. INFORMED CONSENT OBTAINED? no. ETHICAL APPROVAL? yes. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: inadequate. EPOC‐ QUALITY CRITERIA 2002: B. moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: restricted randomisation in blocks of ten was performed using a table of random numbers from a standard statistical text. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square/ logistic regression. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: ED patients. GEOGRAPHIC LOCATION: Australia. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 16‐65+ . GENDER (% MALE): 64. ETHNICITY: not stated. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: various. OTHER HEALTH PROBLEM/S: TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: patients in the intervention group were phoned by one of the authors (MD) or a research nurse 1‐3 days after ED attendance. A general enquiry was made about their health and the importance of medical follow‐up was explained in general terms; if the patient had already made an appointment, they were reminded about that appointment; for those who had not yet scheduled an appointment, they were reminded that they had been advised to do so, and an offer was made to book one for them at that time. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: doctor/nurse. INTERVENTION QUALITY: unclear, FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 1. OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Compliance / self‐developed / no / hospital record / within 3 months. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce anxiety and depression. AIM OF STUDY: the study tests the hypothesis that telephone follow‐up from the ward will reduce patients' anxiety and depression levels in the early post‐discharge period. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: patients admitted during a 21‐day consecutive period were enrolled to a defined group. After completion of this period, no patients were enrolled for 7 days to prevent patient overlap if their discharge was delayed. The process was then repeated with the patients being enrolled to the alternative group. Patients were asked for consent and enrolled into the study on the day they were given a planned discharge date. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: have undergone elective coronary artery bypass grafting or valve replacement. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: unable to give consent/ could not communicate in English/ history of mental illness/ have undergone emergency cardiac surgery. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: inadequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: A convenience sampling model utilizing alternative block selection was used. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: Mann‐Whitney U test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients / surgery patients. GEOGRAPHIC LOCATION: UK. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): unclear. GENDER (% MALE): unclear. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: coronary heart and/or valve disease. OTHER HEALTH PROBLEM/S: not specified. TREATMENT RECEIVED/RECEIVING: CABG‐surgery or valve replacement. OTHER SOCIAL/DEMOGRAPHIC DETAILS: not given. | |
| Interventions | DETAILS OF INTERVENTION: patients in the intervention group received standard discharge advice/information plus 2 additional follow‐up calls from a nurse. Contact was made at 7 and 21 days after discharge. On contact, the callers introduced themselves and patient identity was confirmed. Patients were invited to discuss how they had been since their last contact with the ward and encouraged to raise any concerns or difficulties they had experienced since that time. DETAILS OF CONTROL: standard advice included the patient being offered a selection of information leaflets, a personal exercise plan and an individual discussion with their named nurse over concerns and discharge medications. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 2 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce worry, mood disturbance and enhance well‐being and quality of relationship. AIM OF STUDY: the purpose of this study was to examine the effects of a 13‐month, 3 phase intervention, comparing an experimental group receiving combined individual telephone and in‐person group social support and education treatment with a control group receiving telephone only individual support and education treatment and a control group receiving one time mailed educational information treatment on cancer‐related worry, well‐being, mood disturbance, loneliness and the quality of relationship with a significant other among women newly diagnosed with early stage breast cancer. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: Women were recruited through letters distributed at physician's offices, hospitals and the American Cancer Society Reach to Recover program. Because letters were distributed by personnel at each site, the number of women reached is not known. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: English speaking/ had surgery for nonmetastatic breast cancer within 4 weeks prior to beginning their participation in the study, had no previous cancer diagnosis, no other major medical problems. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: adequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: Using a permuted block design for randomization, when successive cohorts of 4‐8 women had been recruited, the entire cohort was randomly assigned to one of the three treatment groups using the sealed opaque envelope technique. When the next cohort was recruited, that cohort was assigned to one of the two remaining study treatment groups, using the two remaining sealed opaque envelopes. The next cohort was assigned to the remaining study treatment group, after which the process started again. Random assignment was repeated in this manner until the full sample was recruited and assigned. METHOD OF CONCEALMENT OF ALLOCATION: sealed opaque envelopes. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: no. STATISTICAL METHODS AND THEIR APPROPRIATENESS: manova/ anova/kruskall‐wallis/ mann‐whitney U. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: oncology patients / surgery patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 54 (10.8). GENDER (% MALE): 0. ETHNICITY: 89% white. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: breast cancer. OTHER HEALTH PROBLEM/S: not specified. TREATMENT RECEIVED/RECEIVING: lumpectomy (42%), mastectomy (57), chemotherapy (44%), radiation therapy (26%). OTHER SOCIAL/DEMOGRAPHIC DETAILS: 61% married, 43% high school. | |
| Interventions | DETAILS OF INTERVENTION: 3 groups: telephone only, telephone and in‐person group social support,and group 3 one time education by mailing. Women in (for this review experimental group) experienced a less intense form of the focal stimulus by receiving social support and education only by telephone in all 3 phases of the study (see control group 1). the intervention was based on Roy's adaptation model. The individual telephone social support and education were provided by either oncology nurse clinicians or social workers, who were trained by the investigators. Specially developed guides were used during the intervention. Logs of the phone contacts were made, that were periodically reviewed. DETAILS OF CONTROL: This group received the most intense intervention in the form of the focal stimulus of social support and education by receiving combined individual telephone and in‐person group social support and education, which was provided in 3 phases over 13 months. The treatment was designed to provide more intense support during the times of peak need. More specifically weekly phone contacts in first 3 months, weekly in‐person social support in next two months and twice‐monthly phone contacts in next 8 months. Control group 2 received usual care in first 3 months, and one‐time mailing in month 4. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse/social worker. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: good. | |
| Outcomes | NUMBER OF OUTCOMES: 7 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Physical functioning / SF‐36 (Ware, 1992) / yes / telephone interview / 1 and 5 weeks after discharge. Symptom distress / Memorial symptom assessment scale (Portenoy 1994) / yes / telephone interview / 1 and 5 weeks after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / self‐developed / no / telephone interview / 1 and 5 weeks after discharge. ED‐visits / self‐developed / no / telephone interview / 1 and 5 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL NOTE: since intervention group and control group 1 receive the same intervention during first 3 months and they only start differing after this point, these groups can be combined for this review into 1 intervention group and compared to control group 2 (usual care). ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to increase patient satisfaction and patient compliance. AIM OF STUDY: to describe and quantify the benefit that can be derived from an organized system of follow‐up telephone calls. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: During a 10 day period, all patients charts were reviewed and each patient who was discharged home from the ED with one of nine diagnosis was included in the study; included patients then were stratified by disease category and were randomized into two groups. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: Discharged home/ diagnosis of undiagnosed chest pain, undiagnosed abdominal pain, acute infection, vaginal haemorrhage, syncope, acute cervical/lumbar pain, asthma/bronchospasm, allergic reaction, headache. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? no. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: · INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: ED patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 36 (15). GENDER (% MALE): 47. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: acute infection (30%), abdominal pain (20%), acute lumbar pain (14%), chest pain (12%), other. OTHER HEALTH PROBLEM/S: not specified. TREATMENT RECEIVED/RECEIVING: unclear. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: Within 48 to 72 hours after the ED‐visit, attempts were made to call the patients. Calls were made by members of the ED clinical nursing staff. The caller had a copy of the patient's ED chart, and interviewed the patient by following a written scenario that was designed to determine the progression of the patient's symptoms, whether the patient had already sought additional medical consultation, whether the patient eventually would seek follow‐up with the provider recommended by the ED physician and whether the ED instructions for aftercare were clear. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 7 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Physical functioning / SF‐36 (Ware, 1992) / yes / telephone interview / 1 and 5 weeks after discharge. Symptom distress / Memorial symptom assessment scale (Portenoy 1994) / yes / telephone interview / 1 and 5 weeks after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / self‐developed / no / telephone interview / 1 and 5 weeks after discharge. ED‐visits / self‐developed / no / telephone interview / 1 and 5 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reduce pain and improve pain management. AIM OF STUDY: to determine whether telephone consultation influenced patients' perception of and reaction to pain after periodontal surgery. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: during a 4 year period patients who presented to the division of periodontology of a general hospital and who fulfilled inclusion criteria were admitted to the study. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: moderate to severe peridontal disease (class 3 and 4), diagnosed as adult cause‐related peridontitis, root planning and subsequent periodontal surgical pocket reduction or prescribed elective preprosthetic periodontal surgery, systemic health,age 30 to 70 years, no history of mental disease, no medication for at least 1 month prior to the procedure. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? no. ETHICAL APPROVAL? unclear. FUNDING: unclear. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: inadequate. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not done. METHOD OF CONCEALMENT OF ALLOCATION: not done. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: student t‐test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: surgery patients. GEOGRAPHIC LOCATION: Canada. SETTING: discharged home from an acute care setting. NUMBER OF PARTTICIPANTS: AGE: RANGE OR MEAN (SD): 50 (2). GENDER (% MALE): 46. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: periodontitis. OTHER HEALTH PROBLEM/S: none. TREATMENT RECEIVED/RECEIVING: periodontal surgery. OTHER SOCIAL/DEMOGRAPHIC DETAILS: | |
| Interventions | DETAILS OF INTERVENTION: the intervention group patients were called no later than 24 hours after the procedure. The telephone interviewer was either the assisting student or the supervisor dentist, who then systematically inquired about 10 points (well‐being of patient, the return to normal and loss of analgesia, jaw swelling, wound bleeding, whether the wound was painful, acquisition and use of mouthwash and analgesics, the need for a soft balanced diet, necessity of sustained oral hygiene, confirmation of the next week's appointment and reassurance about the reaction and pain). The interviewer was instructed to be sympathetic, to reassure patients that whatever reaction they were having was within the range of expected normal limits and to be positive about a successful outcome. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: dentist. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: unclear. | |
| Outcomes | NUMBER OF OUTCOMES: 3 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Selfcare deficits / self‐developed / no / telephone interview / 6 weeks after discharge. Blood glucose level / Hba1c‐level / unclear / blood sample / 3 months after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Knowledge / Diabetes knowledge scale (Dunn,1984) / yes / telephone interview / 6 weeks after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to improve cardiac surgery recovery following hospital discharge, as reflected in improved health related quality of life, decreased symptom distress, improved satisfaction with discharge and follow‐up care and decreased unplanned contacts with the hospital. AIM OF STUDY: The purpose of the study was to determine if the provision of Advanced Practice Nurse (APN) support, delivered via the telephone, improved cardiac surgery recovery following hospital discharge, as reflected in improved health related quality of life, decreased symptom distress, improved satisfaction with discharge and follow‐up care and decreased unplanned contacts with the hospital. STUDY DESIGN: RCT. METHODS OF RECRUITMENT OF PARTICIPANTS: participants were recruited from the inpatient cardiac surgery unit. Prior to discharge, eligible participants were identified by a research assistant who obtained consent and baseline data. The research assistant informed the APN of consenting patients' discharge. recruited patients were randomized after discharge. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: a) undergone first elective or emergent cardiac surgery, b) no unexpected cardiac complications that necessitated an unexpected stay in ICU, c) oriented to time, place and person, d) no history of acute or chronic psychiatric problems, e) able to read, write and speak English, f) capable of responding over the telephone. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: B.moderate risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: random numbers were generated by a statistical consultant through a computer based randomization schedule and forwarded to the APN who assigned patients. METHOD OF CONCEALMENT OF ALLOCATION: unclear. BLINDING: INTENTION TO TREAT ANALYSIS: yes. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: yes. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test/chi‐square/Mann‐Whitney U test. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients / surgery patients. GEOGRAPHIC LOCATION: Canada. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 64. GENDER (% MALE): 76. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: coronary artery disease. OTHER HEALTH PROBLEM/S: COPD in 20%; peripheral vascular disease in 10%, cerebrovascular disease in 145, renal failure in 3.5%. TREATMENT RECEIVED/RECEIVING: CABG. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 16% single. | |
| Interventions | DETAILS OF INTERVENTION: The intervention group received, in addition to the usual care, active and ongoing follow‐up via nurse‐initiated telephone calls from the APN in cardiac surgery at 3 and 5 days following discharge and then weekly for 4 more weeks. During the calls, the APN continued with the plan of care established in the hospital, provided ongoing information and assessment, assisted with self‐management of common symptoms and facilitated referrals to appropriate healthcare resources. Telephone sessions were individually tailored in response to patient's symptoms, concerns and recovery. All sessions, however, were standardized sufficiently to include evaluation of physical and psychological states and incorporated mutual goal setting for the management of symptoms in the recovery period. The initial TFU were approximately 20 to 30 minutes. DETAILS OF CONTROL: usual care: this included preoperative and discharge preparation by the APN, provision of an education booklet and home care follow‐up if necessary. Patients were provided the contact information for the APN and instruction to call with questions or concerns. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: good. FIDELITY/INTEGRITY: good. | |
| Outcomes | NUMBER OF OUTCOMES: 7 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Physical functioning / SF‐36 (Ware, 1992) / yes / telephone interview / 1 and 5 weeks after discharge. Symptom distress / Memorial symptom assessment scale (Portenoy 1994) / yes / telephone interview / 1 and 5 weeks after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / self‐developed / no / telephone interview / 1 and 5 weeks after discharge. ED‐visits / self‐developed / no / telephone interview / 1 and 5 weeks after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? yes. ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to increase self‐care knowledge, improve metabolic control and reduce self‐care behavioral deficits. AIM OF STUDY: to determine the effect of telephone follow‐up on diabetes self‐care knowledge, blood glucose levels, and changes in self‐care behaviors of the elderly subjects. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: a convenience sample was recruited from inpatients of a diabetic hospital. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: 60 years and older/ diabetes mellitus 2/ successfully completed an inpatient diabetes education program during their hospitalisation/ intact cognitive functioning/ able to perform self‐care activities independently/ are being followed by primary physician in the diabetes clinic. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: complex medical conditions (heart failure, end‐stage renal disease, advanced cancer, major surgery,..). INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? yes. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: t‐test/chi‐square. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: diabetes patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 65 (6.5). GENDER (% MALE): 33. ETHNICITY: 52% white. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: diabetes 2. OTHER HEALTH PROBLEM/S: not specified. TREATMENT RECEIVED/RECEIVING: education program. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 63% had 12 years of education. | |
| Interventions | DETAILS OF INTERVENTION: subjects in the experimental group were contacted by either the primary investigator or a trained research assistant (both nurses) within 24 to 48 hours after discharge from hospital. The telephone calls were repeated at weekly intervals for 3 weeks, thus a total of 4 calls were made. Each call consisted of assessing the diabetic client's self care knowledge and practice of self‐care activities or behaviours; supplemental instructions were provided when indicated. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: good. | |
| Outcomes | NUMBER OF OUTCOMES: 3 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): Selfcare deficits / self‐developed / no / telephone interview / 6 weeks after discharge. Blood glucose level / Hba1c‐level / unclear / blood sample / 3 months after discharge. C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): Knowledge / Diabetes knowledge scale (Dunn,1984) / yes / telephone interview / 6 weeks after discharge. D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | DETAILS OF STUDY AIM OF INTERVENTION: to reinforce education, answer questions, and support the patient and family in regard to the post‐operative recovery process. AIM OF STUDY: to evaluate the telephone follow‐up program; it was sought to compare 2 groups of cardiac surgery patients: those who received usual care and those who received usual care and telephone follow‐up calls for 1 month after discharge, with regard to satisfaction with continuity of care, depression, recidivism, and complications. STUDY DESIGN: CCT. METHODS OF RECRUITMENT OF PARTICIPANTS: all eligible cardiac surgery patients were invited to participate. The resulting convenience sample was then randomly divided to control and intervention group. INCLUSION CRITERIA FOR PARTICIPATION IN STUDY: 21 years and older/ discharged home 3 to 7 days after surgery, able to read, speak and understand English. EXCLUSION CRITERIA FOR PARTICIPATION IN STUDY: not specified. INFORMED CONSENT OBTAINED? yes. ETHICAL APPROVAL? unclear. FUNDING: yes. ASSESSMENT OF STUDY QUALITY ALLOCATION CONCEALMENT: unclear. EPOC‐ QUALITY CRITERIA 2002: C.high risk of bias. METHOD OF GENERATING RANDOMISATION SCHEDULE: not specified. METHOD OF CONCEALMENT OF ALLOCATION: not specified. BLINDING: INTENTION TO TREAT ANALYSIS: not stated. BASELINE COMPARABILITY OF INTERVENTION AND CONTROL GROUPS: not stated. STATISTICAL METHODS AND THEIR APPROPRIATENESS: not stated. CONSUMER INVOLVEMENT: not stated. | |
| Participants | DESCRIPTION: cardiac patients / surgery patients. GEOGRAPHIC LOCATION: USA. SETTING: discharged home from an acute care setting. NUMBER OF PARTICIPANTS: AGE: RANGE OR MEAN (SD): 63. GENDER (% MALE): 70. ETHNICITY: unclear. PRINCIPAL HEALTH PROBLEM OR DIAGNOSIS: coronary artery disease. OTHER HEALTH PROBLEM/S: not specified. TREATMENT RECEIVED/RECEIVING: CABG or valve replacement. OTHER SOCIAL/DEMOGRAPHIC DETAILS: 20% living alone, 55% retired. | |
| Interventions | DETAILS OF INTERVENTION: within 2 days of discharge, a telephone call was made by a cardiovascular stepdown nurse. This nurse called once weekly for one month or more frequently if the needs or concerns of the patient or his family so required. The nurse was allowed to talk with either the patient or a family member. A standardized assessment sheet guided the calls. Areas of focus included respiratory, cardiac, and neurologic systems, fluid status, pain management, sleep, nutrition, elimination, activity, self‐care, psychosocial status, wound management and patient knowledge. DETAILS OF CONTROL: usual care. CO‐INTERVENTION? no. DETAILS OF CO‐INTERVENTIONS: DELIVERY OF INTERVENTION PROVIDERS: nurse. INTERVENTION QUALITY: unclear. FIDELITY/INTEGRITY: good. | |
| Outcomes | NUMBER OF OUTCOMES: 4 OUTCOME / TOOL / TOOL VALIDATED / METHOD OF ASSESSMENT / TIME OF ASSESSMENT B.Physical health of patients (eg. functional status, self‐care, self‐efficacy, independence, ..): C.Other consumer oriented outcomes (eg. treatment adherence, knowledge, adverse events, ..): D. Health service delivery oriented outcomes (eg. hospital readmission, health services utilization, ..): Readmission / self‐developed / no / hospital record / 1 month after discharge. | |
| Notes | ·CHANGES IN TRIAL PROTOCOL ·CONTACT WITH AUTHOR ·POWER CALCULATION? ·RECORD IF THE STUDY WAS TRANSLATED FROM A LANGUAGE OTHER THAN ENGLISH. ·RECORD IF THE STUDY WAS A DUPLICATE PUBLICATION. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cells containing blanks under a heading mean there was no information on that item in the trial report.
No studies reported methods of follow‐up for non‐respondents, or adverse events.
RCT: randomised controlled trial
CCT: controlled clinical trial (quasi‐randomised)
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| The intervention does not take place at least once within the first month after hospital disharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| The intervention does not take place at least once within the first month after hospital disharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| The intervention does not take place at least once within the first month after hospital disharge. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Does not present results of a controlled trial. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Outcomes are not measured at least once within 3 months after discharge. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. | |
| Effects of the TFU can not be isolated and analyzed to some degree. | |
| Does not present results of a controlled trial. | |
| Study does not concern patients discharged from hospital to home. | |
| Does not present results of a controlled trial. | |
| Intervention is not a telephone follow‐up by a hospital based professional to patient. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on anxiety in cardiac surgery patients at appr. 1 month after discharge compared to usual care Show forest plot | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.28, 0.34] |
| Analysis 1.1  Comparison 1 Effect of TFU on anxiety in cardiac surgery patients at appr. 1 month after discharge compared to usual care, Outcome 1 Effect of TFU on anxiety in cardiac surgery patients at appr. 1 month after discharge compared to usual care. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on compliance in cardiac surgery patients compared to usual care Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.59, 4.78] |
| Analysis 2.1  Comparison 2 Effect of TFU on compliance, Outcome 1 Effect of TFU on compliance in cardiac surgery patients compared to usual care. | ||||
| 2 Effect of TFU on compliance (making an appointment) in ED patients compared to usual care Show forest plot | 3 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.92, 3.14] |
| Analysis 2.2  Comparison 2 Effect of TFU on compliance, Outcome 2 Effect of TFU on compliance (making an appointment) in ED patients compared to usual care. | ||||
| 3 Effect of TFU on compliance (keeping an appointment) in ED patients compared to usual care Show forest plot | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.01, 2.48] |
| Analysis 2.3  Comparison 2 Effect of TFU on compliance, Outcome 3 Effect of TFU on compliance (keeping an appointment) in ED patients compared to usual care. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on knowledge in cardiac patients at around 6 weeks post discharge compared to control condition Show forest plot | 3 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 1.44 [‐0.25, 3.13] |
| Analysis 3.1  Comparison 3 Effect of TFU on knowledge in cardiac patients compared to control condition, Outcome 1 Effect of TFU on knowledge in cardiac patients at around 6 weeks post discharge compared to control condition. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on readmissions in cardiac patients compared to usual care Show forest plot | 3 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.36] |
| Analysis 4.1  Comparison 4 Effect of TFU on readmissions, Outcome 1 Effect of TFU on readmissions in cardiac patients compared to usual care. | ||||
| 2 Effect of TFU on readmissions in surgery patients compared to control condition Show forest plot | 4 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.28, 1.55] |
| Analysis 4.2  Comparison 4 Effect of TFU on readmissions, Outcome 2 Effect of TFU on readmissions in surgery patients compared to control condition. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on ED visits in surgery patients compared to control condition Show forest plot | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.53] |
| Analysis 5.1  Comparison 5 Effect of TFU on ED visits in surgery patients compared to control condition, Outcome 1 Effect of TFU on ED visits in surgery patients compared to control condition. | ||||
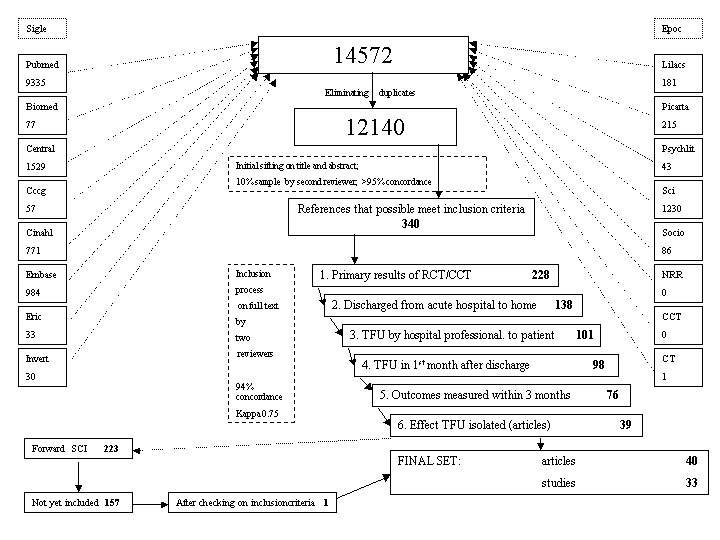
Figure 1: Inclusion Process

Comparison 1 Effect of TFU on anxiety in cardiac surgery patients at appr. 1 month after discharge compared to usual care, Outcome 1 Effect of TFU on anxiety in cardiac surgery patients at appr. 1 month after discharge compared to usual care.
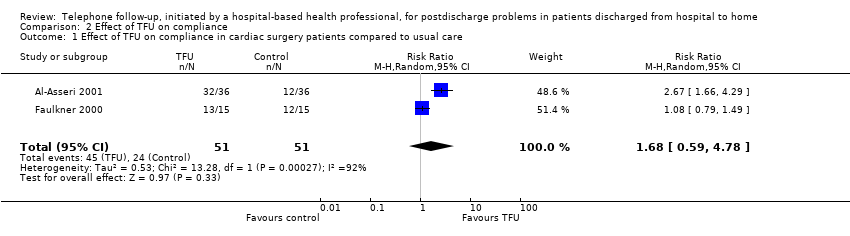
Comparison 2 Effect of TFU on compliance, Outcome 1 Effect of TFU on compliance in cardiac surgery patients compared to usual care.

Comparison 2 Effect of TFU on compliance, Outcome 2 Effect of TFU on compliance (making an appointment) in ED patients compared to usual care.

Comparison 2 Effect of TFU on compliance, Outcome 3 Effect of TFU on compliance (keeping an appointment) in ED patients compared to usual care.
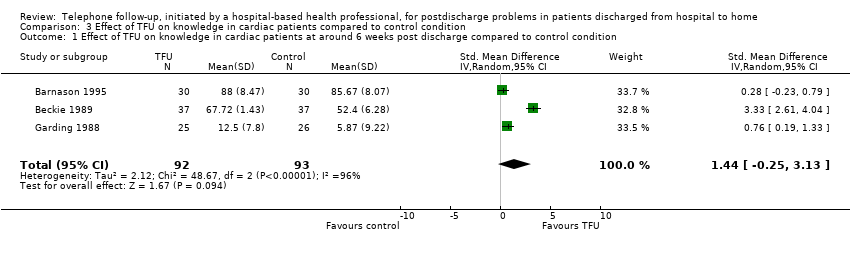
Comparison 3 Effect of TFU on knowledge in cardiac patients compared to control condition, Outcome 1 Effect of TFU on knowledge in cardiac patients at around 6 weeks post discharge compared to control condition.

Comparison 4 Effect of TFU on readmissions, Outcome 1 Effect of TFU on readmissions in cardiac patients compared to usual care.

Comparison 4 Effect of TFU on readmissions, Outcome 2 Effect of TFU on readmissions in surgery patients compared to control condition.

Comparison 5 Effect of TFU on ED visits in surgery patients compared to control condition, Outcome 1 Effect of TFU on ED visits in surgery patients compared to control condition.
| Outcome category | Cardiac patients | Surgery patients | ED patients | Paediatric patients | Neurology patients |
| PSYCHO‐SOCIAL HEALTH OUTCOMES | |||||
| ‐anxiety | 3 | 3 | |||
| ‐satisfaction | 5 | 6 | |||
| ‐depression | 2 | 2 | |||
| OTHER CONSUMER ORIENTED OUTCOMES | |||||
| ‐compliance | 2 | 2 | 4 | 3 | |
| ‐knowledge | 3 | 2 | |||
| HEALTH SERVICES ORIENTED OUTCOMES | |||||
| ‐readmissions | 4 | 5 | 2 | ||
| ‐ED‐visits | 2 | 3 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on anxiety in cardiac surgery patients at appr. 1 month after discharge compared to usual care Show forest plot | 3 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.28, 0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on compliance in cardiac surgery patients compared to usual care Show forest plot | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.59, 4.78] |
| 2 Effect of TFU on compliance (making an appointment) in ED patients compared to usual care Show forest plot | 3 | 1039 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.92, 3.14] |
| 3 Effect of TFU on compliance (keeping an appointment) in ED patients compared to usual care Show forest plot | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.01, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on knowledge in cardiac patients at around 6 weeks post discharge compared to control condition Show forest plot | 3 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 1.44 [‐0.25, 3.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on readmissions in cardiac patients compared to usual care Show forest plot | 3 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.36] |
| 2 Effect of TFU on readmissions in surgery patients compared to control condition Show forest plot | 4 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.28, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effect of TFU on ED visits in surgery patients compared to control condition Show forest plot | 2 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.85, 2.53] |

