Dodatak testosterona na hormonsku terapiju žena u menopauzi
Appendices
Appendix 1. Search string
The MEDLINE, Bio Abstract, CINAHL, and PsycINFO databases were searched using the following subject headings and keywords:
1. menopause/ or perimenopause/ or postmenopause/
2. menopaus$.mp.
3. perimenopaus$.mp.
4. postmenopaus$.mp.
5. peri‐menopaus$.mp.
6. post‐menopaus$.mp.
7. climacteric/ or menopause/
8. climacteric.mp.
9. or/1‐8
10. testosterone.mp. or exp Testosterone/
11. androgen$.mp. or exp Androgens/
12. 10 or 11
13. 9 and 12
14. randomised controlled trial.pt.
15. controlled clinical trial.pt.
16. Randomized controlled trials/
17. random allocation/
18. double‐blind method/
19. single‐blind method/
20. or/14‐19
21. clinical trial.pt.
22. exp clinical trials/
23. (clin$ adj25 trial$).ti,ab,sh.
24. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh.
25. placebos/
26. placebo$.ti,ab,sh.
27. random$.ti,ab,sh.
28. Research design/
29. or/21‐28
30. animal/ not (human/ and animal/)
31. 20 or 29
32. 31 not 30
33. 13 and 32
34. limit 33 to yr="2003"
35. limit 33 to yr="2004"
36. limit 33 to yr="2005"
37. limit 33 to yr="2006"
38. limit 33 to yr="2007"
39. limit 33 to yr="2008"
40. or/34‐39
41. from 40 keep 1‐176
The EMBASE database was searched using the following subject headings and keywords:
1. menopause.mp. or exp MENOPAUSE/ or exp MENOPAUSE RELATED DISORDER/ or exp
"MENOPAUSE AND CLIMACTERIUM"/
2. perimenopaus$.mp.
3. peri‐menopaus$.mp.
4. postmenopaus$.mp.
5. post‐menopaus$.mp.
6. menopaus$.mp.
7. or/1‐6
8. exp TESTOSTERONE/ or testosterone.mp.
9. exp ANDROGEN/ or androgen.mp. or exp ANDROGEN THERAPY/
10. androgen$.mp.
11. or/8‐10
12. 7 and 11
13. Controlled study/ or randomised controlled trial/
14. double blind procedure/
15. single blind procedure/
16. crossover procedure/
17. drug comparison/
18. placebo/
19. random$.ti,ab,hw,tn,mf.
20. latin square.ti,ab,hw,tn,mf.
21. crossover.ti,ab,hw,tn,mf.
22. cross‐over.ti,ab,hw,tn,mf.
23. placebo$.ti,ab,hw,tn,mf.
24. ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf.
25. (comparative adj5 trial$).ti,ab,hw,tn,mf.
26. (clinical adj5 trial$).ti,ab,hw,tn,mf.
27. or/13‐26
28. nonhuman/
29. animal/ not (human/ and animal/)
30. or/28‐29
31. 27 not 30
32. 12 and 31
33. 32 not men.mp. [mp=title, abstract, subject headings, heading word, drug
trade name, original title, device manufacturer, drug manufacturer name]
34. 33 not male$.mp.
35. 34 not infertil$.mp.
36. 35 not prostat$.mp.
37. 36 not review.mp. [mp=title, abstract, subject headings, heading word, drug
trade name, original title, device manufacturer, drug manufacturer name]
38. 37 not meta‐analysis.mp. [mp=title, abstract, subject headings, heading
word, drug trade name, original title, device manufacturer, drug manufacturer
name]
39. 38 not in vitro.mp. [mp=title, abstract, subject headings, heading word,
drug trade name, original title, device manufacturer, drug manufacturer name]
40. 39 not gene.mp. [mp=title, abstract, subject headings, heading word, drug
trade name, original title, device manufacturer, drug manufacturer name]
41. 40 not cross‐section$.mp.
42. limit 41 to yr="2003"
43. limit 41 to yr="2004"
44. limit 41 to yr="2005"
45. limit 41 to yr="2006"
46. limit 41 to yr="2007"
47. limit 41 to yr="2008"
48. or/43‐47
47. from 48 keep 1‐90
Appendix 2. Central search
1 menopause.mp. or exp MENOPAUSE/ or exp MENOPAUSE RELATED DISORDER/ or exp "MENOPAUSE AND CLIMACTERIUM"/ (3495)
2 perimenopaus$.mp. (211)
3 peri‐menopaus$.mp. (20)
4 postmenopaus$.mp. (5544)
5 post‐menopaus$.mp. (591)
6 menopaus$.mp. (3285)
7 or/1‐6 (7362)
8 exp TESTOSTERONE/ or testosterone.mp. (2137)
9 exp ANDROGEN/ or androgen.mp. or exp ANDROGEN THERAPY/ (1215)
10 androgen$.mp. (1727)
11 or/8‐10 (3151)
12 7 and 11 (381)
13 new.uf. (1538)
14 12 and 13 (3)
15 from 14 keep 1‐3 (3)
Appendix 3. EMBASE
1 menopause.mp. or exp MENOPAUSE/ or exp MENOPAUSE RELATED DISORDER/ or exp "MENOPAUSE AND CLIMACTERIUM"/ (42950)
2 perimenopaus$.mp. (1827)
3 peri‐menopaus$.mp. (142)
4 postmenopaus$.mp. (31390)
5 post‐menopaus$.mp. (3648)
6 menopaus$.mp. (32396)
7 or/1‐6 (56480)
8 exp TESTOSTERONE/ or testosterone.mp. (49813)
9 exp ANDROGEN/ or androgen.mp. or exp ANDROGEN THERAPY/ (76848)
10 androgen$.mp. (38802)
11 or/8‐10 (86212)
12 7 and 11 (5534)
13 Controlled study/ or randomized controlled trial/ (2293356)
14 double blind procedure/ (61834)
15 single blind procedure/ (6177)
16 crossover procedure/ (17992)
17 drug comparison/ (81250)
18 placebo/ (91809)
19 random$.ti,ab,hw,tn,mf. (350155)
20 latin square.ti,ab,hw,tn,mf. (1042)
21 crossover.ti,ab,hw,tn,mf. (31613)
22 cross‐over.ti,ab,hw,tn,mf. (11026)
23 placebo$.ti,ab,hw,tn,mf. (139476)
24 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (103309)
25 (comparative adj5 trial$).ti,ab,hw,tn,mf. (5284)
26 (clinical adj5 trial$).ti,ab,hw,tn,mf. (460069)
27 or/13‐26 (2757937)
28 nonhuman/ (2802171)
29 animal/ not (human/ and animal/) (12834)
30 or/28‐29 (2805771)
31 27 not 30 (1615851)
32 12 and 31 (2078)
33 32 not men.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1802)
34 33 not male$.mp. (1631)
35 34 not infertil$.mp. (1529)
36 35 not prostat$.mp. (1512)
37 36 not review.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1195)
38 37 not meta‐analysis.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1183)
39 38 not in vitro.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1100)
40 39 not gene.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1041)
41 40 not cross‐section$.mp. (997)
42 ("200627" or "200628" or "200629").em. (38660)
43 20063$.em. (133700)
44 ("200640" or "200641" or "200642" or "200643" or "200644" or "200645").em. (76537)
45 or/42‐44 (248897)
46 41 and 45 (36)
47 from 46 keep 1‐36 (36)
Appendix 4. MEDLINE
1 menopause/ or perimenopause/ or postmenopause/ (29597)
2 menopaus$.mp. (32496)
3 perimenopaus$.mp. (2062)
4 postmenopaus$.mp. (31923)
5 peri‐menopaus$.mp. (147)
6 post‐menopaus$.mp. (3659)
7 climacteric/ or menopause/ (21082)
8 climacteric.mp. (4416)
9 or/1‐8 (55288)
10 testosterone.mp. or exp Testosterone/ (63018)
11 androgen$.mp. or exp Androgens/ (85657)
12 10 or 11 (95782)
13 9 and 12 (3736)
14 randomized controlled trial.pt. (242141)
15 controlled clinical trial.pt. (78086)
16 Randomized controlled trials/ (50223)
17 random allocation/ (59871)
18 double‐blind method/ (93163)
19 single‐blind method/ (11128)
20 or/14‐19 (410811)
21 clinical trial.pt. (467703)
22 exp clinical trials/ (199836)
23 (clin$ adj25 trial$).ti,ab,sh. (133967)
24 ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh. (91805)
25 placebos/ (26399)
26 placebo$.ti,ab,sh. (116344)
27 random$.ti,ab,sh. (503850)
28 Research design/ (47173)
29 or/21‐28 (897919)
30 animal/ not (human/ and animal/) (3146512)
31 20 or 29 (904514)
32 31 not 30 (831335)
33 13 and 32 (782)
34 200607$.ed. (48685)
35 200608$.ed. (63404)
36 200609$.ed. (55606)
37 200610$.ed. (54157)
38 200611$.ed. (18375)
39 or/34‐38 (240227)
40 33 and 39 (24)
41 from 40 keep 1‐24 (24)

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
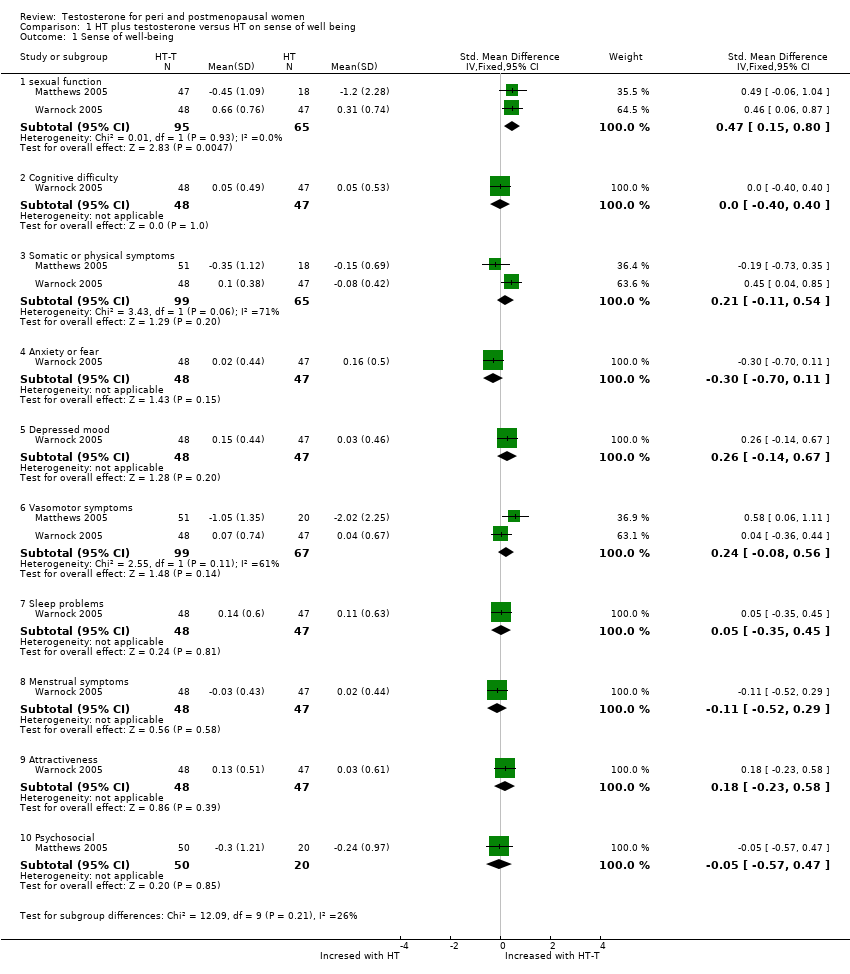
Comparison 1 HT plus testosterone versus HT on sense of well being, Outcome 1 Sense of well‐being.

Comparison 2 HT plus testosterone versus HT on sexual function, Outcome 1 Change scores of sexual function.
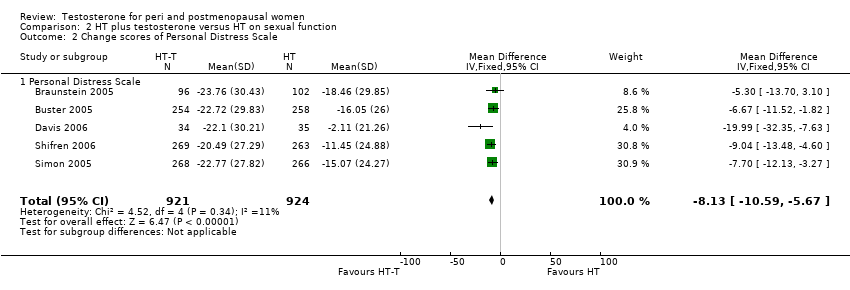
Comparison 2 HT plus testosterone versus HT on sexual function, Outcome 2 Change scores of Personal Distress Scale.

Comparison 3 HT plus testosterone versus HT on bone mineral density, Outcome 1 Lumbar BMDs at 12 months.
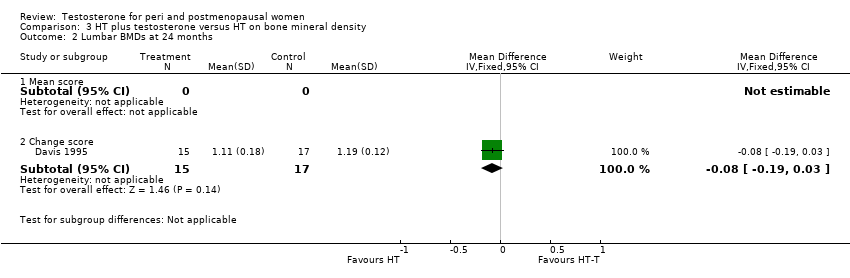
Comparison 3 HT plus testosterone versus HT on bone mineral density, Outcome 2 Lumbar BMDs at 24 months.

Comparison 3 HT plus testosterone versus HT on bone mineral density, Outcome 3 Femur BMDs at 12 months.
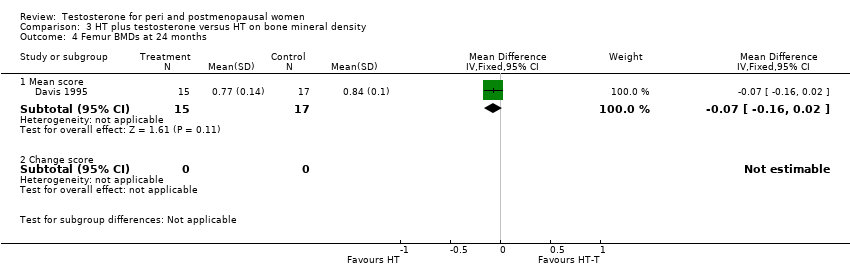
Comparison 3 HT plus testosterone versus HT on bone mineral density, Outcome 4 Femur BMDs at 24 months.
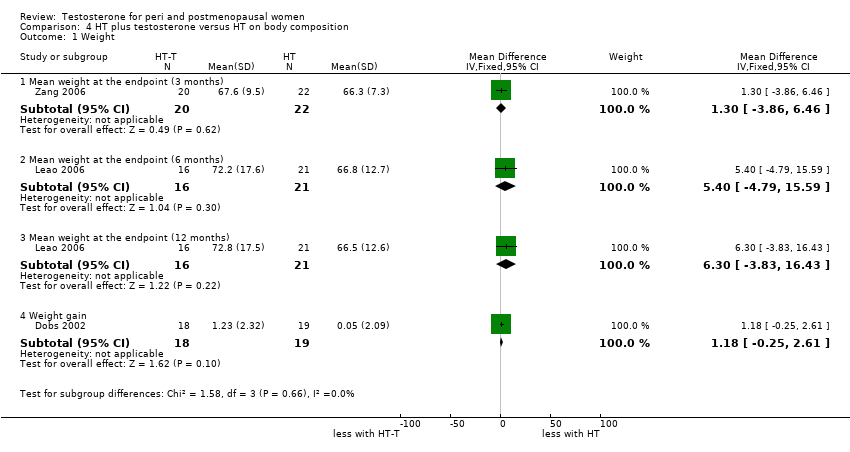
Comparison 4 HT plus testosterone versus HT on body composition, Outcome 1 Weight.
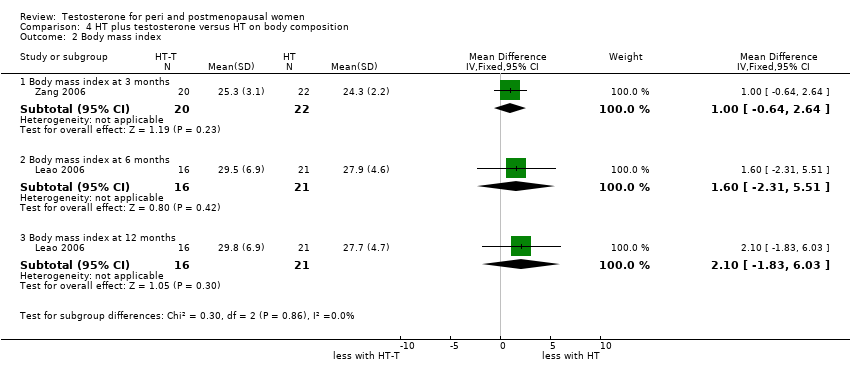
Comparison 4 HT plus testosterone versus HT on body composition, Outcome 2 Body mass index.
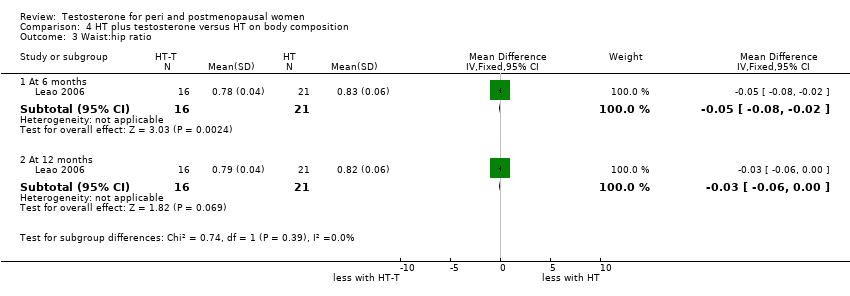
Comparison 4 HT plus testosterone versus HT on body composition, Outcome 3 Waist:hip ratio.
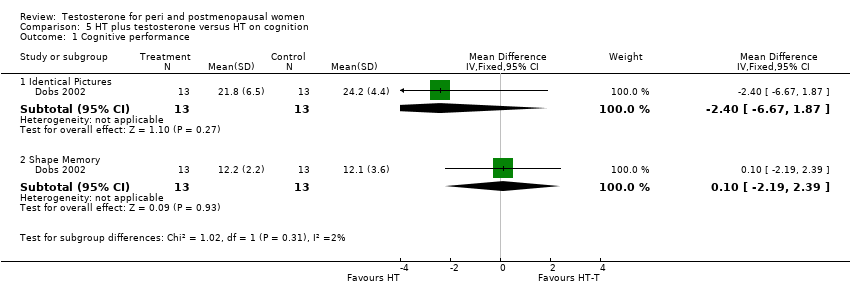
Comparison 5 HT plus testosterone versus HT on cognition, Outcome 1 Cognitive performance.

Comparison 5 HT plus testosterone versus HT on cognition, Outcome 2 Cognition difficulty.

Comparison 6 HT plus testosterone versus HT on menopausal symptoms, Outcome 1 Vasomotor symptom.
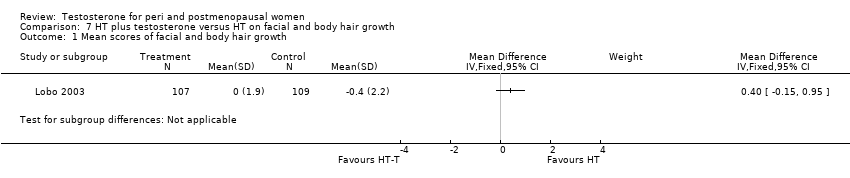
Comparison 7 HT plus testosterone versus HT on facial and body hair growth, Outcome 1 Mean scores of facial and body hair growth.
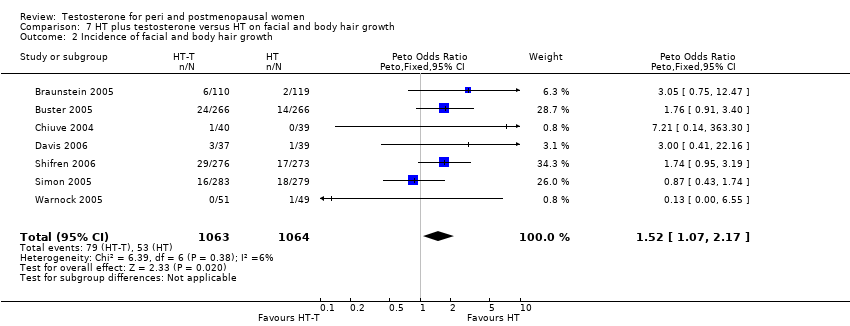
Comparison 7 HT plus testosterone versus HT on facial and body hair growth, Outcome 2 Incidence of facial and body hair growth.
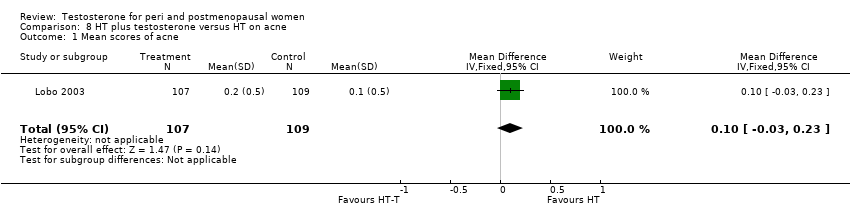
Comparison 8 HT plus testosterone versus HT on acne, Outcome 1 Mean scores of acne.

Comparison 8 HT plus testosterone versus HT on acne, Outcome 2 Incidence of acne.
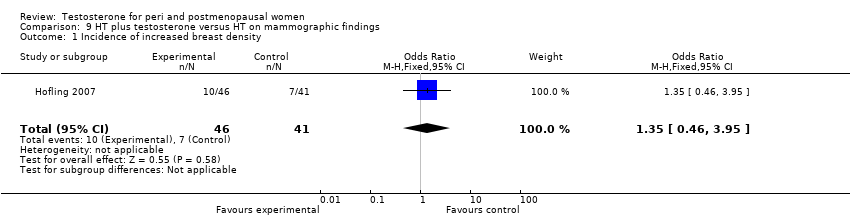
Comparison 9 HT plus testosterone versus HT on mammographic findings, Outcome 1 Incidence of increased breast density.
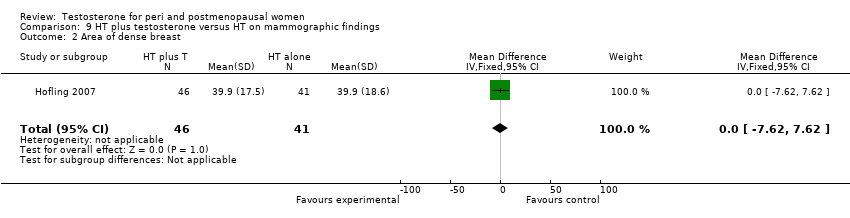
Comparison 9 HT plus testosterone versus HT on mammographic findings, Outcome 2 Area of dense breast.
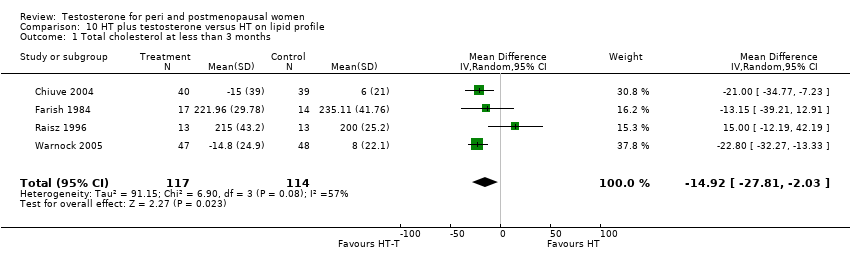
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 1 Total cholesterol at less than 3 months.
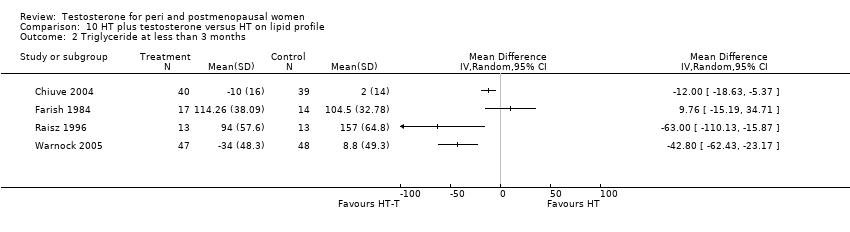
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 2 Triglyceride at less than 3 months.
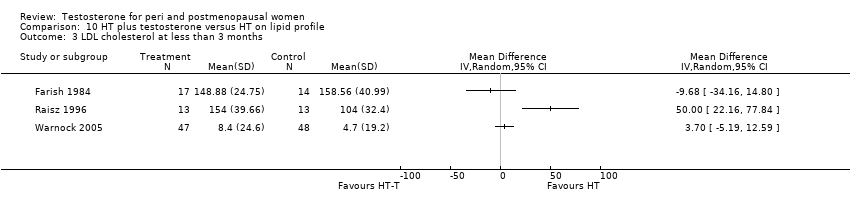
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 3 LDL cholesterol at less than 3 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 4 HDL cholesterol at less than 3 months.
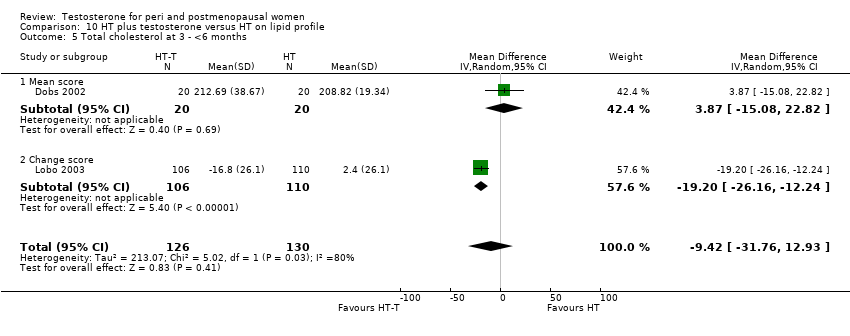
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 5 Total cholesterol at 3 ‐ <6 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 6 Triglyceride at 3 ‐ <6 months.
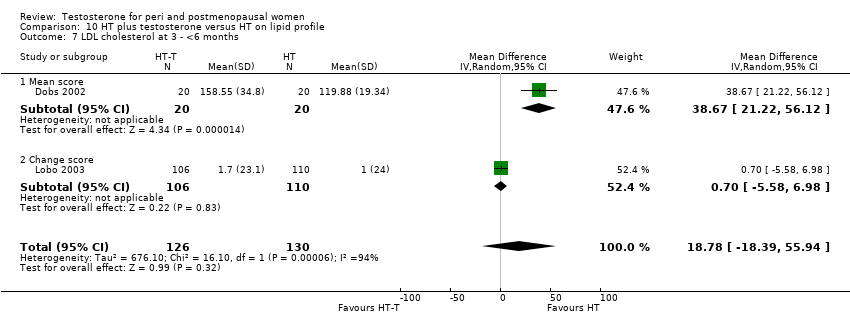
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 7 LDL cholesterol at 3 ‐ <6 months.
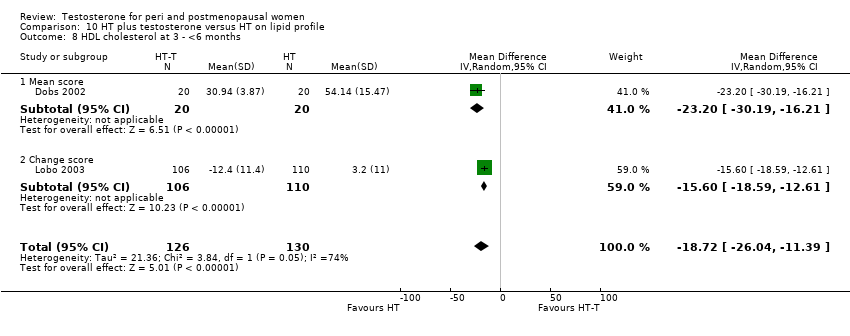
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 8 HDL cholesterol at 3 ‐ <6 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 10 Total cholesterol at 6 ‐ <12 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 11 Triglyceride at 6 ‐ <12 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 12 LDL cholesterol at 6 ‐ <12 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 13 HDL cholesterol at 6 ‐ <12 months.
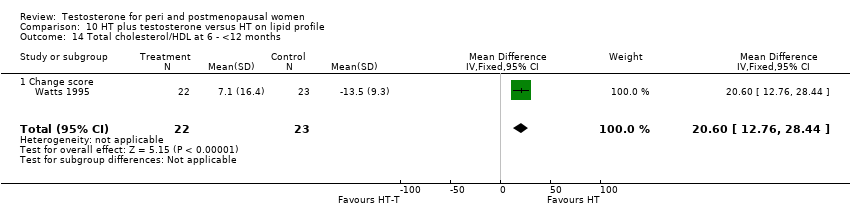
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 14 Total cholesterol/HDL at 6 ‐ <12 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 15 Total cholesterol at 12 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 16 Triglyceride at 12 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 17 LDL cholesterol at 12 months.
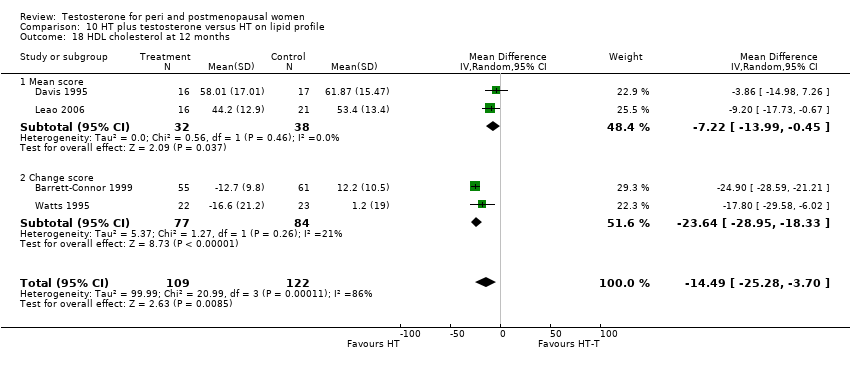
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 18 HDL cholesterol at 12 months.
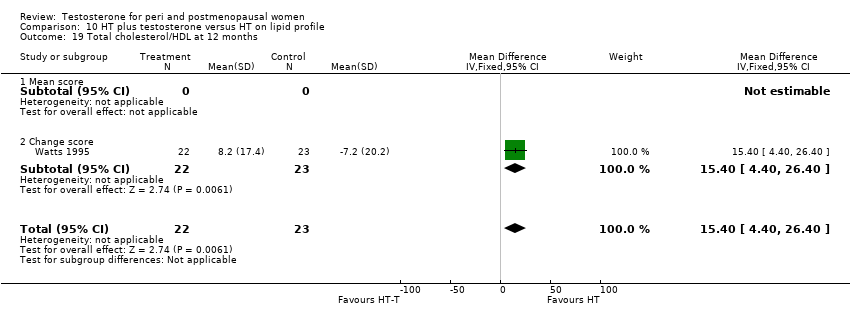
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 19 Total cholesterol/HDL at 12 months.
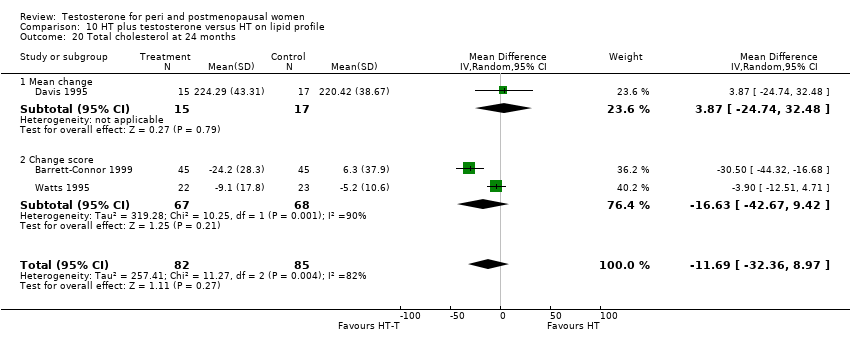
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 20 Total cholesterol at 24 months.
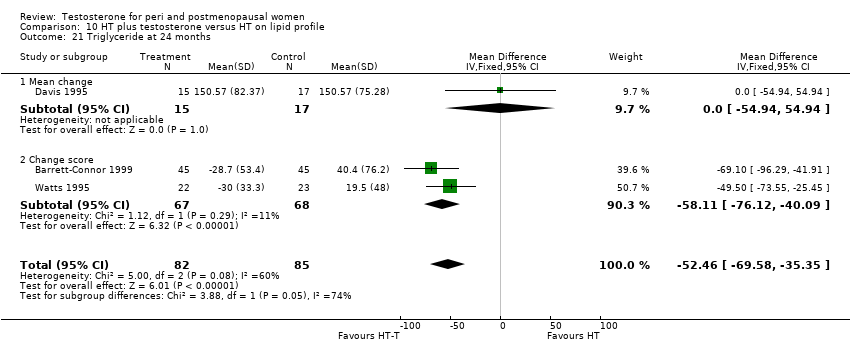
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 21 Triglyceride at 24 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 22 LDL cholesterol at 24 months.

Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 23 HDL cholesterol at 24 months.
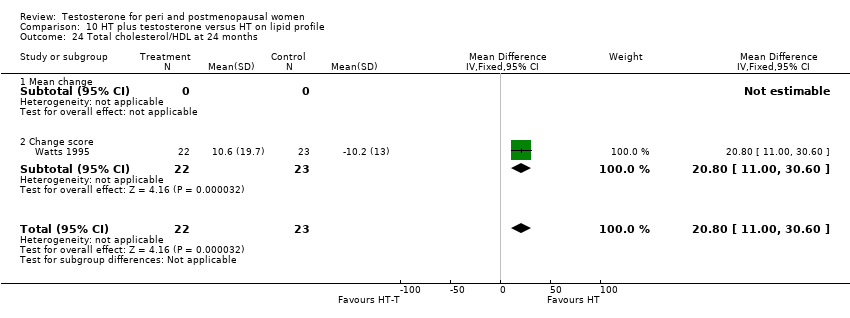
Comparison 10 HT plus testosterone versus HT on lipid profile, Outcome 24 Total cholesterol/HDL at 24 months.
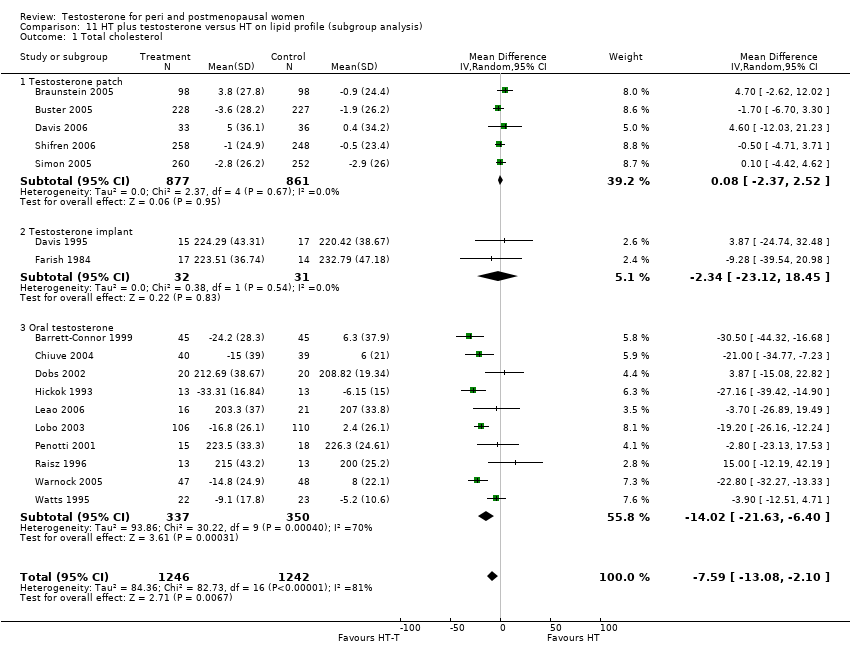
Comparison 11 HT plus testosterone versus HT on lipid profile (subgroup analysis), Outcome 1 Total cholesterol.

Comparison 11 HT plus testosterone versus HT on lipid profile (subgroup analysis), Outcome 2 HDL cholesterol.
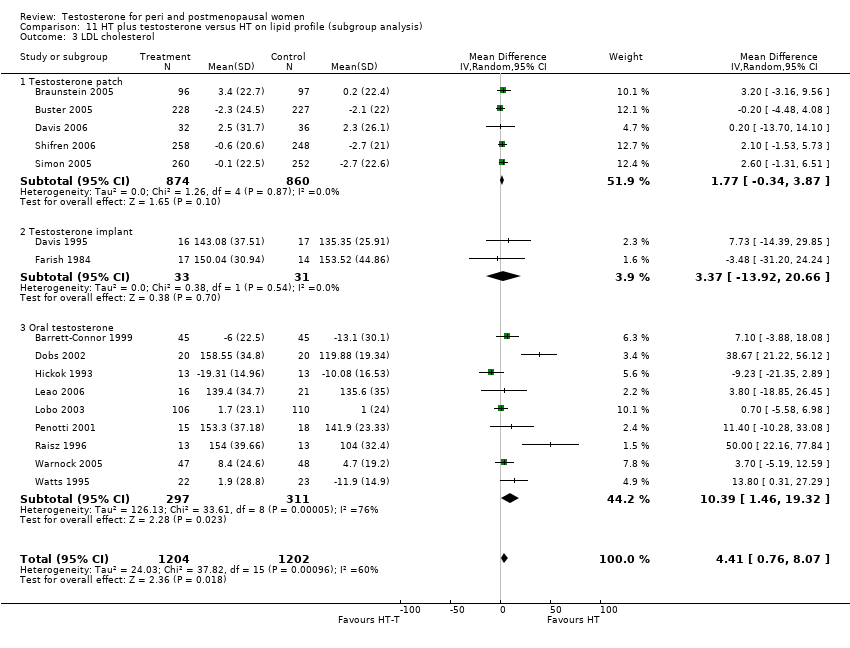
Comparison 11 HT plus testosterone versus HT on lipid profile (subgroup analysis), Outcome 3 LDL cholesterol.

Comparison 11 HT plus testosterone versus HT on lipid profile (subgroup analysis), Outcome 4 Triglyceride.
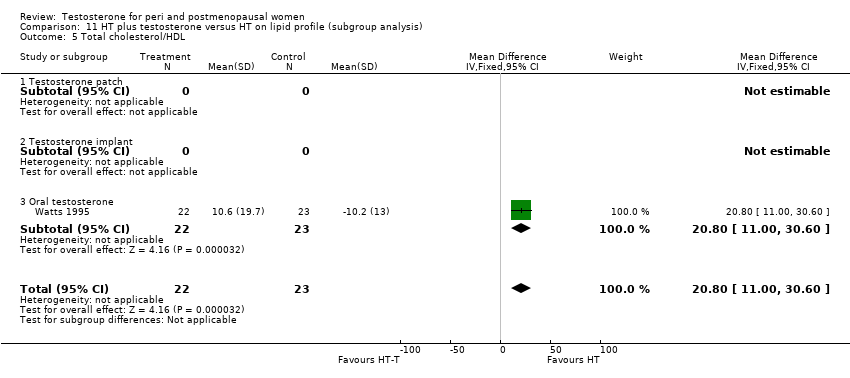
Comparison 11 HT plus testosterone versus HT on lipid profile (subgroup analysis), Outcome 5 Total cholesterol/HDL.

Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 1 Discontinuation rate (overall).
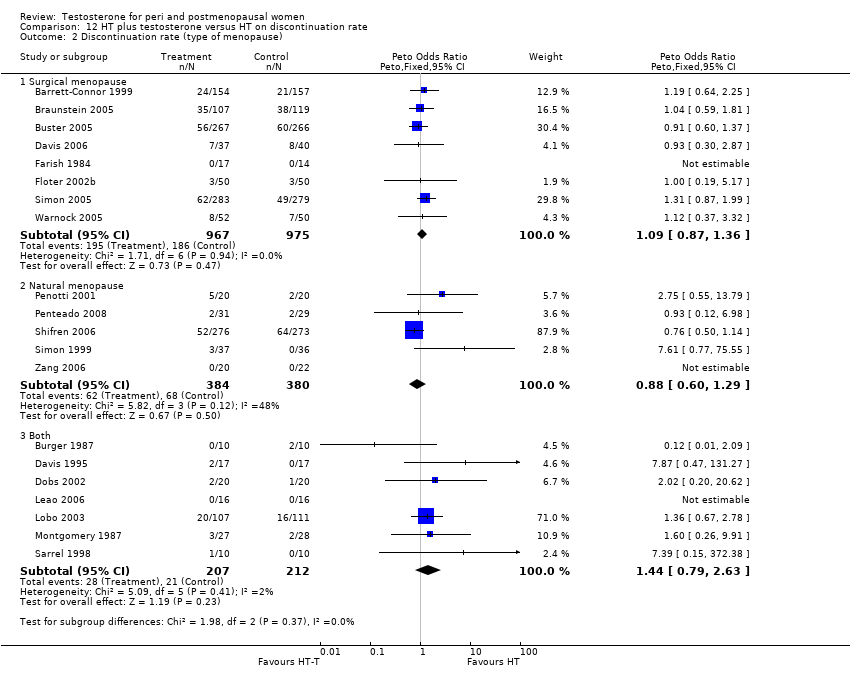
Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 2 Discontinuation rate (type of menopause).

Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 3 Discontinuation rate (menopausal status).

Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 4 Discontinuation rate (route of hormone therapy).
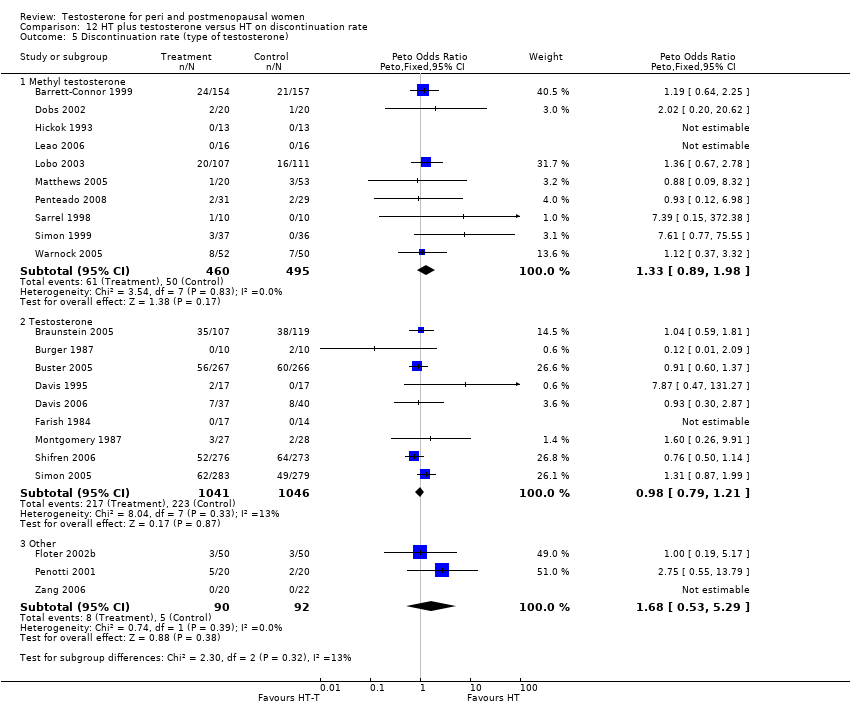
Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 5 Discontinuation rate (type of testosterone).
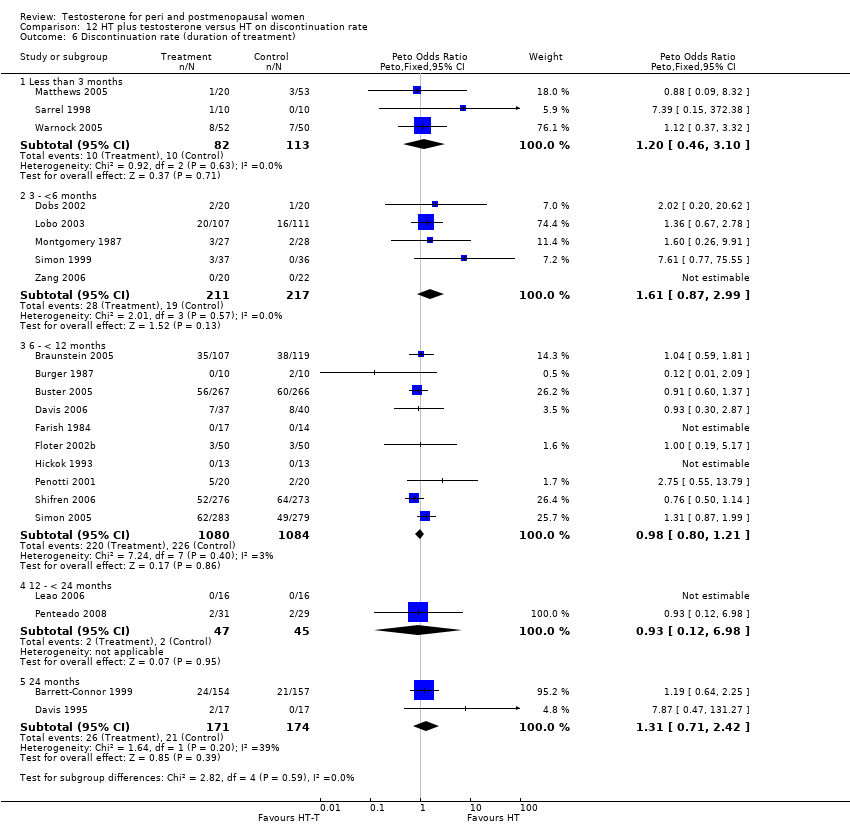
Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 6 Discontinuation rate (duration of treatment).

Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 7 Discontinuation rate (blinding).

Comparison 12 HT plus testosterone versus HT on discontinuation rate, Outcome 8 Discontinuation rate (disease status).
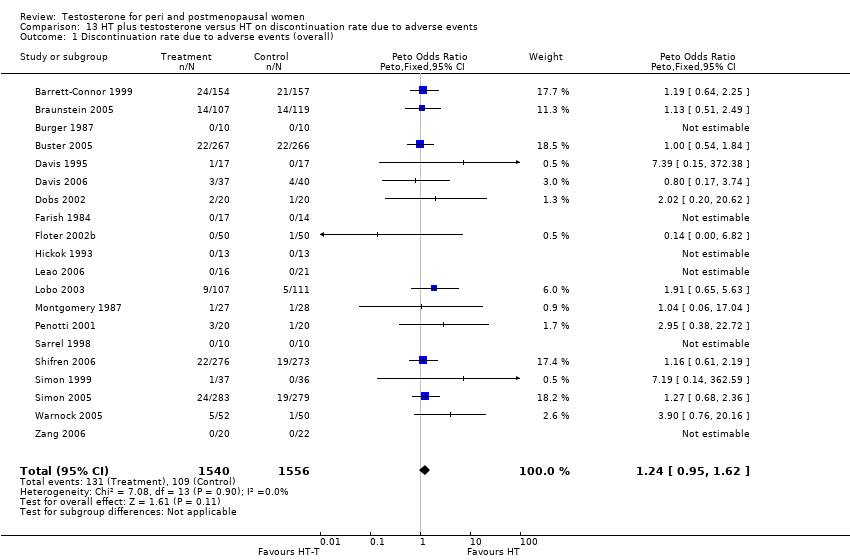
Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 1 Discontinuation rate due to adverse events (overall).

Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 2 Discontinuation rate due to adverse events (type of menopause).

Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 3 Discontinuation rate due to adverse events (menopausal status).

Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 4 Discontinuation rate due to adverse events (route of hormone therapy).

Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 5 Discontinuation rate due to adverse events (type of testosterone).

Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 6 Discontinuation rate due to adverse events (duration of treatment).

Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 7 Discontinuation rate due to adverse events (blinding).
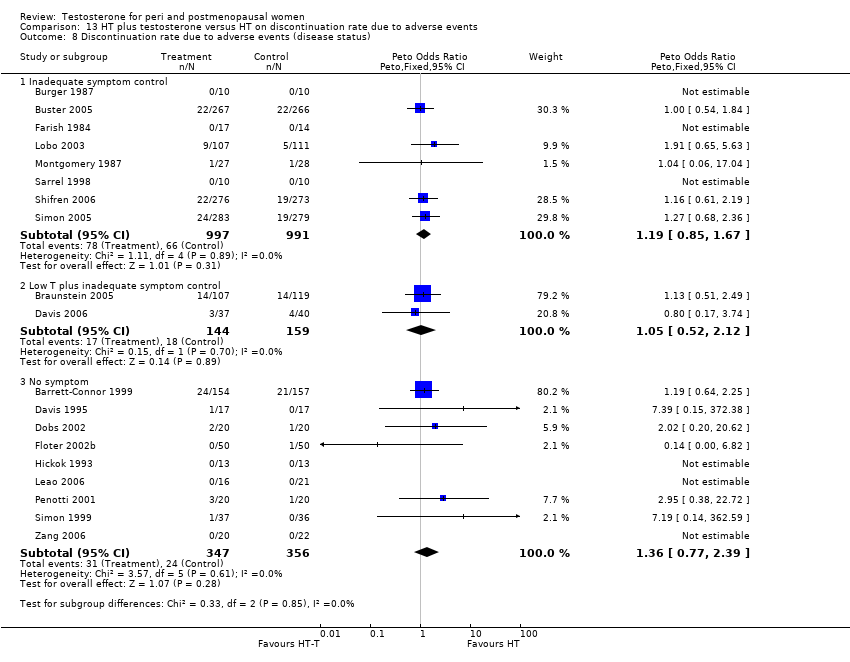
Comparison 13 HT plus testosterone versus HT on discontinuation rate due to adverse events, Outcome 8 Discontinuation rate due to adverse events (disease status).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 1 Discontinuation rate (allocation quality).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 2 Discontinuation rate due to adverse events (allocation quality).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 3 Discontinuation rate (quality of randomisation)).
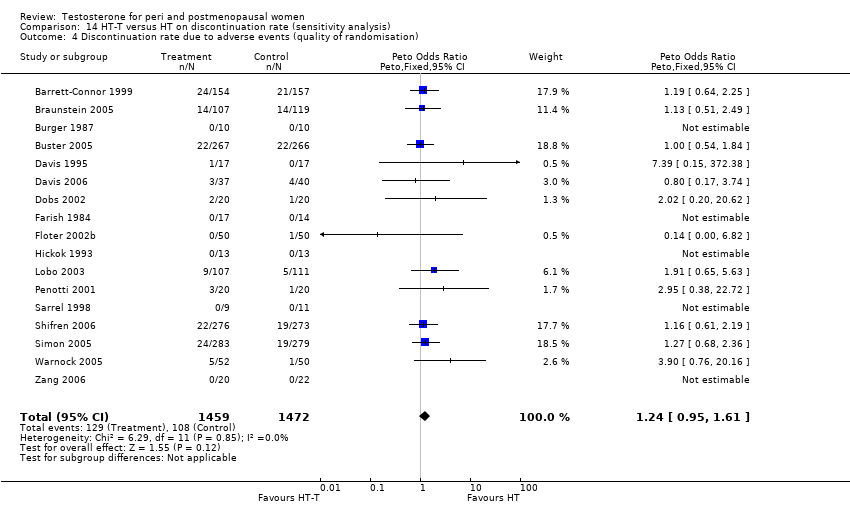
Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 4 Discontinuation rate due to adverse events (quality of randomisation).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 5 Discontinuation rate (blinding method).
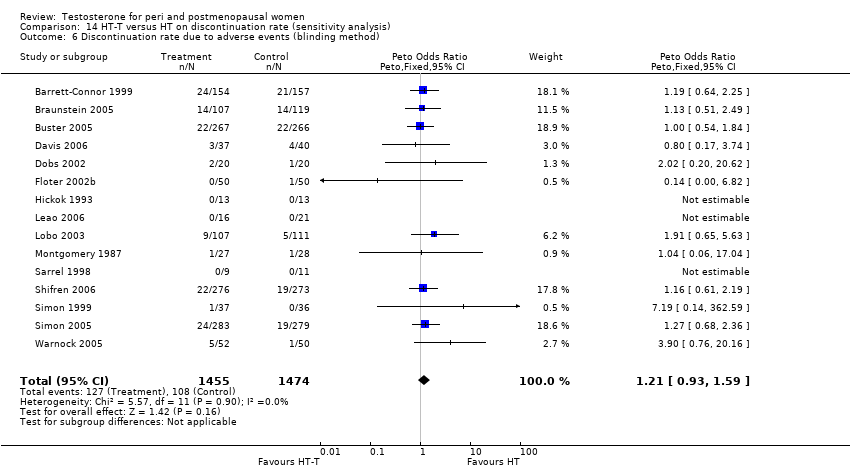
Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 6 Discontinuation rate due to adverse events (blinding method).
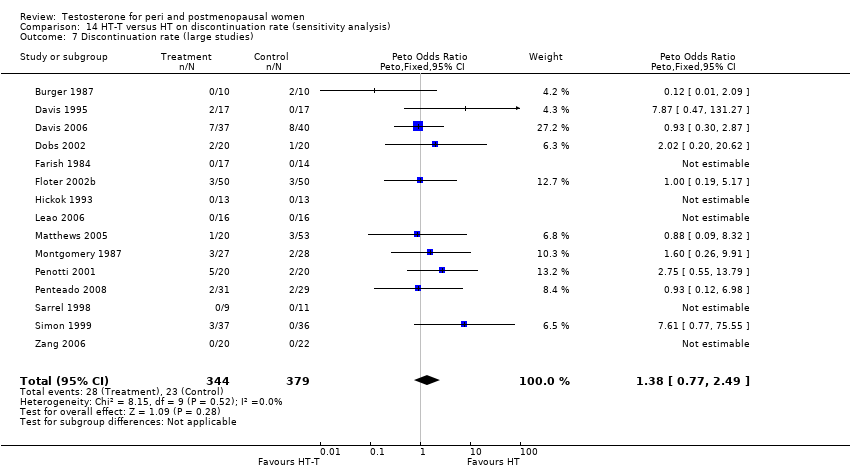
Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 7 Discontinuation rate (large studies).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 8 Discontinuation rate due to adverse events (large studies).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 9 Discontinuation rate (methyl testosterone doses).
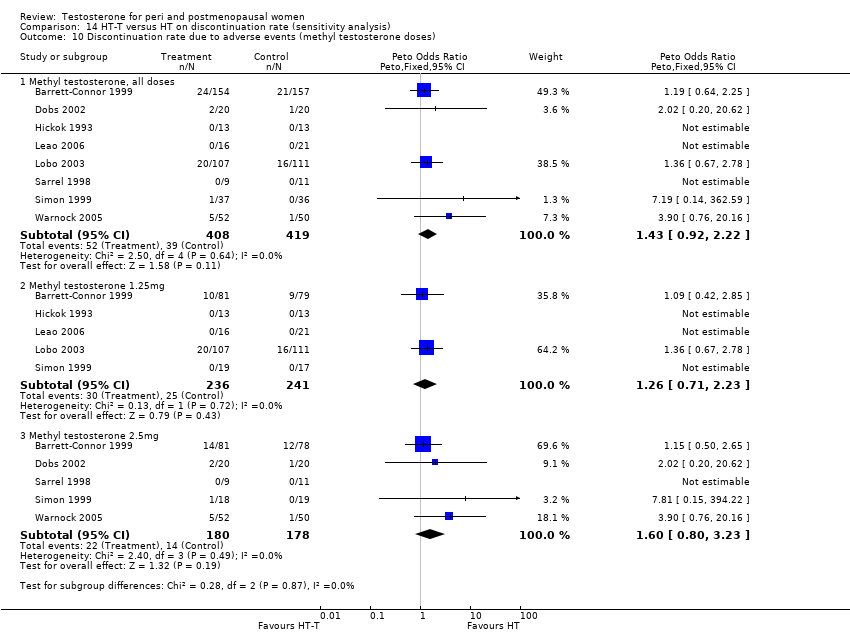
Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 10 Discontinuation rate due to adverse events (methyl testosterone doses).
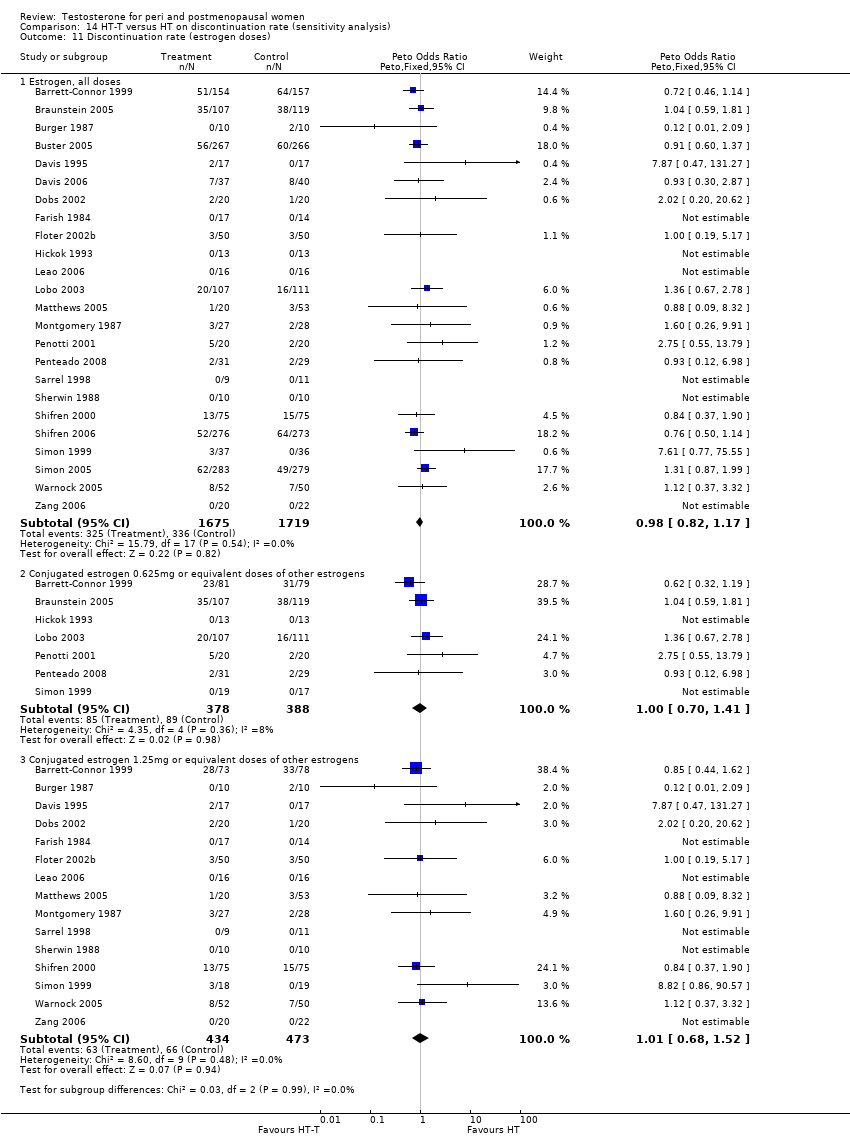
Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 11 Discontinuation rate (estrogen doses).

Comparison 14 HT‐T versus HT on discontinuation rate (sensitivity analysis), Outcome 12 Discontinuation rate due to adverse events (estrogen doses).
| Outcome | Study ID | N | Reason | Conclusion |
| Acne | 291 | The data was not available | Acne of mild or moderate severity was reported by 5 (3%) estrogen‐testosterone treated participants, whereas no participants receiving oestrogen reported acne | |
| Biochemical Markers of bone metabolism | 50 | A crossover study with no washout period | Both treatments had similar effects, with a significant decrease in bone resorption (ICTP) and bone turnover (osteocalcin) after 24 weeks. | |
| Biochemical markers of bone metabolism | 57 | The data was likely to be skewed because the means were smaller than twice the SDs | There were no between group differences noted in baseline Dpd levels(p=0.111), Dpd% change (P=0.338), baseline NTx levels (P=0.112), or NTx % change (P=0.271) | |
| Biochemical markers of bone metabolism | 28 | The data was not available | The effects of oestrogen‐testosterone and oestrogen alone on markers of bone resorption were generally similar. The increase in bone formation markers after oestrogen‐testosterone treatment was significantly different from the effect of oestrogen for all bone formation parameters. | |
| Bone mineral density of lumbar spine and femur | 199 | The data was not available | BMD increased in the estrogen‐testosterone(low dose) were comparable to those in the oestrogen(low dose) group, while the BMD changes at 24 months in the estrogen‐testosterone(high dose) group significantly exceeded those in oestrogen(high dose) group(P=0.014 for lumbar spine, BMD and P=0.009 for total hip BMD) | |
| Bone mineral density | 50 | A crossover study with no washout period. | No changes in BMD were noted in the total body, hip, or lumbar spine with either regimen | |
| Bone mineral density | 50 | The data was not available. | There was no significant differences in bone density at any of the sites measured between women receiving oestrogen alone and those receiving estrogen‐testosterone. No treated subjects had a significant bone loss(more than twice the measurement precision) at either spine or femoral neck at 1 year, but three in each treated group showed a small but non significant decrease at both sites | |
| Bone mineral density of L1‐L4, femur and forearm | 48 | The data was not available | The estrogen‐testosterone showed significant increases in spinal BMD at 12 and 24 months(P<0.01). The estrogen group demonstrated a non significant increase in spinal BMD. The difference between groups was not significant at 12 or 24 months. There were no significant changes in BMD from baseline in either group at the radius, femoral neck, Ward triangle, or greater trochanter | |
| Body composition | 40 | It was unclear with regard to the standard deviation (SD) of the data | ‐ When compared with oestrogen alone, estrogen‐testosterone treatment significantly increased lean body mass in the arms, legs, and trunk. Body fat percentage decreased significantly from baseline in the same arms, legs, and trunk in the oestrogen‐testosterone group but not the oestrogen alone group. When changes in arms, legs, and trunk in each participant were analysed simultaneously, the difference between treatments was significant for lean body mass(P=0.007) and percentage of fat tissue(P=0.077) | |
| Body composition | 50 | A crossover study with no washout period | There was no significant differences in total body fat, total lean body mass, trunk fat, and trunk lean mass between the two treatments | |
| Body composition | 37 | The data was likely to be skewed | When compared to HT alone, T‐HT treatment significantly increased visceral fat area (P = 0.009). However there was no significant difference in subcutaneous fat area between the two groups | |
| Cognition and psychological well being | 42 | A crossover study with no washout period | Switching Attention Test that mean reaction time in the switching condition was faster in the estrogen‐testosterone group than in the estrogen group(t=3.25, df=37, P<0.002, effect size = 0.53 SD). For other conditions of the same test, such as side condition and direction condition, they did not differ between two groups. | |
| Cognition | 49 | The data was not available | There was no comparative effects between oestrogen‐testosterone and oestrogen alone group. | |
| Cognition | 30 | The data was likely to be skewed | No significant interactions were found showing an advantage for oestrogen‐testosterone treated group as contrasted to oestrogen‐treated group | |
| Cognition (Cube Comparisons and Building Memory) | 26 | The data was likely to be skewed | Differences in task performance between women receiving E or E‐T treatment were assessed with a 2‐factor(treatment group x test session), mixed analysis of variance for each cognitive task. Post hoc comparisons were conducted using Tukey's method of multiple comparisons. With regard to Cube Comparisons, performance improved for both groups across test sessions, however this improvement only approached statistical significance (P=0.09). No other effects were significant. Regarding Building Memory, a main effect of test session was observed, with performance declining across sessions for both groups(P<0.01). A treatment x test session interaction was observed(P<0.05). Post hoc comparison revealed that this effect was due to a decrease in the E group(P<0.05) but not The E‐T group(P>0.1) across sessions. | |
| Hematocrit | 199 | The data was not available. | There was no clinically significant difference in haematology | |
| Hematocrit | 50 | A cross‐over study with no washout period | They reported that there was no change in blood counts during the study | |
| Hematocrit | 26 | The data was not available. | ‐ At 6 months, statistically significant between‐group differences were seen for hematocrit. The difference was small in magnitude, remained within the normal ranges, and was not considered clinically significant. | |
| Hematocrit | 67 | A cross‐over study with no washout period | Transdermal testosterone treatment had no significant effects on blood counts | |
| Hematocrit | 48 | The data were not available | No clinically significant changes in hematologic indices | |
| Hirsutism | 199 | The data was not available | Changes in hair growth in the oestrogen‐testosterone(low dose) group were similar to those in the oestrogen(low dose) group, and there were no statistically significant differences in the hirsutism scores between the treatment groups. In the high‐dose groups only four participants treated with oestrogen‐testosterone and two treated with oestrogen reported hirsutism as an adverse event at month 12. At 24 months, 10 oestrogen‐testosterone‐treated and 3 oestrogen‐treated participants reported hirsutism as an adverse event | |
| Hirsutism and acne | 50 | A crossover study with no washout period | Incidences of hirsutism and acne were similar in two treatment groups | |
| Hirsutism and acne | 67 | A crossover study with no washout period | The hirsutism and acne scores did not change significantly during treatment. The mean facial depilation rate increased slightly during treatment with estrogen‐testosterone 300 microgram | |
| Lipid profile | 40 | The data was not available. | After 16 weeks of treatment, significant decreases in total cholesterol, HDL, and triglycerides occurred in the estrogen‐testosterone group. LDL values were virtually unchanged. The oestrogen group demonstrated the opposite effect on lipids, with a significant decrease in LDL and no meaningful change in the other lipid parameters | |
| Lipid profile | 50 | A crossover study with no washout period | Serum levels of total testosterone increased markedly from a baseline mean of 0.8–4.9 mmol/l during testosterone addition. Total and LDL‐cholesterol levels were significantly reduced by both treatments as also were those of Lp‐(a) although the difference was not significant. A 13% reduction in HDL‐cholesterol levels was found when testosterone was added, but no change with oestrogen alone. Triglyceride levels were increased by oestrogen treatment, but not affected by the combination of oestrogen plus testosterone | |
| Lipid profile | 56 | The data was not available | There were significant reductions in total cholesterol and LDL cholesterol in all groups. In estrogen‐testosterone‐treated group triglyceride levels increased 26.0% and HDL cholesterol levels decreased 9.0%. In contrast, with oestrogen therapy triglyceride levels decreased 9.0% and HDL cholesterol levels increased 9.0% | |
| Lipid profile | 57 | The data was likely to be skewed because the means were smaller than twice the SDs | The study found significant reductions in total cholesterol and LDL cholesterol in all groups. Triglyceride levels increased 26.0% and HDL cholesterol levels decreased 9.0% in estrogen‐testosterone‐treated group. In contrast, with oestrogens therapy triglyceride levels decreased 9.0% and HDL cholesterol levels increased 9.0% | |
| Lipid profile | 60 | A crossover study with no washout period | Total cholesterol, triglycerides, HDL and LDL revealed no significant differences between any of the periods or groups | |
| Menopausal symptoms, sense of well being and sexual function | 199 | The data were not available | Women in all treatment groups reported an improvement in menopausal symptoms and quality of life measures at 24 months. There was a non significant trend toward greater improvement in well being and sexual interest and higher scores on the modified menopausal rating scale in the oestrogen‐testosterone groups | |
| Menopausal symptoms and sexual function | 40 | The data were non‐normal distribution | There were no significant differences between treatments on any variable at either 2 months or 6 months after treatment | |
| Menopausal symptoms | 26 | The data were non‐normal distribution | There was no statistically significant difference between two treatments in menopausal symptoms | |
| Menopausal symptoms | 51 | The data were not available | Vasomotor symptoms were reduced by at least 75% after treatment in all groups | |
| Menopausal symptoms | 28 | The data was likely to be skewed | Both treatments significantly decreased somatic symptom scores, but only estrogen‐testosterone treatment provided significant relief of psychosomatic and psychological symptoms | |
| Menopausal symptoms | 20 | The data was not available | There was no statistical difference between the estrogen‐testosterone groups versus the oestrogen group | |
| Menopausal symptoms | 49 | The data was not available | There was no result for the comparative effect on hot flushes between estrogen‐testosterone and oestrogen alone | |
| Menopausal symptoms | 43 | The data were not available | Menopausal index: | |
| Menopausal symptoms | 92 | The data was not available | In general, estrogen‐testosterone therapy provided greater relief from these symptoms than oestrogen therapy. This was most apparent in the finding that the degree of vasomotor symptom relief with low dose estrogen‐testosterone preparation was similar to relief experienced with higher dose estrogen therapy alone. | |
| Menopausal symptoms | 66 | The data were not available | There were no significant differences in somatic symptoms between the oestrogen and estrogen‐testosterone groups at baseline or after treatment. Psychosomatic and psychologic symptom values are not presented because of the small number of evaluable symptomatic participants | |
| Mood (hostility) | 36 | The data were not available | Hostility scores did not differ significantly in the two groups (testosterone‐oestrogen or oestrogen alone) | |
| Sense of well being | 40 | The data were not available. | With regard to QUALMS questionnaire, the oestrogen‐testosterone group showed significant improvement from baseline in somatic symptoms(week 10, P=0.003; week 16, P=0.073). The oestrogen group showed significant improvement from baseline in well being (week 16, P= 0.049) and cognition (week 10, P=0.054) | |
| Sense of well being | 50 | A crossover study with no washout period | There were no significant differences between the treatments in any of the sub scores or total PGWB index | |
| Sense of well being | 84 | The data were likely to be skewed | There was no difference in SRD 30 scores between the two active treatment groups at either 2 or 4 months | |
| Sense of well being | 40 | The data were not available. | No conclusion on psycho‐physical well being. | |
| Sense of well being | 35 | A cross‐over study with no washout period | No significant effects of adding testosterone into hormone therapy | |
| Sense of well being | 43 | The data was not available. | Anxiety: There was no differences among any of the groups across time. | |
| Sense of well being | 65 | A crossover study with no washout period | Adding 300 microgram patch into oral oestrogen has a significant improvement in general well being by means of PGWB (P=0.04). There also were significant increases with oestrogen‐testosterone 300 microgram treatment for sub scales of positive well being and depressed mood. | |
| Sexual function | 20 | The data was not available. | After six weeks the loss of libido in the single implant group remained, while the combined group showed significant symptomatic relief(P<0.01). Eight in the single implant group chose to have a testosterone implant at the first follow up visit at 6 weeks; the other two stopped coming because of dissatisfaction with the treatment | |
| Sexual function | 40 | The data was not available. | The sample size was not powered, nor was entry criteria designed to assess sexual dysfunction parameters; however, there were significant results. In the oestrogen‐testosterone group, BISF‐W mean increases at each visit were statistically significant for frequency/psychosexual(P=0.05) and pleasure/orgasm(P=0.041) domains. The mean composite BISF‐W score increased in the oestrogen‐testosterone group, whereas the mean score in the estrogen group decreased. Although it appeared that the two treatment groups were not well balanced at baseline(the estrogen group seemed to have healthier sexual function at baseline than the estrogen‐testosterone group), the estrogen‐testosterone group showed significant improvement in sexual function compared with the estrogen group. | |
| Sexual function (total McCoy score) | 44 | A crossover study with no washout period | After 24 weeks of treatment, the addition of testosterone had a significantly better effect on the variables 'enjoyment of sex', 'satisfaction with frequency of sexual activity' and 'interest in sex'. The total McCoy score was significantly increased by both treatments, but the addition of testosterone exerted a stronger effect (P<0.05) | |
| Sexual function | 51 | The data was not available | Improvement (P<0.05) in sexual interest, sexual satisfaction, frequency of sexual intercourse and intensity and frequency of orgasm during sexual intercourse were reported in all groups except the estrogen alone group | |
| Sexual function | 60 | A cross‐over study with no washout period | The scores concerning frequency of sexual activity, orgasm and intercourse, sexual arousal, fantasies and enjoyment, satisfaction with orgasms, and interest in sex were all significantly improved for testosterone addition as compared to placebo both before and after crossover | |
| Sexual function(desire and satisfaction) | 33 | The data was not available | No difference between two groups was observed at any of the considered time points. | |
| Sexual function | 30 | The data was likely to be skewed | Oestrogen‐testosterone‐treated participants reported significantly less lack of sexual desire or interest to engage in sexual activity, compared to participants receiving oestrogen alone | |
| Sexual function | 43 | The data was not available | Women who received either of the androgen‐containing preparations had significantly higher scores than women in the estrogen and placebo groups(P<0.01) in association with their higher levels of plasma testosterone. Women in the estrogen‐testosterone and testosterone‐only group experienced a greater number of fantasies during every treatment than did women in the oestrogen and placebo group (P<0.01). During treatment phases, both androgen groups attained higher levels of sexual arousal than did the estrogen and placebo groups(P<0.01) | |
| Sexual function (scores) | 65 | A cross‐over study with no washout period | The mean composite score expressed as a percentage of the mean value for normal women, increased from 52(27) percent at baseline to 72(38) percent during estrogen treatment, 74(37) percent during treatment with estrogen plus 150 microgram of testosterone per day, and 81(37) percent during treatment with estrogen plus 300 microgram of testosterone per day(P=0.05 for the comparison with estrogen‐alone). The scores for thoughts‐desire, frequency of sexual activity, and pleasure‐orgasm were lowest at baseline and increased in a dose‐dependent fashion. With the estrogen plus testosterone 300 microgram, the increases in scores for frequency of sexual activity and pleasure‐orgasm were significantly greater than those with estrogen‐alone (P=0.03 for both comparisons). The score for problems affecting sexual function was 116%(48) of the normative mean at baseline and decreased to 98%(49) during treatment with estrogen plus 300 microgram of testosterone(P=0.07 for the comparison with oestrogen‐alone) | |
| Sexual function (the prevalence of particular types of sexual behavior) | 65 | A crossover study with no washout period | The percentage of women who reported having sexual fantasies at least once a week was 12% at baseline, 10% during oestrogen treatment, 18 percent during estrogen plus testosterone 150 microgram, and 24% during treatment with estrogen plus 300 microgram of testosterone. The percentage of women who reported masturbating at least once a week was 3%, 5% and 10% at baseline, estrogen treatment and estrogen plus testosterone treatment, respectively. Finally, the percentage of women who engaged in sexual intercourse at least once a week was 23% at baseline, 35% during treatment with either oestrogen‐alone or oestrogen plus 150 microgram of testosterone, and 41% during treatment with oestrogen plus 300 microgram of testosterone | |
| Unexplained fatigue (vitality) | 50 | A crossover study with no washout period | There was no significant difference between the treatments in vitality | |
| Unexplained fatigue (vitality) | 67 | A crossover study with no washout period | Vitality improved in women treated with testosterone patch combined with oral conjugated equine oestrogen | |
| Unexplained fatigue and sense of well being | 43 | The data was not available | Women in estrogen alone and placebo groups reported significantly lower ratings of energy level and well being than did those who received either of the androgen‐containing preparations (P<0.01) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sense of well‐being Show forest plot | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 sexual function | 2 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.15, 0.80] |
| 1.2 Cognitive difficulty | 1 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.40, 0.40] |
| 1.3 Somatic or physical symptoms | 2 | 164 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.11, 0.54] |
| 1.4 Anxiety or fear | 1 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.70, 0.11] |
| 1.5 Depressed mood | 1 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.14, 0.67] |
| 1.6 Vasomotor symptoms | 2 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.08, 0.56] |
| 1.7 Sleep problems | 1 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.35, 0.45] |
| 1.8 Menstrual symptoms | 1 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.52, 0.29] |
| 1.9 Attractiveness | 1 | 95 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.23, 0.58] |
| 1.10 Psychosocial | 1 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.57, 0.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change scores of sexual function Show forest plot | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Number of satisfying | 5 | 1893 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.20, 0.38] |
| 1.2 Number of activity | 7 | 1946 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.17, 0.34] |
| 1.3 Number of orgasms | 5 | 1893 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.21, 0.39] |
| 1.4 Libido, desire or interest in sex | 9 | 2215 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.26, 0.43] |
| 1.5 Orgasm | 6 | 1872 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.19, 0.37] |
| 1.6 Arousal | 5 | 1845 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.27, 0.45] |
| 1.7 Pleasure or enjoyment of sex | 6 | 1641 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.22, 0.43] |
| 1.8 Sexual concerns | 5 | 1852 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.22, 0.41] |
| 1.9 Responsiveness | 8 | 2171 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.23, 0.40] |
| 1.10 Sexual self‐image | 5 | 1839 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.16, 0.35] |
| 1.11 Satisfaction | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.98 [0.24, 1.72] |
| 1.12 Fantasy | 1 | 32 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.37 [0.59, 2.15] |
| 1.13 Frequency of desire | 1 | 96 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.19, 0.61] |
| 1.14 Composite score | 3 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.19, 0.63] |
| 2 Change scores of Personal Distress Scale Show forest plot | 5 | 1845 | Mean Difference (IV, Fixed, 95% CI) | ‐8.13 [‐10.59, ‐5.67] |
| 2.1 Personal Distress Scale | 5 | 1845 | Mean Difference (IV, Fixed, 95% CI) | ‐8.13 [‐10.59, ‐5.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lumbar BMDs at 12 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mean score | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.11, 0.00] |
| 1.2 Change score | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.93, 1.13] |
| 2 Lumbar BMDs at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mean score | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.19, 0.03] |
| 3 Femur BMDs at 12 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mean score | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.09, ‐0.01] |
| 3.2 Change score | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.14, 2.66] |
| 4 Femur BMDs at 24 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Mean score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.16, 0.02] |
| 4.2 Change score | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mean weight at the endpoint (3 months) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐3.86, 6.46] |
| 1.2 Mean weight at the endpoint (6 months) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [‐4.79, 15.59] |
| 1.3 Mean weight at the endpoint (12 months) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 6.30 [‐3.83, 16.43] |
| 1.4 Weight gain | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐0.25, 2.61] |
| 2 Body mass index Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Body mass index at 3 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.64, 2.64] |
| 2.2 Body mass index at 6 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐2.31, 5.51] |
| 2.3 Body mass index at 12 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐1.83, 6.03] |
| 3 Waist:hip ratio Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.08, ‐0.02] |
| 3.2 At 12 months | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cognitive performance Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Identical Pictures | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.67, 1.87] |
| 1.2 Shape Memory | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.19, 2.39] |
| 2 Cognition difficulty Show forest plot | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vasomotor symptom Show forest plot | 2 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.18, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean scores of facial and body hair growth Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Incidence of facial and body hair growth Show forest plot | 7 | 2127 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [1.07, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean scores of acne Show forest plot | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.03, 0.23] |
| 2 Incidence of acne Show forest plot | 7 | 2127 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [1.07, 2.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of increased breast density Show forest plot | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.46, 3.95] |
| 2 Area of dense breast Show forest plot | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐7.62, 7.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol at less than 3 months Show forest plot | 4 | 231 | Mean Difference (IV, Random, 95% CI) | ‐14.92 [‐27.81, ‐2.03] |
| 2 Triglyceride at less than 3 months Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 LDL cholesterol at less than 3 months Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 HDL cholesterol at less than 3 months Show forest plot | 4 | 231 | Mean Difference (IV, Random, 95% CI) | ‐17.11 [‐23.47, ‐10.75] |
| 5 Total cholesterol at 3 ‐ <6 months Show forest plot | 2 | 256 | Mean Difference (IV, Random, 95% CI) | ‐9.42 [‐31.76, 12.93] |
| 5.1 Mean score | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.87 [‐15.08, 22.82] |
| 5.2 Change score | 1 | 216 | Mean Difference (IV, Random, 95% CI) | ‐19.2 [‐26.16, ‐12.24] |
| 6 Triglyceride at 3 ‐ <6 months Show forest plot | 2 | 256 | Mean Difference (IV, Fixed, 95% CI) | ‐25.62 [‐38.53, ‐12.72] |
| 6.1 Mean score | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐44.29 [‐85.55, ‐3.03] |
| 6.2 Change score | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐23.6 [‐37.18, ‐10.02] |
| 7 LDL cholesterol at 3 ‐ <6 months Show forest plot | 2 | 256 | Mean Difference (IV, Random, 95% CI) | 18.78 [‐18.39, 55.94] |
| 7.1 Mean score | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 38.67 [21.22, 56.12] |
| 7.2 Change score | 1 | 216 | Mean Difference (IV, Random, 95% CI) | 0.7 [‐5.58, 6.98] |
| 8 HDL cholesterol at 3 ‐ <6 months Show forest plot | 2 | 256 | Mean Difference (IV, Random, 95% CI) | ‐18.72 [‐26.04, ‐11.39] |
| 8.1 Mean score | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐23.2 [‐30.19, ‐16.21] |
| 8.2 Change score | 1 | 216 | Mean Difference (IV, Random, 95% CI) | ‐15.60 [‐18.59, ‐12.61] |
| 9 Total cholesterol/HDL cholesterol at 3 ‐ <6 months | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Total cholesterol at 6 ‐ <12 months Show forest plot | 10 | 1910 | Mean Difference (IV, Random, 95% CI) | ‐2.93 [‐7.18, 1.32] |
| 10.1 Mean score | 3 | 101 | Mean Difference (IV, Random, 95% CI) | ‐6.27 [‐20.41, 7.88] |
| 10.2 Change score | 7 | 1809 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐7.47, 2.00] |
| 11 Triglyceride at 6 ‐ <12 months Show forest plot | 10 | 1909 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐14.25, 2.67] |
| 11.1 Mean score | 3 | 101 | Mean Difference (IV, Random, 95% CI) | 7.00 [‐8.74, 22.74] |
| 11.2 Change score | 7 | 1808 | Mean Difference (IV, Random, 95% CI) | ‐8.64 [‐18.40, 1.11] |
| 12 LDL cholesterol at 6 ‐ <12 months Show forest plot | 10 | 1906 | Mean Difference (IV, Fixed, 95% CI) | 1.86 [‐0.15, 3.87] |
| 12.1 Mean score | 3 | 101 | Mean Difference (IV, Fixed, 95% CI) | 3.24 [‐10.76, 17.23] |
| 12.2 Change score | 7 | 1805 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [‐0.20, 3.86] |
| 13 HDL cholesterol at 6 ‐ <12 months Show forest plot | 10 | 1907 | Mean Difference (IV, Random, 95% CI) | ‐5.84 [‐9.10, ‐2.58] |
| 13.1 Mean score | 3 | 101 | Mean Difference (IV, Random, 95% CI) | ‐9.38 [‐13.64, ‐5.12] |
| 13.2 Change score | 7 | 1806 | Mean Difference (IV, Random, 95% CI) | ‐4.74 [‐8.42, ‐1.07] |
| 14 Total cholesterol/HDL at 6 ‐ <12 months Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 20.6 [12.76, 28.44] |
| 14.1 Change score | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 20.6 [12.76, 28.44] |
| 15 Total cholesterol at 12 months Show forest plot | 4 | 231 | Mean Difference (IV, Random, 95% CI) | ‐7.99 [‐23.45, 7.48] |
| 15.1 Mean score | 2 | 70 | Mean Difference (IV, Random, 95% CI) | 1.75 [‐15.03, 18.52] |
| 15.2 Change score | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐14.35 [‐38.05, 9.35] |
| 16 Triglyceride at 12 months Show forest plot | 4 | 231 | Mean Difference (IV, Random, 95% CI) | ‐23.38 [‐55.53, 8.76] |
| 16.1 Mean score | 2 | 70 | Mean Difference (IV, Random, 95% CI) | 3.92 [‐21.60, 29.43] |
| 16.2 Change score | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐45.29 [‐80.17, ‐10.40] |
| 17 LDL cholesterol at 12 months Show forest plot | 4 | 231 | Mean Difference (IV, Fixed, 95% CI) | 8.84 [2.13, 15.54] |
| 17.1 Mean score | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 5.81 [‐10.02, 21.64] |
| 17.2 Change score | 2 | 161 | Mean Difference (IV, Fixed, 95% CI) | 9.50 [2.10, 16.90] |
| 18 HDL cholesterol at 12 months Show forest plot | 4 | 231 | Mean Difference (IV, Random, 95% CI) | ‐14.49 [‐25.28, ‐3.70] |
| 18.1 Mean score | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐7.22 [‐13.99, ‐0.45] |
| 18.2 Change score | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐23.64 [‐28.95, ‐18.33] |
| 19 Total cholesterol/HDL at 12 months Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 15.40 [4.40, 26.40] |
| 19.1 Mean score | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.2 Change score | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 15.40 [4.40, 26.40] |
| 20 Total cholesterol at 24 months Show forest plot | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐11.69 [‐32.36, 8.97] |
| 20.1 Mean change | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 3.87 [‐24.74, 32.48] |
| 20.2 Change score | 2 | 135 | Mean Difference (IV, Random, 95% CI) | ‐16.63 [‐42.67, 9.42] |
| 21 Triglyceride at 24 months Show forest plot | 3 | 167 | Mean Difference (IV, Fixed, 95% CI) | ‐52.46 [‐69.58, ‐35.35] |
| 21.1 Mean change | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐54.94, 54.94] |
| 21.2 Change score | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐58.11 [‐76.12, ‐40.09] |
| 22 LDL cholesterol at 24 months Show forest plot | 3 | 167 | Mean Difference (IV, Fixed, 95% CI) | 9.15 [1.09, 17.20] |
| 22.1 Mean change | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 3.87 [‐20.94, 28.68] |
| 22.2 Change score | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 9.77 [1.26, 18.29] |
| 23 HDL cholesterol at 24 months Show forest plot | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐17.63 [‐31.45, ‐3.80] |
| 23.1 Mean change | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐11.76, 11.76] |
| 23.2 Change score | 2 | 135 | Mean Difference (IV, Random, 95% CI) | ‐26.34 [‐28.00, ‐22.69] |
| 24 Total cholesterol/HDL at 24 months Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 20.80 [11.00, 30.60] |
| 24.1 Mean change | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Change score | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 20.80 [11.00, 30.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total cholesterol Show forest plot | 17 | 2488 | Mean Difference (IV, Random, 95% CI) | ‐7.59 [‐13.08, ‐2.10] |
| 1.1 Testosterone patch | 5 | 1738 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐2.37, 2.52] |
| 1.2 Testosterone implant | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐23.12, 18.45] |
| 1.3 Oral testosterone | 10 | 687 | Mean Difference (IV, Random, 95% CI) | ‐14.02 [‐21.63, ‐6.40] |
| 2 HDL cholesterol Show forest plot | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Testosterone patch | 5 | 1735 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.98, ‐0.19] |
| 2.2 Testosterone implant | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐5.01 [‐11.72, 1.70] |
| 2.3 Oral testosterone | 10 | 687 | Mean Difference (IV, Random, 95% CI) | ‐18.63 [‐22.18, ‐15.08] |
| 3 LDL cholesterol Show forest plot | 16 | 2406 | Mean Difference (IV, Random, 95% CI) | 4.41 [0.76, 8.07] |
| 3.1 Testosterone patch | 5 | 1734 | Mean Difference (IV, Random, 95% CI) | 1.77 [‐0.34, 3.87] |
| 3.2 Testosterone implant | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 3.37 [‐13.92, 20.66] |
| 3.3 Oral testosterone | 9 | 608 | Mean Difference (IV, Random, 95% CI) | 10.39 [1.46, 19.32] |
| 4 Triglyceride Show forest plot | 17 | 2487 | Mean Difference (IV, Random, 95% CI) | ‐14.80 [‐23.24, ‐6.36] |
| 4.1 Testosterone patch | 5 | 1737 | Mean Difference (IV, Random, 95% CI) | ‐3.64 [‐8.73, 1.44] |
| 4.2 Testosterone implant | 2 | 63 | Mean Difference (IV, Random, 95% CI) | 9.10 [‐16.04, 34.24] |
| 4.3 Oral testosterone | 10 | 687 | Mean Difference (IV, Random, 95% CI) | ‐27.07 [‐41.44, ‐12.70] |
| 5 Total cholesterol/HDL Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 20.80 [11.00, 30.60] |
| 5.1 Testosterone patch | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Testosterone implant | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Oral testosterone | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 20.80 [11.00, 30.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Discontinuation rate (overall) Show forest plot | 21 | 3124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.83, 1.19] |
| 2 Discontinuation rate (type of menopause) Show forest plot | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Surgical menopause | 8 | 1942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| 2.2 Natural menopause | 5 | 764 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.60, 1.29] |
| 2.3 Both | 7 | 419 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.79, 2.63] |
| 3 Discontinuation rate (menopausal status) Show forest plot | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Perimenopausal | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Postmenopausal | 19 | 3076 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.86, 1.25] |
| 3.3 Both | 3 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.26 [0.85, 12.47] |
| 4 Discontinuation rate (route of hormone therapy) Show forest plot | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oral HT | 13 | 1840 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.79, 1.30] |
| 4.2 Non‐oral HT | 7 | 289 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.62, 2.82] |
| 4.3 Oral and non‐oral HT | 2 | 1095 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.82, 1.46] |
| 5 Discontinuation rate (type of testosterone) Show forest plot | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Methyl testosterone | 10 | 955 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.89, 1.98] |
| 5.2 Testosterone | 9 | 2087 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 5.3 Other | 3 | 182 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.68 [0.53, 5.29] |
| 6 Discontinuation rate (duration of treatment) Show forest plot | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Less than 3 months | 3 | 195 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.46, 3.10] |
| 6.2 3 ‐ <6 months | 5 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.61 [0.87, 2.99] |
| 6.3 6 ‐ < 12 months | 10 | 2164 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 6.4 12 ‐ < 24 months | 2 | 92 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.12, 6.98] |
| 6.5 24 months | 2 | 345 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.71, 2.42] |
| 7 Discontinuation rate (blinding) Show forest plot | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Double‐blind | 18 | 3088 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.87, 1.26] |
| 7.2 Open or single‐blind | 4 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.85 [0.53, 6.49] |
| 8 Discontinuation rate (disease status) Show forest plot | 21 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 Inadequate symptom control | 9 | 2048 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.80, 1.25] |
| 8.2 Low T plus inadequate symptom control | 2 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.62, 1.67] |
| 8.3 No symptom | 10 | 771 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.90, 2.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Discontinuation rate due to adverse events (overall) Show forest plot | 20 | 3096 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.95, 1.62] |
| 2 Discontinuation rate due to adverse events (type of menopause) Show forest plot | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Surgical menopause | 8 | 1942 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.85, 1.59] |
| 2.2 Natural menopause | 4 | 704 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.72, 2.39] |
| 2.3 Both | 7 | 424 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.79, 4.78] |
| 3 Discontinuation rate due to adverse events (menopausal status) Show forest plot | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Perimenopausal | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Postmenopausal | 17 | 2948 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.95, 1.61] |
| 3.3 Both | 3 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [0.20, 19.45] |
| 4 Discontinuation rate due to adverse events (route of hormone therapy) Show forest plot | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oral HT | 11 | 1707 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.92, 1.86] |
| 4.2 Non‐oral HT | 7 | 294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.48, 4.20] |
| 4.3 Oral and non‐oral HT | 2 | 606 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.57, 1.92] |
| 5 Discontinuation rate due to adverse events (type of testosterone) Show forest plot | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Methyl testosterone | 8 | 827 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.56 [0.94, 2.57] |
| 5.2 Testosterone | 9 | 2087 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.82, 1.55] |
| 5.3 Other | 3 | 182 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.25, 9.35] |
| 6 Discontinuation rate due to adverse events (duration of treatment) Show forest plot | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Less than 3 months | 2 | 122 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.90 [0.76, 20.16] |
| 6.2 3 ‐ < 6 months | 5 | 428 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.94 [0.79, 4.77] |
| 6.3 6 ‐ < 12 months | 10 | 2164 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.82, 1.54] |
| 6.4 12 ‐ <24 months | 1 | 37 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 24 months | 2 | 345 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.67, 2.33] |
| 7 Discontinuation rate due to adverse events (blinding) Show forest plot | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Double‐blind | 16 | 2960 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.93, 1.59] |
| 7.2 Open or single‐blind | 4 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.59 [0.59, 21.94] |
| 8 Discontinuation rate due to adverse events (disease status) Show forest plot | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 Inadequate symptom control | 8 | 1988 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.85, 1.67] |
| 8.2 Low T plus inadequate symptom control | 2 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.52, 2.12] |
| 8.3 No symptom | 9 | 703 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.77, 2.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Discontinuation rate (allocation quality) Show forest plot | 14 | 2773 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.81, 1.17] |
| 2 Discontinuation rate due to adverse events (allocation quality) Show forest plot | 14 | 2778 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.91, 1.57] |
| 3 Discontinuation rate (quality of randomisation)) Show forest plot | 17 | 2902 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 4 Discontinuation rate due to adverse events (quality of randomisation) Show forest plot | 17 | 2931 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.95, 1.61] |
| 5 Discontinuation rate (blinding method) Show forest plot | 17 | 3057 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.81, 1.17] |
| 6 Discontinuation rate due to adverse events (blinding method) Show forest plot | 15 | 2929 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.93, 1.59] |
| 7 Discontinuation rate (large studies) Show forest plot | 15 | 723 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.77, 2.49] |
| 8 Discontinuation rate due to adverse events (large studies) Show forest plot | 13 | 595 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.57, 3.64] |
| 9 Discontinuation rate (methyl testosterone doses) Show forest plot | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Methyl testosterone, all doses | 10 | 955 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.68, 1.35] |
| 9.2 Methyl testosterone 1.25mg | 5 | 473 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.55, 1.43] |
| 9.3 Methyl testosterone 2 mg | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.12, 6.98] |
| 9.4 Methyl testosterone 2.5mg | 6 | 431 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.57, 1.58] |
| 10 Discontinuation rate due to adverse events (methyl testosterone doses) Show forest plot | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 Methyl testosterone, all doses | 8 | 827 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.92, 2.22] |
| 10.2 Methyl testosterone 1.25mg | 5 | 477 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.71, 2.23] |
| 10.3 Methyl testosterone 2.5mg | 5 | 358 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.80, 3.23] |
| 11 Discontinuation rate (estrogen doses) Show forest plot | 24 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 Estrogen, all doses | 24 | 3394 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.82, 1.17] |
| 11.2 Conjugated estrogen 0.625mg or equivalent doses of other estrogens | 7 | 766 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.70, 1.41] |
| 11.3 Conjugated estrogen 1.25mg or equivalent doses of other estrogens | 15 | 907 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.68, 1.52] |
| 12 Discontinuation rate due to adverse events (estrogen doses) Show forest plot | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 Estrogen, all doses | 22 | 3266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.93, 1.58] |
| 12.2 Conjugated estrogen 0.625mg or equivalent doses of other estrogens | 6 | 706 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.80, 2.24] |
| 12.3 Conjugated estrogen 1.25mg or equivalent doses of other estrogens | 14 | 839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.76, 2.65] |

