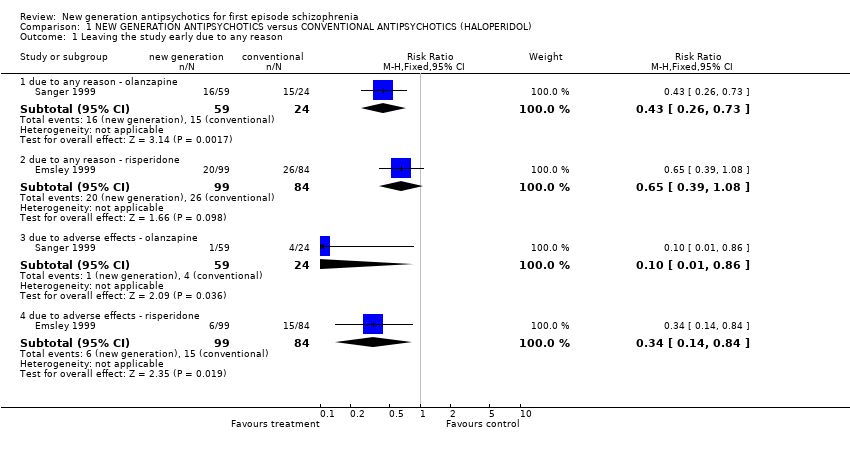
| 1 Leaving the study early due to any reason Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.1 due to any reason ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.26, 0.73] |
| 1.2 due to any reason ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.39, 1.08] |
| 1.3 due to adverse effects ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.86] |
| 1.4 due to adverse effects ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.84] |
| 2 Global state: 1. No clinically significant change (CGI < much improved) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 2.1 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.54] |
| 3 Global state: 2. Need of additional medication (at least one dose of benzodiazepine) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 3.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.52, 1.11] |
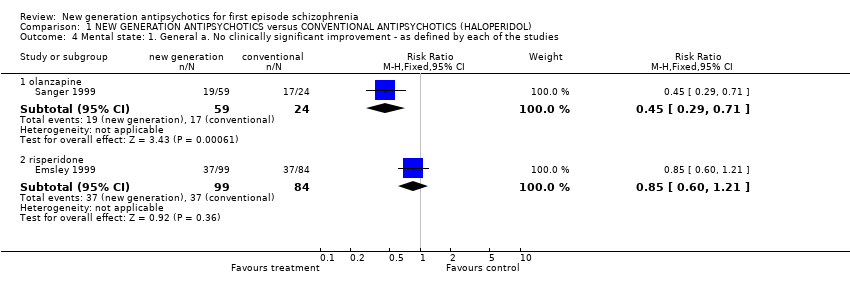
| 4 Mental state: 1. General a. No clinically significant improvement ‐ as defined by each of the studies Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 4.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.71] |
| 4.2 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 5 Mental state: 1. General b. Various measures of change Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 5.1 less than 50% PANSS reduction (risperidone) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 5.2 less than 40% PANSS reduction (olanzapine) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.71] |
| 5.3 less than 50% BPRS reduction (risperidone) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.12] |
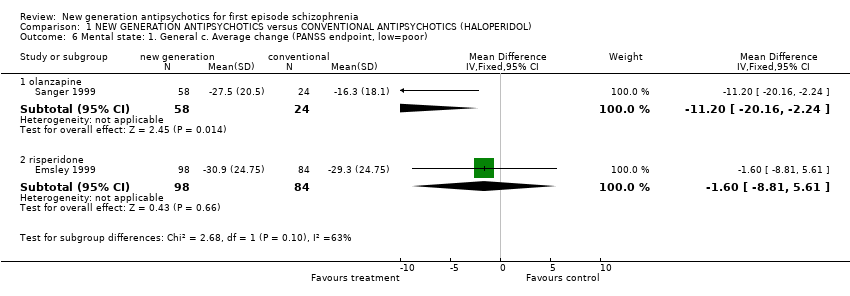
| 6 Mental state: 1. General c. Average change (PANSS endpoint, low=poor) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 6.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐20.16, ‐2.24] |
| 6.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐8.81, 5.61] |
| 7 Mental state: 1. General d. Average change (BPRS endpoint, low=poor) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 7.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐7.80 [‐13.35, ‐2.25] |
| 7.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.27, 3.07] |
| 8 Mental state: 2. Specific a. Positive symptoms ‐ average change (PANSS positive subscore endpoint, low=poor) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 8.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐6.34, ‐0.46] |
| 8.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.18, 1.98] |
| 9 Mental state: 2. Specific b. Positive symptoms ‐ average change (BPRS positive subscore endpoint, low=poor) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 9.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐3.28, 0.68] |
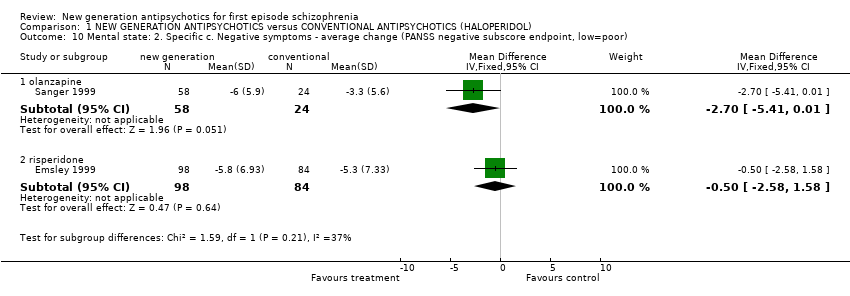
| 10 Mental state: 2. Specific c. Negative symptoms ‐ average change (PANSS negative subscore endpoint, low=poor) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 10.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐5.41, 0.01] |
| 10.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.58, 1.58] |
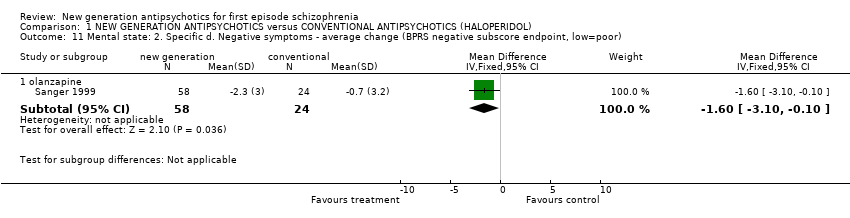
| 11 Mental state: 2. Specific d. Negative symptoms ‐ average change (BPRS negative subscore endpoint, low=poor) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 11.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.10, ‐0.10] |
| 12 Mental state: 2. Specific e. Depressive symptoms ‐ average change (MADRS endpoint, low=poor) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 12.1 olanzapine | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐7.01, 1.21] |
| 13 Adverse events: 1. General a. At least one adverse event Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 13.1 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.76, 0.98] |
| 14 Adverse events: 2. Extrapyramdial problems a. General i. Needing anticholinergic medication at least once Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 14.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.72] |
| 14.2 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.85] |
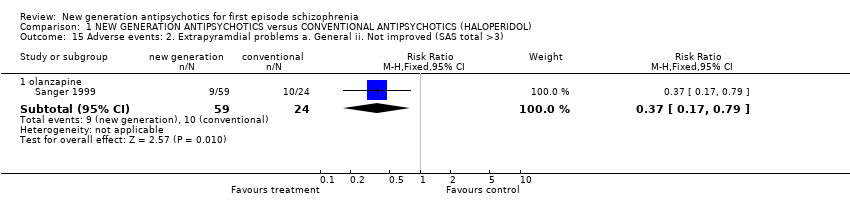
| 15 Adverse events: 2. Extrapyramdial problems a. General ii. Not improved (SAS total >3) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 15.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.79] |
| 16 Adverse events: 2. Extrapyramdial problems a. General iii. Average change (SAS, low=poor) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 16.1 olanzapine | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐8.08, ‐1.92] |
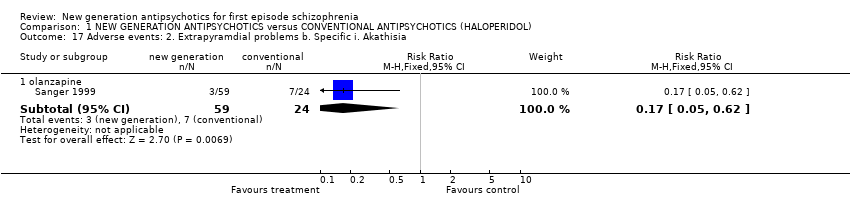
| 17 Adverse events: 2. Extrapyramdial problems b. Specific i. Akathisia Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 17.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.62] |
| 18 Adverse events: 2. Extrapyramdial problems b. Specific ii. Akathisia ‐ average change (BAS, low=poor) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 18.1 olanzapine | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐1.12, ‐0.08] |
| 19 Adverse Events: 2. Extrapyramdial problems b. Specific iii. Others Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 19.1 extrapyramidal syndrome ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.52] |
| 19.2 hypertonia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.83] |
| 19.3 hypokinesia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.83] |
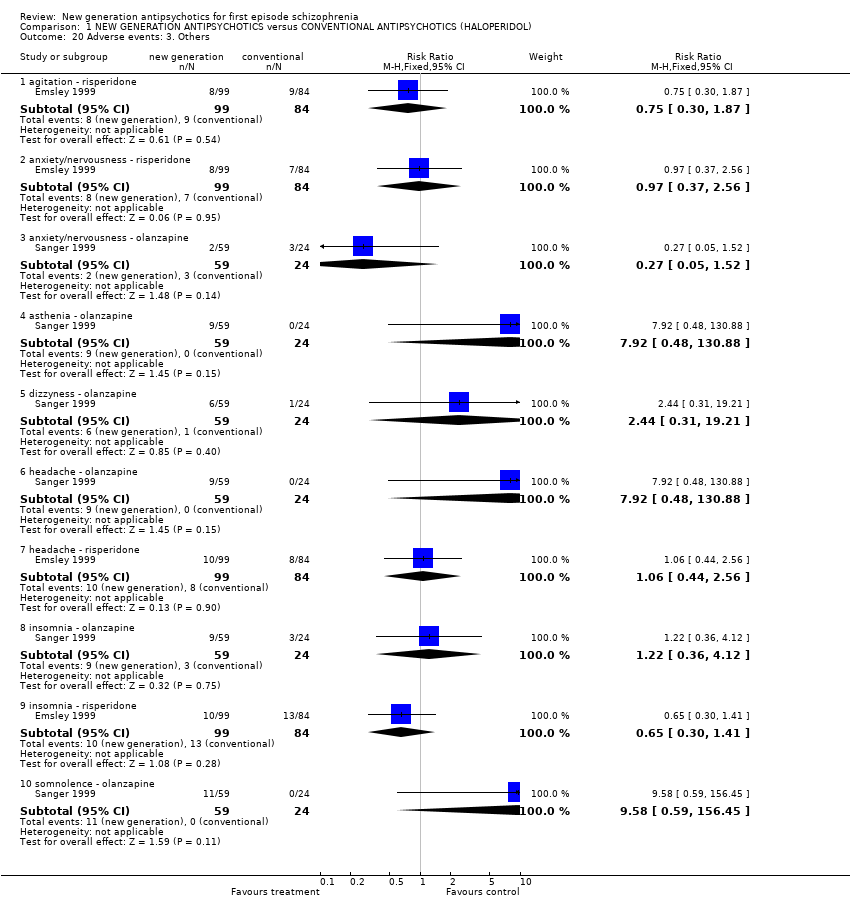
| 20 Adverse events: 3. Others Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 20.1 agitation ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.87] |
| 20.2 anxiety/nervousness ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.37, 2.56] |
| 20.3 anxiety/nervousness ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.52] |
| 20.4 asthenia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [0.48, 130.88] |
| 20.5 dizzyness ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.31, 19.21] |
| 20.6 headache ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [0.48, 130.88] |
| 20.7 headache ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.44, 2.56] |
| 20.8 insomnia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.36, 4.12] |
| 20.9 insomnia ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.41] |
| 20.10 somnolence ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.58 [0.59, 156.45] |