Efavirenz o nevirapina en el tratamiento de combinación de tres fármacos con dos inhibidores nucleósidos de la transcriptasa inversa para el tratamiento inicial de la infección por VIH en individuos que nunca recibieron tratamiento antirretroviral
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | A prospective, open, randomised trial in the department of infectiology of the Hospital de Especialidades in Moterry, Nuevo Leon, Mexico. | |
| Participants | 58 participants. Inclusion criteria: At least 18 years old, of either gender, HIV‐positive, antiretroviral‐naïve. Exclusion criteria: Patients with contraindications to either NVP or EFV, pregnant women, diminished renal or liver functions. | |
| Interventions | AZT 300mg and 3TC 150mg with either NVP 200mg twice daily (N=28) or EFV 600mg at night (N=30) | |
| Outcomes | Viral load, CD4 count, adverse events, AIDS‐defining conditions, death. Follow up was for 48 weeks. | |
| Notes | All patients provided informed consent to participate in the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The author provided this information |
| Allocation concealment? | High risk | The author provided this information |
| Blinding? | High risk | Open‐label study |
| Incomplete outcome data addressed? | Unclear risk | Unclear, ITT analyses conducted but loss to follow‐up was quite high and reasons for drop‐outs were not reported. |
| Free of selective reporting? | Low risk | All outcomes of interest were reported upon |
| Free of other bias? | Low risk | Yes, was funded by the Mexican Ministry of Health (based on author |
| Baseline data reported? | Low risk | Demographic characteristics, clinical stage, CD4 count, viral load |
| Methods | Prospective open‐label randomised, comparative trial in Nonthaburi, Thailand from December 2006 to October 2007 | |
| Participants | Inclusion criteria: HIV‐1 infection in individuals aged 18‐60 years, active TB diagnosed by clinical features plus acid‐fast stain and/or culture positive for Mycobacterium tuberculosis, receipt of treatment with a rifampicin‐ containing anti‐TB regimen 4‐16 weeks before enrolment,naïve to ART, and CD4+ cell count, <350 cells/ mm3. Exclusion criteria: aspartate aminotransferase and alanine aminotransferase levels >5 times the upper limit of normal range;serum creatinine level >12 mg/ dL; receipt of a medication that has drug‐drug interactions with nevirapine or efavirenz;receipt of immunosuppressive drugs; and pregnancy or lactation | |
| Interventions | Efavirenz 600mg or Nevirapine 200mg twice daily with 3TC 150mg/D4T 30 or 40mg BID. Follow up was for 48 weeks. 142 patients with 71 in each arm. | |
| Outcomes | Primary outcome: Proportion of patients achieving a plasma HIV‐RNA level<50 copies/mL after 48 weeks of ART. Secondary outcomes: Proportion of patients with concentrations of NNRTI at 12 hours after dosing, lower than the recommended minimal level, CD4 cell count at week 48 of ART, incidence of NNRTI‐associated adverse reactions | |
| Notes | Written consent was obtained from participants aka N2R study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomised" |
| Allocation concealment? | Unclear risk | "Patients were randomised" |
| Blinding? | High risk | Open‐label study |
| Incomplete outcome data addressed? | Low risk | No missing outcome data |
| Free of selective reporting? | High risk | Primary, but not all secondary outcomes are reported. |
| Free of other bias? | Low risk | Yes, this study was funded by the Thailand Ministry of Public Health, |
| Baseline data reported? | Low risk | Age, sex, body weight, body mass index, site of tuberculosis, time from tuberculosis diagnosis to initiation of ART, CD4 cell count, plasma HIV‐1 RNA level, Hemoglobin concentration, serum alkaline phosphatase, alanine aminotransferase, albumin, creatinine, hepatitis B virus antigen, hepatitis C antibody, cholesterol, triglycerides |
| Methods | A randomised, open‐label, pilot study in Hospital Carlos III in Madrid Span from March 1999 to January 2002 | |
| Participants | Eligibility criteria: HIV‐infected antiretroviral‐naïve adults, aged above 18 years old with CD4 counts >100 cells/mm3 and detectable plasma | |
| Interventions | d4T and ddI with either NVP or EFV at the following doses: NVP 400 mg once a day, d4T 40 mg twice a day, ddI 400 mg once a day, and EFV 600 mg once a day. Follow up was for 48 weeks. | |
| Outcomes | Primary: the proportion of individuals achieving plasma HIV RNA <50 copies/ mL and the proportion developing drug‐related toxicities, which caused cessation of the NNRTI. Secondary: mean changes in CD4+ lymphocyte counts, overall safety, degree of adherence, and adverse events. | |
| Notes | All patients provided informed consent to participate in the study. aka SENC trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Participants were randomised" |
| Allocation concealment? | Unclear risk | "Participants were randomised" |
| Blinding? | High risk | Open‐label study. |
| Incomplete outcome data addressed? | Low risk | No missing outcome data. 3 patients lost to follow up right after enrolment |
| Free of selective reporting? | Low risk | Primary and secondary outcomes are reported. |
| Free of other bias? | Low risk | Yes, not funded by industry. Funded by the Asociacíon Investigacíon y |
| Baseline data reported? | Low risk | Age, gender, HIV transmission, plasma HIV RNA, absolute CD4 count, number with AIDS, positive antiHCV antibody, positive HBsAg |
| Methods | A randomised controlled trial to compare AZT+3TC+NVP vs. AZT+3TC+EFV among 70 HIV‐infected patients in Senegal Age limits not given. | |
| Participants | 70 ART treatment‐naïve patients from Senegal | |
| Interventions | AZT 300 mg,3TC 150 mg and NVP 200 mg (N=35) on one hand versus AZT 300 mg,3TC 150 mg and EFV 600 mg (N=35) | |
| Outcomes | Decrease in viral burden, side‐effects and change in CD4 count. Follow up was for 76 weeks. | |
| Notes | This study was reported in abstract form only, so information and data for abstraction was limited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? | Unclear risk | Not reported |
| Incomplete outcome data addressed? | Unclear risk | Not reported |
| Free of selective reporting? | Unclear risk | Not reported |
| Free of other bias? | Unclear risk | Not reported |
| Baseline data reported? | Unclear risk | Not reported |
| Methods | Open‐label, randomised trial of 127 HIV patients co‐infected with TB in the Tuberculosis Research Centre, Chennai, India, between May 2006 and June 2008. | |
| Participants | HIV positive patients, co‐infected with pulmonary or extra pulmonary tuberculosis receiving treatment including rifampicin. EFV 600mg arm N= 59, NVP 400mg arm, N= 57. Age limits not given. | |
| Interventions | Two arms: EFV 600 mg or NVP 400 mg (after a 14‐day phase with 200 mg) with a DDI 250/400 mg and 3TC 300 mg backbone, all given once‐daily in the morning. Follow up was for 24 weeks. | |
| Outcomes | Sputum smear and mycobacterial, CD4 cell count, viral load (> 400 copies/mL), liver function and death. | |
| Notes | This study was reported in abstract form only, so information and data for abstraction was limited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? | High risk | " Open‐label clinical trial" |
| Incomplete outcome data addressed? | Unclear risk | Not reported |
| Free of selective reporting? | Unclear risk | Not reported |
| Free of other bias? | Unclear risk | Not reported |
| Baseline data reported? | Unclear risk | Not reported |
| Methods | The FIRST study randomised patients into three strategy arms, one of which was NNRTI+NRTI. NNRTI was determined by optional randomisation (NVP or EFV) or by choice. | |
| Participants | 228 antiretroviral‐naïve, HIV‐positive patients, aged at least 13 years. | |
| Interventions | There were 111 participants in the EFV arm (EFV 600mg once daily) and 117 in the NVP arm (NVP 200mg twice daily). Dosing was obtained from the authors. Four different NRTI backbones were used (ABC/3TC, ddI/d4T, AZT/3TC, d4T/3TC) | |
| Outcomes | HIV RNA >50 copies /ml, change in CD4 count or death. Follow up was for 32 weeks. | |
| Notes | All patients provided informed consent to participate in the study. aka FIRST or CPCRA study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 1:1 allocation |
| Allocation concealment? | Low risk | Participants called a hotline to be assigned a treatment |
| Blinding? | Low risk | Yes, in the FIRST paper (2001) the study team was blinded to interm results so for this sub‐study we assumed they were blinded to treatment as well. |
| Incomplete outcome data addressed? | Low risk | ITT analyses were used |
| Free of selective reporting? | Low risk | Yes, all outcomes of interest were reported. |
| Free of other bias? | Low risk | Yes, this study was sponsored by non‐industry funding (NIH). |
| Baseline data reported? | Low risk | Socio‐demographic data, CD4 count, viral load, prior AIDS event, hepatitis B or C and history of injection drug use |
| Methods | Multicentre, open‐label, randomised trial of 1216 ARV naïve patients in North/South America, Australia, Europe, South Africa and Thailand | |
| Participants | Inclusion criteria: ARV naïve patients of both sexes, aged at least 16 years, with plasma RNA > 5000 copies per ml Exclusion criteria: Pregnancy, lactation, HB<6.3mmol/L in males and 5.7mmol/L in females, neutrophils <1 x 109, platelets<75 x 109, serum amylases > 2·0 times the upper limit of normal in combination with serum lipase < 1·5 times the upper limit of normal; aspartate aminotransferase < 5·0 times the upper limit of normal; or bilirubin< 2·5 times the upper limit of normal; history of clinical pancreatitis or neuropathy within the previous 6 months; renal failure necessitating dialysis; radiotherapy, cytotoxic, or immunomodulating treatment within the month preceding the start of study or the expected need for it; infection with HIV‐2; or likely non adherence as judged by the investigator. NVP once daily N=220, NVP twice daily N= 387, EFV N=400. | |
| Interventions | Four arms; only three of interest: d4T 40mg BID and 3TC 150mg BID with either NVP 400mg once daily, NVP 200mg twice daily or EFV 600mg once daily. Follow up for 48 weeks. | |
| Outcomes | Primary: Proportion of patients with treatment failure Secondary: proportion of patients with virological failure (never having a plasma HIV‐1 RNA concentration <50 copies per mL, or two consecutive measurements 50 copies per mL after having had a concentration below the cut‐off), the proportion of patients with plasma HIV‐1 RNA concentrations below 50 copies/mL at each study week; the change in CD4‐positive cells between the start of treatment and week 48; and the frequencies of clinical and laboratory adverse events. | |
| Notes | Ethics: approved by the ethics review bodies in the participating countries, and all patients gave written aka 2NN study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "A treatment allocation sequence was generated by use of the minimisation variables CD4‐positive T‐cell count (350 vs >350 cells per μL) and study region. Treatment allocation was stratified by baseline plasma HIV‐1 RNA concentration (30 000 copies per mL vs >30 000 copies per mL)". |
| Allocation concealment? | Low risk | "Allocation was done at the central study coordination centre, concealed from the investigator before enrolment" |
| Blinding? | High risk | "There was no masking after treatment allocation" |
| Incomplete outcome data addressed? | Low risk | "All analyses were done for the intention‐to‐treat population, including all randomised patients (n=1216)." |
| Free of selective reporting? | Low risk | All outcome measurements were analysed and reported |
| Free of other bias? | High risk | Some of the authors had received travel grants and honoraria from the sponsors. This study was industry funded (Boehringer‐Ingelheim). |
| Baseline data reported? | Low risk | Gender,age, body mass index, geographical region, HIV risk behaviour, CDC class C, CD4 cell count, HIV RNA, co‐infection(hepatitis B, hepatitis C viruses) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised clinical trial, a retrospective cohort study from the EUROSIDA data base. | |
| Not a randomised clinical trial | |
| Not a randomised clinical trial, a retrospective cohort study from 'Initiative Senegalaise d'Acces aux Medicaments Antiretroviraux' (ISAARV) prospective cohort of which the EFV arm was a clinical trial. | |
| Not a randomised clinical trial | |
| Not a randomised clinical trial, a prospective cohort study. | |
| Not a randomised clinical trial,a prospective cohort study. | |
| Not a randomised clinical trial,a prospective cohort study. | |
| Not a randomised clinical trial, a retrospective cohort study from medical records. | |
| Not a randomised clinical trial, an observational study. | |
| Not a randomised clinical trial, an observational study. | |
| Not a randomised clinical trial, an observational study. | |
| Not a randomised clinical trial, a retrospective cohort study from medical records. | |
| Not a randomised clinical trial, a retrospective cohort study from the Aid for AIDS prospective data base in southern Africa. | |
| Not a randomised clinical trial | |
| Not a randomised clinical trial, a prospective cohort study. | |
| Not a randomised clinical trial, a prospective cohort study. Data collected from 2 treatment cohorts. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | A prospective, randomised, open‐label, multi centre comparative trial by the AMADEUS study group in Madrid, Spain. |
| Participants | 69 HIV positive treatment‐naïve patients were included. |
| Interventions | ZDV/3TC versus D4T/ddI plus EFV, NVP or Indinavir/Ritonavir. |
| Outcomes | Viral load < 200 copies/ml, median increase in CD4 count. |
| Notes | aka AMADEUS 01 Study. Full text not available. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 4 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| Analysis 1.1  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 1 Virological success. | ||||
| 2 Change in CD4 count Show forest plot | 5 | 1285 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐23.17, 19.18] |
| Analysis 1.2  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 2 Change in CD4 count. | ||||
| 3 Mortality Show forest plot | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.50, 1.57] |
| Analysis 1.3  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 3 Mortality. | ||||
| 4 Progression to AIDS Show forest plot | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.53, 3.30] |
| Analysis 1.4  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 4 Progression to AIDS. | ||||
| 5 Discontinuation rate Show forest plot | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| Analysis 1.5  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 5 Discontinuation rate. | ||||
| 6 Severe adverse events:Rash Show forest plot | 4 | 1227 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.03] |
| Analysis 1.6  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 6 Severe adverse events:Rash. | ||||
| 7 Severe adverse events:CNS Show forest plot | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.64, 12.79] |
| Analysis 1.7  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 7 Severe adverse events:CNS. | ||||
| 8 Severe adverse events: GIT Show forest plot | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.38] |
| Analysis 1.8  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 8 Severe adverse events: GIT. | ||||
| 9 Severe adverse events: Pyrexia Show forest plot | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.36] |
| Analysis 1.9  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 9 Severe adverse events: Pyrexia. | ||||
| 10 Severe adverse events: Raised transaminases Show forest plot | 2 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.95] |
| Analysis 1.10  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 10 Severe adverse events: Raised transaminases. | ||||
| 11 Severe adverse events: Raised alkaline phosphatase Show forest plot | 1 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.14, 2.41] |
| Analysis 1.11  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 11 Severe adverse events: Raised alkaline phosphatase. | ||||
| 12 Severe adverse events: Raised Amylase Show forest plot | 1 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.54, 2.32] |
| Analysis 1.12  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 12 Severe adverse events: Raised Amylase. | ||||
| 13 Severe adverse events: Raised Triglycerides Show forest plot | 1 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.28, 3.32] |
| Analysis 1.13  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 13 Severe adverse events: Raised Triglycerides. | ||||
| 14 Severe adverse events: Neutropenia Show forest plot | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.76] |
| Analysis 1.14  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 14 Severe adverse events: Neutropenia. | ||||
| 15 All severe adverse events Show forest plot | 4 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.60, 1.70] |
| Analysis 1.15  Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 15 All severe adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 3 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.31] |
| Analysis 2.1  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 1 Virological success. | ||||
| 2 Change in CD4 count Show forest plot | 2 | 687 | Mean Difference (IV, Random, 95% CI) | 8.74 [‐7.60, 25.08] |
| Analysis 2.2  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 2 Change in CD4 count. | ||||
| 3 Mortality Show forest plot | 3 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.94] |
| Analysis 2.3  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 3 Mortality. | ||||
| 4 Progression to AIDS Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.30, 2.04] |
| Analysis 2.4  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 4 Progression to AIDS. | ||||
| 5 Discontinuation rate Show forest plot | 2 | 674 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.15, 1.90] |
| Analysis 2.5  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 5 Discontinuation rate. | ||||
| 6 Severe adverse events: Rash Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.45, 3.07] |
| Analysis 2.6  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 6 Severe adverse events: Rash. | ||||
| 7 Severe adverse events:CNS Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 7.52 [1.14, 49.80] |
| Analysis 2.7  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 7 Severe adverse events:CNS. | ||||
| 8 Severe adverse events: GIT Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.49, 3.44] |
| Analysis 2.8  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 8 Severe adverse events: GIT. | ||||
| 9 Severe adverse events: Pyrexia Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.14, 4.90] |
| Analysis 2.9  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 9 Severe adverse events: Pyrexia. | ||||
| 10 Severe adverse events: Raised transaminases Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.81] |
| Analysis 2.10  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 10 Severe adverse events: Raised transaminases. | ||||
| 11 Severe adverse events: Raised alkaline phosphatase Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.14, 4.90] |
| Analysis 2.11  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 11 Severe adverse events: Raised alkaline phosphatase. | ||||
| 12 Severe adverse events: Raised Amylase Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.78, 6.14] |
| Analysis 2.12  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 12 Severe adverse events: Raised Amylase. | ||||
| 13 Severe adverse events: Raised Triglycerides Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.32, 4.22] |
| Analysis 2.13  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 13 Severe adverse events: Raised Triglycerides. | ||||
| 14 Severe adverse events: Raised cholesterol Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 6.03 [0.75, 48.78] |
| Analysis 2.14  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 14 Severe adverse events: Raised cholesterol. | ||||
| 15 Severe adverse events: Neutropenia Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.30, 2.29] |
| Analysis 2.15  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 15 Severe adverse events: Neutropenia. | ||||
| 16 All severe adverse events Show forest plot | 3 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.93, 1.91] |
| Analysis 2.16  Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 16 All severe adverse events. | ||||
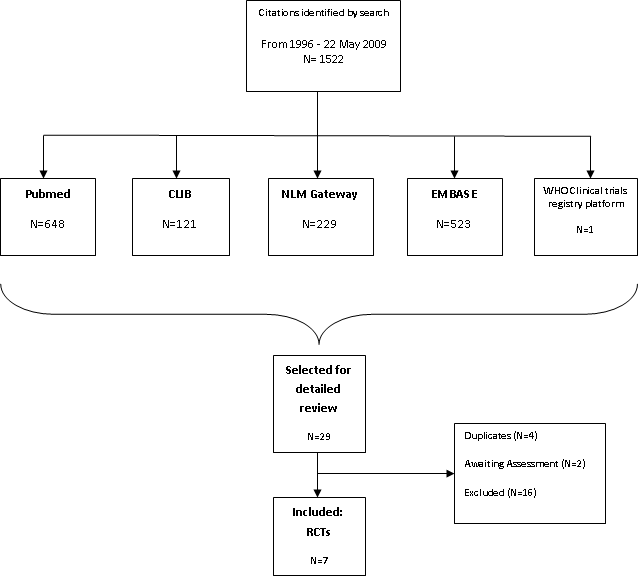
Results of search

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
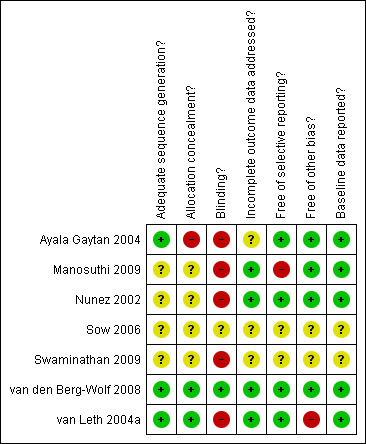
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
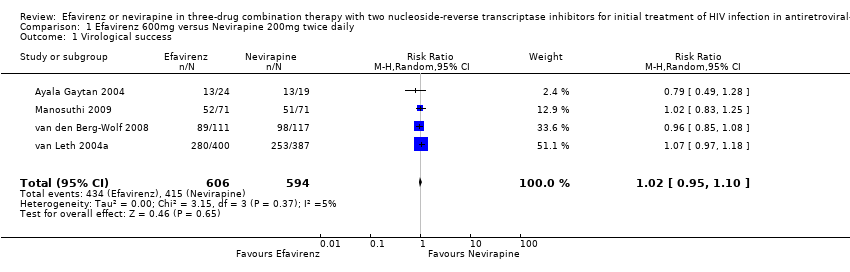
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 1 Virological success.
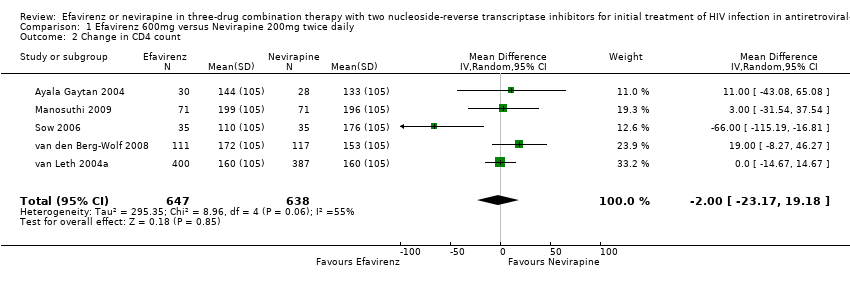
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 2 Change in CD4 count.

Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 3 Mortality.
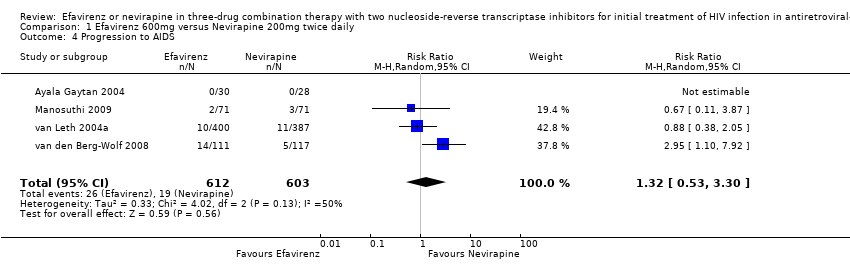
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 4 Progression to AIDS.
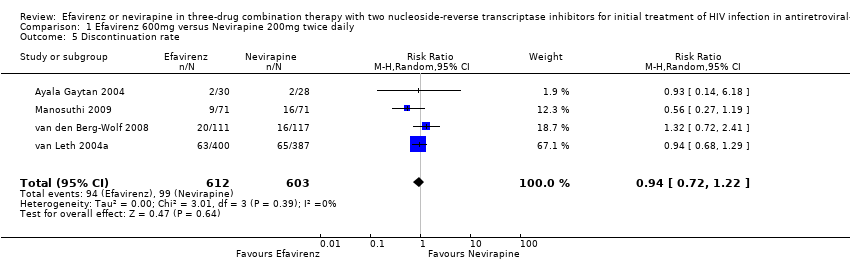
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 5 Discontinuation rate.
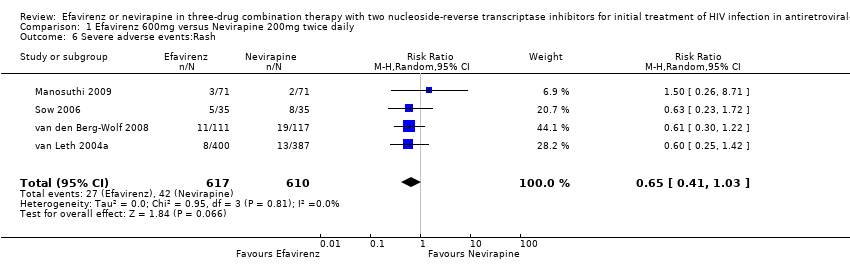
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 6 Severe adverse events:Rash.
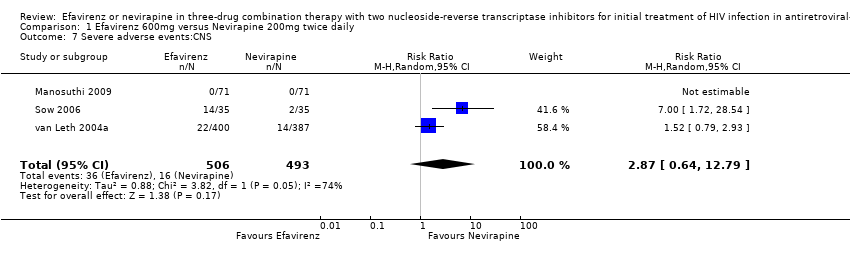
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 7 Severe adverse events:CNS.
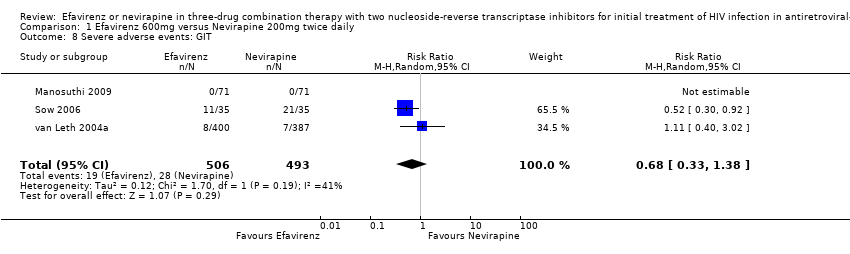
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 8 Severe adverse events: GIT.

Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 9 Severe adverse events: Pyrexia.
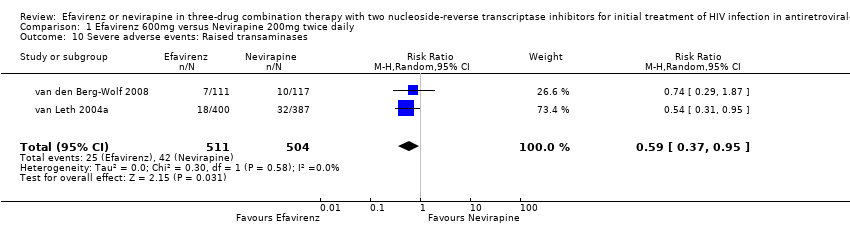
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 10 Severe adverse events: Raised transaminases.

Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 11 Severe adverse events: Raised alkaline phosphatase.

Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 12 Severe adverse events: Raised Amylase.

Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 13 Severe adverse events: Raised Triglycerides.
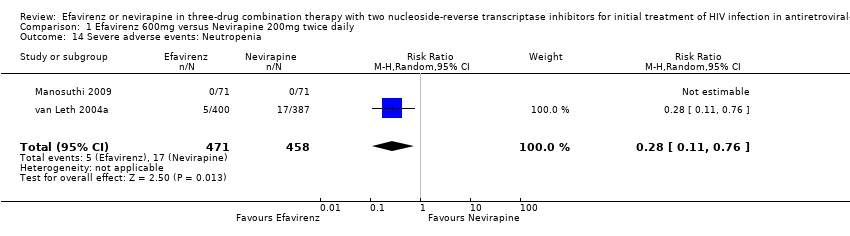
Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 14 Severe adverse events: Neutropenia.

Comparison 1 Efavirenz 600mg versus Nevirapine 200mg twice daily, Outcome 15 All severe adverse events.
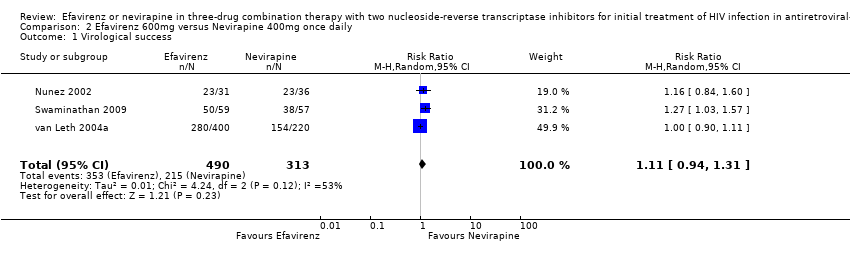
Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 1 Virological success.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 2 Change in CD4 count.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 3 Mortality.
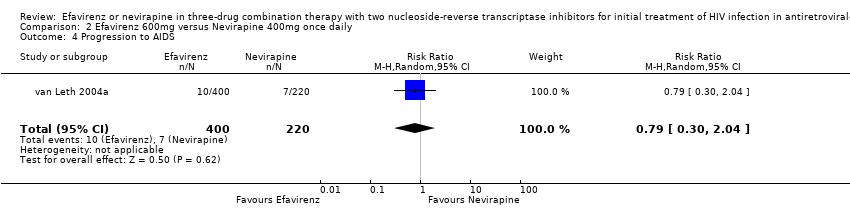
Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 4 Progression to AIDS.
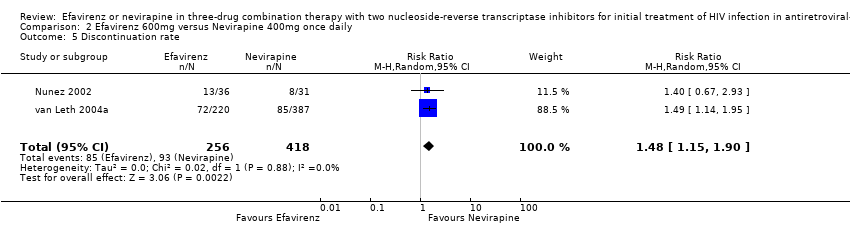
Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 5 Discontinuation rate.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 6 Severe adverse events: Rash.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 7 Severe adverse events:CNS.
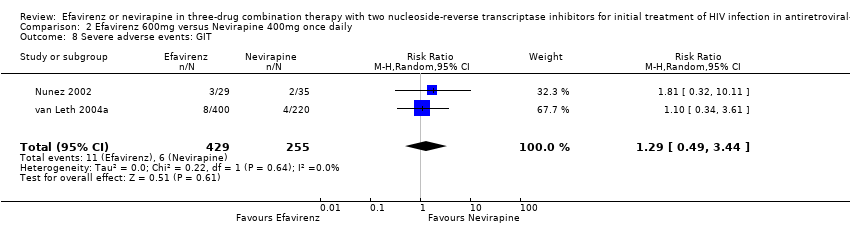
Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 8 Severe adverse events: GIT.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 9 Severe adverse events: Pyrexia.
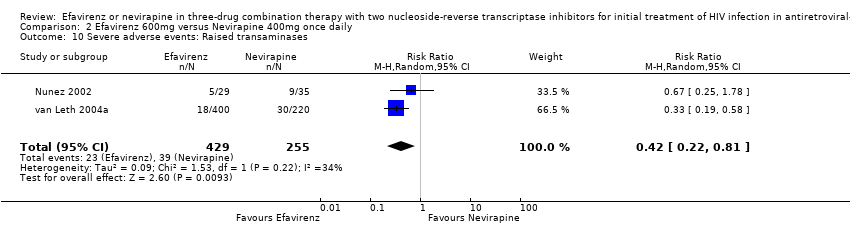
Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 10 Severe adverse events: Raised transaminases.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 11 Severe adverse events: Raised alkaline phosphatase.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 12 Severe adverse events: Raised Amylase.
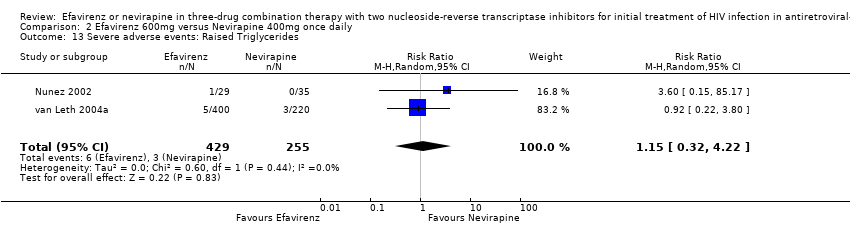
Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 13 Severe adverse events: Raised Triglycerides.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 14 Severe adverse events: Raised cholesterol.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 15 Severe adverse events: Neutropenia.

Comparison 2 Efavirenz 600mg versus Nevirapine 400mg once daily, Outcome 16 All severe adverse events.
| Efavirenz 600mg versus Nevirapine 200mg twice daily for initial treatment of HIV infection in antiretroviral‐naive individuals | ||||||
| Patient or population: patients with initial treatment of HIV infection in antiretroviral‐naive individuals | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Efavirenz 600mg versus Nevirapine 200mg twice daily | |||||
| Virological success | 699 per 1000 | 713 per 1000 | RR 1.02 | 1200 | ⊕⊕⊕⊕ | |
| Change in CD4 count | The mean Change in CD4 count in the intervention groups was | 1285 | ⊕⊕⊕⊕ | |||
| Mortality | 55 per 1000 | 49 per 1000 | RR 0.89 | 1215 | ⊕⊕⊕⊝ | |
| Progression to AIDS | 32 per 1000 | 42 per 1000 | RR 1.32 | 1215 | ⊕⊕⊕⊝ | |
| Discontinuation rate | 164 per 1000 | 154 per 1000 | RR 0.94 | 1215 | ⊕⊕⊕⊝ | |
| All severe adverse events | 201 per 1000 | 203 per 1000 | RR 1.01 | 1054 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 3 of the 7 RCT studies were open‐label (Ayala, Manosuthi, Swaminathan), but studies were not downgraded based on this fact. | ||||||
| Efavirenz 600mg versus Nevirapine 400mg once daily for initial treatment of HIV infection in antiretroviral‐naive individuals | ||||||
| Patient or population: patients with initial treatment of HIV infection in antiretroviral‐naive individuals | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Efavirenz 600mg versus Nevirapine 400mg once daily | |||||
| Virological success | 687 per 1000 | 763 per 1000 | RR 1.11 | 803 | ⊕⊕⊕⊕ | |
| Change in CD4 count | The mean Change in CD4 count in the intervention groups was | 687 | ⊕⊕⊕⊕ | |||
| Mortality | 52 per 1000 | 21 per 1000 | RR 0.41 | 878 | ⊕⊕⊕⊝ | |
| Progression to AIDS | 32 per 1000 | 25 per 1000 | RR 0.79 | 620 | ⊕⊕⊕⊝ | |
| Discontinuation rate | 222 per 1000 | 329 per 1000 | RR 1.48 | 674 | ⊕⊕⊕⊝ | |
| All severe adverse events | 163 per 1000 | 218 per 1000 | RR 1.34 | 803 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 3 of the 7 RCT studies were open‐label (Ayala, Manosuthi, Swaminathan), but studies were not downgraded based on this fact. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 4 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.95, 1.10] |
| 2 Change in CD4 count Show forest plot | 5 | 1285 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐23.17, 19.18] |
| 3 Mortality Show forest plot | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.50, 1.57] |
| 4 Progression to AIDS Show forest plot | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.53, 3.30] |
| 5 Discontinuation rate Show forest plot | 4 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 6 Severe adverse events:Rash Show forest plot | 4 | 1227 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.03] |
| 7 Severe adverse events:CNS Show forest plot | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.64, 12.79] |
| 8 Severe adverse events: GIT Show forest plot | 3 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.38] |
| 9 Severe adverse events: Pyrexia Show forest plot | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.36] |
| 10 Severe adverse events: Raised transaminases Show forest plot | 2 | 1015 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.95] |
| 11 Severe adverse events: Raised alkaline phosphatase Show forest plot | 1 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.14, 2.41] |
| 12 Severe adverse events: Raised Amylase Show forest plot | 1 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.54, 2.32] |
| 13 Severe adverse events: Raised Triglycerides Show forest plot | 1 | 787 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.28, 3.32] |
| 14 Severe adverse events: Neutropenia Show forest plot | 2 | 929 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.76] |
| 15 All severe adverse events Show forest plot | 4 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.60, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Virological success Show forest plot | 3 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.31] |
| 2 Change in CD4 count Show forest plot | 2 | 687 | Mean Difference (IV, Random, 95% CI) | 8.74 [‐7.60, 25.08] |
| 3 Mortality Show forest plot | 3 | 878 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.94] |
| 4 Progression to AIDS Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.30, 2.04] |
| 5 Discontinuation rate Show forest plot | 2 | 674 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.15, 1.90] |
| 6 Severe adverse events: Rash Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.45, 3.07] |
| 7 Severe adverse events:CNS Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 7.52 [1.14, 49.80] |
| 8 Severe adverse events: GIT Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.49, 3.44] |
| 9 Severe adverse events: Pyrexia Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.14, 4.90] |
| 10 Severe adverse events: Raised transaminases Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.81] |
| 11 Severe adverse events: Raised alkaline phosphatase Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.14, 4.90] |
| 12 Severe adverse events: Raised Amylase Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.78, 6.14] |
| 13 Severe adverse events: Raised Triglycerides Show forest plot | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.32, 4.22] |
| 14 Severe adverse events: Raised cholesterol Show forest plot | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 6.03 [0.75, 48.78] |
| 15 Severe adverse events: Neutropenia Show forest plot | 1 | 620 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.30, 2.29] |
| 16 All severe adverse events Show forest plot | 3 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.93, 1.91] |

