Corticosteroides prenatales para acelerar la maduración del pulmón fetal en mujeres con riesgo de parto prematuro
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Instituto Materno‐Infantil de Pernambuco, Recife, state of Pernambuco, Brazil | |
| Interventions | 12 mg betamethasone IM, repeated after 24 hours and weekly thereafter if delivery had not occurred. | |
| Outcomes | Maternal outcomes (death, chorioamnionitis, maternal infection, fever after trial entry requiring antibiotics, intrapartum fever requiring antibiotics, postnatal fever, admission to ICU, glucose intolerance, hypertension), fetal/neonatal outcomes (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, Apgar score < 7, interval between trial entry and delivery, small‐for‐gestational age, admission to NICU, need for mechanical ventilation/CPAP, duration of oxygen supplementation, surfactant use, systemic infection in the first 48 hours of life, proven infection while in the NICU, necrotising enterocolitis), childhood outcomes (death, developmental delay, cerebral palsy) and health service outcomes reported (length of antenatal hospitalisation for women, length of postnatal hospitalisation for women, length of neonatal hospitalisation) | |
| Notes | Further information obtained from the study authors, including substantial unpublished data Dates of the study: April 1997‐June 1998 Funding sources: "supported by Instituto Materno‐Infantil de Pernambuco, Brazil" Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation code kept by the chief pharmacist." |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessors was not described, but it is likely as the authors state, "the data analysis was carried out without knowledge of which of the treatments corresponded to corticosteroid and which to placebo". The code was fully broken only after the analysis was completed. |
| Incomplete outcome data (attrition bias) | Low risk | Two women (1%) in the placebo group voluntarily dropped out after randomisation. |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but study appears to report on all pre‐specified outcomes |
| Other bias | Low risk | The groups were comparable at baseline. The study appears free of other sources of bias. |
| Study characteristics | ||
| Methods | Type of study: open‐label RCT Stratification: none stated | |
| Participants | Location: Chonburi Hospital, Thailand Gestational age range: 34 weeks + 0 days to 36 weeks + 6 days | |
| Interventions | The treatment group received 6 mg dexamethasone IM, up to 4 doses 12 hours apart. The control group received no treatment. | |
| Outcomes | Maternal outcomes (chorioamnionitis, side effects of therapy in women) Fetal/neonatal outcomes (RDS, IVH, birthweight, necrotising enterocolitis, systemic infection in the first 48 hours of life, need for mechanical ventilation/CPAP, Apgar score < 7, admission to NICU) | |
| Notes | Labour augmentation performed if needed even if women had not received full course of steroids. 6 (6%) women in the intervention group received a full course of steroids; most women (75/96 (78%)) in the intervention arm received just 1 dose of dexamethasone. Data for 'maternal local or systemic adverse reactions to treatment' have been included in the review under our outcome of maternal side effects. Data from the trial are available for the following outcomes: low birthweight (not defined); hypoglycaemia in infant; need for respiratory support in infant (6/96 treatment and 14/98 control; (these data are in addition to the need for 'positive pressure ventilation' included in the review outcome 'need for mechanical ventilation'); and maternal length of stay (not separated into intrapartum and postpartum) Contact details: 3803 Qiss Bldg. A2 5,6Fl. Room 501‐2,601‐2 Rama IV Rd., Phra Khanong, Khlong Toei, Bangkok ‐ 10110 Email: [email protected] Information from trialist (received July 2020): "The protocol was developed and approved by our IRB committee prior to the enrolment. This trial prospectively register at Chonburi hospital but do not have online registration number." Dates of the study: March 2013‐March 2014 Funding sources: not stated Declarations of interest: authors declare no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. Method reported as block randomisation only |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label, participants would have been aware of allocation. Delivery nurse not blinded but all other hospital staff delivering care were blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | The data were retrieved from chart review and hospital staff were blinded apart from delivery room nurses. |
| Incomplete outcome data (attrition bias) | Low risk | 5 women in the dexamethasone delivered after 1 week and were included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported |
| Other bias | Low risk | The groups were comparable at baseline. |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Dept of Obstetrics and Gynecology, Hospital of Meram, Faculty of Medicine, Selcuk University, Konya, Turkey Gestational age range: 34 + 0‐36 + 0 weeks. | |
| Interventions | The treatment group received a single dose of 12 mg betamethasone IM. The control group received no treatment. Women who delivered at least 24 hours after betamethasone administration were included in the study. | |
| Outcomes | Apgar score at 1 and 5 minutes, need for resuscitation, development of RDS | |
| Notes | Email: [email protected] or [email protected] Dates of the study: January 2007 to May 2009 Funding sources: not reported Declarations of interest: correspondence from author (Sept 2020) "There is no conflict of interest in terms of funding source for any or any of the authors of the our published article" Correspondence from author (August 2020) confirmed baseline data similarity was due to chance and that there was no prospective trial registration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Generated by a computer" |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Sequential sealed envelopes" not stated if opaque or not |
| Blinding of participants and personnel (performance bias) | High risk | Due to comparison group receiving no treatment and treatment group receiving corticosteroids, blinding of participants and personnel would not have been possible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors is not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol but all outcomes specified in methods are reported in full. |
| Other bias | Low risk | No indication of any other sources of bias. |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Department of Gynecology and Obstetrics at the University of Oklahoma College of Medicine, Oklahoma City, Oklahoma, USA | |
| Interventions | Group A: 12 mg betamethasone IM repeated after 24 hours if delivery had not occurred GRoup C: Control group received 1 mL normal saline IM repeated after 24 hours if delivery had not occurred. If there was evidence of progressive cervical dilatation an alcohol infusion was given in order to attempt to delay delivery for at least 48 hours. In women with PROM delivery was induced if serial white blood cell counts or temperatures became elevated regardless of time elapsed since drug administration. | |
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, need for mechanical ventilation/CPAP) | |
| Notes | Further information was requested from the study authors but there was no reply. Dates of the study: not stated in manuscript, the study is coded as 1970s for the review Funding sources: quote: "supported in part by a grant from Schering Corporation, Kenilworth, New Jersey, USA; and The Upjohn Company, Kalamazoo, Michigan, USA" Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Computer generated randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | Quote:"Consecutively numbered boxes containing randomly selected study drug or placebo." |
| Blinding of participants and personnel (performance bias) | Low risk | Clinicians were never aware of the contents of the coded box. Placeob was saline so it is likely that participants were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | 14 (10%) women delivered elsewhere and were lost to follow‐up. 6 (4%) women were excluded from analyses as they failed to complete the protocol (1 in the betamethasone group, 2 in the methylprednisolone group, and 3 in the control group). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes. |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: 5 university hospitals in the USA | |
| Interventions | 4 doses of 5 mg dexamethasone phosphate IM 12 hours apart | |
| Outcomes | Maternal outcomes (postnatal fever), fetal/neonatal outcomes (fetal death, neonatal death, RDS, birthweight, interval between trial entry and delivery, systemic infection in the first 48 hours of life, proven infection while in the NICU, necrotising enterocolitis), childhood outcomes (death, lung function, developmental delay, intellectual impairment, cerebral palsy) and health service outcomes were reported (length of neonatal hospitalisation) | |
| Notes | Further information was requested from the authors but there was no reply Funding sources: Division of Lung Diseases of the National Heart, Lung and Blood Institute (National Institutes of Health, USA). "Merck, Sharpe and Dohme provided the drug and placebo preparations used in this study. This acknowledgement of appreciation is in no way an endorsement of a particular product." Study dates: March 1977‐March 1980 Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | High risk | Sealed envelope containing the identity of the contents of was attached to each vial quote:"to be opened in emergency only in case of an emergency". The manuscripts do not state how often these were opened. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:"Both placebo and steroid were dispensed as 10 ml clear, colourless solutions which differed only in that one contained the steroid". It is likely that participants were blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 (0.27%) infants in the control arm were lost to RDS follow‐up as neonates. At age 3, 240 (37%) children were lost to follow‐up (124 in the treatment arm and 116 in the control arm), or had died (47 in the treatment arm and 46 in the control arm). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: 6 hospitals in South Africa | |
| Interventions | 2 doses of 12 mg dexamethasone IM 24 hours apart All women also received ampicillin, metronidazole and hexoprenaline if contractions present in < 24 hours | |
| Outcomes | Maternal outcomes (maternal death, chorioamnionitis, endometritis, postnatal fever), fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, IVH, birthweight, need for mechanical ventilation/CPAP, systemic infection in the first 48 hours of life, necrotising enterocolitis) | |
| Notes | Study authors supplied additional data Emailed study authors July 2020 to clarify ethic approval; reply received that all sites had ethics approval from the University in each case. Study dates: not stated in the manuscripts, the study is coded as 1990s for the review Funding sources: quote: "the authors acknowledge the Medical Research Council for funding this study and Donmed Pharmaceuticals for supplying the dexamethasone and saline vials." Declarations of interest: not reported Study was discontinued before target sample size was reached due to increasing body of evidence of the use of corticosteroids in women with PPROM being advantageous to the infants, and it was felt unnecessary to conduct further trials of antenatal corticosteroids in women with PPROM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation. Sequentially‐numbered drug boxes were used |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants likely as identical looking placebo was used. Blinding of study personnel was not described, other than "double blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | 7 (3%) women and infants were excluded from analysis (3 women did not have PROM, 2 women were < 26 weeks at randomisation, 1 woman received off‐protocol corticosteroid, a neonatal bed was not available in 1 case) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: CHU Farhat Hached, Sousse, Tunisia | |
| Interventions | Abstract and full report state slightly different protocols for the intervention arm. The abstract stated that 24 mg betamethasone was given as two 12 mg IM doses at 24 hours apart. The full text states that this regimen was repeated weekly. Women had two doses of 12 mg given 24 hours apart, and this regimen was repeated weekly. | |
| Outcomes | Maternal outcomes (chorioamnionitis, postnatal fever) and fetal/neonatal outcomes reported (neonatal death, RDS, IVH) | |
| Notes | Article in French, abstract in English. Article translated by review authors (La Tunisie Medicale, 2002, Vol 80; No. 5: 260‐265). Further information was requested from the study authors but there was no reply Study dates: January 1998‐June 1999 Funding sources: not stated Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) | High risk | Blinding is unlikely as placebo was not used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of post‐randomisation exclusions not stated |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: 11 hospitals in the UK | |
| Interventions | 6 doses of 4 mg betamethasone phosphate IM 8 hours apart All women with spontaneous labour received IV salbutamol | |
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, IVH, birthweight, systemic infection in the first 48 hours of life) | |
| Notes | Study dates: mid 1975‐February 1978 Funding sources: not reported. Quote: "the compilers would like to acknowledge...Glaxo (Group Research Ltd) for the generous provision of computing facilities and for the supply of betamethasone and placebo and their distribution" Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | It is likely that participants were blinded as placebo was used. Blinding of study personnel was not described other than "double‐blind". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Long Beach Memorial Women's Hospital, California, USA | |
| Interventions | 2 doses of 6 mg betamethasone acetate and 6 mg betamethasone phosphate IM 24 hours apart, repeated weekly if still < 28 weeks and thought likely to deliver within the next week | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, Apgar < 7, need for mechanical ventilation/CPAP, duration of mechanical ventilation/CPAP, proven neonatal infection while in NICU) | |
| Notes | It is not stated how many women received corticosteroids off protocol. Study dates: December 1984‐May 1990 Funding sources: quote: "supported by a grant from the Long Beach Memorial Foundation" Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence by pharmacy |
| Allocation concealment (selection bias) | Unclear risk | The pharmacy provided consecutive sealed envelopes, not stated if envelopes were opaque |
| Blinding of participants and personnel (performance bias) | Low risk | It is likely that participants were blinded as placebo was used. Blinding of study personnel was not described other than "double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | 5 (7%) women delivered elsewhere and were lost to follow‐up (4 in treatment arm and 1 in control arm). |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: double‐blind, RCT Stratification: yes, according to clinical site and gestational age (34‐35 weeks and 36 weeks) | |
| Participants | Location: 17 university‐based clinics in the USA. All centres affiliated with the Maternal–Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. | |
| Interventions | Treatment group: (n = 1429 randomised) 2 IM injections of 12 mg betamethasone (equal parts betamethasone sodium phosphate and betamethasone acetate) administered 24 hors apart Control group received matching placebo "For those enrolled because of an indication for preterm delivery, labor inductions were expected to start by 36 weeks 5 d, and cesarean deliveries were to be scheduled by 36 weeks 6 days and not before 24 hours after randomization." Control: (n = 1402 randomised) placebo IM injections as above Follow up: to 28 d for oxygen dependency outcome | |
| Outcomes | Maternal outcome (maternal death, chorioamnionitis, side effects of therapy in women), fetal/neonatal outcomes (perinatal death, fetal death, neonatal death, RDS, IVH, birthweight, necrotising enterocolitis, proven infection while in NICU, need for mechanical ventilation/CPAP, surfactant use, air leak syndrome, Apgar score < 7, small for gestation age, admission to NICU) We asked study authors to clarify the mechanical ventilation/CPAP data presented in Table 2 of the publication; we are unsure if outcome categories are exclusive or not. We have not included data from this trial in the meta‐analysis for 1.25 due to these concerns; data will be included at the next update if confirmed by study authors. Data from trial are available for following non‐review outcomes: maternal serious adverse events, infant serious adverse events, hypoglycaemia in infant. Length of stay (maternal and infant) reported as median with IQR only. Randomisation to delivery interval reported as median with IQR only | |
| Notes | Supplementary appendix published online with data tables and additional information on trial methods relevant to risk of bias. Contact author confirmed no maternal deaths and blinding of researchers abstracting data from maternal and neonatal charts (24.2.2016 by email) ClinicalTrials.gov number, NCT01222247. Ruptured membranes occurred in 22.1% intervention and 21.7% controls
"A total of 860 of 1429 women (60.2%) in the betamethasone group and 826 of 1402 (58.9%) in the placebo group received the prespecified two doses of study medication. Of the 1145 women who did not receive a second dose, 1083 (94.6%) delivered before 24 hours; 6 women did not receive any of the assigned study medication. (In the placebo group, 3 women who consented to participate in the trial subsequently declined the injection, 1 woman delivered after randomization but before the first dose, and 1 received open label betamethasone. In the betamethasone group, 1 woman was in active labor with complete cervical dilation at the time of randomization)" Study dates: October 2010‐February 2015 Funding sources: National Heart, Lung, and Blood Institute, USA; Eunice Kennedy Shriver National Institute of Child Health and Human Development, USA; National Center for Advancing Translational Sciences, National Institutes of Health, USA Declarations of interest: "No potential conflict of interest relevant to this article was reported." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Independent data‐coordinating centre with the use of the simple urn method, with stratification according to clinical site and gestational age category (34 to 35 weeks vs. 36 weeks)" |
| Allocation concealment (selection bias) | Low risk | Remote centre performed randomisation and packaged intervention and placebo |
| Blinding of participants and personnel (performance bias) | Low risk | Identical treatment and placebo packs prepared remotely. Women and staff blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Trained research staff extracted data from maternal and neonatal staff; authors confirmed by email that these researchers were blinded. Charts of babies admitted to special care were reviewed by blinded staff for respiratory outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Two women in each group lost to follow‐up. Data available for 2827 neonates |
| Selective reporting (reporting bias) | Low risk | Supplementary outcome data published online with paper |
| Other bias | Low risk | Few baseline imbalances apart from mean maternal age (28.6 versus 27.8 years) and Hispanic ethnic background (28.3 versus. 32%) |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: 5 hospitals in Finland | |
| Interventions | 4 doses of 6 mg dexamethasone sodium phosphate IM 12 hours apart | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, chronic lung disease, IVH, birthweight, surfactant use, necrotising enterocolitis, small‐for‐gestational age) and childhood outcomes reported (death, neurodevelopmental delay) | |
| Notes | Efficacy analysis restricted to 91 infants in treatment arm and 88 infants in control arm. 3 infants excluded for protocol violations (1 mother with twins in placebo arm was given corticosteroid, 1 infant in the treatment arm developed RDS but was not given surfactant as it was not available) and 6 infants were excluded because of congenital malformations (2 treatment, 4 placebo) Study dates: April 1989‐October 1991 Funding: Foundation for Pediatric Research, Finland; Orange County Infant Care Specialists, Finland; The Orion Corporation Research Foundation, Finland; Instrumentarium Corporation Research Foundation, Finland; Arvo and Lea Ylppo Foundation, Finland; Rinnekoti Foundation, Finland; and Organon Company, Oss, The Netherlands Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated.Quote: "Randomisation in each participating hospital was performed in blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigators and those who provided care were unaware of the treatment allocation". It is likely that participants were blinded as "ampoules containing betamethasone and placebo were identical". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | 10 (11%) children in the follow‐up study at age 2 (2 in the treatment arm and 8 in the control arm). 1 female placebo‐treated infant born at 27 weeks' gestation died 3 months after the expected date of delivery, 4 infants were lost due to parental refusal, 2 were living overseas, and 3 were in other regions of the country. Efficacy analysis restricted to 91 infants in treatment arm and 88 infants in control arm. 3 infants excluded for protocol violations (1 mother with twins in placebo arm was given corticosteroid, 1 infant in the treatment arm developed RDS but was not given surfactant as it was not available) and 6 infants were excluded because of congenital malformations (2 treatment, 4 placebo); low attrition and not differential. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Louisiana State University Medical Center, Shreveport, Louisiana, USA | |
| Interventions | The treatment group received 12 mg IM betamethasone repeated at 24 hours and weekly if the women had not delivered. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes (neonatal death, RDS, IVH, birthweight, Apgar < 7, interval between trial entry and delivery, admission to NICU, surfactant use, proven neonatal infection while in NICU, necrotising enterocolitis) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Emailed authors July 2020 to enquire about the dates the study took place; awaiting reply. Timeframe: not stated in manuscript, the study is coded as 1990s for the review Funding sources: not stated Declarations of interest not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinical research nurse uninvolved in clinical care generated randomisation sequence by using random‐number table, with a random permuted block size of 10 |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) | High risk | Comparison was "no treatment" so blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) | Low risk | 2 (2%) women left hospital against medical advice after randomisation and were lost to follow‐up (1 women in each arm) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias |
| Study characteristics | ||
| Methods | Type of study: RCT Method of treatment allocation: random‐number table generated randomisation sequence by chief pharmacist. Pharmacy provided coded drug ampoules containing treatment or placebo Stratification: no Placebo: yes, of identical appearance Sample‐size calculation: no Intention‐to‐treat analyses: yes Losses to follow‐up: yes, 54 (18%) children in the follow‐up study at ages 4‐6 years (31 in the treatment arm and 23 in the control arm) and 412 (44%) adults in the follow‐up study at age 30 years (219 in the treatment arm and 193 in the control arm) | |
| Participants | Location: National Women's Hospital, Auckland, New Zealand Eligibility criteria: women with threatened or planned preterm delivery Gestational age range: 24‐36 weeks Exclusion criteria: imminent delivery, contraindication to corticosteroids Total recruited: 1142 women and 1218 infants; 560 women and 601 infants in the treatment arm and 582 women and 617 infants in the control arm | |
| Interventions | The treatment group 2 doses of 6 mg betamethasone phosphate and 6 mg betamethasone acetate IM 24 hours apart. After the first 717 women had enrolled, the treatment intervention was doubled to 2 doses of 12 mg betamethasone phosphate and 12 mg betamethasone acetate IM 24 hours apart. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, cerebroventricular haemorrhage, mean birthweight, Apgar score < 7, mean interval between trial entry and delivery, proven infection while in NICU), childhood outcomes (death, mean weight, mean height, mean head circumference, mean lung function, mean blood pressure, intellectual impairment, cerebral palsy) and adulthood outcomes were reported (death, mean weight, mean height, mean head circumference, mean skin fold thickness, mean blood pressure, glucose impairment, HPA axis function, mean cholesterol, educational achievement, visual impairment, hearing impairment, intellectual impairment) | |
| Notes | Review includes new intention‐to‐treat analysis of the complete study and additional data due to the study authors providing individual participant study records Study dates: December 1969 and February 1974 Funding sources: Health Research Council of New Zealand, Auckland, New Zealand; Auckland Medical Research Foundation, Auckland, New Zealand; and New Zealand Lottery Grants Board, Wellington, New Zealand Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence by chief pharmacist |
| Allocation concealment (selection bias) | Low risk | Pharmacy provided coded drug ampoules containing treatment or placebo |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of study personnel was not described. It is likely that participants were blinded as placebo was of identical appearance to the corticosteroid. |
| Blinding of outcome assessment (detection bias) | Low risk | For the diagnosis of RDS, clinical records and chest radiographs were assessed separately and independently, by 1 of the study authors, and by a radiologist. |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data for 54 (18%) children in the follow‐up study at ages 4‐6 (31 in the treatment arm and 23 in the control arm) and 412 (44%) adults in the follow‐up study at age 30 (219 in the treatment arm and 193 in the control arm) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
| Study characteristics | ||
| Methods | Type of study: RCT Stratification: not stated | |
| Participants | Location: Department of Obstetrics and Gynecology, Faculty of Medicine, National Univeristy of Colombia 40 women, 40 infants | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM, 12 hours apart. The control group received no treatment. | |
| Outcomes | Neonatal mortality, RDS, Apgar score < 7 at 5 minutes, systemic infection in first 48 hours | |
| Notes | Original article in Spanish, translated into English Study dates: August 1983‐December 1985 Funding: not stated Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated other than quote: "patients were classified randomly into groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Comparison is "no treatment" so blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias exist. |
| Study characteristics | ||
| Methods | Type of study: double‐blind RCT Stratification: not stated Double‐blind, randomised controlled trial in Kurdistan University of Medical Sciences, Sanandaj, Iran | |
| Participants | Location: Kurdistan University of Medical Sciences, Sanandaj, Iran | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone, IM. The control group received a placebo of normal saline. | |
| Outcomes | Maternal outcome (maternal death, maternal infections), fetal/neonatal outcomes reported (RDS, birthweight, necrotising enterocolitis, systemic infection in the first 48 hours of life, need for mechanical ventilation/CPAP, Apgar < 7 at 5 minutes, admission to NICU) | |
| Notes | Original article in Persian; we have obtained a truncated translation for this update. Our translator was unable to translate the definition of respiratory distress syndrome but said that the outcome was based on defined symptoms and confirmed by a paediatrician. Additonal outcome data for this trial are: Maternal length of stay > 3 days (equal numbers in treatment arms) is reported narratively above: mean birthweight and SD in kg has been analysed as g. Data for the trial outcome of 'need for respiratory support' has been included in the review analysis 1.26 'need for mechanical ventilation'. We have been unable to confirm whether the trial included only singleton pregnancy, but this is suggested by the equal numbers of women and infants reported. We have included data from this trial in the singleton subgroup. We had no information about membrane status from our translation, and so this trial has been included in the 'not reported or mixed population subgroup.' Maternal length of stay > 3 days (equal numbers in both arms) is reported narratively. We emailed study investigators for clarification and additional information with no reply (2/2016). Study dates: "during 2007" stated Funding: Kurdistan University of medical sciences Declarations of interest: not included in English translation, unclear if reported in original language | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of sequence not stated, but block method specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Trial described as double‐blind. Placebo‐controlled trial, and researchers and women were blind to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Neonatal outcomes extracted by blinded paediatrician. |
| Incomplete outcome data (attrition bias) | Low risk | Data reported for all women randomised |
| Selective reporting (reporting bias) | Low risk | Relevant outcome data reported |
| Other bias | Unclear risk | We have obtained a basic translation, but future correspondence with authors may clarify some of the risk of bias domains above. |
| Study characteristics | ||
| Methods | Type of study: four‐arm randomised controlled trial | |
| Participants | Location: 3 hospitals in Florida, USA | |
| Interventions | 4 treatment arms. Group 1, expectant management. Group 2, expectant management plus 2 doses of 12 mg betamethasone IM 24 hours apart, repeated weekly if the women remained undelivered. Group 3, expectant management plus 2 g ampicillin IV every 6 hours until cervical cultures were negative. Group 4, combination of group 2 and 3 management. We combined Groups 2 and 4 in the treatment arm for the review, and groups 1 and 3 in the control arm for the review. | |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes reported (neonatal death, RDS, chronic lung disease, IVH, birthweight, proven neonatal infection while in NICU, necrotising enterocolitis, duration of mechanical ventilation/CPAP) | |
| Notes | Further information requested from study authors but there was no reply. No information was available on post‐randomisation exclusions Study dates: January 1986‐March 1988 Funding sources: not stated Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes" were used. Not further described |
| Blinding of participants and personnel (performance bias) | High risk | As comparison was expectant management, blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment was not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No losses to follow‐up noted. No information was available on post‐randomisation exclusions as per exclusion criteria |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias exist |
| Study characteristics | ||
| Methods | Parallel group, two‐arm RCT City of Memphis Hospitals, Tennessee, USA Fifth General Hospital, Stuttgart, Germany Stratification: no Placebo: yes Sample size calculation: no Intention‐to‐treat analyses: no | |
| Participants | Inclusion criteria: women in premature labour or had fetomaternal disorders requiring early delivery Exclusion criteria: women with medical or operative disease that interdicted steroid therapy, women receiving immunosuppressive therapy, allergies to corticosteroids, currently taking corticosteroids, women with mature lecithin/sphingomyelin ratios thought to be in premature labour. Women in active labour or with fetomaternal complications were not entered in the study if delivery was anticipated within 24 hours. Gestational age: less than 34 weeks 196 women randomised: unclear how many randomised to each group. Reported numbers for analysis are 67 women, 67 infants in intervention group and 59 women, 59 infants in control group | |
| Interventions | Experimental: hydrocortisone 100 mg per mL. Five ml administered every 12 hours over a 48‐hour period. Control: placebo (lactose and water 100 mg permL. Five mL administered every 12 hours over a 48‐hour period. Participants followed up for six months after delivery. Some followed up for three years but no useable data for those time points | |
| Outcomes | RDS Neonatal death Birthweight Side effects of therapy | |
| Notes | Study dates: July 1972 to December 1975 Study authors’ declarations of interest: not reported Funding sources: not reported. Authors reported participating in a trial sponsored by the National Heart, Lung, and Blood institute, unclear if this refers to the current trial or to a different one, and unclear if the National Heart, Lung, and Blood institute has a funding role. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomized fashion” ‐ not further details reported |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealment was attempted: quote: “After entry to the study, each patient was assigned an identification number by the research technician. The number was recorded in a log book in the perinatal laboratory. A multidose vial bearing the identification number contained 25 ml of the test drug or placebo). These numbered vials were prepared in the Medicinal Chemistry Department and used in both hospitals” |
| Blinding of participants and personnel (performance bias) | Low risk | “Numbered vials prepared in the Medicinal Chemistry Dept and used in both hospitals…were clear and colourless and of the same relative viscosity …after delivery the vial was returned to the research technician so that the remaining liquid could be measured to validate the amount of drug given”. “the master code was retained by a cooperating member of the Dept of Biochemistry…to prevent unblinding” |
| Blinding of outcome assessment (detection bias) | Low risk | The master code for the drug allocation was retained by a cooperating member of the Department of Biochemistry, who did not have access to the L/S ratios, clinical data or follow‐up results. |
| Incomplete outcome data (attrition bias) | High risk | 60/196 were excluded after delivery because of evidence of hypoxia, obstetric factors affecting the neonate, or because delivery did not occur within 7 days. A further 10 women did not return for follow‐up visits and were excluded from the analysis. The group allocation of these 70 women is not reported. |
| Selective reporting (reporting bias) | Low risk | No protocol available (would not be common practice at the time of conducting this trial). Outcomes reported as expected. |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Wake Forest University Medical Center, North Carolina, USA | |
| Interventions | 3 treatment arms. Group 1: 2 doses of 6 mg or 12 mg betamethasone IM 12 hours apart, delivery 24 to 48 hours after PROM and after 24 hours of corticosteroid therapy. Group 2 received similar treatment to group 1 except that no steroids were administered. Delivery 24 to 48 hours after PROM. Group 3, expectant management (no tocolytics or steroids). We did not include Group 3 in the review. | |
| Outcomes | Fetal/neonatal outcomes (neonatal death, RDS, proven neonatal infection while in NICU) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Authors provided further information Study dates: not stated in manuscript, the study is coded as 1980s for the review Funding sources: not stated Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Consecutive, sealed envelopes were used, not stated if opaque |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up or exclusions |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias exist. |
| Study characteristics | ||
| Methods | Two‐arm parallel RCT Setting: Obstetrics and Gynaecology Department in collaboration with the Department of Neonatology at the Jawaharlal Nehru Institute of Postgraduate Medical Education and Research, India | |
| Participants | Inclusion criteria: women admitted to the labor room with a period of gestation between 34 and 36 + 6 weeks with a singleton pregnancy with risk of preterm delivery either spontaneously or requiring termination for maternal (or) fetal indication Exclusion criteria: those who were far advanced in labour (5 cm or more dilated) or had received the previous course of steroids or had chorioamnionitis at admission, women with multiple gestations, gestational diabetes and diabetes mellitus, major congenital malformations in the fetus and those undergoing a pre‐labour scheduled cesarean section Gestational age: 34 weeks to 36 weeks + 6 days Number randomised: 310 women (155 to each group) Number analysed: 309 women, 309 infants (1 stillbirth was not included in analysis) | |
| Interventions | Experimental: dexamethasone 6 mg administered intramuscularly 12 hourly, four doses in total. Control: no treatment All babies followed up until discharge. | |
| Outcomes | Composite respiratory morbidities (transient tachypnoea; respiratory distress syndrome) Quote: “Other complications such as hypoglycemia, hypothermia, poor feeding, sepsis and neonatal mortality were also noted. We followed up all the babies until discharge from the hospital.” | |
| Notes | Study authors’ declarations of interest: not reported Funding sources: not reported Study dates: main trial report states February 2015 to June 2016, 2018 conference abstract states October 2014 to June 2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“computer‐generated random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote:“sequentially numbered, sealed, opaque envelopes, from an independent data coordinating party” |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants (control received no treatment or placebo) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Quote:“There was no loss to follow‐up after randomization. One case in the study group was excluded from analysis because it was stillborn.” |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in trial registry record and in methods are all reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
| Study characteristics | ||
| Methods | Type of study: RCT Stratification: not stated | |
| Participants | Location: Instituto de Medicina Integral Professor Fernando Figueira, Recife, Pernambuco, Brazil | |
| Interventions | The treatment group received 2 doses of 12 mg IM betamethasone 24 hours apart. The control group received IM saline as placebo. | |
| Outcomes | Maternal outcomes (side effects of therapy in women) and fetal/neonatal outcomes (fetal deaths, neonatal deaths, RDS, birthweight, proven infection while in NICU, need for mechanical ventilation/CPAP, mean duration of mechanical ventilation/CPAP, surfactant use, small for gestational age, admission to NICU) | |
| Notes | For infant outcomes, we have used the denominator stated in the published report excluding women who left the trial pregnant. An intention‐to‐treat analysis should have included these women, so for 'Summary of findings' outcomes we carried out a sensitivity analysis to determine if the denominator used made a difference to the overall pooled effect estimate; it did not (data not shown) Attempted to contact trial authors in July 2020 to clarify ethics approval but email address is no longer in use. Study dates: April 2008‐June 2010 Funding sources: quote: "This study was supported by the Instituto de Medicina Integral Prof Fernando Figueira‐IMIP (www.imip.org.br), a private, not for profit healthcare organisation based in Recife, Pernambuco, Brazil, where the study was carried out. The institute did not interfere with study design or analysis and the funding covered all study expenses, including purchase of the drug and placebo." Declarations of interest: All authors declare: "no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was prepared by a statistician not involved in the study, using random allocation software |
| Allocation concealment (selection bias) | Low risk | The hospital pharmacy prepared sealed cardboard boxes numbered according to the random number table, and containing either betamethasone or placebo, identical in appearance, volume and colour. |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators, physicians caring for the women, the women themselves and the statistician were all blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators, physicians caring for the women, the women themselves and the statistician were all blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 43 (13%) women (19 in steroid group and 24 in placebo group) were discharged from hospital still pregnant and were considered post‐randomisation losses to follow‐up. 2 (1%) women were excluded from the placebo group as they were found to be ineligible after randomisation (multiple pregnancy, and term pregnancy). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes appear to have been reported. |
| Other bias | Low risk | Nothing to indicate any other |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: 2 military hospitals in Jordan | |
| Interventions | The treatment group received 4 doses of 6 mg dexamethasone IM 12 hours apart, repeated if women had not delivered after 1 week. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis), fetal/neonatal outcomes (fetal death, neonatal death, RDS, IVH, proven neonatal infection while in NICU, necrotising enterocolitis, Apgar < 7) and health service outcome reported (length of neonatal hospitalisation) | |
| Notes | Study authors contacted for further information but no reply. Discrepancy in number of infants with necrotising enterocolitis in manuscript Study dates: January 1997‐February 1999 Funding: not stated Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel was not possible due to the nature of the comparison. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up or exclusions stated |
| Selective reporting (reporting bias) | Unclear risk | Discrepancy in number of infants with necrotising enterocolitis in manuscript |
| Other bias | Low risk | No indication of any other source of bias |
| Study characteristics | ||
| Methods | Parallel, four‐arm RCT. California, USA | |
| Participants | Inclusion criteria: preterm labour or having premature rupture of the membranes (or both), gestational age 26 weeks to 32 weeks or estimated fetal weight of 750 g to 1750 g, cervical examination had to be > 5 cm Exclusion criteria: women known to have a disease that might significantly affected by glucocorticosteroids or tocolytic agents or both. If bacteria were seen on Gram stain of amniotic fluid, the woman was excluded. Gestational age: 26 weeks to 32 weeks, or estimated fetal weight of 750 to 1750 g. Number randomised:144 women (149 infants). Numbers randomised to each group are not reported. Numbers analysed: hydrocortisone 15 infants, 15 women; methylprednisolone 17 infants, 16 women; betamethasone 34 infants, 32 women; control 31 infants, 29 women. | |
| Interventions | Experimental 1: hydrocortisone 250 mg. Two IM doses, given 24 hours apart. Experimental 2: methylprednisolone 125 mg. Two IM doses, given 24 hours apart. Experimental 3: betamethasone 12 mg. Two IM doses, given 24 hours apart. Control: placebo (saline). Two IM doses, given 24 hours apart. | |
| Outcomes | RDS Neonatal death Birth weight | |
| Notes | Study dates: July 1977 to April 1980 Study authors’ declarations of interest: not reported Funding sources: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the sequence of drugs had been determined by means of a table of random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote:“consecutively numbered opaque bags” “the bags were prepared by the project nurses who then sealed them and kept a record of their use” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:“the record book was not available to those providing patient care or who were evaluating the data” “each dosage consisted of a 2 ml volume prepared by a nurse on the oncology ward, who had no contact with obstetric patients” “the syringe barrel was covered with an opaque label so the care‐givers could not determine visually which drug was being administered.” |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors seem to be care‐givers, who are blinded. |
| Incomplete outcome data (attrition bias) | High risk | 52/144 women and their infants excluded from analysis (28 because of birth weights > 2500 g, 13 due to incomplete maternal or baby information, 6 due to procedural errors, 3 due to mature L/S ratio, 2 stillbirths). The group allocation of these women is not reported. |
| Selective reporting (reporting bias) | High risk | No protocol, most outcomes reported in full but no data reported for endometritis although it is specified in the methods. |
| Other bias | High risk | Quote:“hydrocortisone and methylprednisolone groups discontinued at 24 months to increase group size in remaining two regimens” |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Department of Obstetrics and Gynaecology and Department of Neonatology, Wilhelmina Gasthuis, University of Amsterdam, Amsterdam, the Netherlands. | |
| Interventions | The treatment group received 8 mg betamethasone phosphate and 6 mg betamethasone acetate IM repeated after 24 hours. All women received orciprenaline infusion and bed‐rest until 32 weeks. | |
| Outcomes | Maternal outcomes (death, chorioamnionitis, maternal infections, fever after trial entry requiring antibiotics, intrapartum fever requiring antibiotics, postnatal fever, admission to ICU, side effects of therapy), fetal/neonatal outcomes (fetal death, neonatal death, RDS, IVH, birthweight, Apgar score < 7), childhood outcomes (weight, height, head circumference, lung function, visual impairment, hearing impairment, intellectual impairment, cerebral palsy, behavioural/learning difficulties) and adulthood outcomes were reported (weight, height, head circumference, blood pressure, intellectual impairment, age at puberty) | |
| Notes | Initial study report included a third arm of women (n = 133) and infants (n = 164) who had been excluded from randomisation because they were: 1. already in labour (n = 80) and could not be prolonged for at least 12 hours or were already 33 weeks' gestation, or; 2. (n = 53) contra‐indicated for corticosteroids, or; 3. wrongly excluded (n = 5). These women and infants are not included in the review. Two perinatal deaths in the corticosteroid treatment arm were excluded for: 1. intrauterine fetal death due to solutio placentae, and 2. death due to prolapsed umbilical cord. These deaths have been included in the analyses. Infections in infants are listed in Table 6 of the Schutte 1979 original report. There are deaths associated with these infections, and it is not clear when these infections or deaths occurred, or if they have been included in the reported numbers for neonatal or perinatal deaths. Study dates: April 1974‐April 1977 Funding sources: Dutch Foundation for Research on Prevention (Praeventiefonds Project 28‐1145), the Netherlands Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Coded drug ampoules prepared by pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Trial described as double blind, with pharmacist preparing identical treatment and control ampoules |
| Blinding of outcome assessment (detection bias) | Low risk | Staff were blinded to treatment group |
| Incomplete outcome data (attrition bias) | Unclear risk | Two perinatal deaths in the corticosteroids group were excluded. Data for infant infections specify additional deaths, and it is unclear whether or not these deaths are counted in the overall total for perinatal deaths. The inclusion of these deaths will not change the overall conclusions of meta‐analysis in favour of corticosteroid use. We are unclear as to the impact of exclusions on results, especially for the outcome of perinatal deaths. |
| Selective reporting (reporting bias) | Low risk | Primary outcome of the trial was RDS; this and other important outcomes are reported |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
| Study characteristics | ||
| Methods | Type of study: RCT Stratification: not stated | |
| Participants | Location: Barnes‐Jewish Hospital, St Louis, Missouri, USA | |
| Interventions | The treatment group received either 2 doses of betamethasone 12 mg IM 24 hours apart, or 4 doses of dexamethasone 6 mg IM 12 hours apart. The control group received no treatment. | |
| Outcomes | Maternal outcomes (side effects of therapy in women) and fetal/neonatal outcomes (need for mechanical ventilation/CPAP, admission to NICU) | |
| Notes | This study was stopped early due to difficulties in participant recruitment. Study dates: May 2003‐May 2008 Funding sources: supported in part by a Clinical and Translational Science Award, and by a grant from the National Centre for Research Resources, a component of the National Institute of Health and NIH Roadmap for Medical Research Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomly assigned" not further described |
| Allocation concealment (selection bias) | Unclear risk | Quote:"Sealed envelopes" not further described |
| Blinding of participants and personnel (performance bias) | High risk | Control group received no treatment so blinding of participants and study personnel would not have been possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention is made of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | High risk | 7 (22%) women (3 in the study group and 4 in the control group) delivered within 7 days of their initial testing for fetal lung maturity and were excluded from the analysis. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Hyaline membrane disease is listed as an outcome, but not reported |
| Other bias | High risk | This study was stopped early due to difficulties in patient recruitment |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: Northwestern University Medical School, Chicago, Illinois, USA | |
| Interventions | The treatment group received 4 doses of 5 mg dexamethasone IM 12 hours apart, repeated weekly if the women remained undelivered. All infants born < 30 weeks received prophylactic surfactant at birth. | |
| Outcomes | Maternal outcomes (chorioamnionitis, endometritis) and fetal/neonatal outcomes reported (neonatal death, RDS, chronic lung disease, IVH, small‐for‐gestational age, birthweight, necrotising enterocolitis) | |
| Notes | Those women undelivered after 29 weeks were eligible for corticosteroid outside the study protocol. These women and their infants are not included in the review as it was not possible to separate out control women who subsequently received corticosteroids Study dates: April 1990‐June 1994 Funding sources: not stated Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence used |
| Allocation concealment (selection bias) | Low risk | Pharmacy provided identical syringes labelled with the woman's study number. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:"Clinical personnel and the patient were effectively blinded to study group assignment" |
| Blinding of outcome assessment (detection bias) | Low risk | The severity of RDS, and diagnosis of IVH were quote:"confirmed independently by chart reviews conducted by 1 of the authors blinded to study group assignment" |
| Incomplete outcome data (attrition bias) | Unclear risk | 49 (40%) of the 124 women initially recruited, remained undelivered after 29 weeks and were not included in the review. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other sources of bias. |
| Study characteristics | ||
| Methods | Type of study: RCT | |
| Participants | Location: University of Helsinki, Finland | |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM 24 hours apart. | |
| Outcomes | Fetal/neonatal outcomes reported (RDS, HPA axis function) | |
| Notes | Study dates: not stated in manuscript, the study is coded as 1980s for the review Funding sources: not stated Declarations of interest: not reported The study was discontinued early because the overall incidence of RDS was too low for any meaningful conclusions concerning the efficacy of prevention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Coded drug boxes were used but it is not clear how they were coded, e.g. if they were sequentially numbered. |
| Blinding of participants and personnel (performance bias) | Low risk | It is likely that participants were blinded due to the use of a placebo quote:"similar in appearance" to the corticosteroid. Blinding of study personnel was not described other than quote: "ampoules were administered to the patients using the double‐blind principle". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up or exclusions stated |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but appears to report on all pre‐specified outcomes |
| Other bias | Low risk | Nothing to indicate any other risk of bias |
| Study characteristics | ||
| Methods | Type of study: multicountry, multicentre, individually‐randomised, parallel‐group, double‐blind, placebo‐controlled trial Method of treatment allocation: 1:1 Stratification: site‐stratified individual randomisation with balanced permuted blocks of size 10 were used Placebo: yes Sample size calculation: yes | |
| Participants | Location: Bangladesh, India, Kenya, Nigeria and Pakistan Inclusion criteria: pregnant women (with confirmed live fetuses) who were at risk of preterm birth between 26 weeks 0 days and 33 weeks 6 days; birth planned or expected in the next 48 hours (following preterm prelabour rupture of membranes, spontaneous labor, or provider‐initiated preterm birth). Exclusion criteria: clinical signs of severe infection; major congenital fetal anomalies; concurrent or recent (within the past two weeks) use of systemic glucocorticoids; participation in another trial; or contraindication to glucocorticoids Total recruited: 2852 women (3070 infants) randomised (A 1429 women, 1544 infants; B 1423 women, 1526 infants) Gestational age range: between 26 weeks 0 days and 33 weeks 6 days | |
| Interventions | Group A: 6 mg dexamethasone administered every 12 hours, to a maximum of four doses, or until hospital discharge or birth Group B: placebo administered every 12 hours, to a maximum of four doses, or until hospital discharge or birth Women were eligible for a repeat course if they had not given birth after seven completed days but still met inclusion criteria. The repeat course was identical to the first course, and the same as the initial allocation | |
| Outcomes |
For the neonate:
For the woman:
| |
| Notes | Study dates: December 2017 through November 2019 Funding sources: Quote:“This trial was primarily funded by the Bill and Melinda Gates Foundation (Grant OPP1136821). Additional support was provided by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research; and Department of Maternal, Newborn, Child, Adolescent Health, and Ageing, of the World Health Organization, Geneva, Switzerland.” Declarations of interest: Quote:“The authors declare that they have no competing interests.” Trial stopped early: the Data Safety Monitoring Monitoring board "recommended the trial be stopped for infant mortality benefits, and strong evidence of a graded dose‐response effect, in the context of existing evidence of benefits of antenatal glucocorticoids." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:“The computer‐generated randomization sequence was prepared centrally at WHO” |
| Allocation concealment (selection bias) | Low risk | Quote:“All sites received serially numbered identical treatment packs containing 4mg/mL ampules of dexamethasone or placebo for two full courses” "The assignment schedule was stored at WHO. Once eligibility was confirmed and consent obtained, trained study staff randomized a woman by taking the next numbered treatment pack from the dispenser (which was designed to ensure packs were taken sequentially" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:“Trial participants, care providers, and investigators were not aware of group assignments.” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote:“Trial participants, care providers, and investigators were not aware of group assignments.” |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition. Quote:“Over 99% of randomized women and infants completed follow‐up” |
| Selective reporting (reporting bias) | Low risk | All outcomes that were pre‐specified in the protocol are reported in full |
| Other bias | Low risk | Nothing to indicate any other source of bias |
CPAP: continuous positive airways pressure
GDM: gestational diabetes mellitus
HPA: hypothalamic‐pituitary‐adrenal
ICU: intensive care unit
IM: intramuscular
ITT: intention‐to‐treat
IQR: interquartile range
IUGR: Iintrauterine growth restriction
IV: intravenous
IVH: intraventricular haemorrhage
LMP: last menstrual period
NICU: neonatal intensive care unit
PIH: pregnancy induced hypertension
PROM: premature rupture of membranes
PPROM: prolonged premature rupture of membranes
RCT: randomised controlled trial
RDS: respiratory distress syndrome
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This abstract compares TRH + betamethasone with betamethasone + placebo. | |
| This is a trial of strategies to optimise use of corticosteroids. | |
| This study is quasi‐experimental. | |
| Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. | |
| This abstract compares TRH + betamethasone with betamethasone + placebo. | |
| Not an eligible comparison | |
| This trial compares oral vs IM dexamethasone in the prevention of RDS. It does not meet our entry criteria for inclusion of studies for the review. | |
| This trial compares a policy of corticosteroid therapy followed by elective delivery with a policy of withholding corticosteroids and awaiting delivery so the independent effect of the 2 co‐interventions cannot be evaluated separately. | |
| Not a randomised trial. Outcomes for women who received steroids were compared with those that did not. Information obtained from translation sheet. Original article in Bosnian. | |
| Not a randomised trial. Women were allocated to placebo if they were expected to deliver within 24 hours and to betamethasone if labour was not expected within 24 hours. | |
| Corticosteroid therapy (hydrocortisone) and co‐intervention of elective delivery was compared to expectant management in PROM. The independent effect of the 2 co‐interventions cannot be evaluated separately. | |
| Compared different doses of corticosteroid: 12‐hourly vs 24‐hourly. The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. | |
| The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. | |
| Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. | |
| The study includes a repeat dose of corticosteroids and is eligible for inclusion in a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015.All women received corticosteroids before the beginning of the trial. | |
| Quasi‐randomised study that allocated women according to the in‐patient sequence. Compared the effect of dexamethasone combined with vitamin K, dexamethasone alone and no dexamethasone or vitamin K on periventricular/intraventricular haemorrhage. | |
| This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. | |
| This study appears to be an observational study of 163 premature infants, 80 of whom were exposed to antenatal corticosteroids, and 83 of whom were not. | |
| This trial compares repeat dose corticosteroids and is eligible for inclusion a different review, 'Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes' Crowther 2015. | |
| This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. | |
| Quasi‐randomised using medical record number. | |
| This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. | |
| Trial status: quote: "Withdrawn (Company decided not to pursue this study.)" Seems to have recruited no participants. | |
| Ineligible comparator | |
| Ineligible intervention: weekly repeats of betamethasone. | |
| This is a head‐to‐head trial of 2 different regimens and is eligible for the Cochrane Review entitled 'Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth' Brownfoot 2013. | |
| This study compares the effects of betamethasone vs dexamethasone on antenatal fetal heart rate. | |
| Quasi‐randomised study. Randomised participants are combined with a non‐randomised cohort and cannot be analysed separately. | |
| This trial compares IM betamethasone with IV cortisol. It does not meet our entry criteria for inclusion of studies for the review. |
IM: intramuscular
IV: intravenous
PROM: premature rupture of membranes
RDS: respiratory distress syndrome
TRH: thyrotropin‐releasing hormone
vs: versus
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Type of study: RCT |
| Participants | Location: Hospital Clinic, University of Barcelona, Spain |
| Interventions | Type and dose of corticosteroid used in the treatment group is not stated |
| Outcomes | Fetal/neonatal outcome reported (RDS) |
| Notes | Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
| Methods | Type of study: RCT |
| Participants | Location: University of South Florida Medical School, Tampa, Florida, USA |
| Interventions | 12 mg betamethasone IM repeated after 24 hours and weekly thereafter until delivery or 34 weeks. |
| Outcomes | Maternal outcome (chorioamnionitis), fetal/neonatal outcomes (RDS, birthweight, days of mechanical ventilation/CPAP) and health service outcomes reported (days in NICU, neonatal days in hospital, neonatal hospital cost). However due to lack of SD data only chorioamnionitis and RDS data were included in the review. |
| Notes | This study included a third arm (12 mg betamethasone IM 24‐hourly for 2 doses and 400 mcg methylprednisolone IV 8‐hourly for 6 doses, repeated weekly until delivery or 34 weeks. The data for the review report the betamethasone and control arms only. Further information was requested from the study authors but there was no reply. Study dates: not stated in manuscript, the study is coded as 1990s for the review Funding sources: not stated Declarations of interest: not stated Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
| Methods | Two arm, parallel RCT Setting: Obstetrics and Gynecology Department at the Ataturk University School of Medicine, Turkey |
| Participants | Inclusion criteria: 28–34th gestational week, systolic blood pressure of 140 mmHg, to 160 mmHg, diastolic blood pressure of 90 mmHg, to 100 mmHg, proteinuria 300 mg and < 5 g in 24‐hour urine or ≥1 proteinuria in spot urine test, and diagnosis of mild preeclampsia Exclusion criteria: karyotypic suspicion of anatomic defects, intrauterine growth retardation, or ruptured membranes, and those under treatment with tocolytic agents, magnesium sulphate, or benzodiazepines that may affect BPP and Doppler parameters Number randomised: 40 (20 to each group) |
| Interventions | Experimental: betamethasone 12 mg, two doses, 24 hours apart. Control: placebo (saline). Same volume as intervention group. Followed up until 72 hours after drug administration |
| Outcomes | Fetal biophysical profile score Doppler measurements of arteries |
| Notes | Study dates: May 2009 and September 2010 Declarations of interest: "All authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of this article" Funding sources: not reported Not included in 2020 update: contacted authors in September and October 2020 to query prospective trial registration. |
| Methods | Type of study: RCT |
| Participants | Location: 6 teaching hospitals in Toronto, Canada |
| Interventions | 4 doses of 3 mg betamethasone acetate and 3 mg betamethasone sodium phosphate IM 12 hours apart |
| Outcomes | Fetal/neonatal outcomes were reported (fetal death, neonatal death, RDS, IVH, birthweight, days of mechanical ventilation) |
| Notes | Study dates: January 1975‐June 1978 Funding sources: The Hospital for Sick Children Foundation, Canada; Schering Corporation, Canada; Ontario Ministry of Health Provincial Research Grant PR 279, Canada Not included in 2020 update: no contact details available, unable to get details of randomisation process to explain why 75 were allocated to intervention and 62 to placebo. |
| Methods | Type of study: RCT (abstract) Stratification: not described |
| Participants | Location: Temple University Hospital, Philadelphia, Pennsylvania, USA |
| Interventions | Treatment group received an IM injection of betamethasone. The control group received an IM injection of saline as placebo. |
| Outcomes | Neonatal mortality, RDS |
| Notes | Study dates: July 1976‐July 1978 Funding sources: not stated Declarations of interest: not stated Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
| Methods | Type of study: double‐blind RCT Stratification: none stated |
| Participants | Location: obstetric emergency department of Vali‐e‐Asr,Hospital, Tehran, Iran Eligibility criteria: patients at risk of preterm labor as determined by routine ultrasound examination in the first trimester Gestational age range: 34‐37 weeks quote: "Women with systemic diseases, maternal hypertension before or during pregnancy, uterine tenderness, chorioamnionitis signs, symptomatic vaginal infection, rupture of membranes, current use of antibiotics, induced pregnancy, and history of smoking were excluded." |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM. The control group received placebo of saline as per regimen above. |
| Outcomes | No outcomes available for the review |
| Notes | Data are provided on endocervical cytokine levels in women who delivered within and after 1 week but no outcome data available for the review are presented Study dates: June 2006 to July 2010 Funding sources: quote: "The study was supported by Tehran University of Medial Sciences. The assays were Declarations of interest: not reported Not included in 2020 update: no protocol or prospective registration |
| Methods | Two‐arm, parallel RCT Setting: Shahid Beheshti University of Medical Sciences, Iran |
| Participants | Inclusion criteria: Women with a single pregnancy at 34–36 weeks and 6 days of gestation, with a high probability of late preterm delivery Exclusion criteria: those with dilatation of 4 cm or more, known congenital malformations, antenatal administration of glucocorticoids before the 34th week, fetal death, major fetal anomalies or non survivable fetus, administration of systemic corticosteroid due to other indications, gestational diabetes, prepregnancy diabetes mellitus, maternal contraindication for betamethasone, chorioamnionitis, unwillingness to participate in the study or/and participation in other intervention study Number randomised: 240 (120 per group) Number analysed: 240 women |
| Interventions | Experimental: 12 mg of betamethasone in two doses with an interval of 24 hours Control: no treatment |
| Outcomes | Primary outcome: composite endpoint describing the need for respiratory support by 72 hours of age consisting of one or more of the following continuous positive airway pressure (CPAP) or high flow nasal cannula (HFNC) for at least two consecutive hours, respiratory distress syndrome or need for mechanical ventilation Birthweight First minute Agpar score Fifth minute Agpar score Need for resuscitation Need for oxygen for more than one hour Need for CPAP or continuous high‐flow nasal canula oxygen therapy Need for continuous high oxygen with FiO2 more than 30% Mechanical ventilation Extracorporeal membrane oxygenation RDS Transient tachypnoea of the newborn Apnoea Bronchopulmonary dysplasia Pneumonia Need for surfactant Umbilical cord blood pH Admission to NICU Duration of NICU admission Duration of hospitalisation in neonatal ward after NICU |
| Notes | Study dates: January 2017 to July 2017 Declarations of interest: quote: "No potential conflict of interest was reported by the authors" Funding sources: not reported Not included in 2020 update: contacted authors in September and October 2020 to query prospective trial registration; awaiting reply. |
| Methods | Type of study: RCT |
| Participants | Location: University of Illinois, Chicago, USA |
| Interventions | The treatment group received 2 doses of 12 mg betamethasone IM 12 hours apart repeated weekly until 32 weeks. |
| Outcomes | Fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, systemic infection in the first 48 hours of life, proven neonatal infection while in NICU) |
| Notes | Study dates: not stated in manuscript, the study is coded as 1980s for the review Funding: not stated Declarations of interest: not stated Abstract only: not included in 2020 update of review because there are no contact details available for the trialists and no way to confirm that the data presented in the abstract are the final data. |
| Methods | Type of study: RCT Stratification: yes, by gestational age at entry |
| Participants | Location: 2 hospitals in Boston, USA |
| Interventions | The treatment group received 6 doses of 4 mg dexamethasone phosphate IM 8 hours apart. |
| Outcomes | Maternal outcomes (endometritis, fever after trial entry requiring antibiotics) and fetal/neonatal outcomes reported (fetal death, neonatal death, RDS, chronic lung disease, IVH, proven neonatal infection while in NICU) |
| Notes | Study authors contacted for further information but there was no reply Study dates: January 1975‐March 1977 Funding sources: not stated Not included in 2020 update: no contact details available, unable to get details of randomisation process to explain imbalance between groups (56 infants in intervention group and 71 in placebo group. |
BPP: biophysical profile
CPAP: continuous positive airways pressure
IM: intramuscular
IV: intravenous
IVH: intraventricular haemorrhage
NICU: neonatal intensive care unit
PROM: premature rupture of membranes
RCT: randomised controlled trial
RDS: respiratory distress syndrome
SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
| Study name | The WHO ACTION‐II (Antenatal CorticosTeroids for Improving Outcomes in preterm Newborns) Trial |
| Methods | Randomised controlled trial Setting: hospitals in Bangladesh, India, Kenya, Nigeria, Pakistan Participants, care givers, outcome assessors and data analysts will all be blinded |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size: 22,589 women |
| Interventions | Experimental group: a single course of 6 mg dexamethasone by intramuscular injection, administered every 12 hours, to a total of four (4) doses (time points 0 hours, 12 hours, 24 hours and 36 hours). If the full regimen is completed, the woman would have received a total of 24 mg in divided doses. No repeat course(s) will be used. Control group: Identical placebo (normal saline), administered according to the same regimen as for dexamethasone, with one injection every 12 hours to a total of four doses. Follow‐up of enrolled women and newborns to 28 days postpartum/postnatal |
| Outcomes | Neonatal death (death of a liveborn within 28 completed days of life) Stillbirth or neonatal death (any death of a fetus (post enrolment), or death of a live birth within 28 completed days of life among all enrolled participants. Possible maternal bacterial infection (occurrence of maternal fever, or clinically suspected or confirmed infection, for which therapeutic antibiotics were used) Stillbirth Early neonatal death Neonatal sepsis Severe intraventricular haemorrhage (sIVH) Neonatal hypoglycaemia Apgar score <7 at 5 minutes Maternal death Maternal fever (greater than or equal to 38.0 C ) Chorioamnionitis Postpartum endometritis Wound infection Non‐obstetric infection Major neonatal resuscitation at birth Timing of breast milk feeding initiation Timing to full enteral feeding Use of oxygen therapy Continuous positive airway pressure (CPAP) ventilation Mechanical ventilation Use of therapeutic antibiotics for 5 or more days Surfactant treatment Length of newborn's hospital stay after birth Admission of newborn to a special newborn care unit Newborn readmission for healthcare at facility Maternal therapeutic antibiotic use Any use of antibiotics in an enrolled participant (maternal) while in facility (prophylactic or therapeutic) Length of total maternal hospitalisation for birth Maternal readmission for healthcare at facility Any maternal referral to another facility Compliance with study allocation Total number of treatment or placebo doses received Time from initiation of first dose until birth Gestational age at birth |
| Starting date | Registered October 2017 (prospectively registered) Last update: October 2010 |
| Contact information | Dr A. Metin Gulmezoglu |
| Notes | Funding sources: Bill and Melinda Gates Foundation |
| Study name | Effects of antenatal corticosteroids in twin neonates with late preterm birth (ACTWIN [Antenatal Corticosteroids in TWIN late preterm neonates] trial): study protocol for a randomized controlled trial |
| Methods | Multicentre, randomised, double‐blind placebo‐controlled trial. Participant and care provider will be blinded. Setting: obstetric departments of two hospitals in South Korea, Seoul National University Hospital and Cheil General Hospital and Women’s Healthcare Centre |
| Participants | Estimated enrolment: 808 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions | Intervention women will receive intramuscular betamethasone 12 mg (3 mL) twice in a 24‐hour interval. Comparator: women will receive 3 mL of normal saline twice in a 24‐hour interval. |
| Outcomes | Primary outcomes
Secondary outcomes
Other Outcome Measures Necrotising enterocolitis |
| Starting date | May 2018 Estimated completion date: December 2022 |
| Contact information | Seung Mi Lee |
| Notes |
| Study name | Evaluation of the effect of betamethasone onnNeonatal outcomes in pregnancies |
| Methods | Randomised, double‐blind, parallel group Setting: Kosar Obstetric and Gynecology Hospita, Qazvin, Iran |
| Participants | Target sample size: 140 women Inclusion criteria: pregnant women aged between 18 to 39 years who are candidate for delivery in 34‐36th week of gestational age Exclusion criteria: delivery after 37th week of gestational age; diabetes; fetal anomalies; delivery before receiving the two doses of the drug; multiple pregnancy |
| Interventions | Group 1: betamethasone. Intramuscularly injection of 6 mL of betamethasone solution (4 mg/mL) (divided into two 3 mL injection with a 12‐hour interval) Group 2: placebo. Intramuscularly injection of 6 mL of normal saline solution (9 mg/mL) (divided into two 3 mL injection with a 12‐hour interval) |
| Outcomes | Respiratory distress syndrome Agpar Need for newborn admission |
| Starting date | Registered October 2014 ("registered while recruiting") |
| Contact information | Mahdieh Yousef‐Zanjani Dr. Maryam Jafari |
| Notes |
| Study name | Effect of antenatal steroids for women at risk of late preterm delivery on neonatal respiratory morbidity |
| Methods | Randomised parallel assignment Setting: various hospitals in Lebanon Blinding: participants, care provider, outcome assessor and investigator |
| Participants | Estimated recruitment: 700 participants Inclusion criteria: women between 34 0/7‐ 36 6/7 weeks of gestation, at high risk of preterm birth Exclusion criteria
|
| Interventions | Group 1: betamethasone: a single course of betamethasone (two doses of 12 mg/dose given at 24‐hourly intervals) Group 2: saline: two doses of 2 mL of normal saline given at 24‐hourly intervals |
| Outcomes | Primary outcome Secondary outcomes
|
| Starting date | Study start date: September 2010 Estimated completion date: September 2013 Last update was June 2011 (Status: recruiting) |
| Contact information | Principal Investigator: Khalid Yunis, MD American University of Beirut Medical Center |
| Notes |
| Study name | Effect of antenatal corticosteroids on neonatal morbidity |
| Methods | Randomised parallel assignment three‐arm trial Blinding: participant, care provider, investigator Setting: Ahmadu Bello University Teaching Hospital Shika‐Zaria, Nigeria |
| Participants | 100 participants Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: dexamethasone sodium phosphate injection 12 mg X doses to be given 12 hours apart Group 2: betamethasone sodium phosphate injection12 mg X 2 doses given 12 hours apart Group 3: placebo. two doses of intramuscular injection of water for injection given 12 hours apart |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | Start date: December 2017 Completion date: May 2019 |
| Contact information | Anisah Yahya, MBBS, Ahmadu Bello University, Nigeria |
| Notes |
ACS: antenatal corticosteroids
NICU: neonatal intensive care unit
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||
| 1.1 Perinatal death Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] | ||||||||||||||||||||||||
| Analysis 1.1  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 1: Perinatal death | ||||||||||||||||||||||||||||
| 1.2 Neonatal death Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] | ||||||||||||||||||||||||
| Analysis 1.2  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 2: Neonatal death | ||||||||||||||||||||||||||||
| 1.3 Fetal death Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] | ||||||||||||||||||||||||
| Analysis 1.3  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 3: Fetal death | ||||||||||||||||||||||||||||
| 1.4 Respiratory distress syndrome Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] | ||||||||||||||||||||||||
| Analysis 1.4  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 4: Respiratory distress syndrome | ||||||||||||||||||||||||||||
| 1.5 Moderate/severe respiratory distress syndrome Show forest plot | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] | ||||||||||||||||||||||||
| Analysis 1.5  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 5: Moderate/severe respiratory distress syndrome | ||||||||||||||||||||||||||||
| 1.6 Chronic lung disease Show forest plot | 5 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] | ||||||||||||||||||||||||
| Analysis 1.6  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 6: Chronic lung disease | ||||||||||||||||||||||||||||
| 1.7 Intraventricular haemorrhage Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] | ||||||||||||||||||||||||
| Analysis 1.7  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 7: Intraventricular haemorrhage | ||||||||||||||||||||||||||||
| 1.7.1 Any IVH grade | 5 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.84] | ||||||||||||||||||||||||
| 1.7.2 IVH Grade 3‐4 | 5 | 6269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.88] | ||||||||||||||||||||||||
| 1.7.3 IVH diagnosed at postmortem | 2 | 1486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] | ||||||||||||||||||||||||
| 1.8 Mean birthweight (g) Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] | ||||||||||||||||||||||||
| Analysis 1.8  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 8: Mean birthweight (g) | ||||||||||||||||||||||||||||
| 1.9 Maternal death Show forest plot | 6 | 6244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.36, 3.89] | ||||||||||||||||||||||||
| Analysis 1.9  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 9: Maternal death | ||||||||||||||||||||||||||||
| 1.10 Chorioamnionitis Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] | ||||||||||||||||||||||||
| Analysis 1.10  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 10: Chorioamnionitis | ||||||||||||||||||||||||||||
| 1.11 Endometritis Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] | ||||||||||||||||||||||||
| Analysis 1.11  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 11: Endometritis | ||||||||||||||||||||||||||||
| 1.12 Death in childhood Show forest plot | 4 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.27] | ||||||||||||||||||||||||
| Analysis 1.12  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 12: Death in childhood | ||||||||||||||||||||||||||||
| 1.13 Neurodevelopmental disability in childhood Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.13  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 13: Neurodevelopmental disability in childhood | ||||||||||||||||||||||||||||
| 1.13.1 Developmental delay | 3 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.97] | ||||||||||||||||||||||||
| 1.13.2 Intellectual impairment | 3 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.44, 1.69] | ||||||||||||||||||||||||
| 1.13.3 Hearing impairment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.04, 9.87] | ||||||||||||||||||||||||
| 1.13.4 Visual impairment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.23] | ||||||||||||||||||||||||
| 1.14 Death into adulthood Show forest plot | 1 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.81] | ||||||||||||||||||||||||
| Analysis 1.14  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 14: Death into adulthood | ||||||||||||||||||||||||||||
| 1.15 Neurodevelopmental disability in adulthood Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.15  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 15: Neurodevelopmental disability in adulthood | ||||||||||||||||||||||||||||
| 1.15.1 Visual impairment | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.55] | ||||||||||||||||||||||||
| 1.15.2 Hearing impairment | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.03] | ||||||||||||||||||||||||
| 1.15.3 Intellectual impairment | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.95] | ||||||||||||||||||||||||
| 1.16 Fever in women after trial entry requiring the use of antibiotics Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.36, 1.21] | ||||||||||||||||||||||||
| Analysis 1.16  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 16: Fever in women after trial entry requiring the use of antibiotics | ||||||||||||||||||||||||||||
| 1.17 Intrapartum fever in woman requiring the use of antibiotics Show forest plot | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.49] | ||||||||||||||||||||||||
| Analysis 1.17  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 17: Intrapartum fever in woman requiring the use of antibiotics | ||||||||||||||||||||||||||||
| 1.18 Postnatal fever in woman Show forest plot | 5 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] | ||||||||||||||||||||||||
| Analysis 1.18  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 18: Postnatal fever in woman | ||||||||||||||||||||||||||||
| 1.19 Admission into adult intensive care unit Show forest plot | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.05] | ||||||||||||||||||||||||
| Analysis 1.19  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 19: Admission into adult intensive care unit | ||||||||||||||||||||||||||||
| 1.20 Side effects of therapy in women Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.20  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 20: Side effects of therapy in women | ||||||||||||||||||||||||||||
| 1.20.1 Any side effects at first dose | 1 | 2825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.82] | ||||||||||||||||||||||||
| 1.20.2 Dyspnoea | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] | ||||||||||||||||||||||||
| 1.20.3 Gastrointestinal upset | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.12, 73.37] | ||||||||||||||||||||||||
| 1.20.4 Hyperglycaemia | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] | ||||||||||||||||||||||||
| 1.20.5 Leucocytosis | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] | ||||||||||||||||||||||||
| 1.20.6 Migraine | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.93] | ||||||||||||||||||||||||
| 1.21 Glucose intolerance Show forest plot | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.14, 6.46] | ||||||||||||||||||||||||
| Analysis 1.21  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 21: Glucose intolerance | ||||||||||||||||||||||||||||
| 1.22 Hypertension Show forest plot | 2 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.59, 1.79] | ||||||||||||||||||||||||
| Analysis 1.22  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 22: Hypertension | ||||||||||||||||||||||||||||
| 1.23 Apgar < 7 at 5 minutes Show forest plot | 12 | 5727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.98] | ||||||||||||||||||||||||
| Analysis 1.23  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 23: Apgar < 7 at 5 minutes | ||||||||||||||||||||||||||||
| 1.24 Mean interval between trial entry and birth (days) Show forest plot | 3 | 1513 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.86, 2.32] | ||||||||||||||||||||||||
| Analysis 1.24  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 24: Mean interval between trial entry and birth (days) | ||||||||||||||||||||||||||||
| 1.25 Mean length at birth (cm) Show forest plot | 1 | 2766 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.37, 0.37] | ||||||||||||||||||||||||
| Analysis 1.25  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 25: Mean length at birth (cm) | ||||||||||||||||||||||||||||
| 1.26 Mean head circumference at birth (cm) Show forest plot | 1 | 2766 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.22, 0.22] | ||||||||||||||||||||||||
| Analysis 1.26  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 26: Mean head circumference at birth (cm) | ||||||||||||||||||||||||||||
| 1.27 Small‐for‐gestational age Show forest plot | 5 | 3478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.28] | ||||||||||||||||||||||||
| Analysis 1.27  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 27: Small‐for‐gestational age | ||||||||||||||||||||||||||||
| 1.28 Admission to neonatal intensive care unit Show forest plot | 9 | 6667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.00] | ||||||||||||||||||||||||
| Analysis 1.28  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 28: Admission to neonatal intensive care unit | ||||||||||||||||||||||||||||
| 1.29 Need for mechanical ventilation/CPAP Show forest plot | 11 | 4519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] | ||||||||||||||||||||||||
| Analysis 1.29  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 29: Need for mechanical ventilation/CPAP | ||||||||||||||||||||||||||||
| 1.30 Mean duration of mechanical ventilation/CPAP (days) Show forest plot | 3 | 471 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐4.59, 0.76] | ||||||||||||||||||||||||
| Analysis 1.30  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 30: Mean duration of mechanical ventilation/CPAP (days) | ||||||||||||||||||||||||||||
| 1.31 Median (IQR) duration of mechanical ventilation (hours) Show forest plot | 1 | Other data | No numeric data | |||||||||||||||||||||||||
| Analysis 1.31
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 31: Median (IQR) duration of mechanical ventilation (hours) | ||||||||||||||||||||||||||||
| 1.32 Median (IQR) duration of CPAP (hours) Show forest plot | 1 | Other data | No numeric data | |||||||||||||||||||||||||
| Analysis 1.32
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 32: Median (IQR) duration of CPAP (hours) | ||||||||||||||||||||||||||||
| 1.33 Air leak syndrome Show forest plot | 2 | 2965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.32, 1.80] | ||||||||||||||||||||||||
| Analysis 1.33  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 33: Air leak syndrome | ||||||||||||||||||||||||||||
| 1.34 Mean duration of oxygen supplementation (hours) Show forest plot | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐5.51, ‐0.21] | ||||||||||||||||||||||||
| Analysis 1.34  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 34: Mean duration of oxygen supplementation (hours) | ||||||||||||||||||||||||||||
| 1.35 Median (IQR) duration of oxygen supplementation (hours) Show forest plot | 1 | Other data | No numeric data | |||||||||||||||||||||||||
| Analysis 1.35
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 35: Median (IQR) duration of oxygen supplementation (hours) | ||||||||||||||||||||||||||||
| 1.36 Surfactant use Show forest plot | 6 | 6104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] | ||||||||||||||||||||||||
| Analysis 1.36  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 36: Surfactant use | ||||||||||||||||||||||||||||
| 1.37 Systemic infection in the first 48 hours of life Show forest plot | 7 | 1708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] | ||||||||||||||||||||||||
| Analysis 1.37  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 37: Systemic infection in the first 48 hours of life | ||||||||||||||||||||||||||||
| 1.38 Proven infection while in the neonatal intensive care unit Show forest plot | 10 | 5521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.98] | ||||||||||||||||||||||||
| Analysis 1.38  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 38: Proven infection while in the neonatal intensive care unit | ||||||||||||||||||||||||||||
| 1.39 Necrotising enterocolitis Show forest plot | 10 | 4702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.78] | ||||||||||||||||||||||||
| Analysis 1.39  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 39: Necrotising enterocolitis | ||||||||||||||||||||||||||||
| 1.40 Mean infant HPA axis function (cortisol) Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 3.94 [‐3.12, 11.00] | ||||||||||||||||||||||||
| Analysis 1.40  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 40: Mean infant HPA axis function (cortisol) | ||||||||||||||||||||||||||||
| 1.40.1 In babies born < 24 hours after 1st dose | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 9.00 [‐11.93, 29.93] | ||||||||||||||||||||||||
| 1.40.2 In babies born 24‐48 hours after 1st dose | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐8.68, 8.68] | ||||||||||||||||||||||||
| 1.40.3 In babies born > 48 hours after 1st dose | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 13.00 [‐1.90, 27.90] | ||||||||||||||||||||||||
| 1.41 Mean childhood weight (kg) Show forest plot | 2 | 333 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.39, 1.00] | ||||||||||||||||||||||||
| Analysis 1.41  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 41: Mean childhood weight (kg) | ||||||||||||||||||||||||||||
| 1.41.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.32, 1.12] | ||||||||||||||||||||||||
| 1.41.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.55, 1.75] | ||||||||||||||||||||||||
| 1.41.3 Schutte (males) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐3.88, 3.68] | ||||||||||||||||||||||||
| 1.42 Mean childhood head circumference (cm) Show forest plot | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.08, 0.63] | ||||||||||||||||||||||||
| Analysis 1.42  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 42: Mean childhood head circumference (cm) | ||||||||||||||||||||||||||||
| 1.42.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.11, 0.71] | ||||||||||||||||||||||||
| 1.42.2 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.05, 0.85] | ||||||||||||||||||||||||
| 1.42.3 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.51, 1.71] | ||||||||||||||||||||||||
| 1.43 Mean childhood height (cm) Show forest plot | 2 | 334 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐0.26, 2.29] | ||||||||||||||||||||||||
| Analysis 1.43  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 43: Mean childhood height (cm) | ||||||||||||||||||||||||||||
| 1.43.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.39, 2.39] | ||||||||||||||||||||||||
| 1.43.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐3.08, 6.48] | ||||||||||||||||||||||||
| 1.43.3 Schutte (males) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐3.79, 4.99] | ||||||||||||||||||||||||
| 1.44 Mean childhood systolic blood pressure (mmHg) Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐4.06, 0.86] | ||||||||||||||||||||||||
| Analysis 1.44  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 44: Mean childhood systolic blood pressure (mmHg) | ||||||||||||||||||||||||||||
| 1.45 Cerebral palsy in childhood Show forest plot | 5 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.03] | ||||||||||||||||||||||||
| Analysis 1.45  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 45: Cerebral palsy in childhood | ||||||||||||||||||||||||||||
| 1.46 Behavioural/learning difficulties in childhood Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.09] | ||||||||||||||||||||||||
| Analysis 1.46  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 46: Behavioural/learning difficulties in childhood | ||||||||||||||||||||||||||||
| 1.47 Mean adult weight (kg) Show forest plot | 2 | 538 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐6.41, 4.76] | ||||||||||||||||||||||||
| Analysis 1.47  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 47: Mean adult weight (kg) | ||||||||||||||||||||||||||||
| 1.47.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐6.00 [‐12.93, 0.93] | ||||||||||||||||||||||||
| 1.47.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐9.91, 7.91] | ||||||||||||||||||||||||
| 1.47.3 Liggins | 1 | 458 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐0.72, 5.86] | ||||||||||||||||||||||||
| 1.48 Mean adult head circumference (cm) Show forest plot | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.33, 0.38] | ||||||||||||||||||||||||
| Analysis 1.48  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 48: Mean adult head circumference (cm) | ||||||||||||||||||||||||||||
| 1.48.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.03, 1.03] | ||||||||||||||||||||||||
| 1.48.2 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.37, 0.97] | ||||||||||||||||||||||||
| 1.48.3 Liggins | 1 | 458 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.34, 0.46] | ||||||||||||||||||||||||
| 1.49 Mean adult height (cm) Show forest plot | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.28, 2.10] | ||||||||||||||||||||||||
| Analysis 1.49  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 49: Mean adult height (cm) | ||||||||||||||||||||||||||||
| 1.49.1 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐5.37, 3.37] | ||||||||||||||||||||||||
| 1.49.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐2.30, 8.30] | ||||||||||||||||||||||||
| 1.49.3 Liggins (females) | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [‐0.65, 2.99] | ||||||||||||||||||||||||
| 1.49.4 Liggins (males) | 1 | 224 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐1.03, 2.53] | ||||||||||||||||||||||||
| 1.50 Mean adult skinfold thickness (log values) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.50  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 50: Mean adult skinfold thickness (log values) | ||||||||||||||||||||||||||||
| 1.50.1 Triceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] | ||||||||||||||||||||||||
| 1.50.2 Biceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] | ||||||||||||||||||||||||
| 1.50.3 Subscapular | 1 | 441 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] | ||||||||||||||||||||||||
| 1.50.4 Suprailiac | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] | ||||||||||||||||||||||||
| 1.51 Abnormal lung function measured as forced vital capacity (adult) Show forest plot | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.16, 1.76] | ||||||||||||||||||||||||
| Analysis 1.51  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 51: Abnormal lung function measured as forced vital capacity (adult) | ||||||||||||||||||||||||||||
| 1.52 Mean adult systolic blood pressure (mmHg) Show forest plot | 2 | 545 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐2.81, 1.07] | ||||||||||||||||||||||||
| Analysis 1.52  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 52: Mean adult systolic blood pressure (mmHg) | ||||||||||||||||||||||||||||
| 1.52.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐9.12, 1.12] | ||||||||||||||||||||||||
| 1.52.2 Schutte (males) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐7.17, 1.17] | ||||||||||||||||||||||||
| 1.52.3 Liggins | 1 | 455 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐1.88, 2.98] | ||||||||||||||||||||||||
| 1.53 Mean adult insulin (log values) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.53  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 53: Mean adult insulin (log values) | ||||||||||||||||||||||||||||
| 1.53.1 Fasting | 1 | 435 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] | ||||||||||||||||||||||||
| 1.53.2 30 minutes fasting following a 75 g oral glucose tolerance test | 1 | 412 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.04, 0.28] | ||||||||||||||||||||||||
| 1.53.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] | ||||||||||||||||||||||||
| 1.54 Mean adult glucose Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.54  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 54: Mean adult glucose | ||||||||||||||||||||||||||||
| 1.54.1 Fasting | 1 | 432 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] | ||||||||||||||||||||||||
| 1.54.2 30 minutes fasting following a 75 g oral glucose tolerance test | 1 | 413 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.12, 0.54] | ||||||||||||||||||||||||
| 1.54.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.52, ‐0.02] | ||||||||||||||||||||||||
| 1.55 Mean adult HPA axis function (mean log fasting cortisol) Show forest plot | 1 | 444 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.02, 0.14] | ||||||||||||||||||||||||
| Analysis 1.55  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 55: Mean adult HPA axis function (mean log fasting cortisol) | ||||||||||||||||||||||||||||
| 1.56 Mean age at puberty (years) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 1.56  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 56: Mean age at puberty (years) | ||||||||||||||||||||||||||||
| 1.56.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.94, 0.94] | ||||||||||||||||||||||||
| 1.57 Educational achievement by adulthood (university or polytechnic education) Show forest plot | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] | ||||||||||||||||||||||||
| Analysis 1.57  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 57: Educational achievement by adulthood (university or polytechnic education) | ||||||||||||||||||||||||||||
| 1.58 Mean length of antenatal hospitalisation (days) Show forest plot | 2 | 412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.23, 0.22] | ||||||||||||||||||||||||
| Analysis 1.58  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 58: Mean length of antenatal hospitalisation (days) | ||||||||||||||||||||||||||||
| 1.59 Length of maternal hospital stay Show forest plot | 4 | Other data | No numeric data | |||||||||||||||||||||||||
| Analysis 1.59
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 59: Length of maternal hospital stay | ||||||||||||||||||||||||||||
| 1.60 Mean length of postnatal hospitalisation (days) Show forest plot | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.72, 1.72] | ||||||||||||||||||||||||
| Analysis 1.60  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 60: Mean length of postnatal hospitalisation (days) | ||||||||||||||||||||||||||||
| 1.61 Mean length of neonatal hospitalisation (days) Show forest plot | 5 | 788 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.51, 0.87] | ||||||||||||||||||||||||
| Analysis 1.61  Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 61: Mean length of neonatal hospitalisation (days) | ||||||||||||||||||||||||||||
| 1.62 Length of neonatal hospitalisation Show forest plot | 2 | Other data | No numeric data | |||||||||||||||||||||||||
| Analysis 1.62
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 62: Length of neonatal hospitalisation | ||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Perinatal death ‐ single or multiple pregnancy Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| Analysis 2.1  Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 1: Perinatal death ‐ single or multiple pregnancy | ||||
| 2.1.1 In babies born from singleton pregnancies | 7 | 5492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.99] |
| 2.1.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.41, 1.22] |
| 2.1.3 Mixed population | 7 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 2.2 Neonatal death ‐ single or multiple pregnancy Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Analysis 2.2  Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 2: Neonatal death ‐ single or multiple pregnancy | ||||
| 2.2.1 In babies born from singleton pregnancies | 13 | 8453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 2.2.2 In babies born from multiple pregnancies | 3 | 813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.02] |
| 2.2.3 Mixed population | 9 | 1343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 2.3 Fetal death ‐ single or multiple pregnancy Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.21] |
| Analysis 2.3  Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 3: Fetal death ‐ single or multiple pregnancy | ||||
| 2.3.1 In babies born from singleton pregnancies | 7 | 5492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.46] |
| 2.3.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.40] |
| 2.3.3 Mixed population | 7 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.29] |
| 2.4 Respiratory distress syndrome ‐ single or multiple pregnancy Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| Analysis 2.4  Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 4: Respiratory distress syndrome ‐ single or multiple pregnancy | ||||
| 2.4.1 In babies born from singleton pregnancies | 17 | 6731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.57, 0.74] |
| 2.4.2 In babies born from multiple pregnancies | 4 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.20] |
| 2.4.3 Mixed population | 9 | 4129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.92] |
| 2.5 Intraventricular haemorrhage ‐ single or multiple pregnancy Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| Analysis 2.5  Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 5: Intraventricular haemorrhage ‐ single or multiple pregnancy | ||||
| 2.5.1 In babies born from singleton pregnancies | 6 | 4494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.75] |
| 2.5.2 In babies born from multiple pregnancies | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.08, 2.26] |
| 2.5.3 Mixed population | 6 | 3831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Perinatal death ‐ intact or ruptured membranes Show forest plot | 14 | 9804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| Analysis 3.1  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 1: Perinatal death ‐ intact or ruptured membranes | ||||
| 3.1.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.10] |
| 3.1.2 In babies born from pregnancies with ruptured membranes at 1st dose | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.83] |
| 3.1.3 Not reported or mixed population | 8 | 7784 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.97] |
| 3.2 Neonatal deaths ‐ intact or ruptured membranes Show forest plot | 22 | 10580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Analysis 3.2  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 2: Neonatal deaths ‐ intact or ruptured membranes | ||||
| 3.2.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.05] |
| 3.2.2 In babies born from pregnancies with ruptured membranes at 1st dose | 7 | 1014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.84] |
| 3.2.3 Not reported or mixed population | 12 | 8234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.91] |
| 3.3 Fetal death ‐ intact or ruptured membranes Show forest plot | 14 | 9804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| Analysis 3.3  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 3: Fetal death ‐ intact or ruptured membranes | ||||
| 3.3.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| 3.3.2 In babies born from pregnancies with ruptured membranes at 1st dose | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.61] |
| 3.3.3 Not reported or mixed population | 8 | 7784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.26] |
| 3.4 RDS ‐ intact or ruptured membranes Show forest plot | 26 | 11079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.64, 0.78] |
| Analysis 3.4  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 4: RDS ‐ intact or ruptured membranes | ||||
| 3.4.1 In babies born from pregnancies with intact membranes at 1st dose | 8 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.4.2 In babies born from pregnancies with ruptured membranes at 1st dose | 10 | 1202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.87] |
| 3.4.3 Not reported or mixed population | 13 | 7953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.88] |
| 3.5 IVH ‐ intact or ruptured membranes Show forest plot | 12 | 8446 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| Analysis 3.5  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 5: IVH ‐ intact or ruptured membranes | ||||
| 3.5.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 3.5.2 In babies born from pregnancies with ruptured membranes at 1st dose | 4 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.28, 0.79] |
| 3.5.3 Not reported or mixed population | 5 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.38] |
| 3.6 Birthweight ‐ intact or ruptured membranes Show forest plot | 19 | 9522 | Mean Difference (IV, Fixed, 95% CI) | ‐14.86 [‐34.59, 4.87] |
| Analysis 3.6  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 6: Birthweight ‐ intact or ruptured membranes | ||||
| 3.6.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1301 | Mean Difference (IV, Fixed, 95% CI) | ‐30.27 [‐100.43, 39.89] |
| 3.6.2 In babies born from pregnancies with ruptured membranes at 1st dose | 5 | 835 | Mean Difference (IV, Fixed, 95% CI) | ‐49.72 [‐113.91, 14.46] |
| 3.6.3 Not reported or mixed population | 11 | 7386 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐31.10, 12.30] |
| 3.7 Chorioamnionitis ‐ intact or ruptured membranes Show forest plot | 15 | 8345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.09] |
| Analysis 3.7  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 7: Chorioamnionitis ‐ intact or ruptured membranes | ||||
| 3.7.1 In women with intact membranes at 1st dose | 4 | 1243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.40] |
| 3.7.2 In women with ruptured membranes at 1st dose | 7 | 1129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.72, 1.48] |
| 3.7.3 Not reported or mixed population | 5 | 5973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.09] |
| 3.8 Endometritis ‐ intact or ruptured membranes Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| Analysis 3.8  Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 8: Endometritis ‐ intact or ruptured membranes | ||||
| 3.8.1 In women with intact membranes at 1st dose | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 2.00] |
| 3.8.2 In women with ruptured membranes at 1st dose | 4 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.25] |
| 3.8.3 Not reported or mixed population | 5 | 5998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.73, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Perinatal deaths ‐ hypertension syndrome vs other trials Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| Analysis 4.1  Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 1: Perinatal deaths ‐ hypertension syndrome vs other trials | ||||
| 4.1.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.57, 1.20] |
| 4.1.2 No hypertension syndrome or hypertension syndromes excluded | 1 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 4.1.3 Hypertension not reported separately | 12 | 8397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.94] |
| 4.2 Neonatal deaths ‐ hypertension syndrome vs other trials Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Analysis 4.2  Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 2: Neonatal deaths ‐ hypertension syndrome vs other trials | ||||
| 4.2.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.83] |
| 4.2.2 No hypertension syndrome or hypertension syndromes excluded | 1 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.25] |
| 4.2.3 Hypertension not reported separately | 20 | 9173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.88] |
| 4.3 Fetal deaths ‐ hypertension syndrome vs other trials Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| Analysis 4.3  Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 3: Fetal deaths ‐ hypertension syndrome vs other trials | ||||
| 4.3.1 Women with hypertension syndrome | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.91, 3.28] |
| 4.3.2 No hypertension syndrome or hypertension syndromes excluded | 2 | 1373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.12] |
| 4.3.3 Hypertension not reported separately | 11 | 8129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.83, 1.30] |
| 4.4 Respiratory distress syndrome ‐ hypertension syndrome vs other trials Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| Analysis 4.4  Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 4: Respiratory distress syndrome ‐ hypertension syndrome vs other trials | ||||
| 4.4.1 Hypertension syndrome | 5 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.69] |
| 4.4.2 No hypertension syndrome or hypertension syndromes excluded | 7 | 2511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.48, 0.74] |
| 4.4.3 Hypertension not reported separately | 19 | 8254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Perinatal death ‐ type of steroid Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| Analysis 5.1  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 1: Perinatal death ‐ type of steroid | ||||
| 5.1.1 Dexamethasone | 6 | 4673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.95] |
| 5.1.2 Betamethasone | 8 | 5092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.99] |
| 5.1.3 Methylprednisolone | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.43, 5.43] |
| 5.2 Neonatal death ‐ type of steroid Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Analysis 5.2  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 2: Neonatal death ‐ type of steroid | ||||
| 5.2.1 Dexamethasone | 7 | 4769 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.91] |
| 5.2.2 Betamethasone | 14 | 5593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.89] |
| 5.2.3 Hydrocortisone | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.47] |
| 5.2.4 Methylprednisolone | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.42, 3.12] |
| 5.3 Fetal death ‐ type of steroid Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| Analysis 5.3  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 3: Fetal death ‐ type of steroid | ||||
| 5.3.1 Dexamethasone | 6 | 4673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.25] |
| 5.3.2 Betamethasone | 8 | 5092 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 5.3.3 Methylprednisolone | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.08, 47.36] |
| 5.4 Respiratory distress syndrome ‐ type of steroid Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.78] |
| Analysis 5.4  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 4: Respiratory distress syndrome ‐ type of steroid | ||||
| 5.4.1 Dexamethasone | 8 | 4963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.92] |
| 5.4.2 Betamethasone | 17 | 5973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.55, 0.72] |
| 5.4.3 Hydrocortisone | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.28] |
| 5.4.4 Methylprednisolone | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.56, 2.27] |
| 5.5 Moderate/severe respiratory distress syndrome ‐ type of steroid Show forest plot | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| Analysis 5.5  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 5: Moderate/severe respiratory distress syndrome ‐ type of steroid | ||||
| 5.5.1 Dexamethasone | 2 | 3166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 5.5.2 Betamethasone | 5 | 1655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.37, 0.67] |
| 5.5.3 Hydrocortisone | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.63] |
| 5.5.4 Methylprednisolone | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.26, 5.31] |
| 5.6 Chronic lung disease ‐ type of steroid Show forest plot | 5 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] |
| Analysis 5.6  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 6: Chronic lung disease ‐ type of steroid | ||||
| 5.6.1 Dexamethasone | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.41, 9.16] |
| 5.6.2 Betamethasone | 3 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.42] |
| 5.7 IVH ‐ type of steroid Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| Analysis 5.7  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 7: IVH ‐ type of steroid | ||||
| 5.7.1 Dexamethasone | 4 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 5.7.2 Betamethasone | 8 | 4981 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.68] |
| 5.8 Birthweight ‐ type of steroid Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| Analysis 5.8  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 8: Birthweight ‐ type of steroid | ||||
| 5.8.1 Dexamethasone | 6 | 3972 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐31.09, 38.76] |
| 5.8.2 Betamethasone | 12 | 5401 | Mean Difference (IV, Fixed, 95% CI) | ‐20.40 [‐44.61, 3.81] |
| 5.8.3 Hydrocortisone | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐146.68 [‐371.30, 77.93] |
| 5.8.4 Methylprednisolone | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐121.00 [‐430.59, 188.59] |
| 5.9 Chorioamnionitis ‐ type of steroid Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| Analysis 5.9  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 9: Chorioamnionitis ‐ type of steroid | ||||
| 5.9.1 Dexamethasone | 6 | 3621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.84, 1.71] |
| 5.9.2 Betamethasone | 9 | 4753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 5.10 Endometritis ‐ type of steroid Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| Analysis 5.10  Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 10: Endometritis ‐ type of steroid | ||||
| 5.10.1 Dexamethasone | 4 | 3270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.92, 2.90] |
| 5.10.2 Betamethasone | 6 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Perinatal death ‐ decade of trial Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| Analysis 6.1  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 1: Perinatal death ‐ decade of trial | ||||
| 6.1.1 Trials conducted in 1970s | 5 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.06] |
| 6.1.2 Trials conducted in 1980s | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.59, 2.21] |
| 6.1.3 Trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
| 6.1.4 Trials conducted in 2000s | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.70] |
| 6.1.5 Trials conducted in 2010s | 3 | 6207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.99] |
| 6.2 Neonatal death ‐ decade of trial Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Analysis 6.2  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 2: Neonatal death ‐ decade of trial | ||||
| 6.2.1 Trials conducted in 1970s | 7 | 2743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.04] |
| 6.2.2 Trials conducted in 1980s | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.55, 1.49] |
| 6.2.3 Trials conducted in 1990s | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 6.2.4 Trials conducted in 2000s | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.66] |
| 6.2.5 Trials conducted in 2010s | 4 | 6482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 6.3 Fetal death ‐ decade of trial Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| Analysis 6.3  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 3: Fetal death ‐ decade of trial | ||||
| 6.3.1 Trials conducted in 1970s | 5 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.32] |
| 6.3.2 Trials conducted in 1980s | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [0.37, 31.41] |
| 6.3.3 Trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.49, 2.36] |
| 6.3.4 Trials conducted in 2000s | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.19, 4.50] |
| 6.3.5 Trials conducted in 2010s | 3 | 6207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.30] |
| 6.4 Respiratory distress syndrome ‐ decade of trial Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| Analysis 6.4  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 4: Respiratory distress syndrome ‐ decade of trial | ||||
| 6.4.1 Trials conducted in 1970s | 8 | 2823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.54, 0.78] |
| 6.4.2 Trials conducted in 1980s | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.88] |
| 6.4.3 Trials conducted in 1990s | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.92] |
| 6.4.4 Trials conducted in 2000s | 5 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.26, 0.59] |
| 6.4.5 Trials conducted in 2010s | 4 | 6401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 6.5 IVH ‐ decade of trial Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| Analysis 6.5  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 5: IVH ‐ decade of trial | ||||
| 6.5.1 Trials conducted in 1970s | 1 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.33, 1.12] |
| 6.5.2 Trials conducted in 1980s | 3 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.81] |
| 6.5.3 Trials conducted in 1990s | 4 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 6.5.4 Trials conducted in 2000s | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.73] |
| 6.5.5 Trials conducted in 2010s | 2 | 5897 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.69, 8.31] |
| 6.6 Birthweight ‐ decade of trial Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| Analysis 6.6  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 6: Birthweight ‐ decade of trial | ||||
| 6.6.1 Trials conducted in 1970s | 5 | 1822 | Mean Difference (IV, Fixed, 95% CI) | ‐41.39 [‐110.05, 27.26] |
| 6.6.2 Trials conducted in 1980s | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐19.60 [‐108.55, 69.35] |
| 6.6.3 Trials conducted in 1990s | 4 | 569 | Mean Difference (IV, Fixed, 95% CI) | ‐33.13 [‐102.39, 36.13] |
| 6.6.4 Trials conducted in 2000s | 3 | 573 | Mean Difference (IV, Fixed, 95% CI) | ‐20.77 [‐61.95, 20.41] |
| 6.6.5 Trials conducted in 2010s | 4 | 6307 | Mean Difference (IV, Fixed, 95% CI) | ‐3.82 [‐30.36, 22.72] |
| 6.7 Chorioamnionitis ‐ decade of trial Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| Analysis 6.7  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 7: Chorioamnionitis ‐ decade of trial | ||||
| 6.7.1 Trials conducted in 1970s | 2 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.17] |
| 6.7.2 Trials conducted in 1980s | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.01] |
| 6.7.3 Trials conducted in 1990s | 5 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.89] |
| 6.7.4 Trials conducted in 2000s | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.59, 6.95] |
| 6.7.5 Trials conducted in 2010s | 3 | 5873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.48, 1.11] |
| 6.8 Endometritis ‐ decade of trial Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| Analysis 6.8  Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 8: Endometritis ‐ decade of trial | ||||
| 6.8.1 Trials conducted in 1970s | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.86] |
| 6.8.2 Trials conducted in 1980s | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.88, 6.06] |
| 6.8.3 Trials conducted in 1990s | 4 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.44] |
| 6.8.4 Trials conducted in 2000s | 4 | 6018 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.75, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Perinatal death ‐ protocol with weekly repeats Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| Analysis 7.1  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 1: Perinatal death ‐ protocol with weekly repeats | ||||
| 7.1.1 Single course only | 10 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.04] |
| 7.1.2 Courses including weekly repeats | 4 | 3504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.93] |
| 7.2 Neonatal death ‐ protocol with weekly repeats Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Analysis 7.2  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 2: Neonatal death ‐ protocol with weekly repeats | ||||
| 7.2.1 Single course only | 14 | 6636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.68, 1.02] |
| 7.2.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.86] |
| 7.3 Fetal death ‐ protocol with weekly repeats Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| Analysis 7.3  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 3: Fetal death ‐ protocol with weekly repeats | ||||
| 7.3.1 Single course only | 10 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 7.3.2 Courses including weekly repeats | 4 | 3504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.31] |
| 7.4 Respiratory distress syndrome ‐ protocol with weekly repeats Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| Analysis 7.4  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 4: Respiratory distress syndrome ‐ protocol with weekly repeats | ||||
| 7.4.1 Single course only | 18 | 7210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 7.4.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| 7.5 Moderate/severe respiratory distress syndrome Show forest plot | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| Analysis 7.5  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 5: Moderate/severe respiratory distress syndrome | ||||
| 7.5.1 Single course only | 3 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.88] |
| 7.5.2 Courses including weekly repeats | 4 | 3515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.89] |
| 7.6 IVH ‐ protocol with weekly repeats Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| Analysis 7.6  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 6: IVH ‐ protocol with weekly repeats | ||||
| 7.6.1 Single course only | 4 | 4502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] |
| 7.6.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.43, 0.79] |
| 7.7 Birthweight ‐ protocol with weekly repeats Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| Analysis 7.7  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 7: Birthweight ‐ protocol with weekly repeats | ||||
| 7.7.1 Single course only | 14 | 6165 | Mean Difference (IV, Fixed, 95% CI) | ‐20.90 [‐44.39, 2.60] |
| 7.7.2 Courses including weekly repeats | 5 | 3386 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐33.92, 39.36] |
| 7.8 Chorioamnionitis ‐ protocol with weekly repeats Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| Analysis 7.8  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 8: Chorioamnionitis ‐ protocol with weekly repeats | ||||
| 7.8.1 Single courses only | 7 | 4659 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.11] |
| 7.8.2 Courses including weekly repeats | 8 | 3715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.28] |
| 7.9 Endometritis ‐ protocol with weekly repeats Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| Analysis 7.9  Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 9: Endometritis ‐ protocol with weekly repeats | ||||
| 7.9.1 Single courses only | 4 | 3332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.39] |
| 7.9.2 Courses including weekly repeats | 6 | 3432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.94, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Perinatal death ‐ gestational age at trial entry Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.77, 0.92] |
| Analysis 8.1  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 1: Perinatal death ‐ gestational age at trial entry | ||||
| 8.1.1 Less than or equal to 35 weeks + 0 days | 11 | 6185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| 8.1.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.68, 4.28] |
| 8.2 Neonatal death ‐ gestational age at trial entry Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.86] |
| Analysis 8.2  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 2: Neonatal death ‐ gestational age at trial entry | ||||
| 8.2.1 Less than or equal to 35 weeks + 0 days | 19 | 6961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.69, 0.86] |
| 8.2.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.49, 4.61] |
| 8.3 Fetal death ‐ gestational age at trial entry Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| Analysis 8.3  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 3: Fetal death ‐ gestational age at trial entry | ||||
| 8.3.1 Less than or equal to 35 weeks + 0 days | 11 | 6185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.19] |
| 8.3.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.42, 8.82] |
| 8.4 Respiratory distress syndrome ‐ gestational age at trial entry Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| Analysis 8.4  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 4: Respiratory distress syndrome ‐ gestational age at trial entry | ||||
| 8.4.1 Less than or equal to 35 weeks + 0 days | 20 | 7041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 8.4.2 Greater than or equal to 34 weeks + 0 days | 7 | 4142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.95] |
| 8.5 IVH ‐ gestational age at trial entry Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.74] |
| Analysis 8.5  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 5: IVH ‐ gestational age at trial entry | ||||
| 8.5.1 Less than or equal to 35 weeks + 0 days | 11 | 5412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.72] |
| 8.5.2 Greater than or equal to 34 weeks + 0 days | 2 | 3063 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 102.09] |
| 8.6 Birthweight ‐ gestational age at trial entry Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐13.36 [‐32.99, 6.26] |
| Analysis 8.6  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 6: Birthweight ‐ gestational age at trial entry | ||||
| 8.6.1 Less than or equal to 35 weeks + 0 days | 13 | 5412 | Mean Difference (IV, Fixed, 95% CI) | ‐9.78 [‐40.81, 21.24] |
| 8.6.2 Greater than or equal to 34 weeks + 0 days | 7 | 4139 | Mean Difference (IV, Fixed, 95% CI) | ‐15.75 [‐41.09, 9.58] |
| 8.7 Chorioamnionitis ‐ gestational age at trial entry Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.07] |
| Analysis 8.7  Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 7: Chorioamnionitis ‐ gestational age at trial entry | ||||
| 8.7.1 Less than or equal to 35 weeks + 0 days | 13 | 5132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 8.7.2 Greater than or equal to 34 weeks + 0 days | 3 | 3242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.99] |
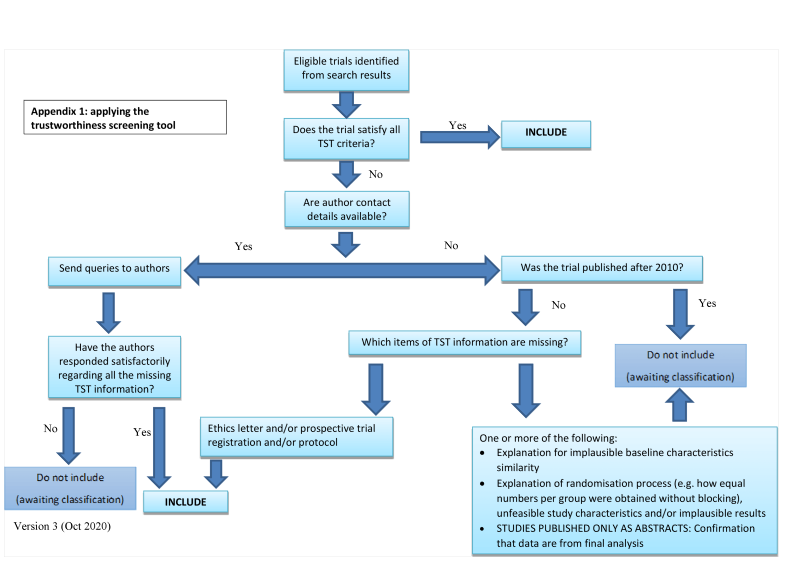
Applying the trustworthiness screening tool criteria

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.4 Perinatal deaths

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.5 Neonatal deaths

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.6 Fetal deaths

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.7 Respiratory distress syndrome

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.7 Intraventricular haemorrhage.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.11 Mean birthweight (g)

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.2 Chorioamnionitis

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.11 Endometritis.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.30 Apgar < 7 at 5 minutes

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.25 Need for mechanical ventilation/CPAP

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.38 Proven infection while in the neonatal intensive care unit.

Funnel plot of comparison: 1 Corticosteroids versus placebo or no treatment, outcome: 1.39 Necrotising enterocolitis.

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 1: Perinatal death

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 2: Neonatal death

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 3: Fetal death

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 4: Respiratory distress syndrome

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 5: Moderate/severe respiratory distress syndrome

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 6: Chronic lung disease

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 7: Intraventricular haemorrhage

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 8: Mean birthweight (g)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 9: Maternal death

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 10: Chorioamnionitis

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 11: Endometritis

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 12: Death in childhood

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 13: Neurodevelopmental disability in childhood

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 14: Death into adulthood

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 15: Neurodevelopmental disability in adulthood

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 16: Fever in women after trial entry requiring the use of antibiotics

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 17: Intrapartum fever in woman requiring the use of antibiotics

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 18: Postnatal fever in woman

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 19: Admission into adult intensive care unit

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 20: Side effects of therapy in women

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 21: Glucose intolerance

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 22: Hypertension

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 23: Apgar < 7 at 5 minutes

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 24: Mean interval between trial entry and birth (days)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 25: Mean length at birth (cm)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 26: Mean head circumference at birth (cm)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 27: Small‐for‐gestational age

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 28: Admission to neonatal intensive care unit

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 29: Need for mechanical ventilation/CPAP

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 30: Mean duration of mechanical ventilation/CPAP (days)
| Median (IQR) duration of mechanical ventilation (hours) | ||
| Study | Corticosteroids | Placebo |
|---|---|---|
| WHO 2020 | 18 hours (12‐48) 83 infants | 18 hours (12‐60) 103 infants |
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 31: Median (IQR) duration of mechanical ventilation (hours)
| Median (IQR) duration of CPAP (hours) | ||
| Study | Corticosteroids | Placebo |
|---|---|---|
| WHO 2020 | 48 hours (24‐96) 265 infants | 48 hours (24‐84) 337 infants |
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 32: Median (IQR) duration of CPAP (hours)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 33: Air leak syndrome

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 34: Mean duration of oxygen supplementation (hours)
| Median (IQR) duration of oxygen supplementation (hours) | ||
| Study | Corticosteroids | Placebo |
|---|---|---|
| WHO 2020 | 36 (18‐96) 726 infants | 48 (12‐93) 756 infants |
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 35: Median (IQR) duration of oxygen supplementation (hours)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 36: Surfactant use

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 37: Systemic infection in the first 48 hours of life

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 38: Proven infection while in the neonatal intensive care unit

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 39: Necrotising enterocolitis

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 40: Mean infant HPA axis function (cortisol)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 41: Mean childhood weight (kg)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 42: Mean childhood head circumference (cm)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 43: Mean childhood height (cm)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 44: Mean childhood systolic blood pressure (mmHg)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 45: Cerebral palsy in childhood

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 46: Behavioural/learning difficulties in childhood

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 47: Mean adult weight (kg)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 48: Mean adult head circumference (cm)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 49: Mean adult height (cm)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 50: Mean adult skinfold thickness (log values)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 51: Abnormal lung function measured as forced vital capacity (adult)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 52: Mean adult systolic blood pressure (mmHg)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 53: Mean adult insulin (log values)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 54: Mean adult glucose

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 55: Mean adult HPA axis function (mean log fasting cortisol)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 56: Mean age at puberty (years)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 57: Educational achievement by adulthood (university or polytechnic education)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 58: Mean length of antenatal hospitalisation (days)
| Length of maternal hospital stay | |||
| Study | Measure | Corticosteroids | Control |
|---|---|---|---|
| Attawattanakul 2015 | Overall length of maternal hospital stay (days) (mean (SD)) | 3.57 (0.87) 96 women | 3.58 (0.75) 98 women |
| Gyamfi‐Bannerman 2016 | Overall length of maternal hospital stay (days) (median (IQR)) | 3 (3 to 5) 1427 women | 3 (3 to 5) 1400 women |
| Mansouri 2010 | Number of women requiring a hospital stay of more than three days | 12/100 | 12/100 |
| WHO 2020 | Overall length of maternal hospital stay (days) (median (IQR)) | 8 (4 to 20) 1323 women | 8 (4 to19) 1322 women |
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 59: Length of maternal hospital stay

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 60: Mean length of postnatal hospitalisation (days)

Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 61: Mean length of neonatal hospitalisation (days)
| Length of neonatal hospitalisation | |||
| Study | Measure | Corticosteroids | Control |
|---|---|---|---|
| Gyamfi‐Bannerman 2016 | Overall length of neonatal hospital stay (days) (median (IQR)) | 7 (4 to 12) 1427 infants | 8 (4 to 13) 1400 infants |
| WHO 2020 | Overall length of neonatal hospital stay (days) (median (IQR)) | 8 (3 to17) 1320 infants | 8 (3 to 17) 1301 infants |
Comparison 1: Corticosteroids versus placebo or no treatment, Outcome 62: Length of neonatal hospitalisation

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 1: Perinatal death ‐ single or multiple pregnancy

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 2: Neonatal death ‐ single or multiple pregnancy

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 3: Fetal death ‐ single or multiple pregnancy

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 4: Respiratory distress syndrome ‐ single or multiple pregnancy

Comparison 2: Corticosteroids versus placebo or no treatment ‐ single or multiple pregnancy, Outcome 5: Intraventricular haemorrhage ‐ single or multiple pregnancy

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 1: Perinatal death ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 2: Neonatal deaths ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 3: Fetal death ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 4: RDS ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 5: IVH ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 6: Birthweight ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 7: Chorioamnionitis ‐ intact or ruptured membranes

Comparison 3: Corticosteroids versus placebo or no treatment ‐ intact membranes versus ruptured membranes at first dose, Outcome 8: Endometritis ‐ intact or ruptured membranes

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 1: Perinatal deaths ‐ hypertension syndrome vs other trials

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 2: Neonatal deaths ‐ hypertension syndrome vs other trials

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 3: Fetal deaths ‐ hypertension syndrome vs other trials

Comparison 4: Corticosteroids versus placebo or no treatment ‐ hypertension syndrome versus all other trials, Outcome 4: Respiratory distress syndrome ‐ hypertension syndrome vs other trials

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 1: Perinatal death ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 2: Neonatal death ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 3: Fetal death ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 4: Respiratory distress syndrome ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 5: Moderate/severe respiratory distress syndrome ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 6: Chronic lung disease ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 7: IVH ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 8: Birthweight ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 9: Chorioamnionitis ‐ type of steroid

Comparison 5: Corticosteroids versus placebo or no treatment ‐ type of steroid, Outcome 10: Endometritis ‐ type of steroid

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 1: Perinatal death ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 2: Neonatal death ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 3: Fetal death ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 4: Respiratory distress syndrome ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 5: IVH ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 6: Birthweight ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 7: Chorioamnionitis ‐ decade of trial

Comparison 6: Corticosteroids versus placebo or no treatment ‐ decade of trial, Outcome 8: Endometritis ‐ decade of trial

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 1: Perinatal death ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 2: Neonatal death ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 3: Fetal death ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 4: Respiratory distress syndrome ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 5: Moderate/severe respiratory distress syndrome

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 6: IVH ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 7: Birthweight ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 8: Chorioamnionitis ‐ protocol with weekly repeats

Comparison 7: Corticosteroids versus placebo or no treatment ‐ weekly repeats, Outcome 9: Endometritis ‐ protocol with weekly repeats

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 1: Perinatal death ‐ gestational age at trial entry

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 2: Neonatal death ‐ gestational age at trial entry

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 3: Fetal death ‐ gestational age at trial entry

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 4: Respiratory distress syndrome ‐ gestational age at trial entry

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 5: IVH ‐ gestational age at trial entry

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 6: Birthweight ‐ gestational age at trial entry

Comparison 8: Corticosteroids versus placebo or no treatment ‐ gestational age at trial entry, Outcome 7: Chorioamnionitis ‐ gestational age at trial entry
| Neonatal and child outcomes: corticosteroids compared to placebo or no treatment for accelerating fetal lung maturation for women at risk of preterm birth | ||||||
| Patient or population: infants born of women at risk of preterm birth | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|
| Without Corticosteroids | With Corticosteroids | Difference | ||||
| Perinatal death (composite of fetal death (in utero death) and neonatal death) | RR 0.85 | Study population | ⊕⊕⊕⊕ | Corticosteroids reduce perinatal death compared with placebo or no treatment. | ||
| 15.6% | 13.3% | 2.3% fewer | ||||
| Neonatal death (infants born with signs of life who die within the first 28 days) | RR 0.78 | Study population | ⊕⊕⊕⊕ | Corticosteroids reduce neonatal death compared with placebo or no treatment. | ||
| 11.9% | 9.3% | 2.6% fewer | ||||
| Respiratory distress syndrome | RR 0.71 | Study population | ⊕⊕⊕⊕ | Corticosteroids reduce respiratory distress syndrome compared with placebo or no treatment. We did not downgrade for risk of bias (two trials) at high risk of bias due to lack of placebo in control) because sensitivity analysis removing those trials made very little difference to the effect estimate. | ||
| 14.8% | 10.5% | 4.3% fewer | ||||
| Intraventricular haemorrhage (IVH) | RR 0.58 | Study population | ⊕⊕⊕⊝ | Corticosteroids probably reduce intraventricular haemorrhage compared with placebo or no treatment. We did not downgrade for risk of bias (four trials infants) at high risk of bias due to lack of placebo in control groups) because sensitivity analysis removing those trials made very little difference to the effect estimate. | ||
| 3.3% | 1.9% | 1.4% fewer | ||||
| Mean birthweight (g) | ‐ | The mean birthweight in the control group ranged from 941 g to 2654 g | ‐ | MD 14.02 lower | ⊕⊕⊕⊕ | Corticosteroids result in little to no difference in mean birthweight compared with placebo or no treatment. We did not downgrade for risk of bias (two trials at high risk of bias due to incomplete outcome data) because sensitivity analysis removing those trials made very little difference to the effect estimate. We did not downgrade for imprecision because the confidence interval showed a difference at most on average of 33 g in weight, which is less than 10% of the lightest mean weight in any trial. |
| Developmental delay in childhood Age at follow‐up: 2 to 12 years. | RR 0.51 (0.27 to 0.97) | 7.7% | 4.0% (2.1 to 7.5) | 3.8% fewer (5.7 fewer to 0.2 fewer) | ⊕⊕⊕⊝ | Corticosteroids probably lead to a slight reduction in developmental delay in childhood compared with placebo or no treatment. Additionally, in three studies (778 children) it was uncertain if corticosteroids had any effect on intellectual impairment (RR 0.86, 95% CI 0.44 to 1.69). In two studies (166 children) it was uncertain if corticosteroids had any effect on the risk of visual impairment (RR 0.55, 95% CI 0.24 to 1.23) and in one study (82 children) it was uncertain if corticosteroids have any effect on hearing impairment (RR 0.64, 95% CI 0.04 to 9.87). Another study reported no children with hearing impairment in either group (84 children). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for indirectness: in some trials only a subset of infants were screened for IVH; some trials diagnosed IVH at postmortem only. 2 Downgraded one level for risk of bias: unclear randomisation, allocation concealment, incomplete outcome data and selective reporting | ||||||
| Maternal outcomes: corticosteroids compared to placebo or no treatment for accelerating fetal lung maturation for women at risk of preterm birth | ||||||
| Patient or population: women at risk of preterm birth | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|
| Without Corticosteroids | With Corticosteroids | Difference | ||||
| Maternal death | RR 1.19 | Study population | ⊕⊕⊕⊝ | Corticosteroids probably result in little to no difference in maternal death, but the wide 95% CI includes possible benefit and possible harm, compared to placebo or no treatment. Four studies (3174 women) reported zero deaths in either arm. | ||
| 0.2% | 0.2% | 0.0% fewer (0.1 fewer to 0.5 more) | ||||
| Chorioamnionitis | RR 0.86 | Study population | ⊕⊕⊕⊝ | Corticosteroids probably make little to no difference to the risk of chorioamnionitis but the wide 95% CI includes possible benefit and possible harm, compared to placebo/no treatment. | ||
| 3.5% | 3.0% | 0.5% fewer | ||||
| Endometritis | RR 1.14 | Study population | ⊕⊕⊕⊝ | Corticosteroids probably make little to no difference to the risk of endometritis but the wide 95% CI includes possible benefit and possible harm, compared to placebo/no treatment. | ||
| 1.8% | 2.1% | 0.3% more | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision: few events and wide 95% CI that includes possible benefit and possible harm 2 Downgraded one level for imprecision: wide 95% CI that includes possible benefit and possible harm | ||||||
| Trial | Minimum (weeks+days) | Maximum (weeks+days) |
| 28+0 | 34+6 | |
| 34+0 | 36+6 | |
| 34+0 | 36+6 | |
| Not reported | 36+6 | |
| 26+0 | 37+0 | |
| 28+0 | 34+6 | |
| 26+0 | 34+6 | |
| Not reported | 34+6 | |
| 24+0 | 27+6 | |
| 34+0 | 36+6 | |
| 24+0 | 31+6 | |
| 24+0 | 34+6 | |
| 24+0 | 36+6 | |
| 27+0 | 35+0 | |
| 35+0 | 36+6 | |
| 26+0 | 34+6 | |
| Not reported | 34+0 | |
| 28+0 | 34+6 | |
| 34+0 | 36+6 | |
| 34+0 | 36+6 | |
| 27+0 | 34+6 | |
| 26+0 | 32+6 | |
| 26+0 | 32+0 | |
| 34+0 | 36+6 | |
| 24+0 | 29+6 | |
| 28+0 | 35+6 | |
| 26+0 | 33+6 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Perinatal death Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 1.2 Neonatal death Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 1.3 Fetal death Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 1.4 Respiratory distress syndrome Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 1.5 Moderate/severe respiratory distress syndrome Show forest plot | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 1.6 Chronic lung disease Show forest plot | 5 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] |
| 1.7 Intraventricular haemorrhage Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 1.7.1 Any IVH grade | 5 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.84] |
| 1.7.2 IVH Grade 3‐4 | 5 | 6269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.88] |
| 1.7.3 IVH diagnosed at postmortem | 2 | 1486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] |
| 1.8 Mean birthweight (g) Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 1.9 Maternal death Show forest plot | 6 | 6244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.36, 3.89] |
| 1.10 Chorioamnionitis Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 1.11 Endometritis Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 1.12 Death in childhood Show forest plot | 4 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.27] |
| 1.13 Neurodevelopmental disability in childhood Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 Developmental delay | 3 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.97] |
| 1.13.2 Intellectual impairment | 3 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.44, 1.69] |
| 1.13.3 Hearing impairment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.04, 9.87] |
| 1.13.4 Visual impairment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.24, 1.23] |
| 1.14 Death into adulthood Show forest plot | 1 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.81] |
| 1.15 Neurodevelopmental disability in adulthood Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 Visual impairment | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.55] |
| 1.15.2 Hearing impairment | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.03] |
| 1.15.3 Intellectual impairment | 2 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.95] |
| 1.16 Fever in women after trial entry requiring the use of antibiotics Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.36, 1.21] |
| 1.17 Intrapartum fever in woman requiring the use of antibiotics Show forest plot | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.49] |
| 1.18 Postnatal fever in woman Show forest plot | 5 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 1.19 Admission into adult intensive care unit Show forest plot | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.26, 2.05] |
| 1.20 Side effects of therapy in women Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.20.1 Any side effects at first dose | 1 | 2825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.59, 0.82] |
| 1.20.2 Dyspnoea | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 1.20.3 Gastrointestinal upset | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.12, 73.37] |
| 1.20.4 Hyperglycaemia | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 1.20.5 Leucocytosis | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.15] |
| 1.20.6 Migraine | 1 | 2828 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.93] |
| 1.21 Glucose intolerance Show forest plot | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.14, 6.46] |
| 1.22 Hypertension Show forest plot | 2 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.59, 1.79] |
| 1.23 Apgar < 7 at 5 minutes Show forest plot | 12 | 5727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 0.98] |
| 1.24 Mean interval between trial entry and birth (days) Show forest plot | 3 | 1513 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.86, 2.32] |
| 1.25 Mean length at birth (cm) Show forest plot | 1 | 2766 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.37, 0.37] |
| 1.26 Mean head circumference at birth (cm) Show forest plot | 1 | 2766 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.22, 0.22] |
| 1.27 Small‐for‐gestational age Show forest plot | 5 | 3478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.28] |
| 1.28 Admission to neonatal intensive care unit Show forest plot | 9 | 6667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.91, 1.00] |
| 1.29 Need for mechanical ventilation/CPAP Show forest plot | 11 | 4519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] |
| 1.30 Mean duration of mechanical ventilation/CPAP (days) Show forest plot | 3 | 471 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐4.59, 0.76] |
| 1.31 Median (IQR) duration of mechanical ventilation (hours) Show forest plot | 1 | Other data | No numeric data | |
| 1.32 Median (IQR) duration of CPAP (hours) Show forest plot | 1 | Other data | No numeric data | |
| 1.33 Air leak syndrome Show forest plot | 2 | 2965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.32, 1.80] |
| 1.34 Mean duration of oxygen supplementation (hours) Show forest plot | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐5.51, ‐0.21] |
| 1.35 Median (IQR) duration of oxygen supplementation (hours) Show forest plot | 1 | Other data | No numeric data | |
| 1.36 Surfactant use Show forest plot | 6 | 6104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 1.37 Systemic infection in the first 48 hours of life Show forest plot | 7 | 1708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 1.38 Proven infection while in the neonatal intensive care unit Show forest plot | 10 | 5521 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.98] |
| 1.39 Necrotising enterocolitis Show forest plot | 10 | 4702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.78] |
| 1.40 Mean infant HPA axis function (cortisol) Show forest plot | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 3.94 [‐3.12, 11.00] |
| 1.40.1 In babies born < 24 hours after 1st dose | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | 9.00 [‐11.93, 29.93] |
| 1.40.2 In babies born 24‐48 hours after 1st dose | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐8.68, 8.68] |
| 1.40.3 In babies born > 48 hours after 1st dose | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | 13.00 [‐1.90, 27.90] |
| 1.41 Mean childhood weight (kg) Show forest plot | 2 | 333 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.39, 1.00] |
| 1.41.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.32, 1.12] |
| 1.41.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.55, 1.75] |
| 1.41.3 Schutte (males) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐3.88, 3.68] |
| 1.42 Mean childhood head circumference (cm) Show forest plot | 2 | 328 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.08, 0.63] |
| 1.42.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.11, 0.71] |
| 1.42.2 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.05, 0.85] |
| 1.42.3 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.51, 1.71] |
| 1.43 Mean childhood height (cm) Show forest plot | 2 | 334 | Mean Difference (IV, Fixed, 95% CI) | 1.02 [‐0.26, 2.29] |
| 1.43.1 Liggins | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.39, 2.39] |
| 1.43.2 Schutte (females) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐3.08, 6.48] |
| 1.43.3 Schutte (males) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐3.79, 4.99] |
| 1.44 Mean childhood systolic blood pressure (mmHg) Show forest plot | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐4.06, 0.86] |
| 1.45 Cerebral palsy in childhood Show forest plot | 5 | 904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.03] |
| 1.46 Behavioural/learning difficulties in childhood Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.09] |
| 1.47 Mean adult weight (kg) Show forest plot | 2 | 538 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐6.41, 4.76] |
| 1.47.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐6.00 [‐12.93, 0.93] |
| 1.47.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐9.91, 7.91] |
| 1.47.3 Liggins | 1 | 458 | Mean Difference (IV, Random, 95% CI) | 2.57 [‐0.72, 5.86] |
| 1.48 Mean adult head circumference (cm) Show forest plot | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.33, 0.38] |
| 1.48.1 Schutte (females) | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.03, 1.03] |
| 1.48.2 Schutte (males) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.37, 0.97] |
| 1.48.3 Liggins | 1 | 458 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.34, 0.46] |
| 1.49 Mean adult height (cm) Show forest plot | 2 | 537 | Mean Difference (IV, Fixed, 95% CI) | 0.91 [‐0.28, 2.10] |
| 1.49.1 Schutte (females) | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐5.37, 3.37] |
| 1.49.2 Schutte (males) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐2.30, 8.30] |
| 1.49.3 Liggins (females) | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [‐0.65, 2.99] |
| 1.49.4 Liggins (males) | 1 | 224 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐1.03, 2.53] |
| 1.50 Mean adult skinfold thickness (log values) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.50.1 Triceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 1.50.2 Biceps | 1 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 1.50.3 Subscapular | 1 | 441 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 1.50.4 Suprailiac | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 1.51 Abnormal lung function measured as forced vital capacity (adult) Show forest plot | 1 | 383 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.16, 1.76] |
| 1.52 Mean adult systolic blood pressure (mmHg) Show forest plot | 2 | 545 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐2.81, 1.07] |
| 1.52.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐9.12, 1.12] |
| 1.52.2 Schutte (males) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐7.17, 1.17] |
| 1.52.3 Liggins | 1 | 455 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐1.88, 2.98] |
| 1.53 Mean adult insulin (log values) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.53.1 Fasting | 1 | 435 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
| 1.53.2 30 minutes fasting following a 75 g oral glucose tolerance test | 1 | 412 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.04, 0.28] |
| 1.53.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 1.54 Mean adult glucose Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.54.1 Fasting | 1 | 432 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 1.54.2 30 minutes fasting following a 75 g oral glucose tolerance test | 1 | 413 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.12, 0.54] |
| 1.54.3 120 minutes following a 75 g oral glucose tolerance test | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.52, ‐0.02] |
| 1.55 Mean adult HPA axis function (mean log fasting cortisol) Show forest plot | 1 | 444 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.02, 0.14] |
| 1.56 Mean age at puberty (years) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.56.1 Schutte (females) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.94, 0.94] |
| 1.57 Educational achievement by adulthood (university or polytechnic education) Show forest plot | 1 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 1.58 Mean length of antenatal hospitalisation (days) Show forest plot | 2 | 412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.23, 0.22] |
| 1.59 Length of maternal hospital stay Show forest plot | 4 | Other data | No numeric data | |
| 1.60 Mean length of postnatal hospitalisation (days) Show forest plot | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐1.72, 1.72] |
| 1.61 Mean length of neonatal hospitalisation (days) Show forest plot | 5 | 788 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.51, 0.87] |
| 1.62 Length of neonatal hospitalisation Show forest plot | 2 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Perinatal death ‐ single or multiple pregnancy Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 2.1.1 In babies born from singleton pregnancies | 7 | 5492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.99] |
| 2.1.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.41, 1.22] |
| 2.1.3 Mixed population | 7 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 2.2 Neonatal death ‐ single or multiple pregnancy Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 2.2.1 In babies born from singleton pregnancies | 13 | 8453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 2.2.2 In babies born from multiple pregnancies | 3 | 813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.02] |
| 2.2.3 Mixed population | 9 | 1343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 2.3 Fetal death ‐ single or multiple pregnancy Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.21] |
| 2.3.1 In babies born from singleton pregnancies | 7 | 5492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.46] |
| 2.3.2 In babies born from multiple pregnancies | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.40] |
| 2.3.3 Mixed population | 7 | 4089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.29] |
| 2.4 Respiratory distress syndrome ‐ single or multiple pregnancy Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 2.4.1 In babies born from singleton pregnancies | 17 | 6731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.57, 0.74] |
| 2.4.2 In babies born from multiple pregnancies | 4 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.20] |
| 2.4.3 Mixed population | 9 | 4129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.92] |
| 2.5 Intraventricular haemorrhage ‐ single or multiple pregnancy Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 2.5.1 In babies born from singleton pregnancies | 6 | 4494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.75] |
| 2.5.2 In babies born from multiple pregnancies | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.08, 2.26] |
| 2.5.3 Mixed population | 6 | 3831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Perinatal death ‐ intact or ruptured membranes Show forest plot | 14 | 9804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 3.1.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.10] |
| 3.1.2 In babies born from pregnancies with ruptured membranes at 1st dose | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.83] |
| 3.1.3 Not reported or mixed population | 8 | 7784 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.97] |
| 3.2 Neonatal deaths ‐ intact or ruptured membranes Show forest plot | 22 | 10580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 3.2.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.05] |
| 3.2.2 In babies born from pregnancies with ruptured membranes at 1st dose | 7 | 1014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.46, 0.84] |
| 3.2.3 Not reported or mixed population | 12 | 8234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.91] |
| 3.3 Fetal death ‐ intact or ruptured membranes Show forest plot | 14 | 9804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 3.3.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.73, 1.64] |
| 3.3.2 In babies born from pregnancies with ruptured membranes at 1st dose | 3 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.46, 1.61] |
| 3.3.3 Not reported or mixed population | 8 | 7784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.80, 1.26] |
| 3.4 RDS ‐ intact or ruptured membranes Show forest plot | 26 | 11079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.64, 0.78] |
| 3.4.1 In babies born from pregnancies with intact membranes at 1st dose | 8 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 3.4.2 In babies born from pregnancies with ruptured membranes at 1st dose | 10 | 1202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.87] |
| 3.4.3 Not reported or mixed population | 13 | 7953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.88] |
| 3.5 IVH ‐ intact or ruptured membranes Show forest plot | 12 | 8446 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 3.5.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 3.5.2 In babies born from pregnancies with ruptured membranes at 1st dose | 4 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.28, 0.79] |
| 3.5.3 Not reported or mixed population | 5 | 6392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.38] |
| 3.6 Birthweight ‐ intact or ruptured membranes Show forest plot | 19 | 9522 | Mean Difference (IV, Fixed, 95% CI) | ‐14.86 [‐34.59, 4.87] |
| 3.6.1 In babies born from pregnancies with intact membranes at 1st dose | 4 | 1301 | Mean Difference (IV, Fixed, 95% CI) | ‐30.27 [‐100.43, 39.89] |
| 3.6.2 In babies born from pregnancies with ruptured membranes at 1st dose | 5 | 835 | Mean Difference (IV, Fixed, 95% CI) | ‐49.72 [‐113.91, 14.46] |
| 3.6.3 Not reported or mixed population | 11 | 7386 | Mean Difference (IV, Fixed, 95% CI) | ‐9.40 [‐31.10, 12.30] |
| 3.7 Chorioamnionitis ‐ intact or ruptured membranes Show forest plot | 15 | 8345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.09] |
| 3.7.1 In women with intact membranes at 1st dose | 4 | 1243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.40] |
| 3.7.2 In women with ruptured membranes at 1st dose | 7 | 1129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.72, 1.48] |
| 3.7.3 Not reported or mixed population | 5 | 5973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.09] |
| 3.8 Endometritis ‐ intact or ruptured membranes Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 3.8.1 In women with intact membranes at 1st dose | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 2.00] |
| 3.8.2 In women with ruptured membranes at 1st dose | 4 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.25] |
| 3.8.3 Not reported or mixed population | 5 | 5998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.73, 1.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Perinatal deaths ‐ hypertension syndrome vs other trials Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 4.1.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.57, 1.20] |
| 4.1.2 No hypertension syndrome or hypertension syndromes excluded | 1 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 4.1.3 Hypertension not reported separately | 12 | 8397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.76, 0.94] |
| 4.2 Neonatal deaths ‐ hypertension syndrome vs other trials Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 4.2.1 Hypertension syndrome | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.83] |
| 4.2.2 No hypertension syndrome or hypertension syndromes excluded | 1 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.25] |
| 4.2.3 Hypertension not reported separately | 20 | 9173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.88] |
| 4.3 Fetal deaths ‐ hypertension syndrome vs other trials Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 4.3.1 Women with hypertension syndrome | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.91, 3.28] |
| 4.3.2 No hypertension syndrome or hypertension syndromes excluded | 2 | 1373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.12] |
| 4.3.3 Hypertension not reported separately | 11 | 8129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.83, 1.30] |
| 4.4 Respiratory distress syndrome ‐ hypertension syndrome vs other trials Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 4.4.1 Hypertension syndrome | 5 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.69] |
| 4.4.2 No hypertension syndrome or hypertension syndromes excluded | 7 | 2511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.48, 0.74] |
| 4.4.3 Hypertension not reported separately | 19 | 8254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Perinatal death ‐ type of steroid Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 5.1.1 Dexamethasone | 6 | 4673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.95] |
| 5.1.2 Betamethasone | 8 | 5092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.99] |
| 5.1.3 Methylprednisolone | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.43, 5.43] |
| 5.2 Neonatal death ‐ type of steroid Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 5.2.1 Dexamethasone | 7 | 4769 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.71, 0.91] |
| 5.2.2 Betamethasone | 14 | 5593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.89] |
| 5.2.3 Hydrocortisone | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.47] |
| 5.2.4 Methylprednisolone | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.42, 3.12] |
| 5.3 Fetal death ‐ type of steroid Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.21] |
| 5.3.1 Dexamethasone | 6 | 4673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.25] |
| 5.3.2 Betamethasone | 8 | 5092 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 5.3.3 Methylprednisolone | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.08, 47.36] |
| 5.4 Respiratory distress syndrome ‐ type of steroid Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.78] |
| 5.4.1 Dexamethasone | 8 | 4963 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.92] |
| 5.4.2 Betamethasone | 17 | 5973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.55, 0.72] |
| 5.4.3 Hydrocortisone | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.28] |
| 5.4.4 Methylprednisolone | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.56, 2.27] |
| 5.5 Moderate/severe respiratory distress syndrome ‐ type of steroid Show forest plot | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 5.5.1 Dexamethasone | 2 | 3166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 5.5.2 Betamethasone | 5 | 1655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.37, 0.67] |
| 5.5.3 Hydrocortisone | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.32, 6.63] |
| 5.5.4 Methylprednisolone | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.26, 5.31] |
| 5.6 Chronic lung disease ‐ type of steroid Show forest plot | 5 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.41, 1.79] |
| 5.6.1 Dexamethasone | 2 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.41, 9.16] |
| 5.6.2 Betamethasone | 3 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.21, 1.42] |
| 5.7 IVH ‐ type of steroid Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 5.7.1 Dexamethasone | 4 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 5.7.2 Betamethasone | 8 | 4981 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.68] |
| 5.8 Birthweight ‐ type of steroid Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 5.8.1 Dexamethasone | 6 | 3972 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐31.09, 38.76] |
| 5.8.2 Betamethasone | 12 | 5401 | Mean Difference (IV, Fixed, 95% CI) | ‐20.40 [‐44.61, 3.81] |
| 5.8.3 Hydrocortisone | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐146.68 [‐371.30, 77.93] |
| 5.8.4 Methylprednisolone | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐121.00 [‐430.59, 188.59] |
| 5.9 Chorioamnionitis ‐ type of steroid Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 5.9.1 Dexamethasone | 6 | 3621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.84, 1.71] |
| 5.9.2 Betamethasone | 9 | 4753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 5.10 Endometritis ‐ type of steroid Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 5.10.1 Dexamethasone | 4 | 3270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.92, 2.90] |
| 5.10.2 Betamethasone | 6 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Perinatal death ‐ decade of trial Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 6.1.1 Trials conducted in 1970s | 5 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.06] |
| 6.1.2 Trials conducted in 1980s | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.59, 2.21] |
| 6.1.3 Trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.97] |
| 6.1.4 Trials conducted in 2000s | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.70] |
| 6.1.5 Trials conducted in 2010s | 3 | 6207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.99] |
| 6.2 Neonatal death ‐ decade of trial Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 6.2.1 Trials conducted in 1970s | 7 | 2743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.04] |
| 6.2.2 Trials conducted in 1980s | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.55, 1.49] |
| 6.2.3 Trials conducted in 1990s | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 6.2.4 Trials conducted in 2000s | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.66] |
| 6.2.5 Trials conducted in 2010s | 4 | 6482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 6.3 Fetal death ‐ decade of trial Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 6.3.1 Trials conducted in 1970s | 5 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.32] |
| 6.3.2 Trials conducted in 1980s | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [0.37, 31.41] |
| 6.3.3 Trials conducted in 1990s | 3 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.49, 2.36] |
| 6.3.4 Trials conducted in 2000s | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.19, 4.50] |
| 6.3.5 Trials conducted in 2010s | 3 | 6207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.30] |
| 6.4 Respiratory distress syndrome ‐ decade of trial Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 6.4.1 Trials conducted in 1970s | 8 | 2823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.54, 0.78] |
| 6.4.2 Trials conducted in 1980s | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.88] |
| 6.4.3 Trials conducted in 1990s | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.65, 0.92] |
| 6.4.4 Trials conducted in 2000s | 5 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.26, 0.59] |
| 6.4.5 Trials conducted in 2010s | 4 | 6401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 6.5 IVH ‐ decade of trial Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 6.5.1 Trials conducted in 1970s | 1 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.33, 1.12] |
| 6.5.2 Trials conducted in 1980s | 3 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.81] |
| 6.5.3 Trials conducted in 1990s | 4 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 6.5.4 Trials conducted in 2000s | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.73] |
| 6.5.5 Trials conducted in 2010s | 2 | 5897 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.69, 8.31] |
| 6.6 Birthweight ‐ decade of trial Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 6.6.1 Trials conducted in 1970s | 5 | 1822 | Mean Difference (IV, Fixed, 95% CI) | ‐41.39 [‐110.05, 27.26] |
| 6.6.2 Trials conducted in 1980s | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐19.60 [‐108.55, 69.35] |
| 6.6.3 Trials conducted in 1990s | 4 | 569 | Mean Difference (IV, Fixed, 95% CI) | ‐33.13 [‐102.39, 36.13] |
| 6.6.4 Trials conducted in 2000s | 3 | 573 | Mean Difference (IV, Fixed, 95% CI) | ‐20.77 [‐61.95, 20.41] |
| 6.6.5 Trials conducted in 2010s | 4 | 6307 | Mean Difference (IV, Fixed, 95% CI) | ‐3.82 [‐30.36, 22.72] |
| 6.7 Chorioamnionitis ‐ decade of trial Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 6.7.1 Trials conducted in 1970s | 2 | 1237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.17] |
| 6.7.2 Trials conducted in 1980s | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.01] |
| 6.7.3 Trials conducted in 1990s | 5 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.89] |
| 6.7.4 Trials conducted in 2000s | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.59, 6.95] |
| 6.7.5 Trials conducted in 2010s | 3 | 5873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.48, 1.11] |
| 6.8 Endometritis ‐ decade of trial Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 6.8.1 Trials conducted in 1970s | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.86] |
| 6.8.2 Trials conducted in 1980s | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.88, 6.06] |
| 6.8.3 Trials conducted in 1990s | 4 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.44] |
| 6.8.4 Trials conducted in 2000s | 4 | 6018 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.75, 2.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Perinatal death ‐ protocol with weekly repeats Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 7.1.1 Single course only | 10 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.04] |
| 7.1.2 Courses including weekly repeats | 4 | 3504 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.93] |
| 7.2 Neonatal death ‐ protocol with weekly repeats Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
| 7.2.1 Single course only | 14 | 6636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.68, 1.02] |
| 7.2.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.86] |
| 7.3 Fetal death ‐ protocol with weekly repeats Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 7.3.1 Single course only | 10 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.31] |
| 7.3.2 Courses including weekly repeats | 4 | 3504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.31] |
| 7.4 Respiratory distress syndrome ‐ protocol with weekly repeats Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 7.4.1 Single course only | 18 | 7210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.64, 0.83] |
| 7.4.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.79] |
| 7.5 Moderate/severe respiratory distress syndrome Show forest plot | 7 | 4874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 7.5.1 Single course only | 3 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.88] |
| 7.5.2 Courses including weekly repeats | 4 | 3515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.89] |
| 7.6 IVH ‐ protocol with weekly repeats Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 7.6.1 Single course only | 4 | 4502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] |
| 7.6.2 Courses including weekly repeats | 8 | 3973 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.43, 0.79] |
| 7.7 Birthweight ‐ protocol with weekly repeats Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐14.02 [‐33.79, 5.76] |
| 7.7.1 Single course only | 14 | 6165 | Mean Difference (IV, Fixed, 95% CI) | ‐20.90 [‐44.39, 2.60] |
| 7.7.2 Courses including weekly repeats | 5 | 3386 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [‐33.92, 39.36] |
| 7.8 Chorioamnionitis ‐ protocol with weekly repeats Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 7.8.1 Single courses only | 7 | 4659 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.11] |
| 7.8.2 Courses including weekly repeats | 8 | 3715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.28] |
| 7.9 Endometritis ‐ protocol with weekly repeats Show forest plot | 10 | 6764 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.82, 1.58] |
| 7.9.1 Single courses only | 4 | 3332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.39] |
| 7.9.2 Courses including weekly repeats | 6 | 3432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.94, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Perinatal death ‐ gestational age at trial entry Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.77, 0.92] |
| 8.1.1 Less than or equal to 35 weeks + 0 days | 11 | 6185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| 8.1.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.68, 4.28] |
| 8.2 Neonatal death ‐ gestational age at trial entry Show forest plot | 22 | 10609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.86] |
| 8.2.1 Less than or equal to 35 weeks + 0 days | 19 | 6961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.69, 0.86] |
| 8.2.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.49, 4.61] |
| 8.3 Fetal death ‐ gestational age at trial entry Show forest plot | 14 | 9833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| 8.3.1 Less than or equal to 35 weeks + 0 days | 11 | 6185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.19] |
| 8.3.2 Greater than or equal to 34 weeks + 0 days | 4 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.42, 8.82] |
| 8.4 Respiratory distress syndrome ‐ gestational age at trial entry Show forest plot | 26 | 11183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 8.4.1 Less than or equal to 35 weeks + 0 days | 20 | 7041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.63, 0.78] |
| 8.4.2 Greater than or equal to 34 weeks + 0 days | 7 | 4142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.95] |
| 8.5 IVH ‐ gestational age at trial entry Show forest plot | 12 | 8475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.74] |
| 8.5.1 Less than or equal to 35 weeks + 0 days | 11 | 5412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.44, 0.72] |
| 8.5.2 Greater than or equal to 34 weeks + 0 days | 2 | 3063 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 102.09] |
| 8.6 Birthweight ‐ gestational age at trial entry Show forest plot | 19 | 9551 | Mean Difference (IV, Fixed, 95% CI) | ‐13.36 [‐32.99, 6.26] |
| 8.6.1 Less than or equal to 35 weeks + 0 days | 13 | 5412 | Mean Difference (IV, Fixed, 95% CI) | ‐9.78 [‐40.81, 21.24] |
| 8.6.2 Greater than or equal to 34 weeks + 0 days | 7 | 4139 | Mean Difference (IV, Fixed, 95% CI) | ‐15.75 [‐41.09, 9.58] |
| 8.7 Chorioamnionitis ‐ gestational age at trial entry Show forest plot | 15 | 8374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.07] |
| 8.7.1 Less than or equal to 35 weeks + 0 days | 13 | 5132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 8.7.2 Greater than or equal to 34 weeks + 0 days | 3 | 3242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.99] |

