Ventilasi mekanikal untuk sklerosis lateral amiotrofik/penyakit neuron motor
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004427.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 octubre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neuromuscular
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Aleksandar Radunovic drafted the first version of the review, with Naveed Mustfa and Djillali Annane. Kate Jewitt edited the protocol and the original version of the review. Muhammad K Rafiq with Djillali Annane selected studies for the first update; the other authors approved the text. Muhammad K Rafiq with Naveed Mustfa assessed studies for the 2017 update of the review, and Ruth Brassington assisted with updating the text and extraction of additional data. All authors approved the final text.
Sources of support
Internal sources
-
Institute of Psychiatry, UK.
-
Guy's, King's & St. Thomas' School of Medicine, King's College London, UK.
-
Hopital Raymond Poincaré, Garches, France.
-
National Institute of Health Research, UK.
External sources
-
Motor Neurone Disease Association, UK.
-
Muscular Dystrophy Association, USA.
Declarations of interest
Aleksandar Radunovic: Member of the Data and Ethics Monitoring Committee for the NIHR Health Technology Assessment RCT of diaphragm pacing in ALS (DiPALS). Member of the NICE Guideline Development Group on management of MND.
Djillali Annane: no conflicts of interest.
Muhammad K Rafiq: no conflicts of interest.
Ruth Brassington: I have no known financial conflicts of interest. I am Managing Editor of Cochrane Neuromuscular, of which the National Institute for Health Research (NIHR) is the largest single funder. Upon joining the author team, I withdrew from the editorial process for this review, in accordance with Cochrane policy. The Motor Neurone Disease Association also supported Cochrane Neuromuscular with a small grant that contributed to my salary.
Naveed Mustfa: no conflicts of interest.
Acknowledgements
We thank Dr EA Oppenheimer for his comments on earlier drafts of the protocol. We are grateful to Professor Nigel Leigh, who developed the protocol for this review and contributed to the original assessment of studies, and Kate Jewitt, former Managing Editor of the Cochrane Neuromuscular Disease Group, who was an author of the original review. Angela Gunn, Information Specialist at Cochrane Neuromuscular, performed the searches.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases and the Motor Neurone Disease Association.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Oct 05 | Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease | Review | Aleksandar Radunovic, Djillali Annane, Muhammad K Rafiq, Ruth Brassington, Naveed Mustfa | |
| 2013 Mar 28 | Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease | Review | Aleksandar Radunovic, Djillali Annane, Muhammad K Rafiq, Naveed Mustfa | |
| 2009 Oct 07 | Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease | Review | Aleksandar Radunovic, Djillali Annane, Kate Jewitt, Naveed Mustfa | |
| 2009 Jul 08 | Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease | Protocol | P Nigel Leigh, Djillali Annane, Kate Jewitt, Naveed Mustfa | |
Differences between protocol and review
P Nigel Leigh withdrew from authorship after protocol publication. Kate Jewitt withdrew following publication of the full review. Muhammad K Rafiq became an author for the previous update, and Ruth Brassington joined as an author at this update.
The review authors assessed risk of bias expressed as ‘low risk’, ‘high risk’, or ‘unclear risk’ of bias in accordance with Higgins 2011.
We included a statement in Types of interventions that we will include comparisons with no intervention or the best standard care, and clarified that adverse events will be collected from included trials. We reworded the review objective in accordance with current guidance. In addition, we clarified that all forms of NIV irrespective of pressure settings and timings are eligible for inclusion.
We added an appendix describing methods that will be used if meta‐analysis becomes possible.
We included a 'Summary of findings' table and searched clinical trials databases, according to current Cochrane requirements.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Quality of Life;
- Amyotrophic Lateral Sclerosis [complications, *mortality];
- Disease Progression;
- Motor Neuron Disease [mortality];
- Randomized Controlled Trials as Topic;
- Respiration, Artificial [methods, *mortality];
- Respiratory Insufficiency [etiology, *mortality, therapy];
- Survival Analysis;
- Time Factors;
Medical Subject Headings Check Words
Humans;
PICO
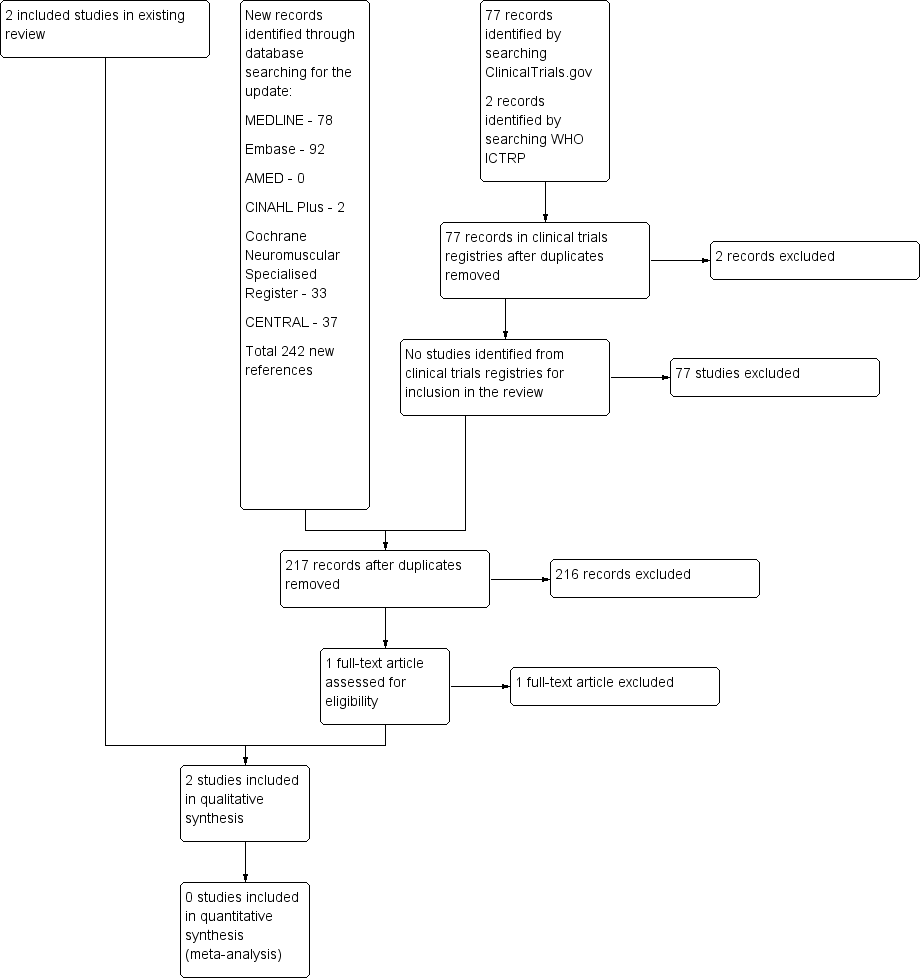
A flow diagram illustrating the study selection process.
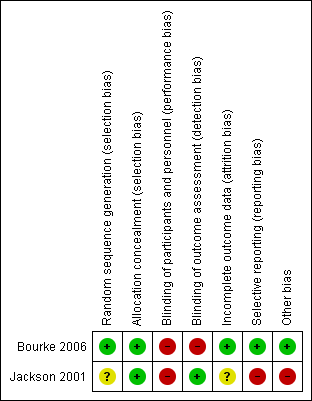
Methodological quality summary: review authors' judgements about each methodological quality item for each included study. A green plus sign indicates low risk of bias; a red minus sign indicates high risk of bias; and a yellow question mark indicates unclear risk of bias.
| Non‐invasive ventilation compared with standard care for amyotrophic lateral sclerosis (ALS) | ||||||
| Patient or population: people with ALS Settings: people with ALS attending a single regional care centre Intervention: non‐invasive ventilation Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Non‐invasive ventilation (NIV) | |||||
| Survival | All participants Median survival was 171 days. | All participants Median survival was 48 days longer (12 to 91 days1 longer). | ‐ | 41 (1 study) | ⊕⊕⊕⊝ Moderate2 | 21 of the 41 participants had poor bulbar function. P = 0.0059 better bulbar function, P = 0.92 poor bulbar function |
| Participants with better (good or moderately impaired) bulbar function Median survival was 11 days. | Participants with better (good or moderately impaired) bulbar function Median survival was 205 days longer (CI not given). | |||||
| Participants with poor bulbar function Median survival was 261 days. | Participants with poor bulbar function Median survival was 39 days shorter (CI not given). | |||||
| Quality of life (SF‐36 MCS) | All participants Median duration that SF‐36 MCS remained above 75% of baseline was 99 days. | All participants Median duration that SF‐36 MCS remained above 75% of baseline was 69 days longer (45 to 667 days longer). | ‐ | 41 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 MCS remained above 75% of baseline was 4 days. | Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 MCS remained above 75% of the baseline was 195 days longer (P = 0.001, CI not given). | |||||
| Participants with poor bulbar function Median duration that SF‐36 MCS remained above 75% of baseline was 164 days. | Participants with poor bulbar function Median duration that SF‐36 MCS remained above 75% of the baseline was 37 days shorter (P = 0.64, CI not given). | |||||
| Quality of life (SF‐36 PCS) | All participants Median duration that SF‐36 PCS remained above 75% of baseline was 81 days. | All participants Median duration that SF‐36 PCS remained above 75% of baseline was 69 days longer (P = 0.004). | ‐ | ‐ | ‐ | CI not given |
| Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 PCS remained above 75% of baseline was 4 days. | Participants with better (good or moderately impaired) bulbar function Median duration that SF‐36 PCS remained above 75% of the baseline was 175 days longer (P < 0.001). | |||||
| Participants with poor bulbar function Median duration that SF‐36 PCS remained above 75% of baseline was 132 days. | Participants with poor bulbar function Median duration that SF‐36 PCS remained above 75% of the baseline was 18 days longer (P = 0.88). | |||||
| Quality of life (SAQLI) | All participants Median duration that SAQLI remained above 75% of baseline was 99 days. | All participants Median duration that SAQLI remained above 75% of baseline was 74 days longer (P = 0.031). | ‐ | 41 (1 study) | ⊕⊕⊝⊝ Low2,3 | CI not given |
| Participants with good or moderately impaired bulbar function Median duration that SAQLI remained above 75% of baseline was 4 days. | Participants with good or moderately impaired bulbar function Median duration that SAQLI remained above 75% of the baseline was 195 days longer (P = < 0.001). | |||||
| Participants with poor bulbar function Median duration that SAQLI remained above 75% of baseline was 132 days. | Participants with poor bulbar function Median duration that SAQLI remained above 75% of the baseline was 29 days shorter (P = 0.77). | |||||
| Adverse events (not reported) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Calculated CIs are approximate. | ||||||
| All participants (n = 41) | Better bulbar function (n = 20) | Poor bulbar function (n = 21) | |||||||
| NIV (n = 22) | Standard care | P | NIV | Standard care | P | NIV (n = 11) | Standard care | P value | |
| SF‐36 MCS | 168 (45 to 1357) | 99 (0 to 690) | 0.0017 | 199 (48 to 552) | 4 (0 to 196) | 0.001 | 127 (45 to 1357) | 164 (2 to 690) | 0.64 |
| SF‐36 PCS | 150 (27 to 908) | 81 (0 to 273) | 0.0014 | 179 (36 to 548) | 4 (0 to 94) | < 0.001 | 150 (27 to 908) | 132 (2 to 273) | 0.88 |
| SAQLI symptoms | 192 (48 to 1357) | 46 (0 to 703) | 0.0013 | 205 (69 to 629) | 4 (0 to 143) | < 0.001 | 143 (48 to 1357) | 100 (2 to 703) | 0.26 |
| SAQLI score | 173 (25 to 1357) | 99 (0 to 645) | 0.031 | 199 (61 to 595) | 4 (0 to 193) | < 0.001 | 103 (25 to 1357) | 132 (2 to 645) | 0.77 |
| Data are median (range). Data from Bourke 2006. Abbreviations: NIV: non‐invasive ventilation; SAQLI: Sleep Apnea Quality of Life Index; SF‐36 MCS: 36‐Item Short‐Form Health Survey Mental Component Summary; SF‐36 PCS: 36‐Item Short‐Form Health Survey Physical Component Summary | |||||||||
| All participants (n = 41) | Better bulbar function (n = 20) | Poor bulbar function (n = 21) | |||||||
| NIV | Standard care (n = 19) | P | NIV (n = 11) | Standard care (n = 9) | P | NIV | Standard care (n = 10) | P value | |
| SF‐36 MCS | 2.31 (0 to 11.54) | 0 (0 to 5.23) | 0.0082 | 2.18 (0 to 11.54) | 0 (0 to 1.39) | 0.0052 | 4.47 (0 to 7.75) | 0.88 (0 to 5.23) | 0.24 |
| SF‐36 PCS | 0.18 (0 to 10.62) | 0 (0 to 6.73) | 0.51 | 0.14 (0 to 10.62) | 0 (0 to 0.39) | 0.031 | 0.21 (0 to 5.41) | 0.48 (0 to 6.73) | 0.37 |
| SAQLI symptoms | 1.07 (0 to 3.20) | 0 (0 to 1.14) | < 0.001 | 1.73 (0.52 to 2.95) | 0 (0 to 0) | < 0.001 | 0.90 (0 to 3.20) | 0.04 (0 to 1.14) | 0.018 |
| SAQLI score | 0.44 (0 to 1.59) | 0 (0 to 0.42) | < 0.001 | 0.50 (0 to 0.88) | 0 (0 to 0.07) | < 0.001 | 0.28 (0 to 1.59) | 0.04 (0 to 0.42) | 0.066 |
| Data are median (range) values of area under the curve above baseline divided by time from randomisation to death. Data from Bourke 2006. Abbreviations: NIV: non‐invasive ventilation; SAQLI: Sleep Apnea Quality of Life Index; SF‐36 MCS: 36‐Item Short‐Form Health Survey Mental Component Summary; SF‐36 PCS: 36‐Item Short‐Form Health Survey Physical Component Summary | |||||||||

