Antipsicóticos de nueva generación para el primer episodio de esquizofrenia
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: random ‐ no further details. | |
| Participants | Diagnosis: provisional schizophreniform disorder or schizophrenia (DSM‐III‐R). | |
| Interventions | 1. Risperidone 4‐16 mg/d. N=99. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: random ‐ in a 2:1 (olanzapine:haloperidol) ratio. | |
| Participants | Diagnosis: schizophrenia, schizophreniform disorder or schizoaffective disorder | |
| Interventions | 1. Olanzapine 5‐20 mg/d. N=59. | |
| Outcomes | Leaving the study early. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
General abbreviations:
SD ‐ Standard Deviation
Diagnostic tools:
DSM‐III‐R ‐ Diagnostic and Statistical Manual of Mental disorders, third edition, revised.
Global effect scales:
CGI ‐ Clinical Global Impression
GAF ‐ Global Assessment of Functioning
Mental state scales:
BPRS ‐ Brief Psychiatric Rating Scale
PANSS ‐ Positive and Negative Symptom Scale
MADRS ‐ Montgomery‐Asberg Depression Rating Scale
Side effect scales:
ESRS ‐ Extrapyramidal Symptom Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details . | |
| Allocation: unclear, probably randomised. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear, probably randomised. | |
| Allocation: unclear. | |
| Allocation: unclear, probably randomised. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear. | |
| Allocation: not randomised. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear. | |
| Allocation: unclear. | |
| Allocation: random ‐ no further details. | |
| Allocation: matched, not randomised. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear, probably randomised. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear, probably randomised. | |
| Allocation: randomised to one of three dosing schedules. | |
| Allocation: unclear. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear, probably randomised. | |
| Allocation: random ‐ no further details. | |
| Allocation: random ‐ no further details. | |
| Allocation: unclear. | |
| Allocation: random ‐ no further details. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Pharmacological long‐term treatment strategies for relapse prevention in first episode schizophrenia. |
| Methods | |
| Participants | First treatment year: 140 patients with first episode in schizophrenia. |
| Interventions | First treatment year: |
| Outcomes | Relapse. |
| Starting date | December 2000 |
| Contact information | Prof. Dr. W. Gaebel, Department of Psychiatry, Heinrich‐Heine‐University Düsseldorf, Rhineland State Clinics Düsseldorf, Bergische Landstr. 2, 40629 Düsseldorf, Germany. |
| Notes |
| Trial name or title | Preventing morbidity in first‐episode schizophrenia |
| Methods | |
| Participants | Patients with a first episode of schizophrenia. |
| Interventions | 1. Olanzapine. |
| Outcomes | Psychopathology (positive, negative and affective symptoms). |
| Starting date | |
| Contact information | John M. Kane, MD, 75‐29 263rd St., Glen Oaks, New York, 11004, United States. |
| Notes |
| Trial name or title | Optimization of acute treatment in first episode schizophrenic patients by new pharmacological treatments. |
| Methods | |
| Participants | 360 people with a first episode of schizophrenia |
| Interventions | 1. Risperidone |
| Outcomes | Psychopathology (HAMD, CDSS, PANSS, SANS, CGI, YMRS). |
| Starting date | November 2000 |
| Contact information | Prof. Dr. Hans‐Jürgen Möller, |
| Notes |
| Trial name or title | Ris‐int‐35 a double blind evaluation of risperidone versus haloperidol on the long‐term morbidity of early psychotic patients. |
| Methods | |
| Participants | 26 people with a diagnosis of early psychotic symptoms. |
| Interventions | 1. Risperidone. |
| Outcomes | Relapse. |
| Starting date | |
| Contact information | Department of Psychiatry, Clinical Sciences Building, University of Leicester, Leicester Royal Infirmary, PO BOX 65, LE2 7LX, United Kingdom. |
| Notes |
| Trial name or title | Olanzapine versus haloperidol in randomised trials of first‐episode patients with schizophrenia. |
| Methods | |
| Participants | People with first‐episode schizophrenia. |
| Interventions | 1. Olanzapine. |
| Outcomes | Neurological and other side effects. |
| Starting date | |
| Contact information | Rosebush, Mazurek, |
| Notes |
| Trial name or title | The FutuRis study ‐ a prospective long‐term evaluation of risperidone versus haloperidol in early psychosis patients. |
| Methods | |
| Participants | 500 people with early psychosis. |
| Interventions | 1. Risperidone 1‐4 mg/d. |
| Outcomes | PANSS. |
| Starting date | |
| Contact information | Dept Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA. |
| Notes |
| Trial name or title | A double‐blind evaluation of risperidone versus haloperidol on the long‐term morbidity of early psychotic patients. |
| Methods | |
| Participants | Approximately 35 people with first episode psychotic disorder. |
| Interventions | 1. Risperidone. |
| Outcomes | |
| Starting date | |
| Contact information | Dept of Psychological Medicine, Instiute of Psychiatry, De Crespigny Park, Denmark Hill, London, SE5 8AF. |
| Notes |
| Trial name or title | The acute and long‐term efficacy of olanzapine in first‐episode psychotic disorders: a randomised double‐blind comparison with haloperidol. |
| Methods | |
| Participants | People with first episode psychotic disorders. |
| Interventions | 1. Olanzapine. |
| Outcomes | |
| Starting date | |
| Contact information | Forest Healthcare NHS Trust, Whipps Cross Hospital, Whipps Cross Road, London, E11 1NR, England. |
| Notes |
Global effect scales:
CGI ‐ Clinical Global Impression
Mental state scales:
PANSS ‐ Positive and Negative Symptoms Scale
SANS ‐ Scale for Assessment of Negative Symptoms
HAMD ‐ Hamilton Depression Scale
CDSS ‐ Calgary Depression Scale for Schizophrenia
YMRS ‐ Young Mania Rating Scale
Side effect scales:
AIMS ‐ Abnormal Involuntary Movement Scale
BAS ‐ Barnes Akathisia Scale
HAS ‐ Hillside Akathisia Scale
SAS ‐ Simpson and Angus Scale
UKU ‐ UKU Side effect Scale
Quality of Life:
LQLP ‐ Lancashire Quality of Life Profile
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early due to any reason Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 1 Leaving the study early due to any reason. | ||||
| 1.1 due to any reason ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.26, 0.73] |
| 1.2 due to any reason ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.39, 1.08] |
| 1.3 due to adverse effects ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.86] |
| 1.4 due to adverse effects ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.84] |
| 2 Global state: 1. No clinically significant change (CGI < much improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 2 Global state: 1. No clinically significant change (CGI < much improved). | ||||
| 2.1 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.54] |
| 3 Global state: 2. Need of additional medication (at least one dose of benzodiazepine) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 3 Global state: 2. Need of additional medication (at least one dose of benzodiazepine). | ||||
| 3.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.52, 1.11] |
| 4 Mental state: 1. General a. No clinically significant improvement ‐ as defined by each of the studies Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 4 Mental state: 1. General a. No clinically significant improvement ‐ as defined by each of the studies. | ||||
| 4.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.71] |
| 4.2 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 5 Mental state: 1. General b. Various measures of change Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 5 Mental state: 1. General b. Various measures of change. | ||||
| 5.1 less than 50% PANSS reduction (risperidone) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 5.2 less than 40% PANSS reduction (olanzapine) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.71] |
| 5.3 less than 50% BPRS reduction (risperidone) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.12] |
| 6 Mental state: 1. General c. Average change (PANSS endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 6 Mental state: 1. General c. Average change (PANSS endpoint, low=poor). | ||||
| 6.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐20.16, ‐2.24] |
| 6.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐8.81, 5.61] |
| 7 Mental state: 1. General d. Average change (BPRS endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 7 Mental state: 1. General d. Average change (BPRS endpoint, low=poor). | ||||
| 7.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐7.80 [‐13.35, ‐2.25] |
| 7.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.27, 3.07] |
| 8 Mental state: 2. Specific a. Positive symptoms ‐ average change (PANSS positive subscore endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 8 Mental state: 2. Specific a. Positive symptoms ‐ average change (PANSS positive subscore endpoint, low=poor). | ||||
| 8.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐6.34, ‐0.46] |
| 8.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.18, 1.98] |
| 9 Mental state: 2. Specific b. Positive symptoms ‐ average change (BPRS positive subscore endpoint, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 9 Mental state: 2. Specific b. Positive symptoms ‐ average change (BPRS positive subscore endpoint, low=poor). | ||||
| 9.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐3.28, 0.68] |
| 10 Mental state: 2. Specific c. Negative symptoms ‐ average change (PANSS negative subscore endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 10 Mental state: 2. Specific c. Negative symptoms ‐ average change (PANSS negative subscore endpoint, low=poor). | ||||
| 10.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐5.41, 0.01] |
| 10.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.58, 1.58] |
| 11 Mental state: 2. Specific d. Negative symptoms ‐ average change (BPRS negative subscore endpoint, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 11 Mental state: 2. Specific d. Negative symptoms ‐ average change (BPRS negative subscore endpoint, low=poor). | ||||
| 11.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.10, ‐0.10] |
| 12 Mental state: 2. Specific e. Depressive symptoms ‐ average change (MADRS endpoint, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 12 Mental state: 2. Specific e. Depressive symptoms ‐ average change (MADRS endpoint, low=poor). | ||||
| 12.1 olanzapine | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐7.01, 1.21] |
| 13 Adverse events: 1. General a. At least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 13 Adverse events: 1. General a. At least one adverse event. | ||||
| 13.1 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.76, 0.98] |
| 14 Adverse events: 2. Extrapyramdial problems a. General i. Needing anticholinergic medication at least once Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 14 Adverse events: 2. Extrapyramdial problems a. General i. Needing anticholinergic medication at least once. | ||||
| 14.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.72] |
| 14.2 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.85] |
| 15 Adverse events: 2. Extrapyramdial problems a. General ii. Not improved (SAS total >3) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 15 Adverse events: 2. Extrapyramdial problems a. General ii. Not improved (SAS total >3). | ||||
| 15.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.79] |
| 16 Adverse events: 2. Extrapyramdial problems a. General iii. Average change (SAS, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 16 Adverse events: 2. Extrapyramdial problems a. General iii. Average change (SAS, low=poor). | ||||
| 16.1 olanzapine | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐8.08, ‐1.92] |
| 17 Adverse events: 2. Extrapyramdial problems b. Specific i. Akathisia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 17 Adverse events: 2. Extrapyramdial problems b. Specific i. Akathisia. | ||||
| 17.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.62] |
| 18 Adverse events: 2. Extrapyramdial problems b. Specific ii. Akathisia ‐ average change (BAS, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 18 Adverse events: 2. Extrapyramdial problems b. Specific ii. Akathisia ‐ average change (BAS, low=poor). | ||||
| 18.1 olanzapine | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐1.12, ‐0.08] |
| 19 Adverse Events: 2. Extrapyramdial problems b. Specific iii. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 19 Adverse Events: 2. Extrapyramdial problems b. Specific iii. Others. | ||||
| 19.1 extrapyramidal syndrome ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.52] |
| 19.2 hypertonia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.83] |
| 19.3 hypokinesia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.83] |
| 20 Adverse events: 3. Others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 20 Adverse events: 3. Others. | ||||
| 20.1 agitation ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.87] |
| 20.2 anxiety/nervousness ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.37, 2.56] |
| 20.3 anxiety/nervousness ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.52] |
| 20.4 asthenia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [0.48, 130.88] |
| 20.5 dizzyness ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.31, 19.21] |
| 20.6 headache ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [0.48, 130.88] |
| 20.7 headache ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.44, 2.56] |
| 20.8 insomnia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.36, 4.12] |
| 20.9 insomnia ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.41] |
| 20.10 somnolence ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.58 [0.59, 156.45] |
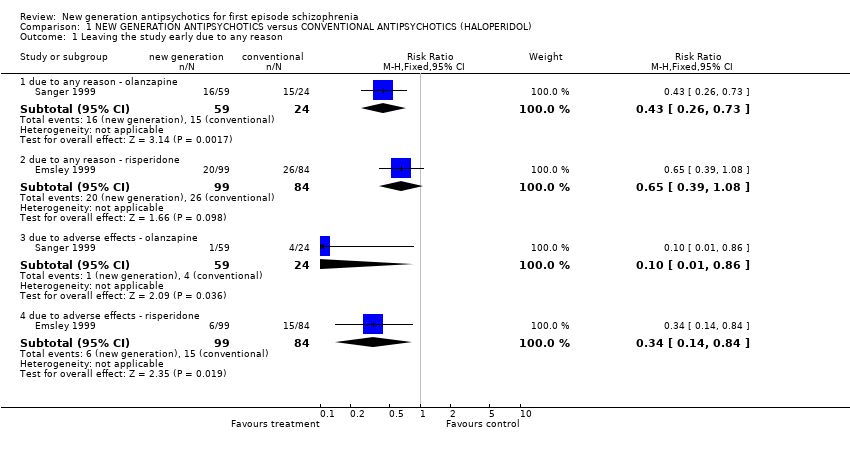
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 1 Leaving the study early due to any reason.

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 2 Global state: 1. No clinically significant change (CGI < much improved).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 3 Global state: 2. Need of additional medication (at least one dose of benzodiazepine).
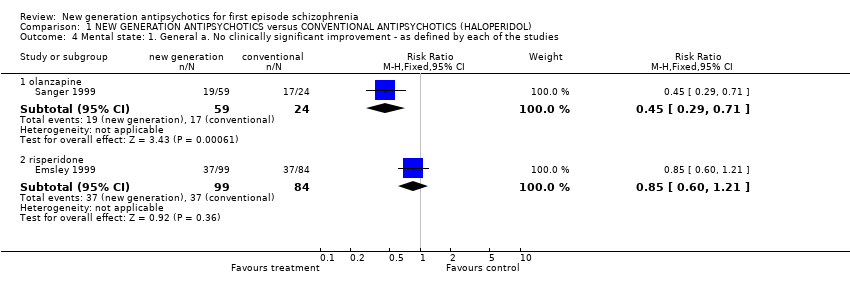
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 4 Mental state: 1. General a. No clinically significant improvement ‐ as defined by each of the studies.

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 5 Mental state: 1. General b. Various measures of change.
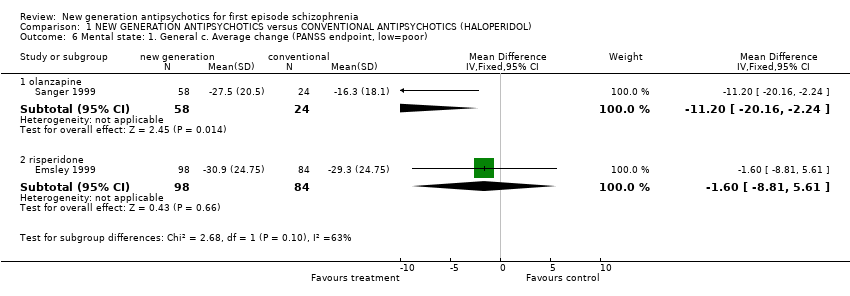
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 6 Mental state: 1. General c. Average change (PANSS endpoint, low=poor).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 7 Mental state: 1. General d. Average change (BPRS endpoint, low=poor).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 8 Mental state: 2. Specific a. Positive symptoms ‐ average change (PANSS positive subscore endpoint, low=poor).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 9 Mental state: 2. Specific b. Positive symptoms ‐ average change (BPRS positive subscore endpoint, low=poor).
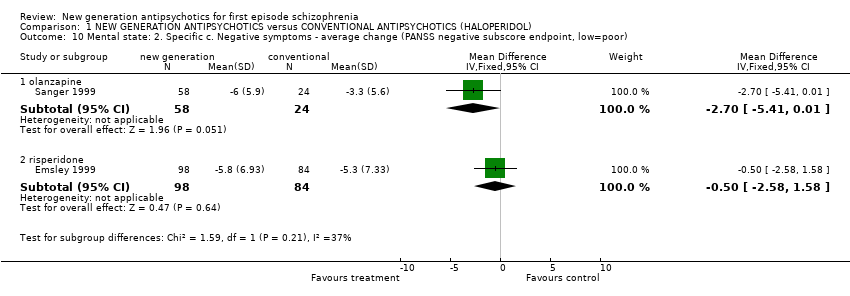
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 10 Mental state: 2. Specific c. Negative symptoms ‐ average change (PANSS negative subscore endpoint, low=poor).
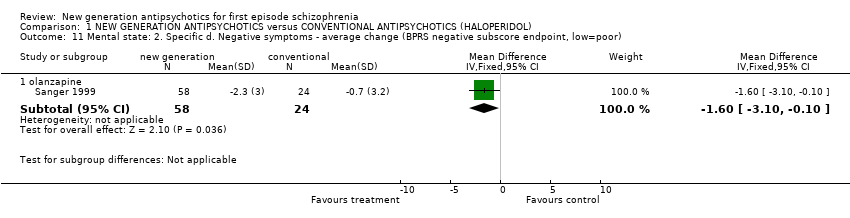
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 11 Mental state: 2. Specific d. Negative symptoms ‐ average change (BPRS negative subscore endpoint, low=poor).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 12 Mental state: 2. Specific e. Depressive symptoms ‐ average change (MADRS endpoint, low=poor).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 13 Adverse events: 1. General a. At least one adverse event.

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 14 Adverse events: 2. Extrapyramdial problems a. General i. Needing anticholinergic medication at least once.
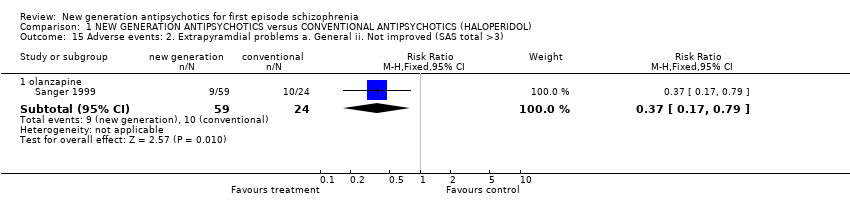
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 15 Adverse events: 2. Extrapyramdial problems a. General ii. Not improved (SAS total >3).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 16 Adverse events: 2. Extrapyramdial problems a. General iii. Average change (SAS, low=poor).
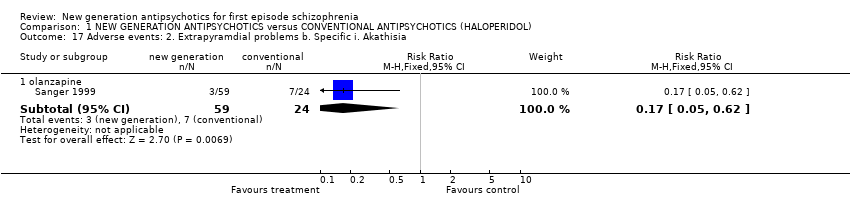
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 17 Adverse events: 2. Extrapyramdial problems b. Specific i. Akathisia.

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 18 Adverse events: 2. Extrapyramdial problems b. Specific ii. Akathisia ‐ average change (BAS, low=poor).

Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 19 Adverse Events: 2. Extrapyramdial problems b. Specific iii. Others.
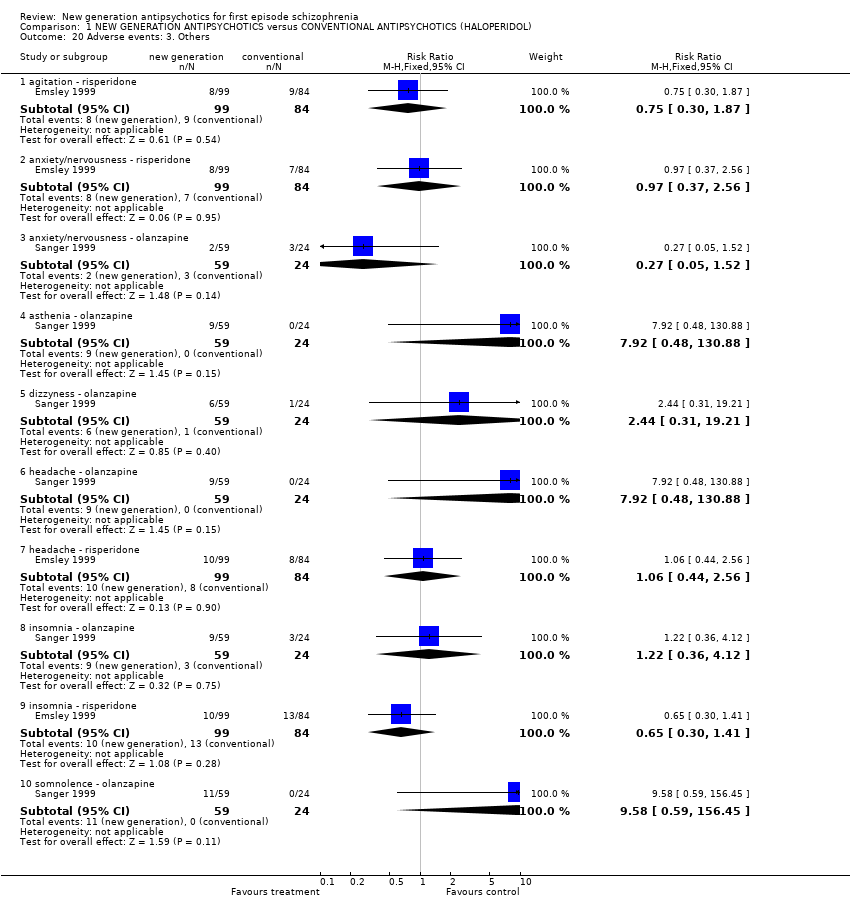
Comparison 1 NEW GENERATION ANTIPSYCHOTICS versus CONVENTIONAL ANTIPSYCHOTICS (HALOPERIDOL), Outcome 20 Adverse events: 3. Others.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early due to any reason Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 due to any reason ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.26, 0.73] |
| 1.2 due to any reason ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.39, 1.08] |
| 1.3 due to adverse effects ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.86] |
| 1.4 due to adverse effects ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.84] |
| 2 Global state: 1. No clinically significant change (CGI < much improved) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.63, 1.54] |
| 3 Global state: 2. Need of additional medication (at least one dose of benzodiazepine) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.52, 1.11] |
| 4 Mental state: 1. General a. No clinically significant improvement ‐ as defined by each of the studies Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.71] |
| 4.2 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 5 Mental state: 1. General b. Various measures of change Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 less than 50% PANSS reduction (risperidone) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 5.2 less than 40% PANSS reduction (olanzapine) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.71] |
| 5.3 less than 50% BPRS reduction (risperidone) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.55, 1.12] |
| 6 Mental state: 1. General c. Average change (PANSS endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐11.2 [‐20.16, ‐2.24] |
| 6.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐8.81, 5.61] |
| 7 Mental state: 1. General d. Average change (BPRS endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐7.80 [‐13.35, ‐2.25] |
| 7.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.27, 3.07] |
| 8 Mental state: 2. Specific a. Positive symptoms ‐ average change (PANSS positive subscore endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐6.34, ‐0.46] |
| 8.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.18, 1.98] |
| 9 Mental state: 2. Specific b. Positive symptoms ‐ average change (BPRS positive subscore endpoint, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐3.28, 0.68] |
| 10 Mental state: 2. Specific c. Negative symptoms ‐ average change (PANSS negative subscore endpoint, low=poor) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.7 [‐5.41, 0.01] |
| 10.2 risperidone | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.58, 1.58] |
| 11 Mental state: 2. Specific d. Negative symptoms ‐ average change (BPRS negative subscore endpoint, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 olanzapine | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.10, ‐0.10] |
| 12 Mental state: 2. Specific e. Depressive symptoms ‐ average change (MADRS endpoint, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 olanzapine | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐7.01, 1.21] |
| 13 Adverse events: 1. General a. At least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.76, 0.98] |
| 14 Adverse events: 2. Extrapyramdial problems a. General i. Needing anticholinergic medication at least once Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.72] |
| 14.2 risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.85] |
| 15 Adverse events: 2. Extrapyramdial problems a. General ii. Not improved (SAS total >3) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.17, 0.79] |
| 16 Adverse events: 2. Extrapyramdial problems a. General iii. Average change (SAS, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 olanzapine | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐8.08, ‐1.92] |
| 17 Adverse events: 2. Extrapyramdial problems b. Specific i. Akathisia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.62] |
| 18 Adverse events: 2. Extrapyramdial problems b. Specific ii. Akathisia ‐ average change (BAS, low=poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 olanzapine | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.6 [‐1.12, ‐0.08] |
| 19 Adverse Events: 2. Extrapyramdial problems b. Specific iii. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 extrapyramidal syndrome ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.52] |
| 19.2 hypertonia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.83] |
| 19.3 hypokinesia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.83] |
| 20 Adverse events: 3. Others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 agitation ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.87] |
| 20.2 anxiety/nervousness ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.37, 2.56] |
| 20.3 anxiety/nervousness ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.52] |
| 20.4 asthenia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [0.48, 130.88] |
| 20.5 dizzyness ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.31, 19.21] |
| 20.6 headache ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [0.48, 130.88] |
| 20.7 headache ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.44, 2.56] |
| 20.8 insomnia ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.36, 4.12] |
| 20.9 insomnia ‐ risperidone | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.41] |
| 20.10 somnolence ‐ olanzapine | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.58 [0.59, 156.45] |

