Fluoxetina versus otros tipos de farmacoterapia para la depresión
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Eight‐week double‐blind, multicentre study | |
| Participants | Outpatients suffering from a major depressive episode according to DSM‐III‐R, with a baseline score on Hamilton Rating Scale for Depression‐17 Item (HDRS‐17) of at least 18, recruited from nine separated psychiatric clinics. | |
| Interventions | Fluoxetine: 56 participants | |
| Outcomes | HDRS‐17 and Hamilton Rating Scale for Anxiety (HAM‐A), Montgomery and Asberg Scale for Depression (MADRS), Zung Self‐Rating Scale for Anxiety, Leeds Sleep Evaluation Questionnaire, Clinical Global Impression Scale, including severity (CGI‐S) and improvement (CGI‐I) | |
| Notes | Seventy‐five per cent of the patients were women. Higher percentage of patients with a family history of psychiatric illness in the fluoxetine group. Higher percentage of patients with severe depression in the fluoxetine group (30.4%) than in the sertraline group (13.7%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double‐blind". No further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind". No further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Incoherence between denominators |
| Selective reporting (reporting bias) | High risk | No follow‐up scores reported |
| Other bias | Unclear risk | Funding: unclear information |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients meeting DSM‐IV diagnostic criteria for major depression, with a minimum baseline score of 20 on the Hamilton Rating Scale for Depression‐17 Item (HDRS‐17). | |
| Interventions | Fluoxetine: 24 participants | |
| Outcomes | Primary outcome: HDRS‐17 | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned". No further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind". No further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "were put in capsules to provide similar appearance with fluoxetine". No further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if raters were independent |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for discontinuation reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Mean endpoint scores and standard deviation reported only in figure. Side effects reported |
| Other bias | Unclear risk | Insufficient information |
| Methods | Eight‐week double‐blind, randomised trial | |
| Participants | Outpatients meeting DSM‐IV diagnostic criteria for major depression, with a minimum baseline score of 18 and not superior to 25 on the Hamilton Rating Scale for Depression‐17 Item (HDRS‐17). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | Primary outcome: HDRS‐17, remission was defined as an endpoint score of 7 or less | |
| Notes | Funding: independent study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to receive capsule of petal of Crocus or fluoxetine in a 1:1 ratio using computer code", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "capsules (...) did not have any different taste compared to fluoxetine", "the person who administered the medication, raters and patients were blind to assignment", not clear if blindness was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "the person who administered the medication, raters and patients were blind to assignment", not clear if blindness was successful |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of responders reported with denominator. Reasons for dropout not reported |
| Selective reporting (reporting bias) | Unclear risk | Mean endpoint scores and standard deviation reported only in figures. Side effects reported |
| Other bias | Low risk | Funding: independent study |
| Methods | Twelve‐week double‐blind study | |
| Participants | Outpatients suffering from a major depressive episode, recurrent depression or disthymia according to DSM‐III‐R, with a score of at least 25 on the HARD Humeur, Agoisse, Ralentissement, Danger (HARD), Ferreri anxiety rating diagram (FARD), Hopkins Symptom check‐list (HSCL). | |
| Interventions | Fluoxetine: 104 participants | |
| Outcomes | HARD and on the FARD scales | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Dropout rate reported with reasons. Scores at endpoint are reported with denominator |
| Selective reporting (reporting bias) | Unclear risk | Full side effects reported. Other outcomes not clearly stated |
| Other bias | High risk | Last author's affiliation was IRIS and this company produces tianeptine |
| Methods | Five‐week double‐blind randomised study | |
| Participants | Inpatients fulfilling DSM‐III criteria for major depressive episode and scoring at least 18 on Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 13 participants | |
| Outcomes | HDRS‐17 score | |
| Notes | Elderly patients only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". No other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind". No further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind". No further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double blind". No further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of dropouts reported, but reasons not specified Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported only in figure. Only most common side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Twelve‐week double‐blind randomised multicentre study | |
| Participants | Outpatients meeting DSM‐IV diagnostic criteria for major depression, with a minimum baseline score of 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21), recruited from three clinical sites. | |
| Interventions | Fluoxetine: 47 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) | |
| Notes | Patients in the fluoxetine group had more chronic histories of depression at baseline. Predominance of females in the whole study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to treatments groups using a balanced randomisation from randomly permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "the investigators were provided individual sealed envelopes" |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Efficacy analysis were performed on ITT basis using a last observation carried forward method (LOCF)", but scores reported without denominator. Reasons and number of dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported (at last 7% incidence). Mean endpoint scores and standard deviation reported |
| Other bias | High risk | First author's affiliation was Wyeth‐Lederle, Portugal, and this company produces venlafaxine |
| Methods | Six‐week double‐blind randomised trial | |
| Participants | In and outpatients meeting DSM‐IV diagnostic criteria for major depression, with a minimum baseline score of 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 18 participants Mirtazapine dose: 30 mg/day | |
| Outcomes | Mean decrease in HDRS‐17 score from baseline | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | "Double blind", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double blind", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double blind", no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "A observed cases analysis over the 6 weeks was the primary efficacy analysis (...) an intention to treat analysis with LOCF was also performed...all discussion of result is based on observed cases (OC) analysis". Number of randomised, and number of lost during the follow‐up reported. Number of responders reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Side‐effects are reported. Vital signs and body weight not reported Mean endpoint scores (HDRS) reported in figures and without standard deviations |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week double‐blind, randomised multicentre study | |
| Participants | In and outpatients meeting DSM‐III‐R diagnostic criteria for major depression, with a minimum baseline score of 22 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21), recruited from 33 clinical sites. | |
| Interventions | Fluoxetine: 127 participants | |
| Outcomes | Primary outcome: absolute change in the HDRS‐21 total score | |
| Notes | Response: decrease of at least 50% in the HDRS total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | "Double blind", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double blind", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double blind", no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of randomised and number of lost during follow‐up reported, but the reasons for dropout were not clear. Only most common side effects were reported Quote: "Statistical analysis was carried out on the intent to treat population" |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects were reported Mean endpoint scores and standard deviation reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind, randomised multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 25 on Montgomery and Asberg Scale for Depression (MADRS) and of at least 4 on Clinical Global Impression Scale (CGI). | |
| Interventions | Fluoxetine: 93 participants | |
| Outcomes | Hamilton Rating Scale for Depression‐24 item (HDRS‐24), MADRS, CGI | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned". No further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double blind", no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for drop‐out reported. Rating scale scores reported without denominator |
| Selective reporting (reporting bias) | High risk | Endpoint scores reported only in figures. Side effects reported |
| Other bias | High risk | Last author's affiliation was Laboratories Pierre Fabre Medicament, and this company produces milnacipran |
| Methods | Eight‐week double‐blind, randomised trial | |
| Participants | Patients meeting DSM‐III‐R diagnostic criteria for non psychotic, moderate to severe major depression disorder and a minimum score of 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). All patients were required to have subjective sleep disturbance (score of 1 or 2 on at least one of the sleep items of the HDRS‐17). Age range: 18‐55 years | |
| Interventions | Fluoxetine: 22 participants | |
| Outcomes | HDRS‐17 total score and sleep parameters | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double dummy capsule‐dosing scheme" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Mean baseline and endpoint scores reported without standard deviations. Number and reasons for dropout specified |
| Selective reporting (reporting bias) | Unclear risk | Side effects not reported |
| Other bias | High risk | This study was supported in part by Bristol‐Myers Squibbs. This company produces nefazodone |
| Methods | Twelve‐week double‐blind, randomised study | |
| Participants | Patients meeting DSM‐III‐R diagnostic criteria for major affective disorder, unipolar and had met the diagnostic criteria for major depressive disorder episode for at least one month before entering the study, with a minimum baseline score of 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17), with a score of two or more on item one. Age range: 23‐54 years | |
| Interventions | Fluoxetine: 9 participants | |
| Outcomes | Response: decrease of at least 50% in the HDRS‐17 total score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Baseline and endpoint scores for each arm not reported. Number and reasons for dropout not specified |
| Selective reporting (reporting bias) | High risk | Side effects not reported. Mean baseline and endpoint score reported only in the whole sample |
| Other bias | High risk | Quote: "this study was supported by grants from SmithKline Beecham", and this company produces paroxetine |
| Methods | Six‐week double‐blind randomised trial | |
| Participants | Patients diagnosed with major depression (MD) or MD‐recurrent according to DSM‐IV diagnostic criteria. | |
| Interventions | Fluoxetine: 21 participants Placebo: 21 participants Venlafaxine dose range: 75‐150 mg/day | |
| Outcomes | Response was defined as a 50% reduction in the index total Hamilton Rating Scale for Depression (HDRS) score | |
| Notes | Funding: by academy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "consequently randomised", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if raters were independent and unclear if blinding was successful |
| Incomplete outcome data (attrition bias) | High risk | Number and reason for dropout not clearly reported. Scores reported without denominator |
| Selective reporting (reporting bias) | High risk | Adverse effects not reported. Number of responders only reported for the whole sample (not for each study arm) |
| Other bias | Low risk | Funding: by academy |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 56 participants | |
| Outcomes | HDRS‐21, Raskin, Covi, Clinical Global Impression Severity and Improvement Scales (CGI) | |
| Notes | Response: decrease of at least 50% in the total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "efficacy data were analysed in accordance with ITT principle", but scores reported without denominator. Study completion rates and reasons for study discontinuations reported |
| Selective reporting (reporting bias) | Unclear risk | Only side‐effect over 5% reported. Vital signs reported |
| Other bias | High risk | Authors' affiliation was Psychopharmacology Division, Lilly Reasearch Laboratories, Eli Lilly and Company, Indianapolis. This company produces fluoxetine |
| Methods | Six‐week double‐blind, randomised multicentre study | |
| Participants | Patients with ICD‐10 depression, with a score between 16 and 24 points on Hamilton Rating Scale for Depression (HDRS‐17). | |
| Interventions | Fluoxetine: 35 participants | |
| Outcomes | HDRS‐17, von Zerssen Depression Scale (VZD), Clinical Global Impression (CGI) Scale | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number of dropouts reported, but reasons not specified. Endpoint scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Side effects not reported. Mean endpoint scores and standard deviation reported |
| Other bias | High risk | Last author's affiliation was PhytoPharm Consulting, Istitute for Phytopharmaceuticals, Berlin; probably this company produces Hypericum perforatum |
| Methods | Six‐week double‐blind, randomised multicentre study | |
| Participants | Outpatients with a diagnosis of major depression or bipolar disorder, depressed, according to DSM‐III‐R, scoring at least 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17), and with a higher on the Raskin Depression Scale than on the Covi Anxiety Scale. | |
| Interventions | Fluoxetine: 144 participants | |
| Outcomes | Primary outcome: HDRS‐17, Clinical Global Impression Severity and Improvement Scales (CGI S‐I) | |
| Notes | Patients with concomitant medical conditions were allowed to participate in the study provided that the conditions were clearly not associated with the illness of the study and that any required medications were not psychoactive agents. One patient attempted suicide in the fluoxetine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned, in equal proportion", no other information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Number and reasons for dropout reported. Scores reported with denominator |
| Selective reporting (reporting bias) | Unclear risk | Scores reported without standard deviations. Only adverse events occurring at least in 3% of the sample reported |
| Other bias | High risk | Quote: "supported by a research grant from international Pharmaceuticals group, Pfizer, Inc, New York". This company produces sertraline |
| Methods | Eight‐week double‐blind, randomised two‐centre study | |
| Participants | Outpatients with a diagnosis of moderate to severe major depressive episode without psychotic features or bipolar disorder of the depressed type according to DSM‐III‐R, with a total score of least 18 points on Hamilton Rating Scale for Depression‐17 item (HDRS‐17) at baseline. | |
| Interventions | Fluoxetine: 37 participants | |
| Outcomes | HDRS‐17, Hamilton Rating Scale for Anxiety, Clinical Global Impression, Patient Global Assessment | |
| Notes | One patient attempted suicide in the fluoxetine group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised". No other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "this was a double‐blind trial", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐dummy", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "this was a double‐blind trial", no further information |
| Incomplete outcome data (attrition bias) | Low risk | Number of dropouts reported. Scores at rating scales were reported with denominator |
| Selective reporting (reporting bias) | Unclear risk | Scores reported without standard deviations. Only adverse effect occurred in at least 10% of the sample were reported |
| Other bias | High risk | Quote: "supported by Bristol‐Myers Squibb Pharmaceutical Research Institute, Wallingford, CT". This company produces nefazodone |
| Methods | Eight‐week double‐blind, randomised study | |
| Participants | Outpatients with a diagnosis of depressive episode less than 2 months duration, according to DSM‐III criteria, with a minimum score of 25 on the Montgomery and Asberg Scale for Depression (MADRS). Age range: 18‐65 years | |
| Interventions | Fluoxetine: 33 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Hamilton Rating Scale for Anxiety (HAM‐A), MADRS | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were allocated at random", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of dropouts reported. Mean endpoint scores not clearly reported. No further information |
| Selective reporting (reporting bias) | Unclear risk | SIde effects not clearly reported. No further information |
| Other bias | High risk | Last author's affiliation was Organon, and this company produces mianserin |
| Methods | Twelve‐week randomised study | |
| Participants | Patients with a diagnosis of major depressive episode, single episode or recurrent, according to DSM‐IV criteria, with a minimum score of 18 on the Hamilton Rating Scale for Depression‐24 item (HDRS‐24). Age range: 30‐50 years Exclusion criteria: DSM‐IV diagnosis of acute or chronic mental disorder, a Mini Mental State Examination score less than 23, concomitant use of any psychotropic drugs (except intermittent use of chloral Hydate or diazepam specifically for sleep), presence of another axis I psychiatric disorder, or any unstable medical condition that might interfere with safety or the interpretation of results. | |
| Interventions | Fluoxetine: 96 participants | |
| Outcomes | HDRS‐24 and Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned". No other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "a double dummy‐procedure was used to preserve the blind". No further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "a double dummy‐procedure was used to preserve the blind". No further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "a double dummy‐procedure was used to preserve the blind". No further information |
| Incomplete outcome data (attrition bias) | High risk | Mean endpoint score not reported. Number and reasons for drop‐out not reported |
| Selective reporting (reporting bias) | High risk | Mean score reported at sixth week and without number of patients. Side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind randomised trial | |
| Participants | Outpatients meeting the DSM‐IV criteria for an acute, recurrent episode of Major Depressive Disorder (MDD) with mild or moderate intensity, aged 18‐70 years, a minimum total score of 21 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21), history of at least two episodes of non‐psychotic MDD, capacity and willingness to give informed consent and to comply with study procedures. | |
| Interventions | Fluoxetine: 57 participants Hypericum: 59 participants Placebo: 58 participants Hypericum dose: 900 mg/day | |
| Outcomes | Primary outcome: change in HDRS‐21 total score Secondary endpoint: change in total Montgomery and Asberg Scale for Depression (MADRS) score and Clinical Global Impression (CGI) score Response was defined as a 50% reduction in the HDRS‐21 total score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "a double dummy technique with matching placebos for each active treatment was applied. Thus, both placebos were identical in shape, weight, colour, smell and taste to their corresponding verum formulations", no other information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "all investigators and personnel, actively involved in the trial, were blinded to group assignment until the database was closed", no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons for and number of dropouts reported. Number of responders in the text is different from number reported in the table. The so called "ITT population" in this study was different from the randomised sample |
| Selective reporting (reporting bias) | Unclear risk | The duration of the study was not clearly reported. Adverse experiences reported |
| Other bias | High risk | Quote: "the study was funded by a grant from Lichtwer Pharma GmbH, Berlin, Germany", and this company produces hypericum extract LI 160 |
| Methods | Eight‐week double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for a major depressive disorder or bipolar disorder. The severity of depression should be 25 or more on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 158 participants | |
| Outcomes | Primary outcome: MADRS score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double dummy" no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Quote: "all efficacy analyses were made on the basis of the efficacy group, whereas the tolerability analyses were made on the basis of the ITT population" |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported only when recorded in at least 5 patients |
| Other bias | High risk | Quote: "sponsored by Lundbeck, Copenaghen. This company also delivered the citalopram tablets and bought (in bulk) the fluoxetine capsules (active and placebo) from Eli LIlly, England, and packed them" |
| Methods | Eight‐week double‐blind, multicentre study | |
| Participants | Outpatients (primary care) fulfilling DSM‐III‐R criteria for a major depressive disorder. The severity of depression should be 22 or more on the Montgomery and Asberg Scale for Depression (MADRS) score. | |
| Interventions | Fluoxetine: 184 participants | |
| Outcomes | Primary outcome: MADRS score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Quote: "all efficacy analyses were made on the basis of the efficacy group, whereas the tolerability analyses were made on the basis of the ITT population" |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported only when recorder in at least 5 patients |
| Other bias | High risk | Quote: "sponsored by Lundbeck, Copenaghen. This company also delivered the citalopram tablets and bought (in bulk) the fluoxetine capsules (active and placebo) from Eli LIlly, England, and packed them" |
| Methods | Six‐week double‐blind, randomised, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a total score of at least 20 on Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 28 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI), Patient self‐rated Global Improvement (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "all medication were prepared in identical capsules and administered by use of the double dummy technique", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale reported without denominator. Number and reasons for dropout reported |
| Selective reporting (reporting bias) | Unclear risk | No standard deviations reported. No endpoint scores. Vital signs measures not reported |
| Other bias | High risk | Quote: "this research was supported in part by a grant from Eli Lilly and Company", this company produces fluoxetine |
| Methods | Twenty‐six‐week double‐blind, randomised, multicentre study | |
| Participants | Outpatients (primary care) fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 20 at Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 120 participants | |
| Outcomes | MADRS and Clinical Global Impression (CGI) | |
| Notes | Response: decrease of at least 50% in the MADRS total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Criteria and number of drop‐out reported. Rating scale reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Scores at rating scales were reported without standard deviations. Side effects reported |
| Other bias | High risk | Last author's affiliation was Pfizer France, Orsay. This company produces sertraline |
| Methods | Five‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria (RDC) criteria for major depressive disorder, with a score of at least 20 on Hamilton Rating Scale for Depression (HDRS), of 8 on Raskin Depression Scale (RDS). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS, RDS, Covi Anxiety scale (CAS), Clinical Global Impressions (CGI) | |
| Notes | Patients over 65 years in the imipramine group only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the study drugs and placebo were supplied as identical capsules". No other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if raters were independent and unclear if blinding was successful |
| Incomplete outcome data (attrition bias) | Unclear risk | Criteria and number of dropouts reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Scores at rating scales were reported without standard deviations |
| Other bias | Low risk | Funding: by academy |
| Methods | Five‐week, double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depression, with a score of at least 20 on Hamilton Rating Scale for Depression (HDRS). Age: not stated | |
| Interventions | Fluoxetine: 18 participants Imipramine: 12 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI) scores | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised schedule". No other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear if raters were independent and unclear if blinding was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number of drop‐out reported, but unclear reasons for dropout. Rating scale scores reported without denominators. Vital signs and side effects not reported |
| Selective reporting (reporting bias) | Unclear risk | No secondary endpoint scores. No standard deviations reported for HDRS score |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind, randomised, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depression (duration of at least 1 month) with a score of at least 20 on Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 32 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI) scores | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was carried out by using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind". No further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "capsules looked identical", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reason for dropout during the study were reported. Withdrawals were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No detailed endpoint scores at CGI. Side effects reported only with percentage, without denominator |
| Other bias | High risk | The study was supported, in part, by Eli Lilly. This company produces fluoxetine |
| Methods | Fifty‐two week double‐blind, randomised, multicentre study | |
| Participants | Outpatients fulfilling ICD‐10 criteria for major depression, with a Mini Mental State Examination (MMSE) score of at least 22 and a Hamilton Rating Scale for Depression (HDRS‐21) score of at least 18. | |
| Interventions | Fluoxetine: 119 participants | |
| Outcomes | HDRS‐21, Clinical Anxiety Scale, Buschke Selective Reminding Test (BSRT), Blessed Information and Memory Test (BIMT), Clifton Assessment Scale (CLAS), Cancellation Task Test (CTT), Wechsler Paried Word Test (WPW), Mini Mental Sate Evaluation (MMSE) and Clinical Global Impression (CGI) | |
| Notes | Depression response: total score less than 10 on the HDRS‐21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear if raters were independent and unclear if blinding was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominator |
| Selective reporting (reporting bias) | High risk | Standard deviations not reported (HDRS). No endpoint scores (CGI, CAS) |
| Other bias | High risk | The study was supported by SmithKline. This company produces paroxetine |
| Methods | Five‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria (RDC) criteria for major depressive disorder, with a score of at least 21 on Hamilton Rating Scale for Depression (HDRS‐17) and of at least 8 on the Raskin Depression Scale (RAS). Age range: 21‐70 years | |
| Interventions | Fluoxetine: 23 participants | |
| Outcomes | Primary outcome: HDRS‐17, Clinical Global Impression (CGI), Efficacy Index‐Side Effects rating (EISE) Secondary outcomes: Hamilton Rating Scale for Anxiety (HAM‐A) and Zung Depression Scale (SDS) | |
| Notes | One patient attempted suicide in the fluoxetine group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned. Assigment was stratified to ensure balanced distribution of male and female patients to each of the two study drug regimen". No other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind conditions in identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without standard deviations. Reasons for withdrawal reported, but withdrawals not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported only with an incidence over 10% |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Twelve‐week double‐blind, randomised, multicentre study | |
| Participants | Patients fulfilling DSM‐III criteria for major depressive disorder, with a score of at least 20 on Hamilton Rating Scale for Depression (HDRS‐21). Age: not stated | |
| Interventions | Fluoxetine: 101 participants | |
| Outcomes | Primary outcomes: HDRS‐21, Clinical Global Impression | |
| Notes | Response: decrease of at least 50% in the HDRS‐21 total score and/or a total score less than 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "the efficacy analysis included all randomised patients who underwent at least one on‐therapy efficacy evaluation". Number of patients responding to treatment reported with denominator. Reason for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Effect side reported only with an incidence over 10% |
| Other bias | High risk | Funding by SmithKline, and this company produces paroxetine |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | In‐ and outpatients fulfilling DSM‐IV criteria for single or recurrent episode of Major Depressive Disorder (MDD), with or without melancholic features, without atypical features, without psychotic features and a score of at least 22 on Hamilton Rating Scale for Depression (HDRS). Morover decrease in HDRS total score should not be more than 20% between start of run‐in and inclusion visit and a severity of illness of at least 4 on CGI. Age range: 18‐59 years | |
| Interventions | Fluoxetine: 137 participants | |
| Outcomes | Primary outcome: HDRS score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Funding: by industry |
| Methods | Six‐week double‐blind, randomised, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐IV criteria for single or recurrent episode of Major Depressive Disorder (MDD), with or without melancholic features, without atypical features, without psychotic features and a score of at least 22 on Hamilton Rating Scale for Depression (HDRS). Age range: 18‐59 years | |
| Interventions | Fluoxetine: 148 participants Agomelatine 50mg: 151 participants | |
| Outcomes | Primary outcome: HDRS score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Funding: by industry |
| Methods | Eight‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 22 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 150 participants | |
| Outcomes | Primary outcome: HDRS score Response: decrease of at least 50% in the HDRS score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | High risk | Number of withdrawals reported, but reasons not clearly described. Mean scores reported only at the baseline and not at the endpoint |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported partially. Baseline mean scores reported without standard deviations |
| Other bias | High risk | Funding by Pharmacia Corporation |
| Methods | Six‐week double‐blind, randomised, multicentre study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with melancholia, with a score of at least 25 on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 34 participants | |
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS‐21), MADRS, Clinical Global Impression Scale (CGI) | |
| Notes | Response: decrease of at least 50% in the HDRS or in the MADRS total scores, or a CGI score of 1 or 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind medication, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind medication, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind medication, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "analysis are on the ITT patients and use the last observation carried forward (LOCF) method" |
| Selective reporting (reporting bias) | Unclear risk | Treatment‐emergent study events reported only if it was reported by three or more patients |
| Other bias | High risk | Quote: "this work was supported by a grant from Wyeth‐Ayerst Research, Paris, France", this company produces venlafaxine |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depressive illness, with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 54 participants | |
| Outcomes | HDRS, Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS), Clinical Global Impression (CGI) Severity and Improvement | |
| Notes | One patient attempted suicide in the fluoxetine group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind study. Placebo and the study drugs were supplied as identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without standard deviations. Reasons for dropout not clear. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported only over 10% |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients (general practice) fulfilling Research Diagnostic Criteria (RDC) criteria for primary unipolar major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS‐17). | |
| Interventions | Fluoxetine: 49 participants | |
| Outcomes | HDRS‐17 score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "a double dummy technique was employed", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominator. Effect side reported. Number of patients randomised and number lost during follow‐up not clear |
| Selective reporting (reporting bias) | High risk | Means and standard deviations reported only in figures |
| Other bias | High risk | Second author had affiliation in Eli Lilly, this company produces fluoxetine |
| Methods | Eight‐week double‐blind, randomised study | |
| Participants | Patients fulfilling DSM‐III‐R criteria for major depression (single or recurrent episode, with or without melancholia and without psychotic features). | |
| Interventions | Fluoxetine: 35 participants | |
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS‐17), Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) Severity | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomised clinical trial”, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "results are reported for the observed‐case analysis, for which no missing data were replaced". Number randomised, and number lost during follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Scores reported without standard deviations. Adverse events were reported with a frequency of at least 10% |
| Other bias | High risk | Authors' affiliation was in Pharmacia&Upjohn Inc, and this company produces pramipexole |
| Methods | Eight‐week double‐blind, randomised, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) and depressive symptoms for at least 1 month before study entry. | |
| Interventions | Fluoxetine: 186 participants | |
| Outcomes | Primary outcomes: HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) Severity and Improvement | |
| Notes | Response: decrease of at least 50% in the HDRS or in the MADRS; or a CGI‐I score of 1 or 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if raters were independent and unclear if blinding was successful |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviations. Side effects reported only if they occurred at least in 5% of the patients |
| Other bias | High risk | Quote: "supported by a grant from Wyeth‐AYerst International". This company produces venlafaxine |
| Methods | Twelve‐week double‐blind, randomised, multicentre study | |
| Participants | Patients fulfilling DSM‐III‐R criteria for major depression (single or recurrent), with a score of at least 20 on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 82 participants | |
| Outcomes | MADRS, Clinical Global Impression (CGI), Mood Anxiety Retardation and Danger (MARD) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random allocation". No further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons of attrition not clear. Ratings scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Data at follow‐up not reported. Adverse effects not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 94 participants | |
| Outcomes | Primary outcome: area under the curve of the change in HDRS‐17 total score from baseline | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons and number of dropouts were not clear. Rating scale scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | Scores reported without standard deviations |
| Other bias | High risk | Quote: "the study was supported by a grant from Solvay Pharmaceuticals". This company produces maprotiline |
| Methods | Six‐week double‐blind, randomised, two‐site study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depressive disorder without psychotic features, with a score of at least 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 30 participants | |
| Outcomes | HDRS‐17, Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS), Clinical Global Impression (CGI) Severity and Improvement | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominator. Number and reasons of dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Vital signs not reported |
| Other bias | High risk | Quote: "the study was supported by Eli Lilly Nederlands". This company produces fluoxetine |
| Methods | Twelve‐week double‐blind, randomised, multicentre study | |
| Participants | Outpatients with a score between 18 and 25 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) and minimum baseline of 8 on the Covi Anxiety Scale (CAS), and considered by the investigator to be moderately depressed. | |
| Interventions | Fluoxetine: 73 participants | |
| Outcomes | Primary outcomes: HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) Severity | |
| Notes | Response: decrease of at least 50% in the HDRS‐21 or in the MADRS total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear if blinding was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominator. Number and reason for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviation |
| Other bias | High risk | Funding by Wyeth, and this company produces venlafaxine |
| Methods | Ten‐week double‐blind, randomised, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 16 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 32 participants | |
| Outcomes | HDRS‐17, Montgomery and Asberg Scale for Depression (MADRS), Covi Anxiety Scale (CAS), Clinical Global Impression (CGI) Severity and Improvement | |
| Notes | Response: decrease of at least 50% in the HDRS‐17 total score or a total score less than 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised trial", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "all ratings were conducted under double blind condition", no further information |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominators. Number and reasons for discontinuation not clear |
| Selective reporting (reporting bias) | Unclear risk | Incidence of adverse effects not clear |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Patients fulfilling DSM‐III criteria for major depression, with a score of at least 18 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 41 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Hopkins Symptoms Check List (HSLC), Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Quote: "the Last Observation Carried Forward (LOCF) data set was used". Scores in follow‐up were reported without denominator. Reasons for withdrawal not clear |
| Selective reporting (reporting bias) | High risk | No follow‐up scores (MADRS, HDRS, HSLC) |
| Other bias | High risk | Funding by Smithkline, and this company produces paroxetine |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 22 participants | |
| Outcomes | HDRS‐21, Inventory for Depressive Symptomatology ‐ Clinician Version (IDS‐C) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported with denominator, but withdrawal not included in analysis. Side effects reported |
| Selective reporting (reporting bias) | Unclear risk | No endpoint scores (IDS‐C). Scores without standard deviation (HDRS) |
| Other bias | High risk | Quote: "supported in part by Ely Lilly". This company produces fluoxetine |
| Methods | Nine‐week double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 15 on the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 35 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI) | |
| Notes | Response: decrease of at least 50% in the HDRS‐21 total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind design", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind design", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double blind design", no further information |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominator. Reasons for dropouts not clear |
| Selective reporting (reporting bias) | Unclear risk | No endpoint scores (CGI). Adverse events not reported |
| Other bias | High risk | Quote: "we are indebted to Eli Lilly Belgium for financial support for the present study", and this company produces fluoxetine |
| Methods | Twenty‐two‐week double‐blind randomised study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depression disorder. | |
| Interventions | Fluoxetine: 42 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS‐17) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind trial", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blind trial", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double blind trial", no further information |
| Incomplete outcome data (attrition bias) | High risk | Mean scores at rating scales only referred to the whole sample (not to each arm). Number and reasons for dropouts not clear |
| Selective reporting (reporting bias) | Unclear risk | The type of adverse events was not reported |
| Other bias | High risk | Quote: "Eli Lilly Benelux provided logistic and material support for this study", and this company produces fluoxetine |
| Methods | Eight‐week randomised, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 75 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression, Symptom Checklist 61 Item (SCL‐61) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Quote: "Open‐label" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Open‐label" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Open‐label" |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominator. Number and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviations. Adverse events reported over 5% |
| Other bias | High risk | Quote: "this study was supported by Wyeth‐Ayerst International, Saint David's, Pennsylvania": Tihs company produces venlafaxine |
| Methods | Eight‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 161 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) scales | |
| Notes | Response: decrease of at least 50% in the HDRS or MADRS total score, or a score of 1 or 2 on the CGI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Scores reported with denominator. Number and reasons for withdrawals reported |
| Selective reporting (reporting bias) | Unclear risk | No CGI endpoint scores reported. Only most common (over 5%) side effects reported |
| Other bias | High risk | Quote: "this study was supported by Wyeth‐Ayerst Research" and this company produces venlafaxine |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depression (unipolar), with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS‐17). | |
| Interventions | Fluoxetine: 30 participants | |
| Outcomes | HDRS, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) Severity and Improvement, Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blind", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "all patients took identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropouts were reported. Scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects were reported. Mean scores were reported without standard deviations. No endpoint scores (MADRS, CGI) |
| Other bias | High risk | In the aknowledgements authors thank Eli Lilly Company. This company produces fluoxetine and probably the study was supported by this industry |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for double depression (dysthymia and major depression), with a score of at least 16 on the Hamilton Rating Scale for Depression (HDRS). Age range: 18‐65 years | |
| Interventions | Fluoxetine: 21 participants | |
| Outcomes | Primary outcomes: percentage of responders defined as decrease of at least 50% in the HDRS | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double blinding was achieved by appropriate drug packaging and formulation", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double blinding was achieved by appropriate drug packaging and formulation", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double blinding was achieved by appropriate drug packaging and formulation", no further information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominators. Mean scores and standard deviations reported only in figure and they were not clear |
| Selective reporting (reporting bias) | Unclear risk | No vital signs and side effects reported. Quote: "no clinically significant changes in vitals signs were recorded" |
| Other bias | High risk | The last author's affiliation was Hoffmann–La Roche Ltd, and this company produces moclobemide |
| Methods | Five‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression (single episode or recurrent). | |
| Interventions | Fluoxetine: 103 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Zung Depression Scale, Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "patients received two bottles of identical capsules". No further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if blinding was successful |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for withdrawal reported. No endpoint scores (CGI, Zung Depression Scale) reported |
| Selective reporting (reporting bias) | Unclear risk | Scores reported without standard deviations. Only most common adverse events were reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients (general practice) fulfilling DSM‐III‐R criteria for major depression. Age range: 18‐70 years | |
| Interventions | Fluoxetine: 42 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Leeds Sleep Evaluation Questionnaire (LSEQ) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "drugs and placebos were packaged in identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominators. Number and reasons for dropouts not clear |
| Selective reporting (reporting bias) | Unclear risk | Only most common adverse events were reported |
| Other bias | High risk | Quote: "the research grant provided by Lilly Industries", and Eli LIlly produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for unipolar major depression (single or recurrent), with the present episode lasting 4 weeks or more and with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). Age: over 62 years | |
| Interventions | Fluoxetine: 14 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI), Treatment Emergent Symptom Scale (TESS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "all capsules were identical", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Scores reported without denominators. Reasons and number of dropouts described |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported. Scores reported for each follow‐up with standard deviations |
| Other bias | High risk | Quote: "this research was supported by a grant from Eli Lilly and Company", and this company produces fluoxetine |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for moderate to moderately severe major depression without a history of mania or hypomania, with a score of at least 18 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21), of at least 8 on the Raskin Depression Scale (and grater than Covi Anxiety Scale (CAS) score). | |
| Interventions | Fluoxetine: 54 participants | |
| Outcomes | HDRS‐21, Covy Anxiety Scale (CAS), Raskin Depression Scale | |
| Notes | Response: decrease of at least 50% in the HDRS‐21 total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "we chose to conduct all analyses with an ITT approach". Number and reasons for dropout reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported. Primary and secondary endpoint reported with standard deviations |
| Other bias | High risk | Quote: "this study was supported by SmithKline Beecham Pharmaceuticals" and this industry produces paroxetine |
| Methods | Ten‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depression or atypical major depression, with a baseline score of at least 16 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 92 participants | |
| Outcomes | Primary outcome: total score on the HDRS‐17 Secondary outcomes: improvement on the Clinical Global Impression (CGI) Severity scale and HDRS sleep disturbance, cognitive disturbance (COG) factors | |
| Notes | Response: decrease of at least 50% in the HDRS‐17 total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Scores reported without denominators. Mean scores and standard deviations reported at each follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Only adverse events reported by at least 10% of the patients described |
| Other bias | High risk | Quote: "supported by a grant from Ely Lilly ", and this company produces fluoxetine |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for current major depression episode of at lest 2 weeks duration, Hamilton Rating Scale for Depression (HDRS) score at least 16. Age range: 18‐65 years Inclusion criteria: negative pregnancy test within 5 days before study start in women of childbearing potential, use of adequate contraception. Exclusion criteria: pregnancy, lactation, or non use of medically accepted contraception, current serious suicide or homicidal risk, serious or instable medical illness, history of seizure disorders, presence of any of the following diagnosis: organic mental disorder, substance use disorder, including alcohol, active within the last 6 months, schizophrenia, delusional disorder, psychotic disorders not elsewhere classified, bipolar disorder, antisocial personality disorder; history of multiple adverse drug reactions or allergy to the study drugs; mood congruent or mood incongruent psychotic features, concomitant use of other psychotropic drugs within 14 days before baseline, other investigational psychotropic drug within 40 days, fluoxetine within 40 days or any other investigational drug within 1 month, hypothyroidism; failure to respond during the course of current MDE to at least 2 adequate antidepressant trials; any other condition which, in the investigator judgement, may pose significant risk to the patient's health or may decrease the chances of obtaining reliable data to achieve the objectives of the study; mental condition rendering the patients unable to understand nature, scope and risk of the study; history or suspicion of unreality, poor cooperation, or non compliance with medical treatment. | |
| Interventions | Fluoxetine: 47 participants St John's wort dose: 900 mg/day | |
| Outcomes | Primary outcome: total score on the HDRS Secondary outcome: improvement on the Clinical Global Impression (CGI) scale and Beck Depression Inventory (BDI) score | |
| Notes | Response: decrease of at least 50% in the HDRS total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Number and reasons for dropout reported. Primary and secondary endpoint scores reported |
| Selective reporting (reporting bias) | Low risk | Only most frequent (at least 10%) adverse events reported. Scores reported with standard deviations |
| Other bias | High risk | Quote: "the study was supported by a grant of Lichtwer Pharma AG (Berlin, Germany)", and this company produces St. John's wort |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for unipolar major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression for Severity and Improvement (CGI S‐I), Patient Global Impression (PGI) | |
| Notes | Improvement: a decrease of at least 50% on the total HDRS‐21 score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "(patients) were randomly assigned to fluoxetine treatment", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Mean endpoint scores reported without denominators. Adverse events not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Mean endpoint scores and standard deviations reported (HDRS, CGI) |
| Other bias | High risk | Supported by Eli Lilly, and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for unipolar major depression (single or recurrent episode), with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS) and Raskin Depression Scale (RDS) score of at least 8 and equal or greater to the Covi Anxiety Scale (CAS) score. | |
| Interventions | Fluoxetine: 78 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI) Severity, RDS, CAS scores | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the study drugs and placebo were supplied in identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominators. Reasons and number of early termination not clear |
| Selective reporting (reporting bias) | High risk | Adverse effects were described only when reported by more than 10% of the sample. Mean scores reported without standard deviations |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Five‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria criteria for unipolar major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS) and Raskin Depression Scale (RDS) score of at least 8. | |
| Interventions | Fluoxetine: 22 participants | |
| Outcomes | HDRS, RAS, Covi Anxiety Scale (CAS), Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "to ensure the double blind, study drugs were divided into daytime and bedtime doses", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Mean endpoint score and standard deviation at CGI not reported. Number and reasons for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Mean endpoint scores (HDRS, RDS, CAS) reported without standard deviations. Side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for unipolar major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS) and Raskin Depression Scale (RDS) score of at least 8 and equal or greater to the Covi Anxiety score (CAS). | |
| Interventions | Fluoxetine: 61 participants | |
| Outcomes | HDRS, RDS, CAS, Clinical Global Impression (CGI), Patient Global Improvement Scale (PGI) | |
| Notes | Improvement: a moderately or markedly improved on the CGI or a decrease of at least 50% on the total HDRS score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Categorical endpoint not reported |
| Selective reporting (reporting bias) | Unclear risk | Number and reasons of early termination were reported. Side effects reported by more than 10% of the sample described |
| Other bias | High risk | Quote: "(study) performed at the Feighner Research Institute". This institute usually receives founds by pharmacological industries |
| Methods | Six‐week randomised, double‐blind two‐centre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for non‐psychotic major depressive episode, lasting between 4 weeks to 2 years, single or recurrent, which was not secondary to another pre‐existing psychiatric or medical condition, with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 62 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI) Severity and Improvement, Hamilton Rating Scale for Anxiety (HAM‐A) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "a double dummy technique was used to maintain the double blind", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Responders denominator was different from the number of randomised. Reasons for discontinuation were unclear |
| Selective reporting (reporting bias) | High risk | Scores reported without standard deviations. Most frequent (reported at least 5%) adverse events reported |
| Other bias | High risk | Quote: "supported by a grant from Burroghs Wellcome, Co.", and this industry produces bupropion |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depression, with a score between 18 and 25 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) . | |
| Interventions | Fluoxetine: 31 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) Severity | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "(treatments) were administered in identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Scores reported without denominators. Number and reasons for early termination reported |
| Selective reporting (reporting bias) | Unclear risk | End‐point scores reported without standard deviations. Side effects not clearly reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 18 on the Hamilton Rating Scale for Depression (HDRS‐24) . | |
| Interventions | Fluoxetine: 33 participants | |
| Outcomes | HDRS‐24, Hamilton Rating Scale for Anxiety (HAM‐A), Clinical Global Impression (CGI) Severity and Improvement, Profile Of Mood States (POMS), Quality of Life Enjoyment and satisfaction Questionnaire (Q‐LES‐Q). | |
| Notes | Response: decrease of at least 50% in the HDRS‐24 total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Responders at endpoint reported without denominators. Number and reasons for withdrawal were reported |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported. Primary endpoint scores reported in figures and without standard deviations |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 18 on the Hamilton Rating Scale for Depression‐ 21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 45 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Hamilton Rating Scale for Anxiety (HAM‐A) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " double dummy technique", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons for withdrawal were not clear. Denominator of responders was different from number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Scores were reported without standard deviations. Only adverse events occurred over 10% were reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Four‐week randomised, double‐blind, two‐centre study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 34 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI) | |
| Notes | Response: decrease of at least 50% in the HDRS‐17 total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double dummy, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominators. Response rate was based on patients completed the trials and not on randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Number and reasons for dropouts reported. Endpoint scores at CGI not reported. Side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depression without psychotic features, with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 25 participants | |
| Outcomes | Final score of less than 10 or a decrease of at least 50% from baseline on the HDRS‐17, Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote:"patients received capsules of identical appearance and taste", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | End‐point scores reported without denominators. Analysis of HDRS scores were based on completers, instead of on randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Number and reasons of withdrawals reported. Adverse effects reported |
| Other bias | High risk | Last author's affiliation was Roche Industry and this company produces moclobemide |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS). | |
| Interventions | Fluoxetine: 52 participants | |
| Outcomes | Endpoint score on HDRS‐17, on Montgomery–Åsberg Depression Rating Scale (MADRS) and on Clinical Global Impression (CGI) | |
| Notes | Funding: industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of drop‐out was reported, but reasons for withdrawn were reported only partially |
| Selective reporting (reporting bias) | Unclear risk | Mean end point score not reported. Side effects reported partially |
| Other bias | High risk | Funding: by industry |
| Methods | Eight‐week randomised, double‐blind study | |
| Participants | Patients fulfilling DSM‐IV criteria for major depression disorder. | |
| Interventions | Fluoxetine: 19 participants Benzodiazepines allowed when needed for anxiety, agitation or sleep | |
| Outcomes | Priamry outcome: fasting blood glucose (FBG) levels | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Initial number of randomised patients was different from the sum of the number of the patients in the different groups. Result on primary outcome (FBG) reported |
| Selective reporting (reporting bias) | High risk | Number and reasons for withdrawals not clearly reported. Adverse effects not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for non‐psychotic, moderate to severe major depressive disorder, with a score of at least 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS‐17, Inventory of Depressive Symptomatology (IDS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no information about randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐dummy dosing scheme", no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Endpoint scores reported without denominators. Number and reasons for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Only the most frequently occurring adverse events (at least in 10% of patients) reported |
| Other bias | High risk | Quote: "this study was supported by Bristol‐Myers‐Squibb Pharmaceutical Research Institute", that produces nefazodone |
| Methods | Eight‐week randomised, double‐blind study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depression with melancholia, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 28 participants | |
| Outcomes | HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Covi Anxiety Scale (CAS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | End‐point scores and standard deviations reported. Dropouts not included in the analysis |
| Selective reporting (reporting bias) | High risk | Number but not reasons for dropouts reported. Side effects occurred on at least 3 occasion reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for non‐psychotic major depressive disorder, with a score of at least 15 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and at least 4 on the Clinical Global Impression‐Severity of Illness (CGI). | |
| Interventions | Fluoxetine: 33 participants | |
| Outcomes | Primary outcome: HDRS‐17 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: " this study used double‐blind placebo lead in such that investigators and patients did not know when randomisation occurred and when active study drug was first administered", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " this study used double‐blind placebo lead in such that investigators and patients did not know when randomisation occurred and when active study drug was first administered", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: " this study used double‐blind placebo lead in such that investigators and patients did not know when randomisation occurred and when active study drug was first administered", further information |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominators. Number and reasons for dropout not clearly reported |
| Selective reporting (reporting bias) | High risk | Mean scores reported without standard deviations. Only common treatment emergent adverse events reported |
| Other bias | High risk | First author's affiliation was Lilly Corporate Center and this company produces fluoxetine |
| Methods | Eight‐week, multicentre, randomised, double‐blind study | |
| Participants | Patients suffering from a major depressive episode according to DSM‐III‐R, with a baseline score on Hamilton Rating Scale for Depression‐17 Item (HDRS‐17) of at least 18, and an HDRS item 10 score of 1 or more. | |
| Interventions | Fluoxetine: 70 participants | |
| Outcomes | Primary outcomes: HDRS and Hamilton Rating Scale for Anxiety (HAM‐A) Secondary outcomes: Clinical Global Impression Scale (CGI), including severity and improvement | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double‐blind". No further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double‐blind". No further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | All ITT analyses used last observation carried forward (LOCF) data set. Number and reasons for dropout reported. Mean endpoint scores (HDRS) reported with standard deviations |
| Selective reporting (reporting bias) | Unclear risk | Most frequent side effects reported. Number of responders not reported |
| Other bias | Unclear risk | Funding: unclear information |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depression for less than 3 months, with a score of at least 22 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 100 participants | |
| Outcomes | Primary outcome: change in the total score on the HDRS‐17 | |
| Notes | Response: decrease of at least 50% in the MADRS and HDRS‐17 total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominators. Number and reasons for withdrawal were reported |
| Selective reporting (reporting bias) | Unclear risk | Scores reported without standard deviations. Endpoint scores at MADRS not reported |
| Other bias | High risk | Quote: "this study was sponsored by Pierre Fabbre Medicament, Boulogne, France" and this company produces milnacipram |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients (general practice) fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 25 on the Montgomery and Asberg Scale for Depression (MADRS) and a Mini Mental State Examination (MMSE) of at least 24. | |
| Interventions | Fluoxetine: 122 participants | |
| Outcomes | Primary outcome: change in the total score on the MADRS Secondary outcomes: total number of responders at endpoint, total number of remissions at endpoint, mean variation on the Geriatric Depression Scale (GDS) | |
| Notes | Response: decrease of at least 50% in the MADRS total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "deux groupes de traitment ont été constitués par tirage au sorte", randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Mean endpoint scores reported with denominators. Dropouts reported with reasons |
| Selective reporting (reporting bias) | Low risk | Side effects reported |
| Other bias | High risk | Author's affiliation was Eli Lilly and this company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients, aged 18 to 65 years and presented with MDD of severe intensity according to DSM‐IV‐TR criteria, with a score of at least 25 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and a Clinical Global Impression (CGI) score of at least 4. Exclusion criteria: seasonal pattern, psychotic features, post‐partum onset, suicidal intent and/or known suicidal tendencies for the current episode, bipolar disorder, anxiety symptoms (such as panic attacks, obsessive compulsive disorder, PTSD) drug abuse or dependency, resistant to fluoxetine for current episode, treatment with electroconvulsive therapy or formal psychotherapy within 3 months, or light therapy started within the earlier two weeks, not responders to the administration of an appropriate dose of two different early antidepressant treatments for at least four weeks each, patients with neurologic disorders or severe uncontrolled organic disorders. | |
| Interventions | Fluoxetine: 263 participants Agomelatine: 252 participants | |
| Outcomes | Primary outcomes: responders to treatment were defined by a decrease of at least 50% in total score from baseline Secondary outcomes: patients with a score of 1 or 2 at CGI scale were considered responders | |
| Notes | Response: decrease of at least 50% in the HDRS total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "both investigator and patients were blind (...) during the entire duration of the study, all patients two capsules orally in the morning and one in the evening, irrespective the treatment and daily dosage allocated...the appearance and taste of the study treatment were the same from the inclusion to the end of the study for all patients. The packaging and labelling were identical" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation clearly reported. Mean endpoint scores reported with standard deviation, but without denominator |
| Selective reporting (reporting bias) | Low risk | Adverse events reported for more than 2% of the subjects. Secondary outcome scores (CGI) reported |
| Other bias | High risk | Quote: "this study was supported by Servier. Authors have received honoraria, research grants or both, from Servier". This company produces agomelatine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients (general practice) fulfilling ICD‐10 criteria for mild depressive episode, with a Mini Mental State Examination (MMSE) of at least 25. | |
| Interventions | Fluoxetine: 79 participants | |
| Outcomes | Primary outcomes: change in the total score on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) | |
| Notes | Response: decrease of at least 50% in the HDRS‐17 total score or a total score of less than 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomised in blocks of four for a total of 32 patients at each centre, it was ensured that equal numbers of patients from each sample and with each degree of severity were randomly allocated to each trial centre" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "patients were asked to take coated tablets twice daily...", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominator. Number and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviation. Adverse drug reactions reported |
| Other bias | High risk | The last author's affiliation was Dr Loges co.gmbh and this company produces St John's wort extract LoHyp‐57 |
| Methods | Twenty four‐week randomised, double‐blind study | |
| Participants | Patients with a diagnosis of Major Depressive Disorder (MDD), who responded to the drugs in 8 weeks. | |
| Interventions | Fluoxetine: 49 participants | |
| Outcomes | Primary outcome: change in the total score of Beck Depression Inventory (BDI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "both patients and the evaluating team were unaware of treatment allocation", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "both patients and the evaluating team were unaware of treatment allocation", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "both patients and the evaluating team were unaware of treatment allocation", no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported with standard deviations, but without denominators |
| Selective reporting (reporting bias) | Unclear risk | Adverse drug reactions reported. Number of withdrawal reported only in the total sample and without specify reasons for discontinuation |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive episode (lasting between 1 week and 1 year), with a score of at least 15 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 66 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominator. Mean scores reported without standard deviations |
| Selective reporting (reporting bias) | Unclear risk | Only main reasons for premature discontinuation reported. Adverse events reported for more than 5% of the subjects |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Four‐week, randomised and open study | |
| Participants | Hospitalized patients. Diagnoses for inclusion (according to the ICD‐10) were: bipolar affective disorder, most recent episode depressed (8 participants); major depressive episode, single (44 participants), major depressive episode, recurrent (38 participants). Average age: 44.5 years (SD: 14.3) | |
| Interventions | Citalopram: 29 participants | |
| Outcomes | Mean change on Hamilton Depression Rating Scale‐21 item (HDRS‐21) | |
| Notes | Study report published only in Czech Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the subjects were randomised to the study antidepressant using computer randomisation program (Excel) at the beginning of the initial hospitalisation at the Dpt. of Psychiatry in Hradec Kralovc" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | No information provided |
| Selective reporting (reporting bias) | High risk | No information provided |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐IV criteria for major depressive episode without psychotic features, with a score between 18 and 26 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 50 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI) scores | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Denominator of the responders was different from the number of randomised patients. Number and reasons for withdrawal were not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Endpoint score at CGI and vital signs not reported. Side effects not clearly described |
| Other bias | High risk | Funding: unclear, probably funded by a pharmaceutical company that produces maprotiline |
| Methods | Six‐week randomised, open study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder. | |
| Interventions | Fluoxetine: 100 participants | |
| Outcomes | Primary outcomes: improvement greater than 60% from baseline on the Montgomery and Asberg Scale for Depression (MADRS) (response) and 2 months sustained improvement (recovery) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Not double blind |
| Blinding of participants and personnel (performance bias) | High risk | Not double blind |
| Blinding of outcome assessment (detection bias) | High risk | Not double blind |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominators. Number of drop‐out reported, but reasons for withdrawal not clearly described |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores at SCL‐90 and Simpson‐Angus Scale (SAS) not reported. Side effects not reported |
| Other bias | High risk | Quote: "this project also received a grant from Lottery Health and an unrestricted grant from Eli Lilly (New Zealand)". Eli Lilly produces fluoxetine |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder (1 month minimum duration of episode), with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 30 participants | |
| Outcomes | HDRS‐17 score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "medication was given in matching capsules". Unclear if raters were independent and unclear if blinding was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominators. Number and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported. Endpoint scores reported with maximum, minimum, mean and standard deviation |
| Other bias | High risk | Quote: "the authors are grateful to Eli Lilly, Australia for financial support for this study". Eli Lilly company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind, multicentre study | |
| Participants | Patients from general practice and specialist settings fulfilling DSM‐IV‐R criteria for major depressive disorder, with a score of at least 22, and maximum 40 on the Montgomery and Asberg Scale for Depression (MADRS), and at least 22 on the Mini Mental State Exams (MMSE) at the screening visit. | |
| Interventions | Fluoxetine: 164 participants Escitalopram: 174 participants | |
| Outcomes | Changes from baseline in MADRS total score at final assessment Response: at least 50% reduction on the MADRS total score from baseline Secondary outcome: changes in Clinical Global Impression (CGI) score | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominators and only in percentage. Number and reasons for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported only in figure and without standard deviations. Side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R or DIS criteria for unipolar major depression, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | Diagnostic Interview Schedule, HDRS‐21, Beck Depression Inventory (BDI), Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "study drugs were packaged as identical capsules" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Rating scale scores reported without denominators. Number and reasons for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported with standard deviations. Only 12 most common side effects reported |
| Other bias | High risk | Quote: "the study was supported by Eli Lilly, Canada Inc.". Eli Lilly Company produces fluoxetine |
| Methods | Ten‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for Major Depressive Episode (MDD), had experienced depressive symptoms for at least 1 month prior to the start of the study, and had a recurrent depression (at least 3 episode of MMD, with at least 2 episode in the past 5 years and a interval of at least 2 months between the end of the previous episode and the beginning of the current episode) and a total score on Hamilton Rating Scale for Depression‐17 item (HDRS‐17) of at least 20 at screening and 18 at the randomisation. | |
| Interventions | Fluoxetine: 275 participants Venlafaxine : 821 participants | |
| Outcomes | Primary outcome: HDRS‐17 Secondary outcomes: Hamilton Rating Scale for Anxiety (HAM‐A), Inventory of Depressive Symptomatology (IDS), Clinical Global Impression (CGI) Response: at least 50% reduction from baseline HDRS‐17 total score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout not clearly reported. Denominatosr of the responders was different from the number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported. Mean endpoint score (HDRS) reported with standard deviations |
| Other bias | High risk | Quote: "this clinical trial and analysis were sponsored by Wyeth Research, Collegeville", and this company produces venlafaxine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Inpatients fulfilling Research Diagnostic Criteria for major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 16 participants | |
| Outcomes | HDRS, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI) Severity and Improvement, Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated, according to predetermined schedule", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Denominator reported for responders was different from the number of randomised patients. Reasons for withdrawal were not reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events were reported. Mean scores were reported with standard deviations |
| Other bias | High risk | Last author's affiliation was Eli Lilly Benelux and this company produces fluoxetine |
| Methods | Five‐week randomised, double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling Research Diagnostic Criteria for unipolar major depressive episode, with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and 8 on the Raskin Depression Scale (RDS). | |
| Interventions | Fluoxetine: 24 participants | |
| Outcomes | HDRS, RDS, Covi Anxiety Scale (CAS), Patient Global Impression (PGI), Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviations. Only most predominant (4%) adverse events reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for unipolar major depression, drug free for a minimum of 2 weeks. Mean age: 44,31 years (SD=9,31) | |
| Interventions | Twenty participants were randomly assigned to a 6‐weeks of treatment with fluoxetine or amitriptyline No other information about the interventions | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS) was assessed at baseline and at the end of the 6th week Response: a reduction of at least 50% of the HDRS score | |
| Notes | Funding: by academy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned", no other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "randomly assigned by an another psychiatrist who was blind to the rating of HDRS", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Randomly assigned by an another psychiatrist who was blind to the rating of HDRS", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Only the total number of randomised patients was reported, the number of participants in each group was not reported. Number and reason for dropout not reported |
| Selective reporting (reporting bias) | High risk | Side effects not reported. Baseline and endpoint score reported for the whole group |
| Other bias | Low risk | Quote: "this work was supported by grant N°. 02‐94‐158 from the Seoul National University Hospital research found" |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 18 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) and 20 on the Mini Mental State Examination (MMSE). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS‐21, Geriatric Depression Scale (GDS), Geriatric Rating Scale (GRS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Quote: "the statistical evaluation was conducted on the patients who completed the trial". The analysis was not conducted on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Number and reasons of adverse events reported. Endpoint scores reported without standard deviations |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Five‐week randomised, double‐blind study | |
| Participants | Outpatients with depressive syndrome with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS) and 8 on the Raskin Depression Scale (RDS). | |
| Interventions | Fluoxetine: 63 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI), RDS , Covi Anxiety Scale (CAS), Patient Global Impression (PGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "four identical capsules were given" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Rating scale scores reported without denominators. Reasons for withdrawals not clearly described |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported without standard deviations. Side effects partially reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 18 on the first 17 items of the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 62 participants | |
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HDRS‐21) | |
| Notes | Response: decrease of at least 50% in the MADRS total score and a total score of less than 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Denominator reported for responders was different from the number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Number and reasons for premature termination reported. Only most frequently reported adverse events were described |
| Other bias | High risk | Quote: "this study was supported by Hoffmann‐La Roche", and this company produces moclobemide |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS) total score and over 21 on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 62 participants | |
| Outcomes | Primary outcomes: HDRS score and MADRS score Response: a decrease of 50% or more of the initial score on the HDRS or MADRS, or a Clinical Global Impression (CGI) rating of "remarkably improved" or "moderately improved" | |
| Notes | Remission: a score of 7 or less on HDRS or a score of 8 or less on MADRS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout reported. ITT number was different from the number of randomised |
| Selective reporting (reporting bias) | Unclear risk | Mean baseline and end‐point scores reported with standard deviations. Adverse events reported |
| Other bias | High risk | Quote: "funding for this study was provided by Bukwang Pharm, Seoul, Korea", and this company produces milnacipran |
| Methods | Six‐week randomised, double‐blind, two‐centre study | |
| Participants | In‐ and outpatients fulfilling Research Diagnostic Criteria for major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 30 participants | |
| Outcomes | HDRS, Montgomery and Asberg Scale for Depression (MADRS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated", no other information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | High risk | Denominator reported for responders was different from the number of randomised number. Number and reasons for withdrawals not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviations. Side effects not clearly reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for non‐psychotic depressive episode (no longer than 5 months), with a score of at least 21 on the Hamilton Rating Scale for Depression (HDRS‐17) and no more than 2 previous antidepressive drugs given for the current episode and no medication for 3‐5 days before first assessment. | |
| Interventions | Fluoxetine: 8 participants | |
| Outcomes | HDRS‐17 and Clinical Global Impression | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no information about randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Quote: "Both the psychiatrist and the patient knew the name of the medication, but this information was withheld from examiner", no further information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Both the psychiatrist and the patient knew the name of the medication, but this information was withheld from examiner", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Both the psychiatrist and the patient knew the name of the medication, but this information was withheld from examination", not clear if blinding of the examinator was successful |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for withdrawals was not clearly reported. Rating scale scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported with standard errors. Side effects not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Five‐week randomised, double‐blind study | |
| Participants | Patients fulfilling DSM‐III criteria for major depressive episode, with a score of at least 18 on the first 17 items of the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 15 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for discontinuation not reported. Scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | End point scores reported without standard deviations. Adverse events not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for predominantly major depressive disorder, with a score of at least 16 on Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 107 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI), Montgomery and Asberg Scale for Depression (MADRS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the study drugs were supplied in identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation reported. Scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported. Mean cores reported with standard deviations |
| Other bias | High risk | Last author's affiliation was Roche OY, Espoo, Finland. This company produces moclobemide |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling ICD‐10 criteria for depressive episode, recurrent depressive disorder, or bipolar affective disorder (depressive), with a score of at least 25 on the Montgomery and Asberg Scale for Depression (MADRS), requiring an antidepressant treatment. | |
| Interventions | Fluoxetine: 196 participants | |
| Outcomes | Primary outcome: MADRS global score | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation reported, but withdrawal was not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only serious adverse events reported. End point mean scores reported with standard deviations |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Five‐week randomised, double‐blind study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 18 on the first 17 items of Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 15 participants | |
| Outcomes | HDRS, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI), Zung Self‐Rating for Depression | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout not reported. Scores reported without denominators |
| Selective reporting (reporting bias) | High risk | Endpoint scores were reported only in figures and they were not clear. Side effects reported only in percentage without denominators |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week randomised, parallel group, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 18 on the first 17 items of Hamilton Rating Scale for Depression (HDRS) and a score of at least 4 on Clinical Global Impression‐Severity (CGI). Age range: 18‐65 years | |
| Interventions | Fluoxetine: 117 participants | |
| Outcomes | Primary outcome: change in HDRS total score Secondary outcome: change in Montgomery and Asberg Scale for Depression (MADRS) total score Response: at least 50% decrease from HDRS baseline score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " we administered treatments in a double blind fashion using a double‐dummy design", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation clearly reported. Denominator reported for responders was different from the number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported. Secondary endpoint scores (MADRS, CGI) reported |
| Other bias | High risk | Quote: "contract grant sponsor: Xian‐Janssen Pharmaceutical Company (honoraria to authors for conducting this trial)". This company produces escitalopram |
| Methods | Ten‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 16 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and a summary score of the HDRS items (agitation, psychic anxiety and somatic anxiety) higher than 5 or the score of at least one of the above items higher than 3. | |
| Interventions | Fluoxetine: 67 participants | |
| Outcomes | Change in HDRS‐17 total score, in agitation/anxiety score and in the response rate | |
| Notes | Response: decrease of at least 50% in the HDRS‐17 total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation reported. Scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported. Secondary end point scores not reported |
| Other bias | High risk | Two author's affiliation was Eli LIlly Italia, Sesto Fiorentino, Italy. This company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind, four‐centre study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for non‐psychotic major depression, with a score of at least 18 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 59 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression‐Severity (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "to maintain the blind, all patients took three capsules of study medication every day", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for discontinuation were not reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported with standard deviation. Side effects not reported |
| Other bias | High risk | Quote: "this work was sponsored by Eli Lilly and Company". This company produces fluoxetine |
| Methods | Twelve‐week randomised, open study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for Major Depressive Disorder (MDD), with a score of at least 16 on the Patient Health Questionnaire (PHQ‐9) at baseline and a score of at least 20 on Quick Inventory of Depression Syntomatology Self‐Report scale (QIDS‐SR) at visit 1 and 2. | |
| Interventions | Fluoxetine: 57 participants | |
| Outcomes | Primary outcome: change in QIDS‐SR total score Secondary measures: Hamilton Rating Scale for Depression (HDRS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Quote: "non‐blinded study" |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "non‐blinded study" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "non‐blinded study" |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation clearly reported. Endpoint mean score reported as a class |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported only when occurred in more than 5% of the sample. Mean endpoint scores reported with standard deviation and denominator |
| Other bias | High risk | Quote: "funded by Lilly USA, Indianapolis", and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive illness, with a score of at least 20 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17), a score of at least 8 on the Raskin Depression Scale (RDS) and greater than the Covi Anxiety Scale (CAS) score. | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS‐17, RDS , CAS, Clinical Global Impression (CGI) Improvement and Severity, Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "capsules that looked identical were supplied to all patients in two bottles marked 'morning dose' and 'bedtime dose'", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for early termination reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported. End point scores reported without standard deviation |
| Other bias | High risk | Quote: "supported by a grant from Eli Lilly & Company", this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind, multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for depressive episode (lasting between 1 to 8 months), without psychotic features, with a score of at least 22 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 89 participants Chloral hydrate (0.5‐1 mg) for sleep | |
| Outcomes | Primary outcome: change in the HDRS total score, number of patients showing response (decrease of at least 50% in HDRS total score) and remission (a final score of 10 or less) Seconday outcomes: Clinical Global Impression‐Severity (CGI‐S), Montgomery and Asberg Scale for Depression (MADRS), Patient Global Impression (PGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number of withdrawals reported, but reasons for dropout not clearly described. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | SIde effects not clearly reported. Mean endpoint scores reported with standard deviation |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Ten‐week randomised, double‐blind, multicentre study | |
| Participants | Patients fulfilling DSM‐IV criteria for major depressive episode, lasting for at least 1 month and having Columbia criteria for atypical depression. | |
| Interventions | Fluoxetine: 49 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "all patients received identical capsules", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of drop out was reported; reasons for dropout were not clearly stated Quote: "in the analyses to follow‐up, we will focus on the ITT group to estimate treatment effects without bias related to attrition", unclear how this analysis was carried out |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported. Only endpoint adjusted scores reported |
| Other bias | High risk | Quote: "supported by Eli Lilly and Company", this company produces fluoxetine |
| Methods | Twelve‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for depressive episode, with a score of at least 17 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 14 participants | |
| Outcomes | Primary outcome: change in the HDRS‐21 total score | |
| Notes | Funding: by academy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | High risk | Only the total number of randomised patients reported, not the number of the single group. Number and reasons for dropout not reported |
| Selective reporting (reporting bias) | Unclear risk | Side effects not reported. Mean baseline and endpoint score (HDRS) reported |
| Other bias | Low risk | Funding: by academy |
| Methods | Eight‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for mild to moderate, non psychotic major depressive disorder, with a baseline score of at least 10 and a maximum score of 24 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | Primary outcome: change in the HDRS total score Seconday outcomes: Clinical Global Impression (CGI) Severity, Montgomery and Asberg Scale for Depression (MADRS), UKU side effects rating scale (UKU) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information. |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons of withdrawals not clearly reported. Endpoint scores and responders reported only in figures |
| Selective reporting (reporting bias) | Unclear risk | SIde effects not reported. Secondary endpoint not reported |
| Other bias | High risk | Quote: "the authors are grateful to Marjan, for the financial support and donation of hypericum perforatum, and to Eli Lilly Brazil, for the donation of fluoxetine", this two pharmaceutical companies produce the two study drugs |
| Methods | Eight‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depression disorder, based on a structured clinical interview. | |
| Interventions | Fluoxetine: 36 participants Nortriptyline : 20 participants | |
| Outcomes | Primary outcomes: change in Hamilton Rating Scale for Depression (HDRS) and in Clinical Global Impression (CGI) total scores | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the investigator was blind as to medications and he did not personally manage any patients in the study", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number of dropouts was reported, but the reasons were not clearly described. Endpoint scores not reported (HDRS, CGI) |
| Selective reporting (reporting bias) | High risk | Side effects not reported. Item in which there was significant improvement reported without mean and standard deviation |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling Research Diagnostic Criteria for major depressive disorder or bipolar illness, with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 26 participants | |
| Outcomes | HDRS‐17, CGI, Montgomery and Asberg Scale for Depression (MADRS), Patient Global Impression (PGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation reported. Score reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only severe and moderate side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients with moderate to severe depression (DSM: single episode or recurrent), with a score of at least 18 in the first 17 items of Hamilton Rating Scale for Depression‐21 item (HDRS‐21) both at the screening and baseline visit, the HDRS score could not decrease by more than 25% between screen and baseline visit. The Raskin Depression Scale (RDS) score had to be at least 8 at baseline and must have exceeded the Covi Anxiety Score (CAS). | |
| Interventions | Fluoxetine: 289 participants Paroxetine: 284 participants | |
| Outcomes | Changes in HDRS total score, RDS and Clinical Global Impression (CGI) Severity of illness and Improvement; CAS, Symptom Checklist‐90 (SCL‐90), Global Assessment of Functioning (GAF) Scale Response: at least 50% reduction on the HDRS‐21 total score from baseline or a score under 10 at HDRS‐21 at the endpoint | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Mean rating scale scores not reported, both at baseline and at endpoint. Number and reasons for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Only most frequent side effects reported; baseline and endpoint scores at rating scales were not reported |
| Other bias | High risk | Funding: by Glasko Smith Kline, and this company produces paroxetine |
| Methods | Twelve‐week randomised, double‐blind, multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for Major Depressive Disorder (MDD), with a total score of at least 18 on the first 17 items of the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). Total score could not have decreased by more than 25% between the screen and the baseline visits. | |
| Interventions | Fluoxetine: 351 participants | |
| Outcomes | Primary outcome: HDRS‐21 Response: a decrease of 50% from baseline in the HDRS‐21 total score at any time during the 12‐week study | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout reported. Mean score at endpoint (HDRS) not reported. Responders were reported in percentage, and without denominator |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported. Secondary outcome measures reported only at endpoint, and not at the baseline |
| Other bias | High risk | Funding by Glasko Smithkline, and this company produces paroxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Inpatients with unipolar non‐psychotic major depression, with a score of at least 18 on the Hamilton Rating Scale for Depression (HDRS) after at least one week in the hospital without medication. | |
| Interventions | Fluoxetine: 14 participants Fluoxetine + desipramine dose: 20 mg/day fluoxetine + 98,1 (SD: 45.0) desipramine | |
| Outcomes | HDRS, Montgomery and Asberg Scale for Depression (MADRS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout reported without distinguish the study arms. Score reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Side effects not reported. End point score on HDRS and MADRS reported with standard deviation |
| Other bias | Low risk | Quote: "this research was supported in part by National Institute of Mental Health Grants R01‐MH‐47894 and MH‐30020" |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive episode, the symptoms is present for at least 1 month, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 104 participants Placebo: 102 participants | |
| Outcomes | Primary outcome: reduction in total score and item 1 of the HDRS‐21, Montgomery and Asberg Scale for Depression (MADRS), Clinical Global Impression (CGI), Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons of dropout reported. Denominator reported for responders was different from the number of randomised patients. Mean endpoint scores (HDRS‐21, MADRS, CGI) reported only in figures and without standard deviations |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported, but not clear the number of patients reporting at least one side effects |
| Other bias | High risk | Quote: "this study was funded by a series of grants to the participating research sites from Wyeth Research, Collegeville", and this company produces venlafaxine |
| Methods | Twelve‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive episode (single or recurrent), without psychotic features, with a score of at least 18 on the Hamilton Rating Scale for Depression‐ 24 item (HDRS‐24). | |
| Interventions | Fluoxetine: 119 participants | |
| Outcomes | Primary outcome: HDRS‐24 (total and factor scores), Clinical Global Impression (CGI) Severity, Efficacy | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double dummy procedure was used to ensured patients and physician blindness to treatment assignment", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominator. Number and reasons for dropout not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported. End point scores reported without standard deviation |
| Other bias | High risk | Quote: "supported by a grant from Pfizer", this company produces sertraline |
| Methods | Eight‐week double‐blind, randomised study | |
| Participants | Outpatients fulfilling DSM‐III and Bech‐Rafaelsen Melancholia Scale criteria for major depressive disorder, with a score of at least 18 on the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 29 participants | |
| Outcomes | HDRS‐21, Bech‐Rafaelsen Melancholia Scale (MES), Clinical Global Impression (CGI), Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Reasons and numbers of dropouts reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most frequent side effects reported. End point scores and standard deviation not clearly reported |
| Other bias | High risk | One of the authors' affiliation was Eli Lilly, Denmark. This company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Patients fulfilling DSM‐III criteria for major depressive disorder, with a score of at least 17 on the first 17 items of the Hamilton Rating Scale for Depression‐21 item (HDRS‐21), a score of at least 8 on the Raskin Depression Scale (RDS), greater than Covi Anxiety Scale (CAS). | |
| Interventions | Fluoxetine: 60 participants | |
| Outcomes | HDRS‐21, CAS, RDS, Patient Global Impression (PGI), Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly allocated", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " apparently identical capsules in seven doubles enveloped marked 'morning' and 'midday' dose", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported. Baseline and mean changes in efficacy measures reported with standard deviation |
| Other bias | High risk | One of the authors' affiliation was Eli Lilly Spain, Madrid; this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind clinical trial | |
| Participants | Outpatients fulfilling DSM‐IV criteria for depressive episode, with a score of at least 18 on the Hamilton Rating Scale for Depression (HDRS) and to be free of psychotropic medication for at least 4 weeks before study entry. | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | Primary outcome: change in the HDRS total score | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised in a 1:1 ratio using a computer‐generated code", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "throughout the study the person who administered the medications, rater and patients were blind to assignments", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " throughout the study the person who administered the medications, rater and patients were blind to assignments", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: " throughout the study the person who administered the medications, rater and patients were blind to assignments", no further information |
| Incomplete outcome data (attrition bias) | High risk | Number of withdrawals reported, but reasons for drop‐out not clearly described. Baseline and end‐point scores reported only in figures |
| Selective reporting (reporting bias) | Unclear risk | Side effects not clearly reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐IV criteria for major depressive disorder, (single or recurrent), without psychotic features, with or without melancholia, or bipolar II disorder, current episode depressed, moderate or severe without psychotic features with or without melancholia, with a score of at least 25 on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 91 participants | |
| Outcomes | Primary outcome: MADRS total score | |
| Notes | Response: decrease of at least 50% in the MADRS total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for drop out reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores (MADRS, Clinical Global Impression [CGI]) reported only in figures and without standard deviation. Only most frequent adverse events reported |
| Other bias | High risk | Quote: "this work was supported by Servier", and this company produces tianeptine |
| Methods | Four‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 7 participants | |
| Outcomes | Primary outcome: HDRS | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | End point scores reported for each patient. All patients complete the study |
| Selective reporting (reporting bias) | Low risk | Side effects not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind two‐centre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 18 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 61 participants | |
| Outcomes | Primary outcome: change from baseline on the HDRS‐21 total score at endpoint | |
| Notes | Response: decrease of at least 50% in the HDRS‐21 total score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "all active and placebo medication was supplied as identical coloured capsules", no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Only principal reasons for withdrawals reported. Endpoint scores reported with standard deviation |
| Selective reporting (reporting bias) | Unclear risk | Only adverse events reported for more than 5% of the sample reported. Secondary outcome not reported |
| Other bias | High risk | Quote: "paroxetine was supplied by SmithKline Beecham, Mexico" |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 18 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 21 participants | |
| Outcomes | HDRS‐21, Hamilton Rating Scale for Anxiety (HAM‐A), Clinical Global Impression (CGI), Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS), Symptom Checklist‐90 (SCL‐90) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation reported. Scores reported without standard deviation |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported. Vital signs reported. Scores reported without standard deviation (HDRS, RDS, CAS, SCL‐90) |
| Other bias | High risk | Last author affiliation was Laboratories Eli Lilly y Cia; this company produces fluoxetine |
| Methods | Four‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling Kielholz/Poeldinger scheme for depression, with a score of at least 11 on the Hamilton Rating Scale for Depression (HDRS‐14). | |
| Interventions | Fluoxetine: 46 participants | |
| Outcomes | HDRS‐14, Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Only completers were analysed |
| Selective reporting (reporting bias) | Unclear risk | No figures for percentages |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder or dysthymic disorder or depressive disorder NOS and Columbia criteria for atypical depression, with a score of at least 10 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI), Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double dummy", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for drop‐out were reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most frequent (at least 20%) treatment emergent adverse events reported. Vital signs described |
| Other bias | High risk | Quote: "supported by a research grant from Eli Lilly & Company, Indianapolis" and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depression (lasting more than 1 month), with a score of at least 20 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 21 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout were reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most frequent (at least 10%) adverse events reported Vital signs described |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Five‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling ICD 9 criteria for major unipolar or bipolar depression, with a score of at least 17 on the HDRS, a score of at least 8 on the Raskin Depression Scale (RDS), greater than Covi Anxiety Scale (CAS) score. | |
| Interventions | Fluoxetine: 51 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS‐17), Clinical Global Impression (CGI), RDS, CAS | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | It was unclear which was the last observation carried forward (LOCF) population |
| Selective reporting (reporting bias) | Unclear risk | Three rating scales for depression were listed in methods, but only one was reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Four‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling Kielholz/Poeldinger scheme for depression, with a score of at least 14 on the Hamilton Rating Scale for Depression (HDRS‐14). | |
| Interventions | Fluoxetine: 73 participants | |
| Outcomes | HDRS‐14, Clinical Global Impresison (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons for dropout not clearly reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | End point scores not reported (CGI). Only most common side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depression (lasting more than 1 month), with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 30 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "fluoxetine, amitriptyline and placebo were identical in appearance", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not reported. Endpoint scores reported with mean and standard deviation |
| Other bias | High risk | Quote: "this work was supported in part by Eli Lilly and Company" and this company produces fluoxetine |
| Methods | Seven‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for current major depressive episode, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) and with a minimum score of 2 on the depressive mood item. | |
| Interventions | Fluoxetine: 49 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI), Hamilton Rating Scale for Anxiety (HAM‐A), Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS), Symptom Checklist‐90 (SCL‐90) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no other information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Study medication was provided in identical‐appearing green capsules", no other information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no other information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "the ITT‐LOCF patients sample was the patients sample analysed for efficacy with at least one valid efficacy assessment after baseline determination". Rating scale scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviation. Only adverse events reported for more than 20% of the sample. |
| Other bias | High risk | Quote: "this study was supported by grants from Solvay Pharmaceuticals", and this company produces fluvoxamine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III criteria for current major depressive episode, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 38 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI), Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS), Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " medication was dispensed in opaque gelatine capsules containing either placebo, fluoxetine or doxepine (...) in a dose‐dispensing system administered by a pharmacist", not clear if blindness was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported |
| Other bias | High risk | Quote: "the authors gratefully acknowledge (...) Eli Lilly of Canada for financial support", this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder (lasting 1 month or more), with a score of at least 20 on the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 26 participants | |
| Outcomes | HDRS‐21, Clinical Global Impression (CGI), Patient Global Impression (PGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "medication was dispensed in opaque gelatine capsules containing either placebo, fluoxetine or doxepine...in a dose‐dispensing system administered by a pharmacist", not clear if blindness was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout reported but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | End point scores not clearly reported. Only most common side effects reported |
| Other bias | High risk | Quote: "the authors wish to aknowledge (...) Eli Lilly for financial support", this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 16 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 50 participants | |
| Outcomes | Primary outcomes: HDRS‐17, Clinical Global Impression (CGI) | |
| Notes | Response: decrease of at least 50% in the total score or a score of maximum 10 on the HDRS‐17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout were reported. Denominator reported for responders was different from the number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcomes not reported. Side effects and vital signs not clearly reported |
| Other bias | High risk | Last author's affiliation is Roche S. A., Brussels, Belgium and this company produces moclobemide |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder or bipolar disorder (currently depressive), with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 90 participants | |
| Outcomes | HDRS‐17, Montgomery and Asberg Scale for Depression (MADRS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "blindness was maintained by having each subject on lofepramine taking placebo‐fluoxetine in addiction and vice versa", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons and number of dropouts reported. Scores reported with denominator |
| Selective reporting (reporting bias) | Unclear risk | Adverse events experienced by more than five subjects in either group were reported. Scores reported only in figures and without standard deviation |
| Other bias | High risk | One author's affiliation was Lilly Industries Ltd, and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for current major depressive disorder, with a score between 18 and 25 on the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 71 participants | |
| Outcomes | HDRS‐21 | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons for discontinuation not clearly reported. Analysis not based on the ITT number |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported only in figures and without standard deviation. Only side effects reported by more than 3 patients |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for current major depressive disorder, with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 103 participants | |
| Outcomes | Primary outcomes: HDRS‐21 total score and depressed mood items, Montgomery–Åsberg Depression Rating Scale (MADRS) total score, Clinical Global Impression (CGI) | |
| Notes | Response: decrease of at least 50% in the total score from baseline on HDRS and MDRS or a CGi score of 1 or 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised in blocks of six using a table of random numbers..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most common adverse events reported. End point scores reported without standard deviation |
| Other bias | High risk | Quote: "Wyeth‐Ayerst Research provided financial support and the study drugs to the investigators". This company produces venlafaxine |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III criteria for moderate to severe major depressive disorder, non psychotic, with a score of at least 18 on the first 17 items of the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 61 participants | |
| Outcomes | HDRS‐17 total score, Inventory of Depressive Symptomatology (IDS), Clinical Global Impression (CGI) Improvement | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "double dummy dosing regimen was employed", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported. Scores reported with denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported |
| Other bias | High risk | Quote: "this study was sponsored by Bristol‐Myers Squibb Pharmaceutical research Institute", this company produces nefazodone |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depressive disorder, with a score of at least 18 on the Hamilton Rating Scale for Depression (HDRS‐17). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS‐17 | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no other information about the randomisation procedures |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "in a double blind manner", no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "in a double blind manner", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "in a double blind manner", no further information |
| Incomplete outcome data (attrition bias) | High risk | Denominator reported for responders was different from the number of randomised. Number and reasons for dropout not clearly described |
| Selective reporting (reporting bias) | Unclear risk | End‐point scores not reported. Side effects not reported |
| Other bias | High risk | Quote: "the original study of cardiac effects of fluoxetine and doxepin was supported by Eli Lilly, Canada". Eli Lilly Company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for unipolar depression (single or recurrent, nonpsychotic) with a current episode of at least 4 weeks in duration; had a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 100 participants Venlafaxine: 104 participants Placebo: 96 participants | |
| Outcomes | Decrease in HDRS‐21 score, Montgomery–Åsberg Depression Rating Scale (MADRS) score and Clinical Global Impression (CGI) score. Response: decrease of at least 50% HDRS score from baseline to endpoint | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation was by number in six‐patients unit with equal numbers of each treatment", no other information about the randomisation procedures |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "medication for each patient was packaged individually and code‐labelled with the study number and a unique patient randomisation number. Units were distributed to study sites according to the lowest available randomisation number", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "medication for each patient was packaged individually and code‐labelled with the study number and a unique patient randomisation number. Units were distributed to study sites according to the lowest available randomisation number", no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout described. Number of responders is reported only in figures and in percentage. Endpoint scores not reported |
| Selective reporting (reporting bias) | Unclear risk | Baseline scores reported without standard deviation. Side effects reported |
| Other bias | High risk | Quote: "funding for this study provided by Wyeth Research", and this company produces venlafaxine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 18 on the first 17 items of the Hamilton Rating Scale for Depression (HDRS‐21). | |
| Interventions | Fluoxetine: 52 participants | |
| Outcomes | HDRS‐21, Montgomery–Åsberg Depression Rating Scale (MADRS), Clinical Global Impression (CGI), Mini Mental State Examination (MMSE), Sandoz Clinical Assessment‐Geriatric (SCAG) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Scores reported without denominator. Number and reasons for dropout not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Only those adverse events occurred in at least 5% of participants were reported. Endpoint score at HDRS‐21 was reported only in figures and without standard deviation |
| Other bias | High risk | Quote: "this research was supported by SmithKline Beecham Pharmaceuticals" and this company produces paroxetine |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling ICD 10 criteria for mild to moderate depression, with a score between 16 and 24 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 114 participants | |
| Outcomes | Primary outcome: change from baseline to endpoint on the HDRS‐21 | |
| Notes | Response: decrease of at least 50% in the total score or a score of maximum 10 on the HDRS‐21 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: " treatment blindness was assured by 'double dummies', whereby hypericum active and placebo tablets were dispensed together with capsules containing fluoxetine or placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout clearly reported. Primary outcome scores (HDRS‐21) and secondary reported |
| Selective reporting (reporting bias) | Unclear risk | Only those adverse events occurred in at least 2% of participants were reported. Endpoint scores reported with standard deviation |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Twenty‐four‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 20 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 120 participants | |
| Outcomes | Change from baseline to endpoint on the HDRS‐17 and Clinacal Global Impression (CGI), Covi Anxiety Scale (CAS), Hamilton Rating Scale for Anxiety (HAM‐A) | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS‐17 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | End point scores reported without denominators. Number and reasons for discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Mean scores reported without standard deviations. Treatment‐related adverse events were reported |
| Other bias | High risk | Second author's affiliation was Pfizer, and this company produces sertraline |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Inpatients fulfilling DSM‐IV criteria for the melancholic subtype of MDD of at least 1 month duration, with a score of at least 24 on the first 21 items of the Hamilton Rating Scale for Depression (HDRS‐21). (In patients at the moment of randomisation, then patients could complete the protocol as outpatients if, in the opinion of the investigator, the response to treatment was sufficient to allow discharge from hospital). | |
| Interventions | Fluoxetine: 99 participants Venlafaxine: 95 participants Venlafaxine dose range: 225‐375 mg/day | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS‐21) total score, Clinical Global Impression (CGI) Improvement , Montgomery–Åsberg Depression Rating Scale (MADRS) total score Response: decrease of at least 50% in the total score on the HDRS and MADRS, or a score of 1‐2 on the CGI‐I | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were enrolled by investigators and assigned to blinded treatment by a computerized randomisation process generated by the sponsor. Medication was randomised in blocks of six patients, lots containing equal numbers of each treatment." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Number and reasons for withdrawal reported. Scores reported with denominators. Mean scores (HDRS, MADRS, CGI) and standard deviations reported clearly at baseline and at the endpoint |
| Selective reporting (reporting bias) | Unclear risk | Only most common (10%) side effects reported |
| Other bias | High risk | Quote: "this study was funded by Wyeth Pharmaceuticals and administered by Quitiles", and this company produces venlafaxine |
| Methods | Twelve‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 20 on the first 17 items on the Hamilton Depression (HDRS‐21) and a score of at least 8 on the Covi Anxiety Scale (CAS) and symptoms of depression for at least 1 month before study entry. | |
| Interventions | Fluoxetine: 119 participants | |
| Outcomes | Primary outcomes: HDRS‐21, Hamilton Rating Scale for Anxiety (HAM‐A) and Clinical Global Impression (CGI) Improvement | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS and HAM‐A, or a score of 1 on the CGI‐I | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for withdrawal reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects reported. Endpoint scores reported without standard deviation |
| Other bias | High risk | Quote: "This study was supported by Wyeth‐Ayerst Research, Philadelphia". This company produces venlafaxine |
| Methods | Twelve‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for dysthymia or a single episode of major depression partial remission, with a score between 14 and 26 on the Montgomery–Åsberg Depression Rating Scale (MADRS). | |
| Interventions | Fluoxetine: 139 participants Fluoxetine dose: 20 mg/day | |
| Outcomes | Primary outcome: a reduction of at least 50% on the MADRS total score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Quote: "a total of 281 patients were included. The intention to treat analysis consisted of 268 patients". Scores at rating scales were reported without denominators. Number and reasons for dropout were reported |
| Selective reporting (reporting bias) | Unclear risk | Treatment emergent adverse events reported. Endpoint scores not reported |
| Other bias | High risk | Synthelabo Clinical Research (Limito di Pioltello, Milano) participated at the study, and this company produces amisulpride |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 31 participants | |
| Outcomes | Global Assessment of Severity (GAS), Clinical Global Impression (CGI), HDRS‐17, Beck and Rafaelsen Mania Scale (MAS), Montgomery–Åsberg Depression Rating Scale (MADRS) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "placebo tablets resembled the opposite active medication", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | End point scores (MADRS, CGI, MAS) not reported Quote: "there was no significant differences between numbers remaining in the trial in the two groups at any time point". No ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Number and reasons for attrition reported. Only main side effects reported. Vital signs not reported |
| Other bias | High risk | Quote: "we would like to thank (...) Eli Lilly", and this company produces fluoxetine |
| Methods | Twenty‐week randomised, double‐blind study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive disorder, without melancholia, with a score of at least 21 on the Hamilton Rating Scale for Depression (HDRS‐24) and a score of at least 2 on the item 1 of HDRS‐21 and a score of maximum 18 on the Hamilton Rating Scale for Anxiety (HAM‐A), a score of at least 8 on the Raskin Depression Scale (RDS) and a total Covi Anxiety Scale (CAS) less than RDS. | |
| Interventions | Fluoxetine: 72 participants | |
| Outcomes | HDRS‐21, Montgomery–Åsberg Depression Rating Scale (MADRS), Clinical Global Impression (CGI), HAM‐A | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "patients in the ABT‐200 group received one placebo capsule which resembled fluoxetine", not clear if blindness was successful |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported, but included in the analysis only by estimation of outcome |
| Selective reporting (reporting bias) | Unclear risk | End point scores (MADRS, CGI) not reported. Only side effects reported by at least 20% of the sample reported |
| Other bias | High risk | Funding: by industry. (Abbott Laboratories, Abbott Park) |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III criteria for major depressive disorder (with a duration of illness of at least 4 weeks), with a score of at least 20 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) and a score of at least 8 on the Raskin Depression Scale (RDS). | |
| Interventions | Fluoxetine: 185 participants | |
| Outcomes | HDRS‐21, RDS, Covi Anxiety Scale (CAS), Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Responders reported without denominators. Number and reasons for dropout reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported only if they occurred at least in 5% of the sample. Endpoint mean scores and standard deviations reported |
| Other bias | High risk | Both authors' affiliation was Lilly Research laboratories, and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Patients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 22 on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 51 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), MADRS, Brief Psychiatric Rating Scale (BPRS), Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "double dummy", no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons and number of dropouts not clearly reported. Statistical analysis was not on an ITT basis |
| Selective reporting (reporting bias) | Unclear risk | End‐point mean scores and standard deviations reported. Side effects not clearly reported |
| Other bias | High risk | First author's affiliation was Eli Lilly, and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Patients with atypical depression according to Quitkin et al (1988) criteria | |
| Interventions | Fluoxetine: 14 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Clinical Global Impression (CGI), Covi Anxiety Scale (CAS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals were reported, but not included in the analysis. Scores reported without denominators. Side effects not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Endpoint mean scores and standard deviations reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, single‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 17 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 15 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, quote "the secret codes were only known to the dispenser", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Single blind, no further information |
| Blinding of participants and personnel (performance bias) | High risk | Single blind, no further information |
| Blinding of outcome assessment (detection bias) | High risk | Single blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | Endpoint mean scores and standard deviation reported. Scores reported with denominators |
| Selective reporting (reporting bias) | Unclear risk | Effects side not clearly reported |
| Other bias | High risk | Quote: "the drugs used in this study were provided by Mess Eli Lilly Company and Hoffman La Roche Company", and these companies produce study drugs |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for unipolar major depressive disorder, with a score of at least 14 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 18 participants | |
| Outcomes | Primary outcome: a HDRS‐21 score of maximum 7 or a Clinical Global Impression (CGI) score of maximum 2 at endpoint (remission) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Sinlge blind, with a blinder rater, no further information |
| Blinding of participants and personnel (performance bias) | High risk | Sinlge blind, with a blinder rater, no further information |
| Blinding of outcome assessment (detection bias) | High risk | Sinlge blind, with a blinder rater, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Scores reported with denominators, but withdrawals not included in the analysis. Reasons and number of discontinuation reported |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported without standard deviations. Side effects not reported |
| Other bias | High risk | Quote: "supported in part from Eli Lilly and Company", this company produces fluoxetine |
| Methods | Five‐week randomised, double‐blind study | |
| Participants | In‐ and outpatients fulfilling RDC (Research Diagnostic Criteria) for unipolar major depressive disorder with a score of at least 17 on the first 17 items of the Hamilton Rating Scale for Depression (HDRS) and a score of at least 8 and equal to or higher than the Covi Anxiety Scale (CAS) score. Age mean: 40.7 (fluoxetine); 42.7 (doxepin). Exclusion criteria: history of drug abuse, concurrent administration of other psychotropic drugs including lithium. | |
| Interventions | Fluoxetine: 26 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI), Raskin Depression Scale (RDS), CAS | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "the study drugs and placebo were supplied in identical capsules" , no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Endpoint mean scores reported without standard deviations. Only most common side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week single‐blind randomised study | |
| Participants | Outpatients fulfilling DSM‐IV criteria for major depressive disorder lasting at least 1 month and for atypical depression. Exclusion criteria: significant suicide risk, pregnancy, lactation or unwillingness to use effective birth control in women, unstable and serious physical illness, a history of seizures, psychosis or organic mental syndrome, substance abuse disorders within the past 6 months, except for nicotine dependence, history of mania, antisocial personality disorder and use of an antidepressant over the previous month. | |
| Interventions | Fluoxetine: 21 participants Reboxetine dose range: 4‐10 mg/day | |
| Outcomes | Change in the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) score and in Clinical Global Impression (CGI) score | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Single blind, no further information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "both psychiatrists, one rated the tests and the other who performed the drug follow‐up, were blinded to each other until the end of the study", no further information |
| Blinding of outcome assessment (detection bias) | High risk | Single blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout reported, mean endpoint scores (HDRS‐17, CGI, Global Assessment of Functioning [GAF]) reported, but data in the text was different from tables |
| Selective reporting (reporting bias) | High risk | Side effects were described, but the whole number of patients reporting side effects was unclear |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Five‐week randomised double‐blind study | |
| Participants | Outpatients with diagnosis of neurotic or reaction depressive disorder on the ICD, with a score of at least 17 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | HDRS, Clinical Global Impression (CGI), Symptom Check List of Taneri, Patient Global Impression (PGI), Zung Depression Scale (SDS) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Scores reported without denominators. Reasons and number of dropouts reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | End point mean scores and standard deviations reported. Only most common side effects reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Twelve‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients (general practice) DSM‐III‐R criteria for major unipolar depression, with a score of at least 12 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 76 participants | |
| Outcomes | Primary outcomes (all were dichotomised as above or below 80% of full compliance): pill count, patient completed questionnaire, Medication Event Monitoring System | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Open label, no further information |
| Blinding of participants and personnel (performance bias) | High risk | Open label, no further information |
| Blinding of outcome assessment (detection bias) | High risk | Open label, no further information |
| Incomplete outcome data (attrition bias) | High risk | Only main reason for dropout reported. Score reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly described. End point scores reported without standard deviations |
| Other bias | High risk | Quote: "this study was carried out by Eli Lilly and Company", and this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Inpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 24 on the Montgomery and Asberg Scale for Depression (MADRS). | |
| Interventions | Fluoxetine: 87 participants | |
| Outcomes | MADRS, Hamilton Rating Scale for Anxiety (HAM‐A), Hospital Anxiety and Depression (14 items), Clinical Global Impression (CGI) Severity | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Denominators of responders were different from number of randomised patients. Reasons and number of dropouts not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Only most common adverse events are reported. Endpoint scores reported without standard deviations |
| Other bias | High risk | Quote: "this research was supported by SmithKline Beecham Pharmaceuticals" and this company produces paroxetine |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling DSM‐III‐R criteria for major depressive unipolar disorder for at least 1 month, non‐psychotic, and subtype as agitated according Research Diagnostic Criteria, with a score of at least 14 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and a score of 2 or more on at least 2 items of the Agitation Rating Scale (ARS). | |
| Interventions | Fluoxetine: 62 participants | |
| Outcomes | Primary outcome: change on Hamilton Rating Scale for Depression (HDRS) from baseline to endpoint | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS‐17 during at least 4 weeks of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reason for withdrawals reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Adverse events reported. Mean endpoint scores reported with standard deviations |
| Other bias | High risk | Quote: "study funding was provided through a research grant from Lilly Research Laboratories, a division of Eli Lilly and Company" and this company produces fluoxetine |
| Methods | Twelve‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients (general practice) fulfilling DSM‐IV criteria for major depressive disorder, with a score of at least 19 on the Montgomery–Åsberg Depression Rating Scale (MADRS). | |
| Interventions | Fluoxetine: 170 participants | |
| Outcomes | Primary outcome: endpoint score on MADRS and Clinical Global Impression (CGI), and Hamilton Rating Scale for Depression (HDRS) | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS or MADRS and a CGI Improvement of 1 or 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible patients were randomised by the permuted blocks method", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "identical capsules" and "In order to maintain blinding, a matched placebo was taken in the evening by patients randomised to received fluoxetine", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for discontinuation reported. Denominator reported for responders was different from the number of randomised patients |
| Selective reporting (reporting bias) | High risk | Adverse events reported only if they occurred in at least 5% of the sample. Endpoint scores reported without standard deviations |
| Other bias | High risk | Quote: "this study was funded by Wyeth Laboratories Ltd." This company produces venlafaxine |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Inpatients fulfilling DSM‐IV criteria for major depression, with melancholia and symptoms lasting at least 1 month before study entry, with a score of at least 25 on the Montgomery–Åsberg Depression Rating Scale (MADRS). | |
| Interventions | Fluoxetine: 54 participants Temazepam and oxazepam were allowed for sleep | |
| Outcomes | Primary outcomes: Hamilton Rating Scale for Depression (HDRS), MADRS, Clinical Global Impression (CGI), Severity and improvement scores at each assessment | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS or MADRS and a CGI improvement of 1 or 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for discontinuation reported. Scores reported without denominator |
| Selective reporting (reporting bias) | Unclear risk | Only most common adverse events were reported. Mean endpoint scores reported without standard deviations |
| Other bias | High risk | Quote: "this study was supported by a grant from Wyeth‐Ayerst International". This company produces venlafaxine |
| Methods | Four‐week randomised, double‐blind study | |
| Participants | Depressed outpatients | |
| Interventions | Fluoxetine: 11 participants | |
| Outcomes | Efficacy data not reported. Only dropout rate | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reason for dropouts reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Endpoint scores reported with standard deviation. Adverse events not reported |
| Other bias | High risk | Quote: "we thank Lilly Industries Ltd for financial support and drug assays", this company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for moderate to severe major depression, with a score of at least 18 on the first 17 items of Hamilton Rating Scale for Depression (HDRS) and a score of at least 3 on the Clinical Global Impression. | |
| Interventions | Fluoxetine: 82 participants | |
| Outcomes | HDRS, MADRS, Clinical Global Impression (CGI) | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS or MADRS, or a score less than 10 on the HDRS Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout reported. Responders denominator is different from number of randomised patients |
| Selective reporting (reporting bias) | Unclear risk | Side effects reported only when occurred at least at 4% of the sample. Endpoint scores reported without standard deviations |
| Other bias | High risk | Quote: "we would like to acknowledge the financial and logistic support of Pfizer Belgium", this company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | Inpatients fulfilling DSM‐IV criteria for major depression, with a score of at least 18 on the first 17 items on the HDRS‐21 and a score of at least 18 on the Hamilton Rating Scale for Anxiety (HAM‐A). | |
| Interventions | Fluoxetine: 77 participants | |
| Outcomes | HDRS‐21, HAM‐A, Raskin Depression Scale (RDS), Covi Anxiety Scale (CAS), Clinical Global Impression (CGI), Patient Global Impression (PGI) | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS and a decrease of at least 25% in the total score on the HAM‐A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reason for dropout reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only most frequent side effects reported. Mean endpoint scores reported with standard deviation |
| Other bias | High risk | Authors' affiliation was Eli lilly Venezuela, and this company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐IV criteria for major depressive episode, with a score of at least 25 on the first 17 items of the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 152 participants | |
| Outcomes | Primary outcome: change in the HDRS score Secondary outcomes: MADRS score and Clinical Global Impression (CGI) score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | High risk | Reasons and number of dropouts not clearly reported. Only baseline scores at rating scales (HDRS, MADRS, CGI) reported |
| Selective reporting (reporting bias) | High risk | Adverse events occurring in more 5% reported. Number of responders reported only in figure and without denominators |
| Other bias | High risk | Quote: "this study was supported by Organon NV, The Netherlands", and this company produces mirtazepine |
| Methods | Five‐week randomised, double‐blind, parallel group study | |
| Participants | In and out‐patients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 16 on Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 20 participants | |
| Outcomes | Primary outcome: HDRS‐17 Secondary outcomes: Montgomery–Åsberg Depression Rating Scale (MADRS), the Adjective Mood Scale (AMS), Clinical Global Impression (CGI), Patient Global Impression (PGI) | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS or a total score on HDRS lower than 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of dropouts reported, but the reasons were unclear. Last observation carried forward analysis (LOCF) was used to report responders |
| Selective reporting (reporting bias) | Low risk | Primary and secondary endpoint scores reported with standard deviations. Side effects reported |
| Other bias | High risk | Quote: "this work was supported by Lilly Deutschland, Bad Homburg, Germany", and this company produces fluoxetine |
| Methods | Eight‐week randomised, double‐blind, multicentre study | |
| Participants | Out‐patients fulfilling DSM‐IV criteria for recurrent major depression, with a score of at least 20 on Hamilton Rating Scale for Depression‐21 item (HDRS‐21) on day ‐1 and ‐7, were currently experiencing a recurrent major depressive episode and had a normal sexual functioning. Mean age Fluoxetine: 37.1 years (SD: 10.7) Mean age Bupropion: 38.6 years (SD: 12.0) | |
| Interventions | Fluoxetine: 155 participants | |
| Outcomes | Primary outcomes: change in HDRS‐21; incidence of the orgasm dysfunction | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS‐21 between baseline and endpoint Remission: HDRS‐21 total score dropped to less than 8 between baseline and endpoint | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported. Mean score at baseline and endpoint not reported |
| Selective reporting (reporting bias) | Unclear risk | Most frequent side effects reported |
| Other bias | High risk | Funding probably by SmithKline, and this company produces bupropion |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depressive episode, with a score of at least 21 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and a score of at least 2 on the HDRS‐17 item 1. | |
| Interventions | Fluoxetine: 67 participants | |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI), Quality of Life Enjoyment and Satisfaction Questionnaire (Q‐LES‐Q) | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated to treatment with either mirtazepine or fluoxetine, according to the centrally prepared randomisation list", no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "active medication was prepared as indistinguishable looking tablets and packaging was performed using a double dummy technique", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Scores reported without denominators. Reason and numbers of dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Adverse events occurred in more than 5% of the sample reported. Endpoint scores not reported |
| Other bias | High risk | Quote: "supported by a clinical research grant from NV Organon, Oss, The Netherlands" and this company produces mirtazapine |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III criteria for major depressive episode, with a score of at least 17 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21). | |
| Interventions | Fluoxetine: 60 participants | |
| Outcomes | Primary outcome: HDRS‐21 Secondary outcome: Clinical Global Impression (CGI) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported, but not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Only most common side effects were reported. Mean endpoint scores not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Eight‐week double‐blind randomised study | |
| Participants | Patients fulfilling DSM‐IV criteria for major depressive disorder (based on a semi structured clinical interview), a score of at least 18 on the Hamilton Rating Scale for Depression‐21 item (HDRS‐21) and a score of at least 4 on the 3 HDRS‐21 sleep item. Age range: 18‐75 years Exclusion criteria: patients with an history of primary sleep disorder, significant medical problems, current alcohol or substance abuse or dependence, psychosis, or suicidal ideation. Psychotropic drugs were discontinued at least 1 week before study initiation and no subject received any prolonged‐acting central nervous system agent during the previous month. | |
| Interventions | Fluoxetine: 8 participants Mirazapine dose range: 15‐45 mg/day | |
| Outcomes | Change in the HDRS‐21 score and in Clinical Global Impression (CGI) score | |
| Notes | Funding: by industry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, double dummy, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, double dummy, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, double dummy, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout not clearly reported Only HDRS baseline scores reported |
| Selective reporting (reporting bias) | High risk | Side effects not reported. Number of responders not reported |
| Other bias | High risk | Quote: "funds for this study were provided through on unrestricted educational grant from Organon, Inc" and this industry produces venlafaxine |
| Methods | Five‐week randomised, double‐blind two‐centre study | |
| Participants | In‐ and outpatients fulfilling DSM‐III‐R criteria for major depression, with a score of at least 16 on the Hamilton Rating Scale for Depression‐17 item (HDRS‐17). | |
| Interventions | Fluoxetine: 10 participants | |
| Outcomes | HDRS‐17, Montgomery and Asberg Scale for Depression (MADRS) | |
| Notes | This study focuses on sleep related problems | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "since investigators were blind with regard to treatment", no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported. Scores reported without denominators |
| Selective reporting (reporting bias) | Unclear risk | Side effects not clearly reported. Mean endpoint scores and standard deviation reported |
| Other bias | High risk | Quote: "this work was supported by Lilly Deutschland", this company produces fluoxetine |
| Methods | Six‐week randomised, double‐blind multicentre study | |
| Participants | Outpatients fulfilling RDC criteria for moderate‐severe major depression, with a score of at least 18 on the Hamilton Rating Scale for Depression (HDRS). | |
| Interventions | Fluoxetine: 25 participants | |
| Outcomes | HDRS, Hamilton Rating Scale for Anxiety (HAM‐A), Beck Depression Inventory Scale (BDI) | |
| Notes | Most patients taking sedatives during study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "both drugs and placebo were identically formulated", no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: " an independent assessor scored the patients", no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | Number and reasons for dropout reported, but not included in the analysis |
| Selective reporting (reporting bias) | High risk | Only most common side effects were reported. Endpoint scores (HRSD, HAM‐A) not reported |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, double‐blind study | |
| Participants | Patients with serious depressive disorder | |
| Interventions | Fluoxetine: 8 participants | |
| Outcomes | Hamilton Rating Scale for Depression (HDRS), Hamilton Rating Scale for Anxiety (HAM‐A) | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind, no further information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double blind, no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | No information provided, impossible to evaluate |
| Selective reporting (reporting bias) | Unclear risk | No information provided, impossible to evaluate |
| Other bias | Unclear risk | Funding: unclear |
| Methods | Six‐week randomised, open label trial | |
| Participants | Patients fulfilling DSM‐IV criteria for major depression, with a score of at least 18 on Hamilton Rating Scale for Depression‐17 item (HDRS‐17) and a HDRS retardation score of at least 8 (assessed using the score of the first item of depressed mood, the 7th item of interest and activity, the 8th item of retardation and 14 item of decreased sexuality in the HDRS scale. | |
| Interventions | Fluoxetine: 61 participants | |
| Outcomes | Reduction in HDRS‐17 and reduction in HDRS‐17 retardation factor score; total score of Symptom Checklist‐90‐R (SCL‐90‐R) energy related 5 items; medical outcomes study‐Short Form (SF‐36); 4) cognitive function | |
| Notes | Response: decrease of at least 50% in the total score on the HDRS‐17 from baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) | High risk | Open label trial, no further information |
| Blinding of participants and personnel (performance bias) | High risk | Open label trial, no further information |
| Blinding of outcome assessment (detection bias) | High risk | Open label trial, no further information |
| Incomplete outcome data (attrition bias) | High risk | Number and reasons for dropout not clearly reported. Number of the responders not reported |
| Selective reporting (reporting bias) | High risk | Endpoint scores reported with standard deviations. Side effects reported only with percentage |
| Other bias | High risk | Quote: "this study was financially supported by Eli Lilly Asia, Inc", and this company produces fluoxetine |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Wrong design trial | |
| Wrong design trial | |
| Not RCT | |
| Not meeting inclusion criteria | |
| Wrong design trial | |
| Wrong design trial: randomisation to different doses of fluoxetine, without any drug comparator | |
| Not fulfilled inclusion criteria | |
| Randomisation to fluoxetine or placebo, no drug comparator | |
| Randomisation to fluoxetine or placebo, no drug comparator | |
| Wrong diagnosis | |
| Wrong design trial: not RCT | |
| Wrong drug comparison: neither antidepressant nor herbal product | |
| Wrong design trial | |
| Wrong design trial: not RCT | |
| Long‐term treatment of depression | |
| Wrong inclusion criteria: diagnosis of dysthymia | |
| Not meeting inclusion criteria | |
| Not meeting inclusion criteria | |
| Not meeting inclusion criteria | |
| Wrong design trial |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Six‐week randomised, double‐blind, double dummy clinical trial |
| Participants | Patients with depressive disorder |
| Interventions | 48 participants randomised to fluoxetine or reboxetine |
| Outcomes | Hamilton Depression Rating Scale (HDRS) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Randomised, double‐blind study |
| Participants | Patients with major depression with associated anxiety |
| Interventions | Fluoxetine and paroxetine |
| Outcomes | Montgomery and Asberg Scale for Depression (MADRS), Hamilton Rating Scale for Anxiety (HAM‐A) |
| Notes | Waiting for translation from Portuguese to English |
| Methods | Eight‐week, randomised study |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | Citalopram: 30 participants |
| Outcomes | Hamilton Depression Rating Scale (HDRS), Clinical Global Impression (CGI) and Treatment Emergent Symptom Scale (TESS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week, randomised study |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | Citalopram: 26 participants |
| Outcomes | Hamilton Depression Rating Scale 17‐Item (HDRS‐17) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised, double‐blind, double dummy multicentre trial |
| Participants | Patients with depressive disorder |
| Interventions | Fluoxetine: 145 participants Kaiyuanshen: 144 participants |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised, double‐blind, double dummy multicentre trial |
| Participants | Patients with depressive disorder |
| Interventions | 144 participants randomised to fluoxetine or bupropion 61 patients in Bupropion group and 64 in fluoxetine group completed the study |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised, double‐blind, double dummy trial |
| Participants | Patients with depressive disorder, with a score of at least 18 at Hamilton Depression Rating Scale (HDRS) and a score of 14 or greater at the Hamilton Anxiety Scale (HAM‐A) |
| Interventions | 137 participants randomised to fluoxetine or reboxetine 64 patients in Reboxetine group and 68 in fluoxetine group completed the study |
| Outcomes | Hamilton Depression Rating Scale (HDRS), Hamilton Rating Scale for Anxiety (HAM‐A) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised, double‐blind, double dummy trial |
| Participants | Patients with depressive disorder |
| Interventions | 228 participants randomised to fluoxetine or reboxetine |
| Outcomes | Hamilton Depression Rating Scale (HDRS) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Four‐week, randomised study |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | Sixty patients randomised to fluoxetine or reboxetine Fluoxetine dose range: 4‐8 mg Reboxetine dose range: 20‐40 mg |
| Outcomes | Hamilton Depression Rating Scale (HDRS) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Eight‐week, (likely) randomised study |
| Participants | In‐ and outpatients with depression according to CCMD‐III criteria |
| Interventions | Citalopram: 30 participants |
| Outcomes | Change in Hamilton Depression Rating Scale 24 Item (HDRS‐24) from baseline to endpoint, number of patients who responded to treatment |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Eight‐week randomised study |
| Participants | Out‐patients fulfilling DSM‐IV criteria for unipolar major depressive disorder, with a score of at least 18 on the Hamilton Depression Rating Scale‐21 item (HDRS‐21) Exclusion criteria: active suicidal risk or history of life‐threatening suicide attempts |
| Interventions | 272 participants randomised to fluoxetine or desipramine |
| Outcomes | HDRS‐21. |
| Notes | Funding:unclear. |
| Methods | Randomised double‐blind, double dummy multicentre trial |
| Participants | Patients with depressive disorder |
| Interventions | 228 participants randomised to fluoxetine or bupropion |
| Outcomes | Hamilton Depression Rating Scale‐17 item (HDRS‐17) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Randomised, double‐blind, controlled study |
| Participants | Patients with depressive disorder |
| Interventions | Participants randomised to fluoxetine or venlafaxine Fluoxetine: 10 participants Venlafaxine: 17 participants |
| Outcomes | Mood and Anxiety Symptoms Questionnaire (MASQ‐AD), Hamilton Depression Rating Scale (HDRS); fMRI response to an emotional regulation task |
| Notes | Funding: by National Grants and industry |
| Methods | Eight‐week randomised single‐blind trial |
| Participants | First episode depressive patients |
| Interventions | Eighty participants randomised to fluoxetine or venlafaxine Fluoxetine dose range: 20‐40 mg/day Venlafaxine dose range: 75‐225 mg/day |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Randomised (unpublished) multi‐site trial |
| Participants | Out‐patients with depression according to DSM‐IV criteria Age range: 18‐60 years |
| Interventions | Fluoxetine: 173 participants Sertraline: 177 participants Fluoxetine range dose: 20‐ 80 mg/day Sertraline range dose: 50‐150mg/day |
| Outcomes | Hamilton Depression Rating Scale‐24 item (HDRS‐24) |
| Notes | Funding: by industry |
| Methods | Randomised clinical trial |
| Participants | Patients with depression according to DSM‐IV criteria |
| Interventions | Fluoxetine: 40 participants Imipramine: 40 participants Fluoxetine dose: 20 mg/day Imipramine dose: 100 mg/day |
| Outcomes | Unclear |
| Notes | Waiting for translation from Arabic to English (only abstract available in English) |
| Methods | Randomised double‐blind, multicentre trial |
| Participants | Patients with depressive disorder |
| Interventions | Fluoxetine: 113 participants Reboxetine: 109 participants Fluoxetine dose: 20 mg/day Reboxetine dose: 8 mg/day |
| Outcomes | Hamilton Depression Rating Scale‐17 item (HDRS‐17), Hamilton Rating Scale for Anxiety (HAM‐A), Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised study |
| Participants | Patients fulfilling DSM‐III criteria for major depressive disorder, with a score of at least 15 on the Hamilton Depression Rating Scale‐17 item (HDRS‐17). |
| Interventions | Fluoxetine: 440 participants |
| Outcomes | HDRS‐17, Clinical Global Impression (CGI) |
| Notes | Funding: probably by industry |
| Methods | Twelve‐week, (likely) randomised study |
| Participants | Patients with first episode major depression |
| Interventions | Fluoxetine: 40 participants Venlafaxine: 40 participants |
| Outcomes | Hamilton Depression rating scale‐17 item (HDRS‐17), Hamilton Rating Scale for Anxiety (HAM‐A), Wechsler Adult Intelligence Scale (WAIS), Wisconsin Card Sorting Test (WCST) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week, randomised study |
| Participants | Patients with depression |
| Interventions | Fluoxetine: 51 participants Venlafaxine: 51 participants Fluoxetine dose‐rage: 20‐40 mg/day Venlafaxine dose‐rage: 50‐200 mg/day |
| Outcomes | Hamilton Depression Rating Scale (HDRS) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Eight‐week, (likely) randomised study |
| Participants | Patients with depression |
| Interventions | Sixty participants randomised to fluoxetine or venlafaxine |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Randomised, double‐blind study |
| Participants | Patients with depressive disorder |
| Interventions | Eighteen participants randomised to fluoxetine or amitriptyline |
| Outcomes | Unclear |
| Notes | Waiting for translation from Chinese to English |
| Methods | Six‐week, randomised study |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | Sixty patients randomised to fluoxetine or venlafaxine |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Unclear |
| Participants | Patients with depressive disorder |
| Interventions | Participants assigned to fluoxetine or paroxetine |
| Outcomes | Quality of life |
| Notes | Waiting for translation from Chinese to English |
| Methods | Six‐week, randomised, double‐blind, double dummy study |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | 48 participants randomised to fluoxetine or bupropion |
| Outcomes | Hamilton Depression rating scale (HDRS), Hamilton Rating Scale for Anxiety (HAM‐A) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Randomised, double‐blind, double dummy multicentre study |
| Participants | Patients with mild or moderate depression |
| Interventions | High‐dose of Morinda Officinalis Oligose capsule: 119 participants Low‐dose of Morinda Officinalis Oligose capsule: 119 participants Fluoxetine: 118 participants |
| Outcomes | Hamilton Depression Rating Scale‐17 item (HDRS‐17) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised study |
| Participants | Out‐patients with major depressive disorder (MDD) |
| Interventions | 117 participants randomised to fluoxetine or venlafaxine |
| Outcomes | Hamilton Depression Rating Scale‐21 item (HDRS‐21) |
| Notes | Abstract from conference |
| Methods | Six‐week, (likely) randomised study |
| Participants | Patients with depression according to CCMD‐II‐R criteria |
| Interventions | Sixty participants randomised to fluoxetine or venlafaxine |
| Outcomes | Hamilton Depression rating scale (HDRS) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Randomised, open trial |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | Sixty patients randomised to fluoxetine or escitalopram Fluoxetine dose range: 20‐40 mg/day Escitalopram dose range: 10‐20bmg/day |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised study |
| Participants | Patients with senile depressive disorder |
| Interventions | 50 participants randomised to fluoxetine or citalopram |
| Outcomes | Hamilton Depression Rating Scale‐17 item (HDRS‐17) and Clinical Global Impression (CGI) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Eight‐week randomised, single‐blind trial |
| Participants | Patients with depression according to CCMD‐III criteria |
| Interventions | Citalopram: 30 participants |
| Outcomes | Hamilton Depression Rating Scale‐24 item (HDRS‐24) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Eight‐week randomised, single‐blind trial |
| Participants | First episode depressive patients aged 60 years or over |
| Interventions | Sixty‐four patients randomised to fluoxetine or venlafaxine |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised trial |
| Participants | Elderly patients with depression |
| Interventions | Sixty patients randomised to fluoxetine or mirtazapine |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
| Methods | Six‐week randomised trial |
| Participants | Patients with depression according to CCMD‐III criteria, aged 60 years or over |
| Interventions | Fluoxetine: 23 participants Mirtazapine: 23 participants |
| Outcomes | Hamilton Depression Rating Scale (HDRS) |
| Notes | Waiting for translation from Chinese to English (only abstract available in English) |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | Eight‐week, randomised, double‐blind, multicentre study with parallel groups |
| Participants | Outpatients suffering from moderate to severe Major Depressive Disorder, according DSM‐IV‐TR criteria Age range: 18 to 65 years Exclusion criteria: all types of depression other than major depressive disorder and all other psychiatric disorders. Pregnancy, breastfeeding or possibility of becoming pregnant during the study without an effective contraception. |
| Interventions | Agomelatine: 314 participants Fluoxetine: 314 participants Agomelatine dosage range: 25‐50 mg Fluoxetine dosage range: 20‐40 mg |
| Outcomes | Change from baseline to end‐point on Depression‐Hamilton Depression Rating Scale‐17 items (HDRS‐17), Clinical Global (CGI) Improvement, Leeds Sleep Evaluation Questionnaire (LSEQ) and Hamilton Anxiety Rating Scale (HAM‐A) |
| Starting date | August 2006 |
| Contact information | Shu Liang, Prof, +86 10 65610341‐308, shu‐[email protected] |
| Notes | Funding: by industry |
| Trial name or title | |
| Methods | Eight week, randomised, open‐label study |
| Participants | Patients fulfilling DSM‐IV criteria for Major Depression Disorder, with a score of at least 20 on the Hamilton Depression Rating Scale‐17 item (HDRS‐17), a score of 2 in the first item of the HDRS‐17 at screening and baseline and a score of at least 4 on Clinical Global Impression of Severity (CGI‐S) at the enrolment visit. Exclusion criteria: history of bipolar disorder (I or II), schizophrenia, schizoaffective disorder, eating disorder or obsessive‐compulsive disorder. Patients recording more than 20% reduction on HDRS‐17 score at baseline (at the time of study allocation) as against the same recorded at the time of screening. Patients not responding to the administration of an appropriate dose of two different earlier antidepressant treatments (including fluoxetine) for at least 4 weeks each, for the current and earlier episodes, or not responding to fluoxetine monotherapy for at least 4 weeks. Substance or alcohol abuse in the last 30 days or dependence in the last 6 months,or with a risk of suicidal behavior (scoring 3 on item N°3 of HDRS‐17). Concomitant psychotropic medication, neurologic disorders or serious or uncontrolled diseases, hepatic insufficiency or renal insufficiency, clinically significant abnormalities on physical examination or laboratory test. Patients having participated in any type of clinical study within in the last one month of the screening date, pregnancy, breastfeeding, absence of adequate contraception measures. |
| Interventions | Fluoxetine dose range: 20‐40 mg IN‐ASTR‐001 dose range: 25‐50 mg |
| Outcomes | HDRS‐17 and CGI‐S |
| Starting date | April 2011 |
| Contact information | Kanhei Charan Sahoo, MD, 07966523302, [email protected] |
| Notes | Funding: by industry |
| Trial name or title | |
| Methods | Randomised, single‐blind study. |
| Participants | Patients fulfilling DSM‐IV criteria for Major Depression Disorder, with a score of at least 14 on the Hamilton Depression Rating Scale‐17 item (HDRS‐17) and resistant to a SSRI (administered at correct dose for at least 6 weeks). Exclusion criteria: treatment with any antidepressant drugs or psychotherapy, and to be resistant to any investigational drug. |
| Interventions | Fluoxetine dose: 20 mg Venlafaxine range dose: 75‐150 mg Nortriptyline dose: 25 mg |
| Outcomes | HDRS‐17 |
| Starting date | February 2008 |
| Contact information | https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2007‐002130‐11 |
| Notes | Funding: by academy |
| Trial name or title | |
| Methods | Six‐week open‐label, randomised trial |
| Participants | Patients fulfilling DSM‐IV criteria for Major Depression Disorder, with a score of at least 16 on the Hamilton Depression Rating Scale (HDRS). |
| Interventions | Fluoxetine range dose: 20‐80 mg Venlafaxine range dose 75‐ 225 mg |
| Outcomes | HDRS |
| Starting date | March 2007 |
| Contact information | Po See Chen, MD, +886‐6‐2353535 ext 5213, [email protected] |
| Notes | Funding: by academy |
| Trial name or title | |
| Methods | Eight week, randomised, double‐blind study |
| Participants | Patients fulfilling DSM‐IV criteria for major depression disorder, with a minimum duration of 4 weeks. Age range: 18‐65 years old. |
| Interventions | Fluoxetine dose range: 20‐60 mg F2695 SR dose range: 40‐120 mg |
| Outcomes | Clinical Global Impression (CGI) Severity, Patient Global Impression (PGI) Severity |
| Starting date | April 2011 |
| Contact information | Carl Gommoll, MS, Forest Laboratories |
| Notes | Funding: by industry |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 24 | 2124 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| Analysis 1.1  Comparison 1 Fluoxetine versus TCAs, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Amitriptyline | 11 | 777 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.68, 1.28] |
| 1.2 Fluoxetine vs Clomipramine | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.45] |
| 1.3 Fluoxetine vs Desipramine | 2 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.56, 5.15] |
| 1.4 Fluoxetine vs Dothiepin/dosulepin | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.08, 4.20] |
| 1.5 Fluoxetine vs Doxepine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| 1.6 Fluoxetine vs Imipramine | 5 | 761 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.41, 1.35] |
| 1.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.55, 1.78] |
| 1.8 Fluoxetine vs Trimipramine | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.56, 7.45] |
| 2 End‐point score on rating scale Show forest plot | 50 | 3393 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.07, 0.14] |
| Analysis 1.2  Comparison 1 Fluoxetine versus TCAs, Outcome 2 End‐point score on rating scale. | ||||
| 2.1 Fluoxetine vs Amiptriptyline | 19 | 1023 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.09, 0.29] |
| 2.2 Fluoxetine vs Clomipramine | 5 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.10] |
| 2.3 Fluoxetine vs Desipramine | 4 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.32, 0.86] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 4 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.27, 0.59] |
| 2.5 Fluoxetine vs Imipramine | 12 | 1063 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.16, 0.42] |
| 2.7 Fluoxetine vs Nomifensine | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.12, 0.38] |
| 2.8 Fluoxetine vs Nortriptyline | 2 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.20, 0.24] |
| 2.9 Fluoxetine vs Trimipramine | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.10, 0.92] |
| 3 Failure to complete ‐ Total Show forest plot | 49 | 4194 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.65, 0.96] |
| Analysis 1.3  Comparison 1 Fluoxetine versus TCAs, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs Amitriptyline | 18 | 1089 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.85] |
| 3.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.14] |
| 3.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 3.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.92, 2.69] |
| 3.5 Fluoxetine vs Doxepine | 4 | 323 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.49, 1.32] |
| 3.6 Fluoxetine vs Imipramine | 12 | 1225 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.21] |
| 3.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 3.8 Fluoxetine vs Nomifensine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 6.33 [0.67, 60.16] |
| 3.9 Fluoxetine vs Nortriptyline | 3 | 448 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.47] |
| 3.10 Fluoxetine vs Trimipramine | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 2.0 [0.41, 9.78] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 33 | 2911 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.96, 1.72] |
| Analysis 1.4  Comparison 1 Fluoxetine versus TCAs, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs Amitriptyline | 13 | 835 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.44, 1.88] |
| 4.2 Fluoxetine vs Clomipramine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [0.37, 145.75] |
| 4.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.35] |
| 4.4 Fluoxetine vs Dothiepin/dosulepin | 3 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.49, 3.66] |
| 4.5 Fluoxetine vs Doxepine | 3 | 283 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.60, 4.92] |
| 4.6 Fluoxetine vs Imipramine | 10 | 1093 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.97, 2.05] |
| 4.7 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.03] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 40 | 3647 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.40, 0.75] |
| Analysis 1.5  Comparison 1 Fluoxetine versus TCAs, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs Amitriptyline | 16 | 1038 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.71] |
| 5.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.79] |
| 5.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.68] |
| 5.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.59, 7.16] |
| 5.5 Fluoxetine vs Doxepine | 3 | 283 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 5.6 Fluoxetine vs Imipramine | 10 | 1093 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.86] |
| 5.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.38] |
| 5.8 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Fluoxetine versus heterocyclics, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [0.91, 4.18] |
| 1.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.27, 2.38] |
| 2 End‐point score on rating scale Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Fluoxetine versus heterocyclics, Outcome 2 End‐point score on rating scale. | ||||
| 2.1 Fluoxetine vs Maprotiline | 5 | 433 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.15, 0.23] |
| 2.2 Fluoxetine vs Mianserin | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.38, 1.23] |
| 3 Failure to complete ‐ Total Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Fluoxetine versus heterocyclics, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs Maprotiline | 4 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.90, 3.41] |
| 3.2 Fluoxetine vs Mianserin | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.18, 2.25] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Fluoxetine versus heterocyclics, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs Maprotiline | 3 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 2.54 [0.33, 19.19] |
| 4.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 2.27 [0.38, 13.63] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Fluoxetine versus heterocyclics, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs Maprotiline | 3 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.15, 1.93] |
| 5.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Fluoxetine versus other SSRIs, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.20, 1.79] |
| 1.2 Fluoxetine vs Escitalopram | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.56, 1.85] |
| 1.3 Fluoxetine vs Fluvoxamine | 1 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.74] |
| 1.4 Fluoxetine vs Paroxetine | 9 | 1574 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.93, 1.65] |
| 1.5 Fluoxetine vs Sertraline | 6 | 1188 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [1.08, 1.74] |
| 2 End‐point score on rating scales Show forest plot | 21 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Fluoxetine versus other SSRIs, Outcome 2 End‐point score on rating scales. | ||||
| 2.1 Fluoxetine vs Citalopram | 3 | 661 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.10, 0.21] |
| 2.2 Fluoxetine vs Escitalopram | 1 | 231 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.19, 0.33] |
| 2.3 Fluoxetine vs Paroxetine | 11 | 2061 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.26, 0.24] |
| 2.4 Fluoxetine vs Sertraline | 7 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.20] |
| 3 Failure to complete ‐ Total Show forest plot | 25 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Fluoxetine versus other SSRIs, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs Citalopram | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.27] |
| 3.2 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.00, 2.37] |
| 3.3 Fluoxetine vs Fluvoxamine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.37] |
| 3.4 Fluoxetine vs Paroxetine | 10 | 1848 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.20] |
| 3.5 Fluoxetine vs Sertraline | 9 | 1591 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.93, 1.49] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 13 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Fluoxetine versus other SSRIs, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs Citalopram | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.48, 1.56] |
| 4.2 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.46, 6.53] |
| 4.3 Fluoxetine vs Paroxetine | 4 | 1005 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 4.4 Fluoxetine vs Sertraline | 5 | 1056 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.68, 1.77] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Fluoxetine versus other SSRIs, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs Citalopram | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.20] |
| 5.2 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.12] |
| 5.3 Fluoxetine vs Fluvoxamine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 7.71] |
| 5.4 Fluoxetine vs Paroxetine | 9 | 1509 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.16] |
| 5.5 Fluoxetine vs Sertraline | 9 | 1591 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Fluoxetine versus SNRIs, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Duloxetine | 1 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.61, 3.25] |
| 1.2 Fluoxetine vs Milnacipran | 2 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.84] |
| 1.3 Fluoxetine vs Venlafaxine | 12 | 3387 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [1.10, 1.51] |
| 2 End‐point score on rating scale Show forest plot | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Fluoxetine versus SNRIs, Outcome 2 End‐point score on rating scale. | ||||
| 2.1 Fluoxetine vs Milnacipran | 2 | 213 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.63, ‐0.08] |
| 2.2 Fluoxetine vs Venlafaxine | 13 | 3097 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [0.00, 0.19] |
| 3 Failure to complete ‐ Total Show forest plot | 19 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Fluoxetine versus SNRIs, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs Duloxetine | 2 | 532 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.53, 1.52] |
| 3.2 Fluoxetine vs Milnacipran | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.42] |
| 3.3 Fluoxetine vs Venlafaxine | 14 | 2683 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.06] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Fluoxetine versus SNRIs, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs Duloxetine | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 3.33 [0.92, 12.11] |
| 4.2 Fluoxetine vs Milnacipran | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.68, 2.30] |
| 4.3 Fluoxetine vs Venlafaxine | 13 | 2640 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.91, 1.89] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Fluoxetine versus SNRIs, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs Duloxetine | 2 | 532 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.23] |
| 5.2 Fluoxetine vs Milnacipran | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.81, 2.76] |
| 5.3 Fluoxetine vs Venlafaxine | 13 | 2640 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Agomelatine | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.99, 2.09] |
| 1.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.04] |
| 1.3 Fluoxetine vs Moclobemide | 7 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.92, 1.75] |
| 1.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.34] |
| 1.5 Fluoxetine vs Reboxetine | 3 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.10] |
| 2 End‐point score on rating scales Show forest plot | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 2 End‐point score on rating scales. | ||||
| 2.1 Fluoxetine vs Agomelatine | 3 | 1213 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.18, 0.23] |
| 2.2 Fluoxetine vs Mirtazapine | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.15, 1.29] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 540 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.30] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.67, 0.57] |
| 2.5 Fluoxetine vs Reboxetine | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.40] |
| 3 Failure to complete ‐ Total Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.49] |
| 3.2 Fluoxetine vs Mirtazapine | 3 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.68] |
| 3.3 Fluoxetine vs Moclobemide | 7 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.70, 1.47] |
| 3.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 3.5 Fluoxetine vs Reboxetine | 4 | 764 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.88] |
| 4.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 2.96] |
| 4.3 Fluoxetine vs Moclobemide | 6 | 679 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.32, 1.56] |
| 4.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Fluoxetine vs Reboxetine | 3 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.47, 1.77] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.73, 3.08] |
| 5.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.66] |
| 5.3 Fluoxetine vs Moclobemide | 7 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 5.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 5.5 Fluoxetine vs Reboxetine | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.10, 1.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Amineptine | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.04] |
| 1.2 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
| 1.3 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.24, 1.26] |
| 1.4 Fluoxetine vs Tianeptine | 1 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.75, 1.67] |
| 1.5 Fluoxetine vs Trazodone | 3 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.86] |
| 2 End‐point score on rating scales Show forest plot | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 2 End‐point score on rating scales. | ||||
| 2.1 Fluoxetine vs ABT‐200 | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐2.25, ‐1.45] |
| 2.2 Fluoxetine vs Amisulpride | 1 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.07, 0.41] |
| 2.3 Fluoxetine vs Nefazodone | 4 | 271 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.30, 0.18] |
| 2.4 Fluoxetine vs Tianeptine | 3 | 730 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 2.5 Fluoxetine vs Trazodone | 4 | 203 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.76, 0.26] |
| 3 Failure to complete ‐ Total Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs ABT‐200 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.08, 0.39] |
| 3.2 Fluoxetine vs Amineptine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.17, 2.21] |
| 3.3 Fluoxetine vs Amisulpride | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.38] |
| 3.4 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.48] |
| 3.5 Fluoxetine vs Nefazodone | 3 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.31] |
| 3.6 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.42] |
| 3.7 Fluoxetine vs Tianeptine | 3 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.69, 1.33] |
| 3.8 Fluoxetine vs Trazodone | 4 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.13] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.4  Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs ABT‐200 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 2.20] |
| 4.2 Fluoxetine vs Amineptine | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.19, 5.57] |
| 4.3 Fluoxetine vs Amisulpride | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.10] |
| 4.4 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.33, 4.10] |
| 4.5 Fluoxetine vs Nefazodone | 3 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.05, 10.71] |
| 4.6 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.05, 4.51] |
| 4.7 Fluoxetine vs Tianeptine | 3 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.27, 2.53] |
| 4.8 Fluoxetine vs Trazodone | 2 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.51] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.5  Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs ABT‐200 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.27] |
| 5.2 Fluoxetine vs Amineptine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.03, 7.82] |
| 5.3 Fluoxetine vs Amisulpride | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.33, 1.82] |
| 5.4 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.25] |
| 5.5 Fluoxetine vs Nefazodone | 4 | 286 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.81] |
| 5.6 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.50] |
| 5.7 Fluoxetine vs Tianeptine | 3 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.71, 1.80] |
| 5.8 Fluoxetine vs Trazodone | 3 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.20, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 1 Failure to respond ‐ HDRS (‐50%). | ||||
| 1.1 Fluoxetine vs Crocus Sativus | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.60] |
| 1.2 Fluoxetine vs Hypericum | 6 | 717 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.55, 1.73] |
| 2 End‐point score on rating scales Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.2  Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 2 End‐point score on rating scales. | ||||
| 2.1 Fluoxetine vs Hypericum | 5 | 648 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.02, 0.29] |
| 3 Failure to complete ‐ Total Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.3  Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 3 Failure to complete ‐ Total. | ||||
| 3.1 Fluoxetine vs Crocus Sativus | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 17.18] |
| 3.2 Fluoxetine vs Hypericum | 5 | 679 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.58, 1.85] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.4  Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 4 Failure to complete ‐ Inefficacy. | ||||
| 4.1 Fluoxetine vs Hypericum | 2 | 401 | Odds Ratio (M‐H, Random, 95% CI) | 4.70 [0.22, 99.39] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 7.5  Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 5 Failure to complete ‐ Side Effects. | ||||
| 5.1 Fluoxetine vs Hypericum | 5 | 679 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.56, 2.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 5 | 341 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.35, 1.27] |
| Analysis 8.1  Comparison 8 Subgroup analysis for fluoxetine versus TCAs: failure to respond, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Amitriptyline | 3 | 207 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.65] |
| 1.2 Fluoxetine vs Clomipramine | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.45] |
| 1.3 Fluoxetine vs Imipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.34] |
| 2 follow‐up 6‐16 weeks Show forest plot | 18 | 1742 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.31] |
| Analysis 8.2  Comparison 8 Subgroup analysis for fluoxetine versus TCAs: failure to respond, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Amitriptyline | 8 | 570 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.50] |
| 2.2 Fluoxetine vs Desipramine | 2 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.56, 5.15] |
| 2.3 Fluoxetine vs Dothiepin/dosulepin | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.08, 4.20] |
| 2.4 Fluoxetine vs Doxepine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| 2.5 Fluoxetine vs Imipramine | 4 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.34] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.55, 1.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 12 | 541 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.15, 0.50] |
| Analysis 9.1  Comparison 9 Subgroup analysis for fluoxetine versus TCAs: endpoint score, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Amiptriptyline | 6 | 290 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.25, 1.02] |
| 1.2 Fluoxetine vs Clomipramine | 2 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.36, 0.35] |
| 1.3 Fluoxetine vs Desipramine | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.72, 0.82] |
| 1.4 Fluoxetine vs Imipramine | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐1.31, 1.22] |
| 1.5 Fluoxetine vs Trimipramine | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.24, 1.00] |
| 2 follow‐up 6‐16 weeks Show forest plot | 36 | 2727 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| Analysis 9.2  Comparison 9 Subgroup analysis for fluoxetine versus TCAs: endpoint score, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Amiptriptyline | 13 | 733 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.07, 0.22] |
| 2.2 Fluoxetine vs Clomipramine | 3 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 2.3 Fluoxetine vs Desipramine | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.47, 1.12] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 4 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.27, 0.59] |
| 2.5 Fluoxetine vs Imipramine | 10 | 1003 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.16, 0.42] |
| 2.7 Fluoxetine vs Nortriptyline | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.44, 0.20] |
| 2.8 Fluoxetine vs Trimipramine | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.44, 1.39] |
| 3 follow‐up >16 weeks Show forest plot | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.27, ‐0.44] |
| Analysis 9.3  Comparison 9 Subgroup analysis for fluoxetine versus TCAs: endpoint score, Outcome 3 follow‐up >16 weeks. | ||||
| 3.1 Fluoxetine vs Nortriptyline | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.27, ‐0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 11 | 663 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.05] |
| Analysis 10.1  Comparison 10 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Amitriptyline | 6 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.13] |
| 1.2 Fluoxetine vs Doxepine | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.29, 5.31] |
| 1.3 Fluoxetine vs Imipramine | 3 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.10, 3.13] |
| 1.4 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.44, 1.35] |
| 2 follow‐up 6‐16 weeks Show forest plot | 36 | 3450 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| Analysis 10.2  Comparison 10 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Amitriptyline | 12 | 780 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.90] |
| 2.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.14] |
| 2.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.92, 2.69] |
| 2.5 Fluoxetine vs Doxepine | 3 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.40] |
| 2.6 Fluoxetine vs Imipramine | 9 | 1127 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.51, 1.27] |
| 2.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 2.8 Fluoxetine vs Nortriptyline | 2 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 3.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 5 | 401 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.50] |
| Analysis 11.1  Comparison 11 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Amitriptyline | 2 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.09] |
| 1.2 Fluoxetine vs Doxepine | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Fluoxetine vs Imipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 1.4 Fluoxetine vs Nortriptyiline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.03] |
| 2 follow‐up 6‐16 weeks Show forest plot | 28 | 2510 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [1.02, 1.87] |
| Analysis 11.2  Comparison 11 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ inefficacy, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Amitriptyline | 11 | 730 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.50, 2.47] |
| 2.2 Fluoxetine vs Clomipramine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [0.37, 145.75] |
| 2.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.35] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 3 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.49, 3.66] |
| 2.5 Fluoxetine vs Doxepine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.60, 4.92] |
| 2.6 Fluoxetine vs Imipramine | 9 | 1053 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.96, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 7 | 554 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.46, 1.43] |
| Analysis 12.1  Comparison 12 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Amitriptyline | 4 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.82] |
| 1.2 Fluoxetine vs Doxepine | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 3.13 [0.30, 32.31] |
| 1.3 Fluoxetine vs Imipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.24] |
| 1.4 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.77] |
| 2 follow‐up 6‐16 weeks Show forest plot | 33 | 3093 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.72] |
| Analysis 12.2  Comparison 12 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Amitriptyline | 12 | 780 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.61] |
| 2.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.79] |
| 2.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.68] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.59, 7.16] |
| 2.5 Fluoxetine vs Doxepine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.42, 1.28] |
| 2.6 Fluoxetine vs Imipramine | 9 | 1053 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.25, 0.87] |
| 2.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 13.1  Comparison 13 Subgroup analysis for fluoxetine versus heterocyclics: endpoint score, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Maprotiline | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.34, 0.46] |
| 2 follow‐up 6‐16 weeks Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 13.2  Comparison 13 Subgroup analysis for fluoxetine versus heterocyclics: endpoint score, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Maprotiline | 3 | 252 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| 2.2 Fluoxetine vs Mianserin | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.38, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 14.1  Comparison 14 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Maprotiline | 2 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.54 [0.63, 3.75] |
| 2 follow‐up 6‐16 weeks Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 14.2  Comparison 14 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.75, 5.63] |
| 2.2 Fluoxetine vs Mianserin | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.18, 2.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 15.1  Comparison 15 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Maprotiline | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 follow‐up 6‐16 weeks Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 15.2  Comparison 15 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ inefficacy, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 2.54 [0.33, 19.19] |
| 2.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 2.27 [0.38, 13.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 16.1  Comparison 16 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Maprotiline | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.49] |
| 2 follow‐up 6‐16 weeks Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 16.2  Comparison 16 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.11, 4.38] |
| 2.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.1  Comparison 17 Subgroup analysis for fluoxetine versus other SSRIs: failure to respond, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.20, 1.79] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.2  Comparison 17 Subgroup analysis for fluoxetine versus other SSRIs: failure to respond, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Fluvoxamine | 1 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.74] |
| 2.2 Fluoxetine vs Paroxetine | 9 | 1574 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.93, 1.65] |
| 2.3 Fluoxetine vs Sertraline | 5 | 950 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [1.00, 1.71] |
| 2.4 Fluoxetine vs Escitalopram | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.56, 1.85] |
| 3 follow‐up >16 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 17.3  Comparison 17 Subgroup analysis for fluoxetine versus other SSRIs: failure to respond, Outcome 3 follow‐up >16 weeks. | ||||
| 3.1 Fluoxetine vs Sertraline | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.97, 2.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 18.1  Comparison 18 Subgroup analysis for fluoxetine versus other SSRIs: endpoint score, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Citalopram | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.48, 0.61] |
| 2 follow‐up >16 weeks Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 18.2  Comparison 18 Subgroup analysis for fluoxetine versus other SSRIs: endpoint score, Outcome 2 follow‐up >16 weeks. | ||||
| 2.1 Fluoxetine vs Paroxetine | 1 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.01, 0.49] |
| 2.2 Fluoxetine vs Sertraline | 1 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.08, 0.53] |
| 3 follow‐up 6‐16 weeks Show forest plot | 18 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 18.3  Comparison 18 Subgroup analysis for fluoxetine versus other SSRIs: endpoint score, Outcome 3 follow‐up 6‐16 weeks. | ||||
| 3.1 Fluoxetine vs Citalopram | 2 | 610 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.10, 0.21] |
| 3.2 Fluoxetine vs Paroxetine | 10 | 1819 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.31, 0.24] |
| 3.3 Fluoxetine vs Sertraline | 6 | 992 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.19] |
| 3.4 Fluoxetine vs Escitalopram | 1 | 231 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.19, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 19.1  Comparison 19 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.22, 4.27] |
| 2 follow‐up 6‐16 weeks Show forest plot | 21 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 19.2  Comparison 19 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Citalopram | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.59, 1.27] |
| 2.2 Fluoxetine vs Fluvoxamine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.37] |
| 2.3 Fluoxetine vs Paroxetine | 9 | 1606 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.25] |
| 2.4 Fluoxetine vs Sertraline | 7 | 1111 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.85, 1.48] |
| 2.5 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.00, 2.37] |
| 3 follow‐up >16 weeks Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 19.3  Comparison 19 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ total, Outcome 3 follow‐up >16 weeks. | ||||
| 3.1 Fluoxetine vs Paroxetine | 1 | 242 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 3.2 Fluoxetine vs Sertraline | 2 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.84, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 20.1  Comparison 20 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.43] |
| 2 follow‐up >16 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 20.2  Comparison 20 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ inefficacy, Outcome 2 follow‐up >16 weeks. | ||||
| 2.1 Fluoxetine vs Sertraline | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.47, 2.07] |
| 3 follow‐up 6‐16 weeks Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 20.3  Comparison 20 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ inefficacy, Outcome 3 follow‐up 6‐16 weeks. | ||||
| 3.1 Fluoxetine vs Citalopram | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.49, 1.65] |
| 3.2 Fluoxetine vs Paroxetine | 4 | 1005 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 3.3 Fluoxetine vs Sertraline | 4 | 818 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.61, 2.29] |
| 3.4 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.46, 6.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 21.1  Comparison 21 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 1.5 [0.23, 9.70] |
| 2 follow‐up 6‐16 weeks Show forest plot | 20 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 21.2  Comparison 21 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Citalopram | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.12] |
| 2.2 Fluoxetine vs Fluvoxamine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 7.71] |
| 2.3 Fluoxetine vs Paroxetine | 9 | 1509 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.16] |
| 2.4 Fluoxetine vs Sertraline | 7 | 1111 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.71] |
| 2.5 Fluoxetina vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.12] |
| 3 follow‐up >16 weeks Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 21.3  Comparison 21 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ side effects, Outcome 3 follow‐up >16 weeks. | ||||
| 3.1 Fluoxetine vs Sertraline | 2 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.72, 2.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 22.1  Comparison 22 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to respond, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.39, 2.60] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 22.2  Comparison 22 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to respond, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Agomelatine | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.99, 2.09] |
| 2.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.04] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.90, 1.89] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.34] |
| 2.5 Fluoxetine vs Reboxetine | 3 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 23.1  Comparison 23 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: endpoint score, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Moclobemide | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.66, 0.42] |
| 2 follow‐up 6‐16 weeks Show forest plot | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 23.2  Comparison 23 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: endpoint score, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Agomelatine | 3 | 1213 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.18, 0.23] |
| 2.2 Fluoxetine vs Mirtazapine | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.15, 1.29] |
| 2.3 Fluoxetine vs Moclobemide | 5 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.33] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.67, 0.57] |
| 2.5 Fluoxetine vs Reboxetine | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 24.1  Comparison 24 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.31, 2.76] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 24.2  Comparison 24 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetina vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.49] |
| 2.2 Fluoxetine vs Mirtazapine | 3 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.68] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.53] |
| 2.4 Fluoxetine vs Reboxetine | 4 | 764 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 25.1  Comparison 25 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.20, 5.68] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 25.2  Comparison 25 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ inefficacy, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.88] |
| 2.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 2.96] |
| 2.3 Fluoxetine vs Moclobemide | 5 | 609 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.65] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Fluoxetine vs Reboxetine | 3 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.47, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 26.1  Comparison 26 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks. | ||||
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 26.2  Comparison 26 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks. | ||||
| 2.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.73, 3.08] |
| 2.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.66] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 2.5 Fluoxetine vs Reboxetine | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.10, 1.61] |
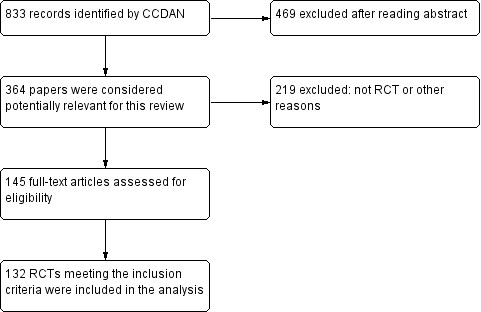
Study flow diagram, 2005 version.

Study flow diagram, 2012 version.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fluoxetine versus TCAs, Outcome 1 Failure to respond ‐ HDRS (‐50%).

Comparison 1 Fluoxetine versus TCAs, Outcome 2 End‐point score on rating scale.

Comparison 1 Fluoxetine versus TCAs, Outcome 3 Failure to complete ‐ Total.
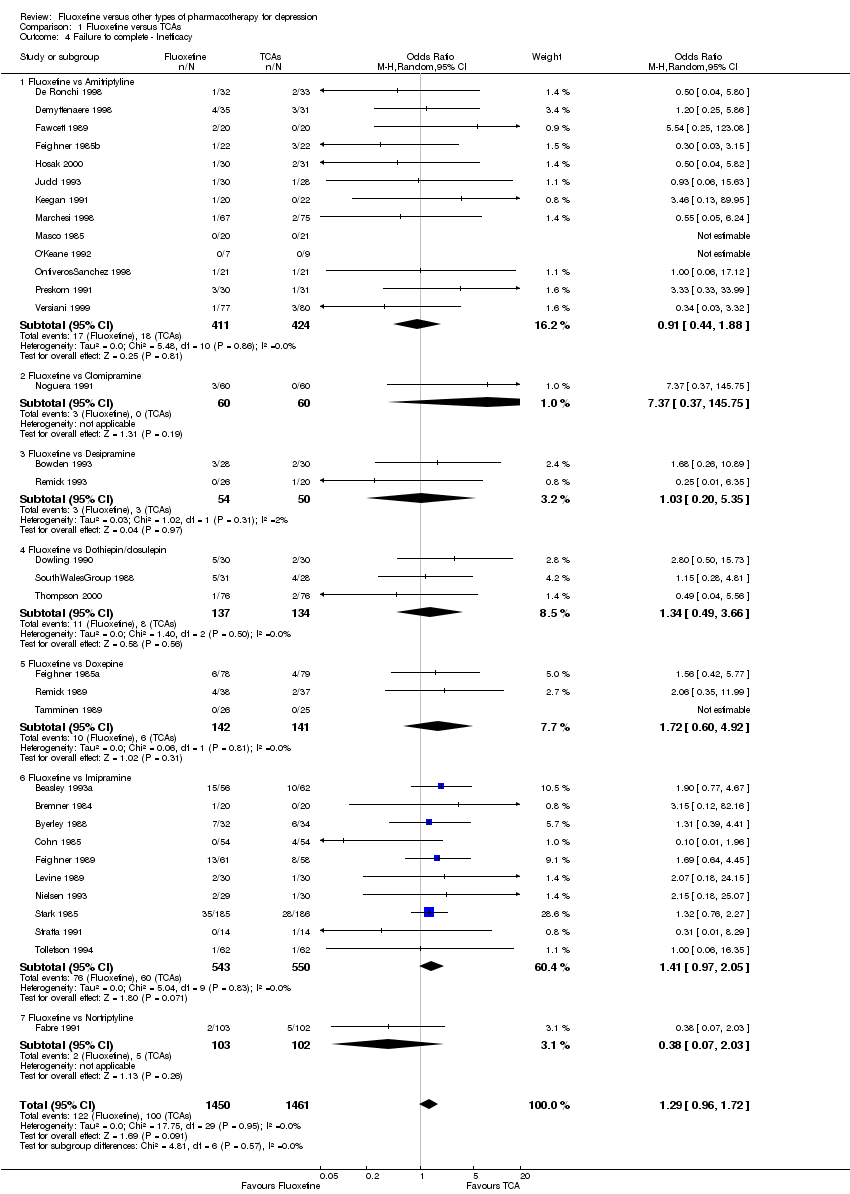
Comparison 1 Fluoxetine versus TCAs, Outcome 4 Failure to complete ‐ Inefficacy.

Comparison 1 Fluoxetine versus TCAs, Outcome 5 Failure to complete ‐ Side Effects.
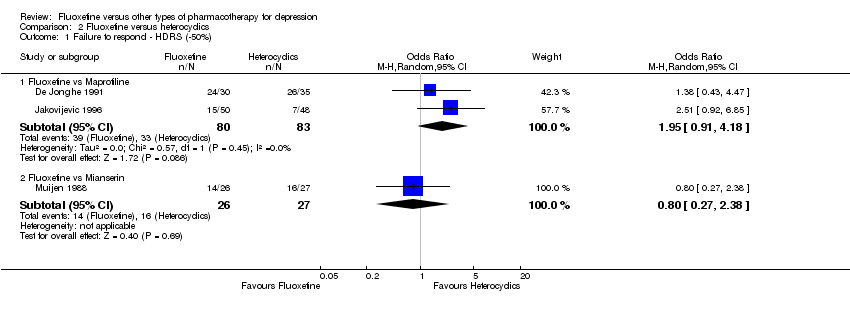
Comparison 2 Fluoxetine versus heterocyclics, Outcome 1 Failure to respond ‐ HDRS (‐50%).

Comparison 2 Fluoxetine versus heterocyclics, Outcome 2 End‐point score on rating scale.

Comparison 2 Fluoxetine versus heterocyclics, Outcome 3 Failure to complete ‐ Total.

Comparison 2 Fluoxetine versus heterocyclics, Outcome 4 Failure to complete ‐ Inefficacy.

Comparison 2 Fluoxetine versus heterocyclics, Outcome 5 Failure to complete ‐ Side Effects.

Comparison 3 Fluoxetine versus other SSRIs, Outcome 1 Failure to respond ‐ HDRS (‐50%).

Comparison 3 Fluoxetine versus other SSRIs, Outcome 2 End‐point score on rating scales.
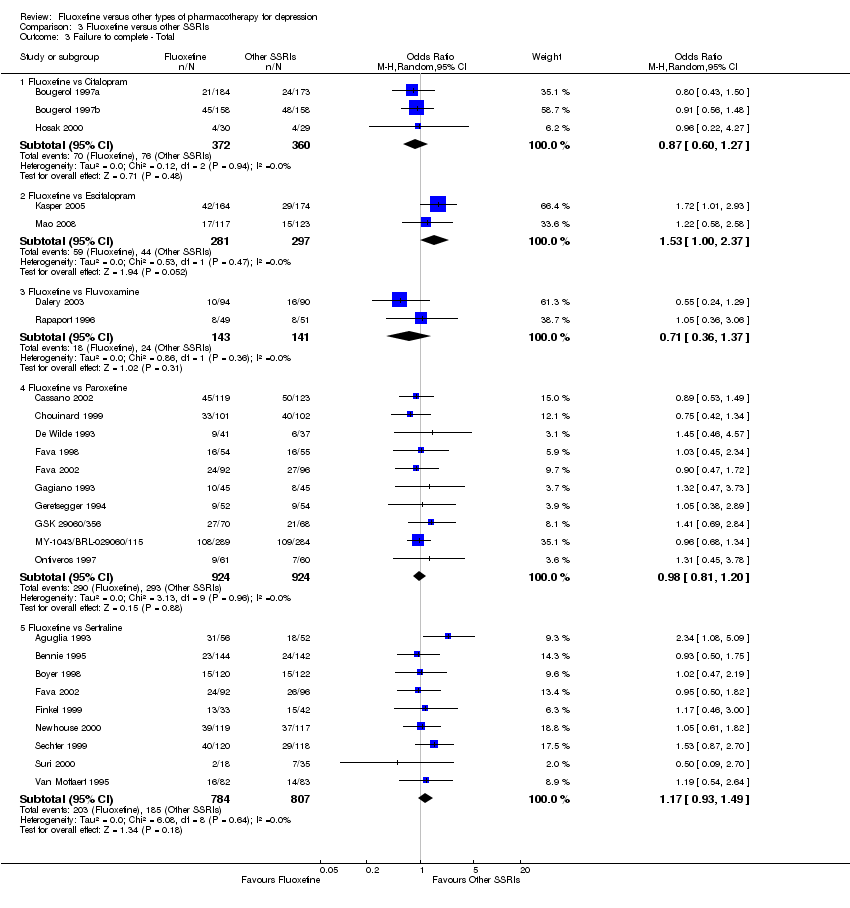
Comparison 3 Fluoxetine versus other SSRIs, Outcome 3 Failure to complete ‐ Total.

Comparison 3 Fluoxetine versus other SSRIs, Outcome 4 Failure to complete ‐ Inefficacy.
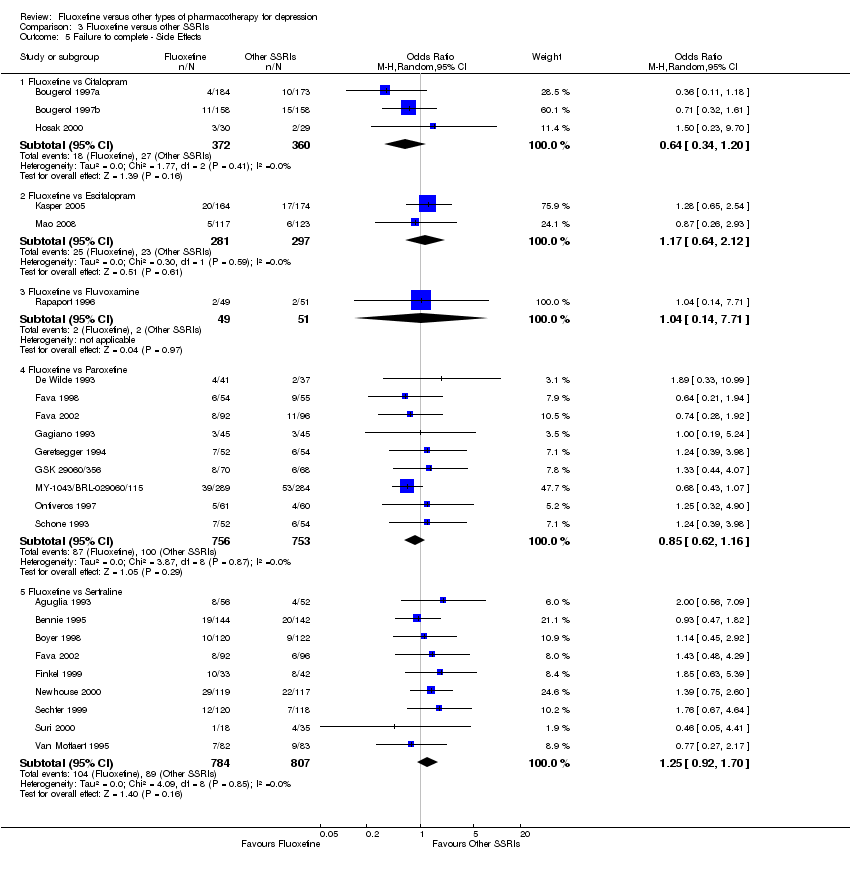
Comparison 3 Fluoxetine versus other SSRIs, Outcome 5 Failure to complete ‐ Side Effects.
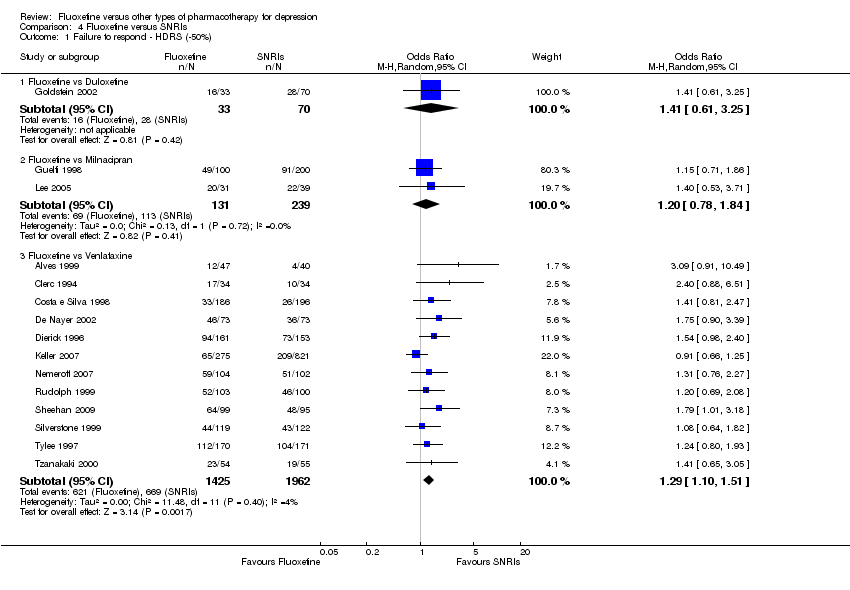
Comparison 4 Fluoxetine versus SNRIs, Outcome 1 Failure to respond ‐ HDRS (‐50%).
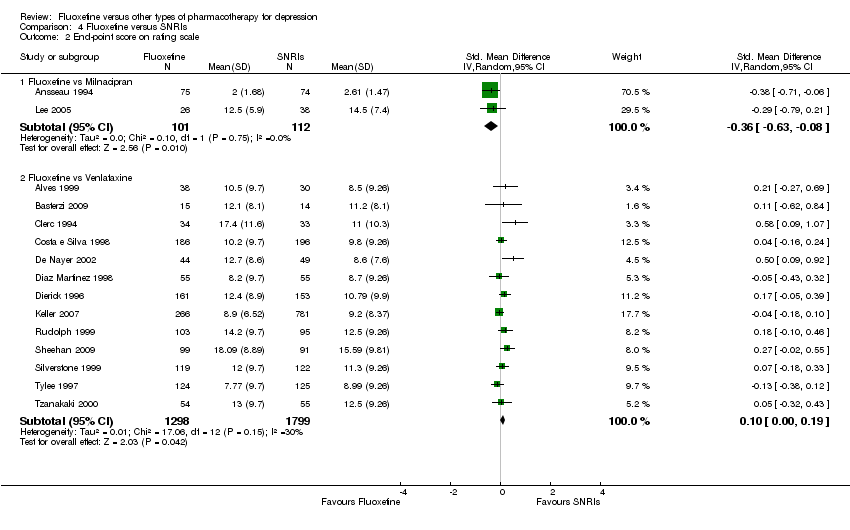
Comparison 4 Fluoxetine versus SNRIs, Outcome 2 End‐point score on rating scale.

Comparison 4 Fluoxetine versus SNRIs, Outcome 3 Failure to complete ‐ Total.
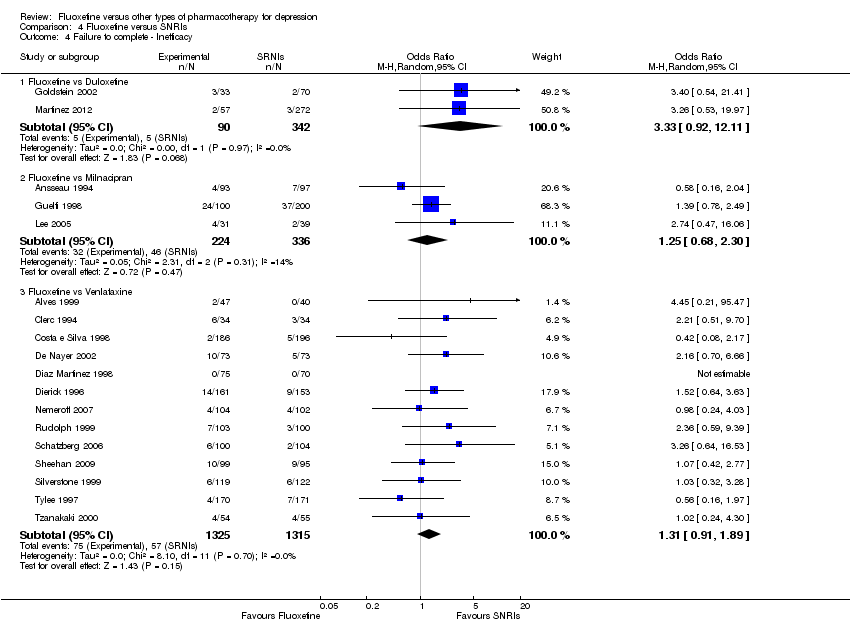
Comparison 4 Fluoxetine versus SNRIs, Outcome 4 Failure to complete ‐ Inefficacy.

Comparison 4 Fluoxetine versus SNRIs, Outcome 5 Failure to complete ‐ Side Effects.

Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 1 Failure to respond ‐ HDRS (‐50%).

Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 2 End‐point score on rating scales.

Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 3 Failure to complete ‐ Total.
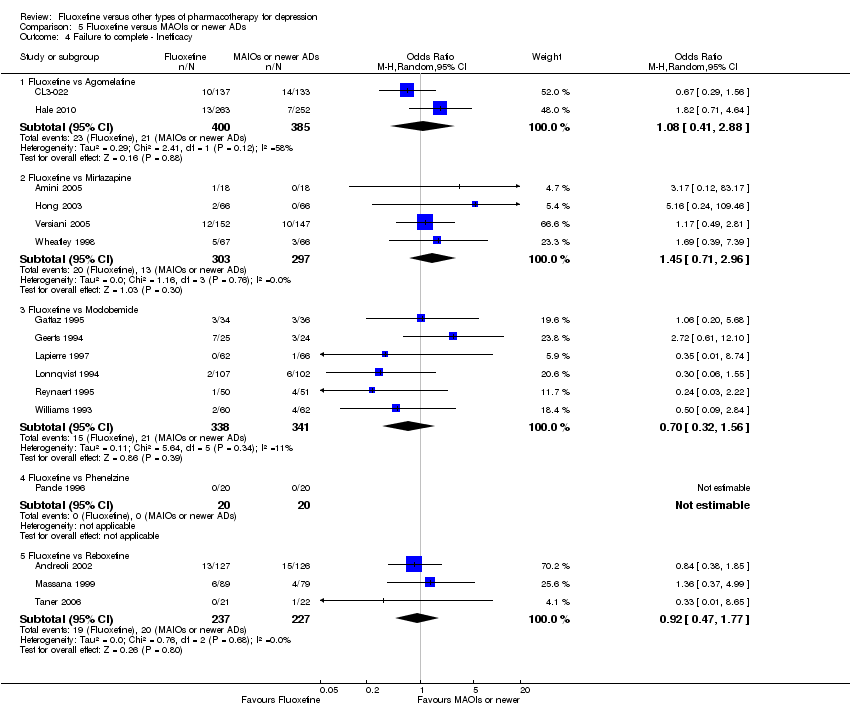
Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 4 Failure to complete ‐ Inefficacy.
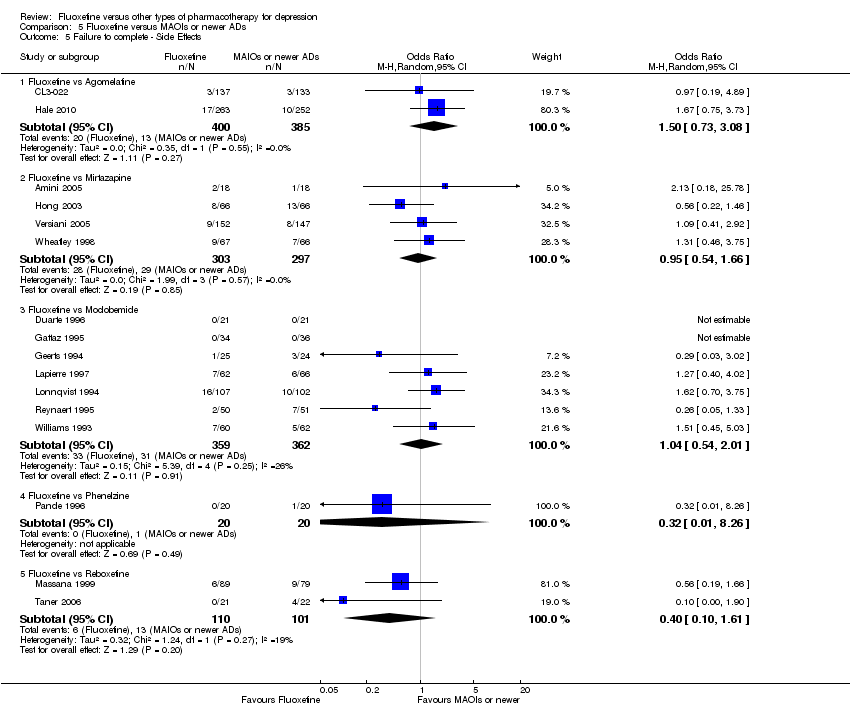
Comparison 5 Fluoxetine versus MAOIs or newer ADs, Outcome 5 Failure to complete ‐ Side Effects.
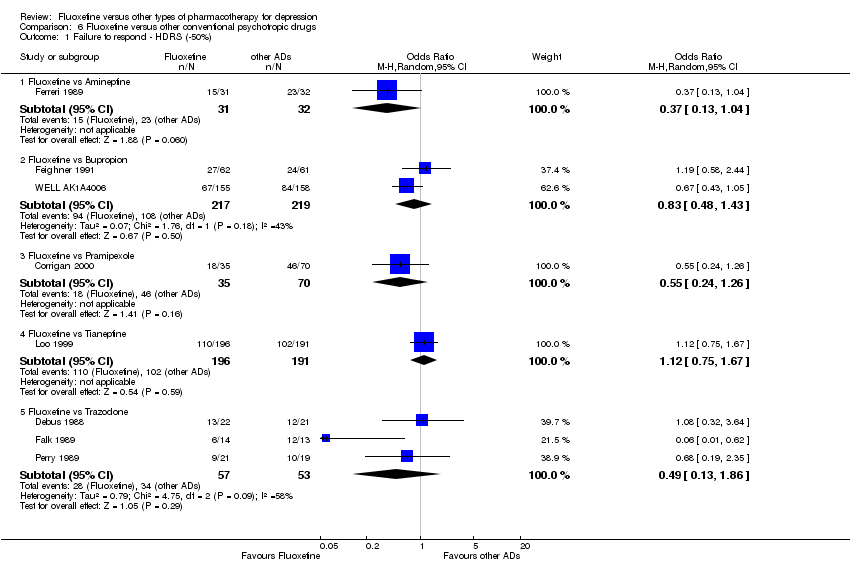
Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 1 Failure to respond ‐ HDRS (‐50%).
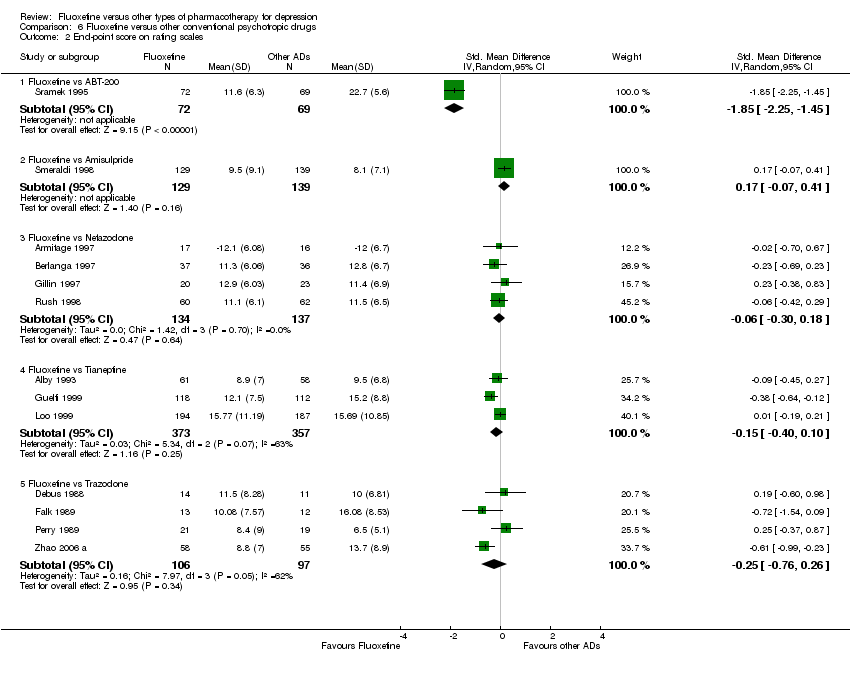
Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 2 End‐point score on rating scales.

Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 3 Failure to complete ‐ Total.

Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 4 Failure to complete ‐ Inefficacy.
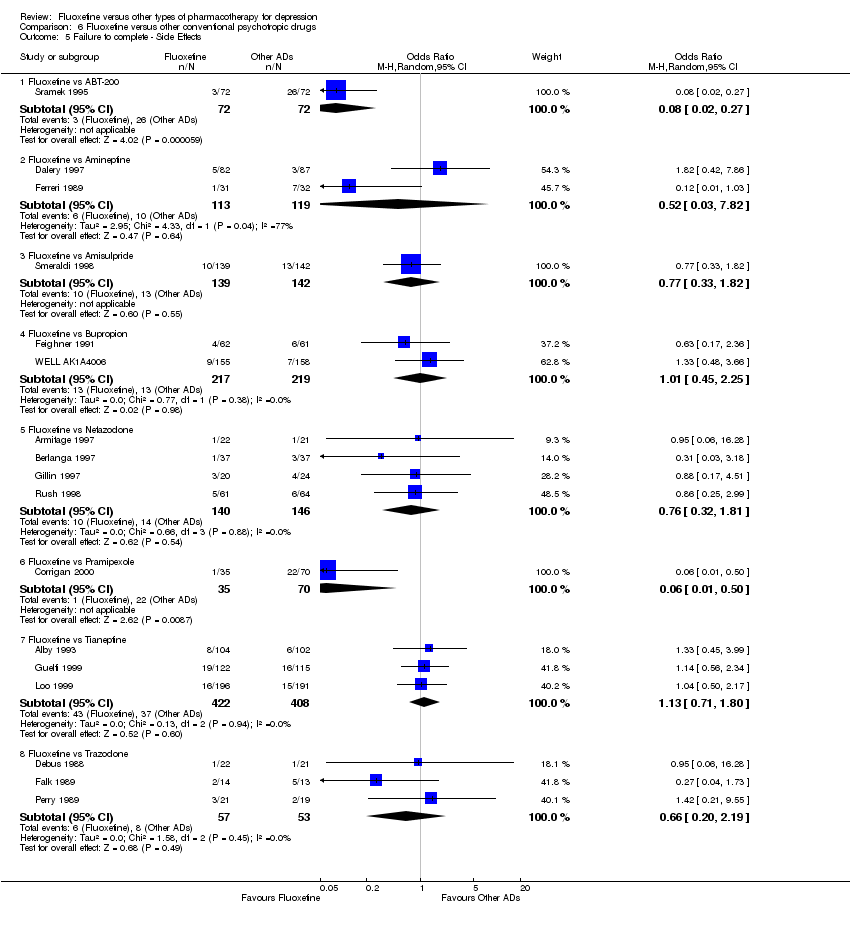
Comparison 6 Fluoxetine versus other conventional psychotropic drugs, Outcome 5 Failure to complete ‐ Side Effects.

Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 1 Failure to respond ‐ HDRS (‐50%).

Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 2 End‐point score on rating scales.

Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 3 Failure to complete ‐ Total.
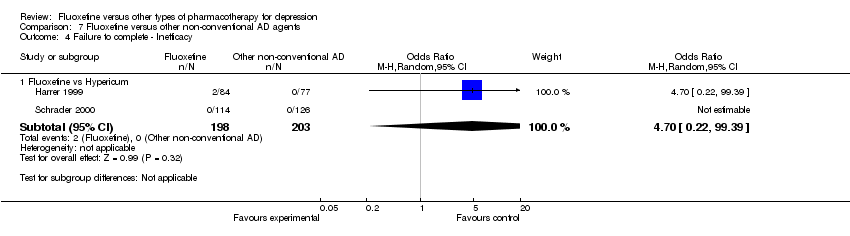
Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 4 Failure to complete ‐ Inefficacy.
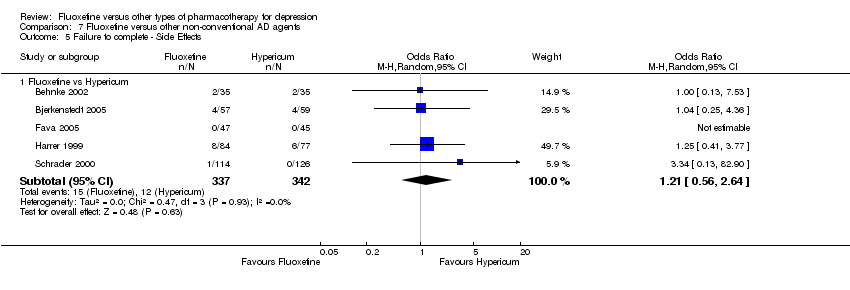
Comparison 7 Fluoxetine versus other non‐conventional AD agents, Outcome 5 Failure to complete ‐ Side Effects.

Comparison 8 Subgroup analysis for fluoxetine versus TCAs: failure to respond, Outcome 1 follow‐up <6 weeks.

Comparison 8 Subgroup analysis for fluoxetine versus TCAs: failure to respond, Outcome 2 follow‐up 6‐16 weeks.

Comparison 9 Subgroup analysis for fluoxetine versus TCAs: endpoint score, Outcome 1 follow‐up <6 weeks.

Comparison 9 Subgroup analysis for fluoxetine versus TCAs: endpoint score, Outcome 2 follow‐up 6‐16 weeks.

Comparison 9 Subgroup analysis for fluoxetine versus TCAs: endpoint score, Outcome 3 follow‐up >16 weeks.
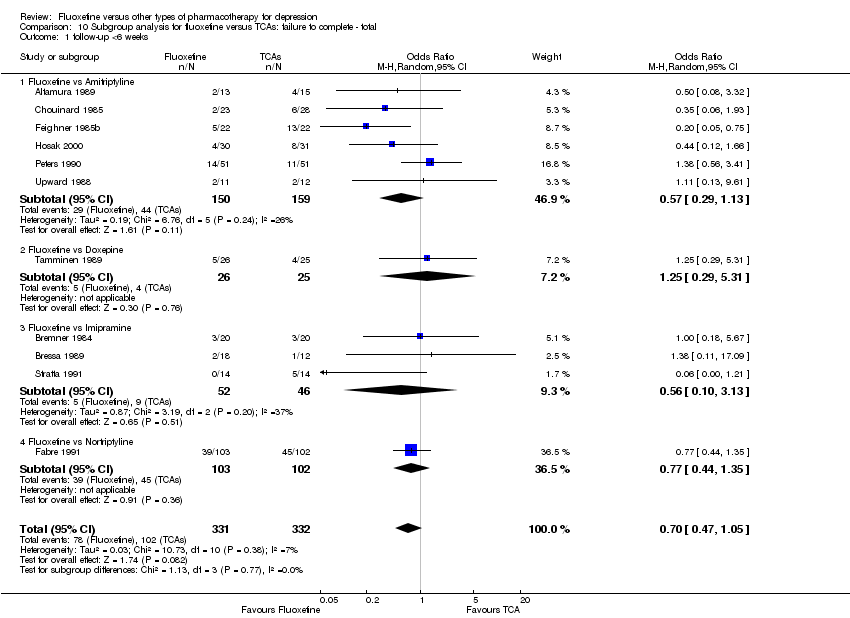
Comparison 10 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks.

Comparison 10 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks.

Comparison 11 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks.
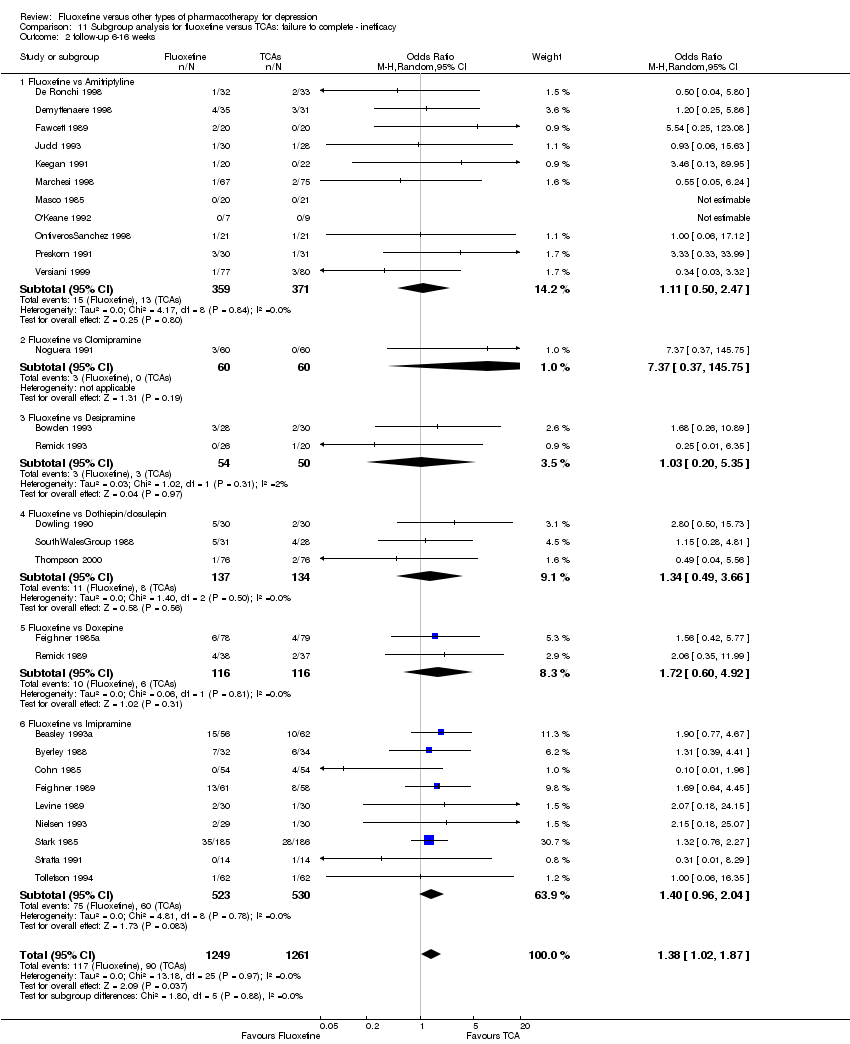
Comparison 11 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ inefficacy, Outcome 2 follow‐up 6‐16 weeks.

Comparison 12 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks.

Comparison 12 Subgroup analysis for fluoxetine versus TCAs: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks.

Comparison 13 Subgroup analysis for fluoxetine versus heterocyclics: endpoint score, Outcome 1 follow‐up <6 weeks.

Comparison 13 Subgroup analysis for fluoxetine versus heterocyclics: endpoint score, Outcome 2 follow‐up 6‐16 weeks.
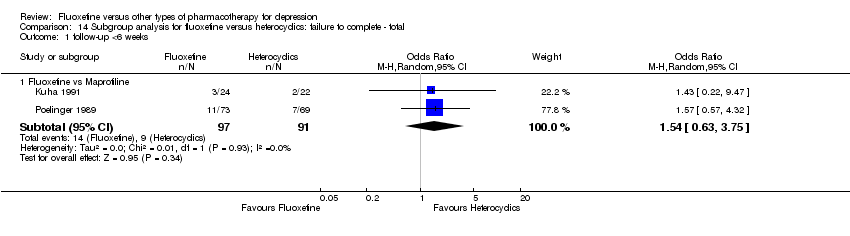
Comparison 14 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks.
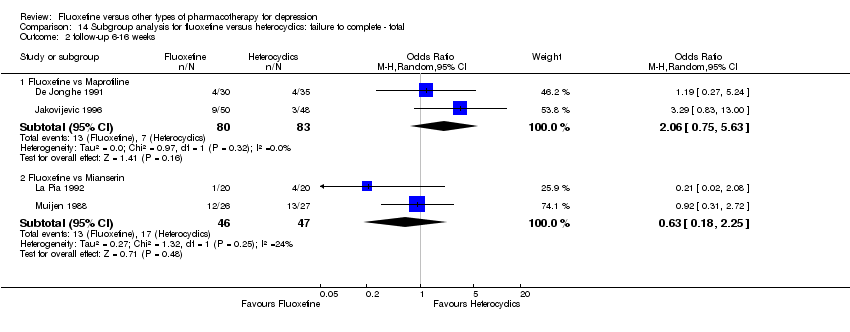
Comparison 14 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks.
Comparison 15 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks.

Comparison 15 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ inefficacy, Outcome 2 follow‐up 6‐16 weeks.

Comparison 16 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks.
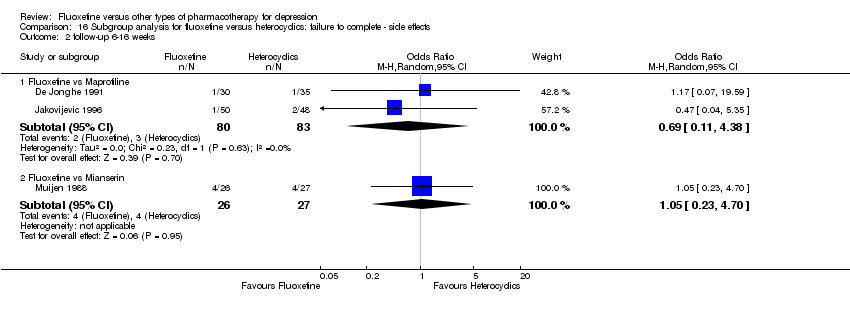
Comparison 16 Subgroup analysis for fluoxetine versus heterocyclics: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks.
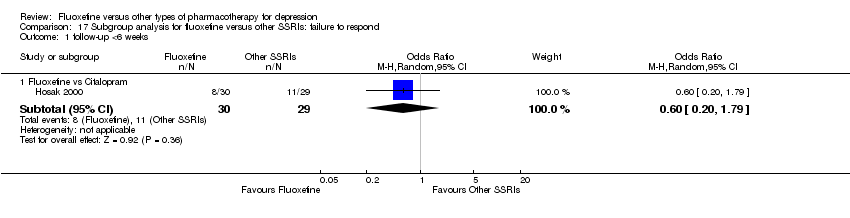
Comparison 17 Subgroup analysis for fluoxetine versus other SSRIs: failure to respond, Outcome 1 follow‐up <6 weeks.

Comparison 17 Subgroup analysis for fluoxetine versus other SSRIs: failure to respond, Outcome 2 follow‐up 6‐16 weeks.
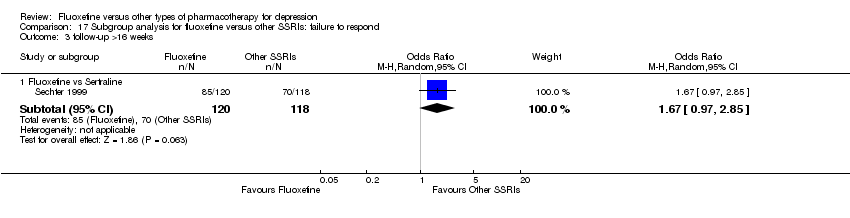
Comparison 17 Subgroup analysis for fluoxetine versus other SSRIs: failure to respond, Outcome 3 follow‐up >16 weeks.

Comparison 18 Subgroup analysis for fluoxetine versus other SSRIs: endpoint score, Outcome 1 follow‐up <6 weeks.

Comparison 18 Subgroup analysis for fluoxetine versus other SSRIs: endpoint score, Outcome 2 follow‐up >16 weeks.

Comparison 18 Subgroup analysis for fluoxetine versus other SSRIs: endpoint score, Outcome 3 follow‐up 6‐16 weeks.

Comparison 19 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks.
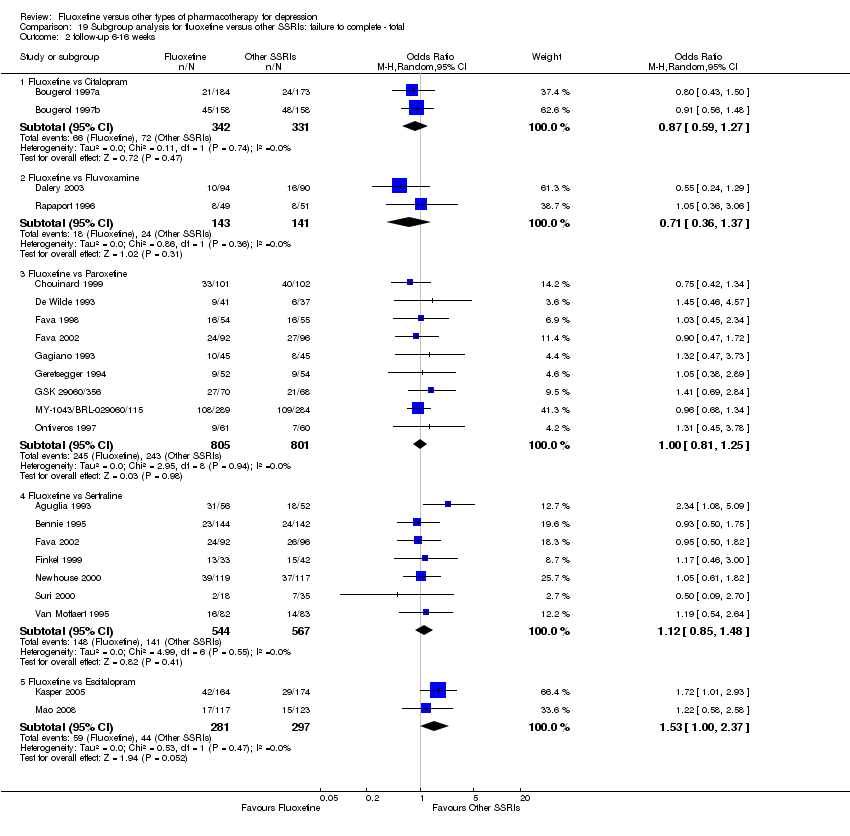
Comparison 19 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks.
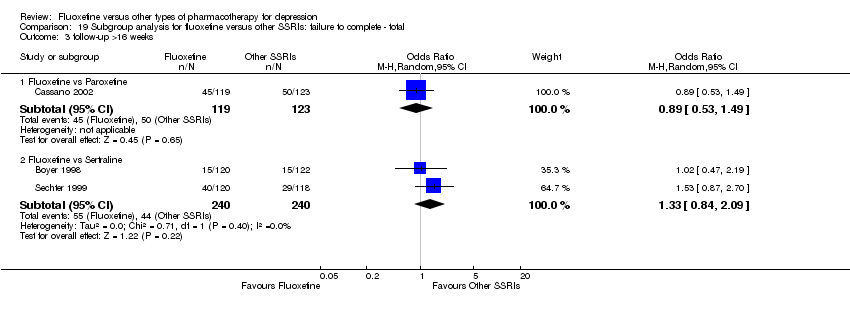
Comparison 19 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ total, Outcome 3 follow‐up >16 weeks.

Comparison 20 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks.
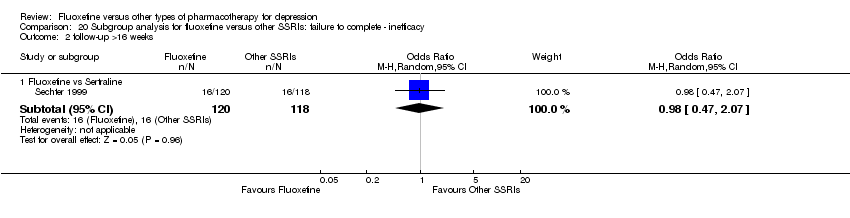
Comparison 20 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ inefficacy, Outcome 2 follow‐up >16 weeks.

Comparison 20 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ inefficacy, Outcome 3 follow‐up 6‐16 weeks.
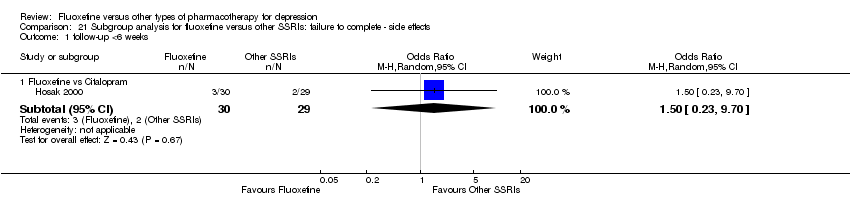
Comparison 21 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks.

Comparison 21 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks.
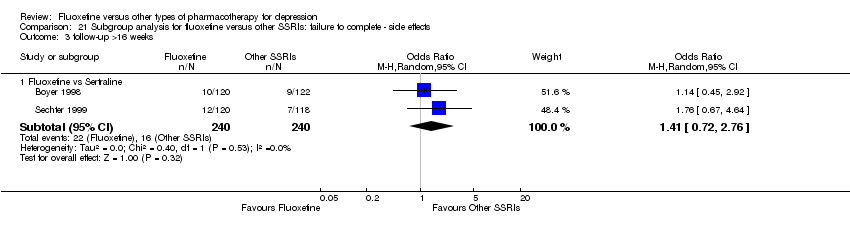
Comparison 21 Subgroup analysis for fluoxetine versus other SSRIs: failure to complete ‐ side effects, Outcome 3 follow‐up >16 weeks.
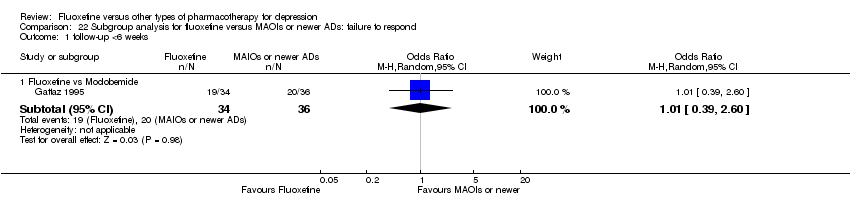
Comparison 22 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to respond, Outcome 1 follow‐up <6 weeks.

Comparison 22 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to respond, Outcome 2 follow‐up 6‐16 weeks.
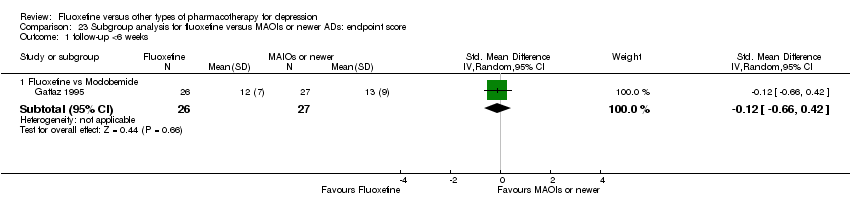
Comparison 23 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: endpoint score, Outcome 1 follow‐up <6 weeks.
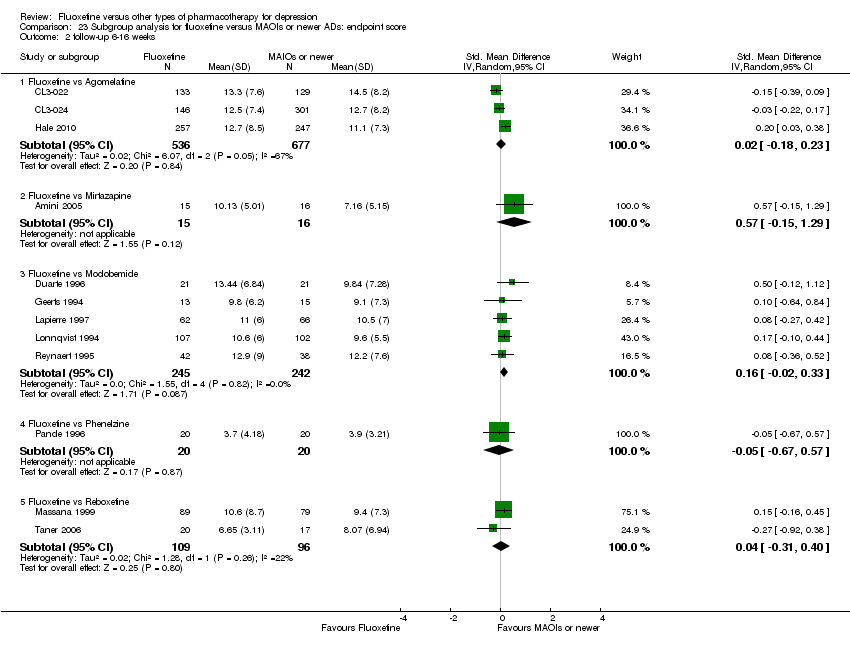
Comparison 23 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: endpoint score, Outcome 2 follow‐up 6‐16 weeks.
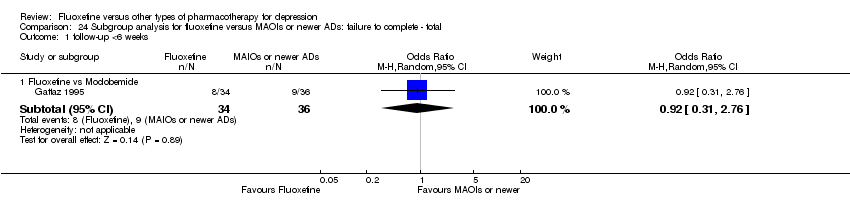
Comparison 24 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ total, Outcome 1 follow‐up <6 weeks.

Comparison 24 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ total, Outcome 2 follow‐up 6‐16 weeks.
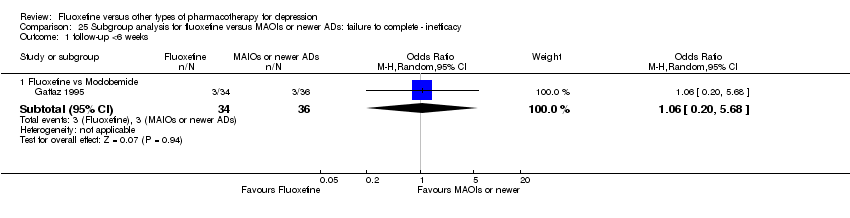
Comparison 25 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ inefficacy, Outcome 1 follow‐up <6 weeks.

Comparison 25 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ inefficacy, Outcome 2 follow‐up 6‐16 weeks.
Comparison 26 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ side effects, Outcome 1 follow‐up <6 weeks.
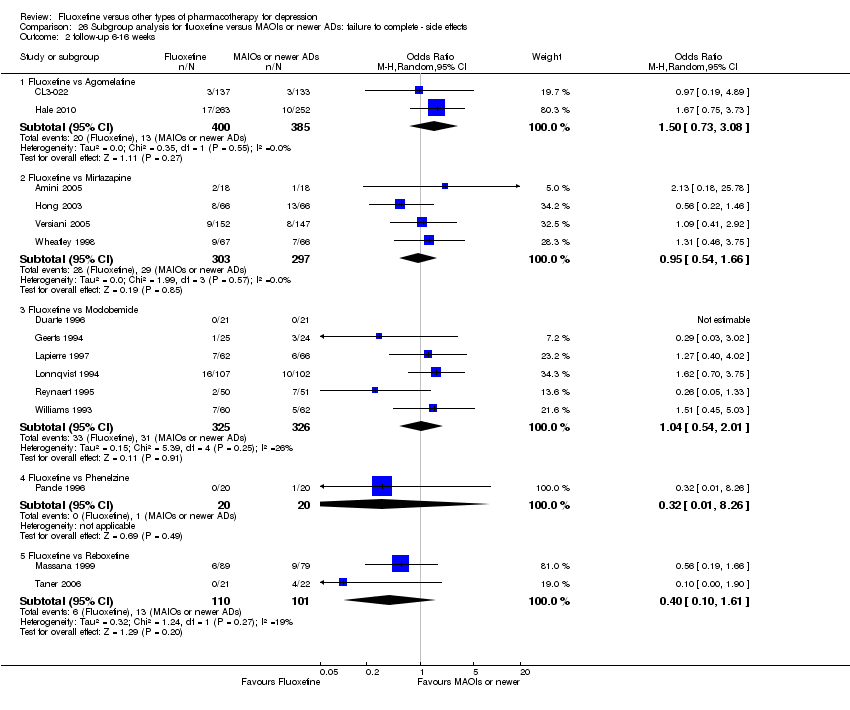
Comparison 26 Subgroup analysis for fluoxetine versus MAOIs or newer ADs: failure to complete ‐ side effects, Outcome 2 follow‐up 6‐16 weeks.
| Fluoxetine compared to TCAs | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| TCAs | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 471 per 1000 | 463 per 1000 | OR 0.97 | 2124 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 3393 | ⊕⊕⊕⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 335 per 1000 | 284 per 1000 | OR 0.79 | 4194 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 193 per 1000 | 116 per 1000 | OR 0.55 | 3647 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 68 per 1000 | 87 per 1000 | OR 1.29 | 2911 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in study design: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to ABT 200 | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| ABT 200 | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 0 per 1000 | Not estimable | 0 (0) | |||
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 141 | ⊕⊕⊝⊝ | This corresponds to a large effect according | ||
| Failure to complete ‐ total ‐ | 528 per 1000 | 167 per 1000 | OR 0.18 | 144 | ⊕⊕⊝⊝ | |
| Failure to complete ‐ inefficacy ‐ | 56 per 1000 | 14 per 1000 | OR 0.24 | 144 | ⊕⊕⊝⊝ | |
| Failure to complete ‐ side effects ‐ | 361 per 1000 | 43 per 1000 | OR 0.08 | 144 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in study design: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. Only one study included in the analysis. | ||||||
| Fluoxetine compared to agomelatine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Agomelatine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 282 per 1000 | 361 per 1000 | OR 1.44 | 515 | ⊕⊕⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 1213 | ⊕⊕⊕⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 135 per 1000 | 170 per 1000 | OR 1.31 | 785 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 55 per 1000 | 59 per 1000 | OR 1.08 | 785 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 34 per 1000 | 50 per 1000 | OR 1.51 | 785 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in this analysis. | ||||||
| Fluoxetine compared to amineptine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Amineptine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 719 per 1000 | 486 per 1000 | OR 0.37 | 63 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 0 (0) | No data available on this outcome | |||
| Failure to complete ‐ total ‐ | 210 per 1000 | 140 per 1000 | OR 0.61 | 232 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 94 per 1000 | 97 per 1000 | OR 1.04 | 63 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ (Copy) | 84 per 1000 | 46 per 1000 | OR 0.52 | 232 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to amisulpride | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Amisulpride | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 0 per 1000 | Not estimable | 0 (0) | |||
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 268 | ⊕⊕⊝⊝ | This corresponds to a very small effect according | ||
| Failure to complete ‐ total ‐ | 225 per 1000 | 288 per 1000 | OR 1.39 | 281 | ⊕⊕⊝⊝ | |
| Failure to complete ‐ inefficacy ‐ | 56 per 1000 | 65 per 1000 | OR 1.16 | 281 | ⊕⊕⊝⊝ | |
| Failure to complete ‐ side effects ‐ | 92 per 1000 | 72 per 1000 | OR 0.77 | 281 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. Only one study included in the analysis. | ||||||
| Fluoxetine compared to bupropion | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Bupropion | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 493 per 1000 | 447 per 1000 | OR 0.83 | 436 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 0 (0) | No data available on this outcome | |||
| Failure to complete ‐ total ‐ | 356 per 1000 | 356 per 1000 | OR 1.00 | 436 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 23 per 1000 | 0 per 1000 | OR 1.16 | 436 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 59 per 1000 | 60 per 1000 | OR 1.01 | 436 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to citalopram | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Citalopram | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 379 per 1000 | 268 per 1000 | OR 0.60 | 59 | ⊕⊝⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 661 | ⊕⊕⊕⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 211 per 1000 | 189 per 1000 | OR 0.87 | 732 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 75 per 1000 | 66 per 1000 | OR 0.87 | 732 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 75 per 1000 | 49 per 1000 | OR 0.64 | 732 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in the analysis and less than 100 patients. | ||||||
| Fluoxetine compared to Crocus sativus | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Crocus sativus | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 250 per 1000 | 150 per 1000 | OR 0.53 | 40 | ⊕⊝⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 0 (0) | No data available on this outcome | |||
| Failure to complete ‐ total ‐ | 50 per 1000 | 50 per 1000 | OR 1.00 | 40 | ⊕⊝⊝⊝ | |
| Failure to complete ‐ inefficacy ‐ | 0 per 1000 | Not estimable | 0 (0) | |||
| Failure to complete ‐ side effects ‐ | 0 per 1000 | Not estimable | 0 (0) | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. Only one study included in the analysis and less than 50 patients. | ||||||
| Fluoxetine compared to for duloxetine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluoxetine | ||||||
| Failure to respond (reduction ≥ 50% on HDRS) | 400 per 1000 | 485 per 1000 | OR 1.41 | 103 | ⊕⊕⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 0 (0) | No data available on this outcome | |||
| Failure to complete ‐ total ‐ | 281 per 1000 | 260 per 1000 | OR 0.90 | 532 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 15 per 1000 | 47 per 1000 | OR 3.33 | 432 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 66 per 1000 | 19 per 1000 | OR 0.28 | 532 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in the analysis. | ||||||
| Fluoxetine compared to escitalopram | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Escitalopram | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 236 per 1000 | 239 per 1000 | OR 1.02 | 240 | ⊕⊕⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 231 | ⊕⊕⊝⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 148 per 1000 | 210 per 1000 | OR 1.53 | 578 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 13 per 1000 | 23 per 1000 | OR 1.74 | 578 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 77 per 1000 | 89 per 1000 | OR 1.17 | 578 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in the analysis. | ||||||
| Fluoxetine compared to fluvoxamine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fluvoxamine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 605 per 1000 | 592 per 1000 | OR 0.95 | 177 | ⊕⊕⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 0 (0) | No data available on this outcome | |||
| Failure to complete ‐ total ‐ | 170 per 1000 | 936 per 1000 | OR 071 | 284 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 0 per 1000 | Not estimable | 0 (0) | |||
| Failure to complete ‐ side effects ‐ | 39 per 1000 | 41 per 1000 | OR 1.04 | 100 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in the analysis. | ||||||
| Fluoxetine compared to hypericum | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hypericum | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 490 per 1000 | 485 per 1000 | OR 0.98 | 717 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 648 | ⊕⊕⊕⊝ | This corresponds to a very small effect according | ||
| Failure to complete ‐ total ‐ | 129 per 1000 | 133 per 1000 | OR 1.04 | 679 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | OR 4.70 | 401 | ⊕⊕⊕⊝ | |||
| Failure to complete ‐ side effects ‐ | 35 per 1000 | 42 per 1000 | OR 1.21 | 679 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to maprotiline | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Maprotiline | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 398 per 1000 | 563 per 1000 | OR 1.95 | 163 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 433 | ⊕⊕⊕⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 92 per 1000 | 151 per 1000 | OR 1.75 | 351 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 67 per 1000 | 36 per 1000 | OR 0.53 | 209 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 19 per 1000 | 47 per 1000 | OR 2.54 | 209 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to mianserin | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mianserin | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 593 per 1000 | 538 per 1000 | OR 0.80 | 53 | ⊕⊝⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 128 | ⊕⊕⊕⊝ | This corresponds to a small effect according | ||
| Failure to complete ‐ total ‐ | 362 per 1000 | 263 per 1000 | OR 0.63 | 93 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 74 per 1000 | 154 per 1000 | OR 2.27 | 53 | ⊕⊝⊝⊝ | |
| Failure to complete ‐ side effects ‐ | 148 per 1000 | 154 per 1000 | OR 1.05 | 53 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in the analysis and less than 100 patients. | ||||||
| Fluoxetine compared to milnacipran | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Milnacipran | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 473 per 1000 | 518 per 1000 | OR 1.20 | 370 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 213 | ⊕⊕⊕⊝ | This corresponds to a small effect according | ||
| Failure to complete ‐ total ‐ | 411 per 1000 | 406 per 1000 | OR 0.98 | 560 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 137 per 1000 | 165 per 1000 | OR 1.25 | 560 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 71 per 1000 | 103 per 1000 | OR 1.50 | 560 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to mirtazapine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mirtazapine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 354 per 1000 | 444 per 1000 | OR 1.46 | 600 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 31 | ⊕⊝⊝⊝ | This corresponds to a medium effect according | ||
| Failure to complete ‐ total ‐ | 327 per 1000 | 304 per 1000 | OR 0.90 | 301 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 44 per 1000 | 62 per 1000 | OR 1.45 | 600 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 98 per 1000 | 93 per 1000 | OR 0.95 | 600 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. 2 Only one study included in the analysis and less than 100 patients. | ||||||
| Fluoxetine compared to moclobemide | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Moclobemide | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 436 per 1000 | 496 per 1000 | OR 1.27 | 721 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 540 | ⊕⊕⊕⊝ | This corresponds to a very small effect according | ||
| Failure to complete ‐ total ‐ | 207 per 1000 | 209 per 1000 | OR 1.01 | 721 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 62 per 1000 | 44 per 1000 | OR 0.70 | 679 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 86 per 1000 | 91 per 1000 | OR 1.07 | 721 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to nefazodone | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Nefazodone | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 0 per 1000 | Not estimable | 0 (0) | |||
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 271 | ⊕⊕⊕⊝ | This effects approaches zero | ||
| Failure to complete ‐ total ‐ | 220 per 1000 | 132 per 1000 | OR 0.54 | 161 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 24 per 1000 | 17 per 1000 | OR 0.71 | 161 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 96 per 1000 | 75 per 1000 | OR 0.76 | 286 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to paroxetine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Paroxetine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 426 per 1000 | 477 per 1000 | OR 1.23 | 1574 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 2061 | ⊕⊕⊕⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 317 per 1000 | 313 per 1000 | OR 0.98 | 1848 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 52 per 1000 | 39 per 1000 | OR 0.75 | 1005 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 133 per 1000 | 115 per 1000 | OR 0.85 | 1509 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to phenelzine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenelzine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 150 per 1000 | 200 per 1000 | OR 1.42 | 40 | ⊕⊝⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 40 | ⊕⊝⊝⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 100 per 1000 | 20 per 1000 | OR 0.18 | 40 | ⊕⊝⊝⊝ | |
| Failure to complete ‐ inefficacy ‐ | 0 per 1000 | Not estimable | 0 (0) | |||
| Failure to complete ‐ side effects ‐ | 50 per 1000 | 17 per 1000 | OR 0.32 | 40 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. Only one study included in the analysis and less than 50 patients. | ||||||
| Fluoxetine compared to pramipexole | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pramipexole | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 657 per 1000 | 513 per 1000 | OR 0.55 | 105 | ⊕⊕⊝⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 0 (0) | No data available on this outcome | |||
| Failure to complete ‐ total ‐ | 443 per 1000 | 87 per 1000 | OR 0.12 | 105 | ⊕⊕⊝⊝ | |
| Failure to complete ‐ inefficacy ‐ | 57 per 1000 | 29 per 1000 | OR 0.49 | 105 | ⊕⊕⊝⊝ | |
| Failure to complete ‐ side effects ‐ | 314 per 1000 | 27 per 1000 | OR 0.06 | 105 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. Only one study included in the analysis. | ||||||
| Fluoxetine compared to reboxetine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Reboxetine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 566 per 1000 | 501 per 1000 | OR 0.77 | 721 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 205 | ⊕⊕⊕⊝ | This effect approaches zero | ||
| Failure to complete ‐ total ‐ | 361 per 1000 | 253 per 1000 | OR 0.60 | 764 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 88 per 1000 | 82 per 1000 | OR 0.92 | 464 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 129 per 1000 | 57 per 1000 | OR 0.41 | 211 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to sertraline | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sertraline | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 416 per 1000 | 494 per 1000 | OR 1.37 | 1188 (6 studies) | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 1160 | ⊕⊕⊕⊝ | This corresponds to a very small effect according | ||
| Failure to complete ‐ total ‐ | 229 per 1000 | 258 per 1000 | OR 1.17 | 1591 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 70 per 1000 | 76 per 1000 | OR 1.09 | 1056 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 110 per 1000 | 134 per 1000 | OR 1.25 | 1591 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to tianeptine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tianeptine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 534 per 1000 | 562 per 1000 | OR 1.12 | 387 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 730 | ⊕⊕⊕⊝ | This corresponds to a very small effect according | ||
| Failure to complete ‐ total ‐ | 225 per 1000 | 218 per 1000 | OR 0.96 | 830 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 47 per 1000 | 39 per 1000 | OR 0.82 | 830 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 91 per 1000 | 101 per 1000 | OR 1.13 | 830 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to trazodone | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Trazodone | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 642 per 1000 | 467 per 1000 | OR 0.49 | 110 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 203 | ⊕⊕⊕⊝ | This corresponds to a small effect according | ||
| Failure to complete ‐ total ‐ | 250 per 1000 | 145 per 1000 | OR 0.51 | 230 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 147 per 1000 | 38 per 1000 | OR 0.23 | 70 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 151 per 1000 | 105 per 1000 | OR 0.66 | 110 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Fluoxetine compared to venlafaxine | ||||||
| Patient or population: patients with depression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Venlafaxine | Fluoxetine | |||||
| Failure to respond (reduction ≥ 50% on HDRS) | 341 per 1000 | 400 per 1000 | OR 1.29 | 3387 | ⊕⊕⊕⊝ | |
| Endpoint score (HDRS or MADRS) | The mean endpoint score in the intervention groups was | 3097 | ⊕⊕⊕⊝ | This corresponds to a very small effect according | ||
| Failure to complete ‐ total ‐ | 256 per 1000 | 234 per 1000 | OR 0.89 | 2683 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ inefficacy ‐ | 43 per 1000 | 56 per 1000 | OR 1.31 | 2640 | ⊕⊕⊕⊝ | |
| Failure to complete ‐ side effects ‐ | 116 per 1000 | 87 per 1000 | OR 0.72 | 2640 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in studies designs: no details on randomisation procedures and allocation concealment. Blinding stated but not tested. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 24 | 2124 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 1.1 Fluoxetine vs Amitriptyline | 11 | 777 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.68, 1.28] |
| 1.2 Fluoxetine vs Clomipramine | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.45] |
| 1.3 Fluoxetine vs Desipramine | 2 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.56, 5.15] |
| 1.4 Fluoxetine vs Dothiepin/dosulepin | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.08, 4.20] |
| 1.5 Fluoxetine vs Doxepine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| 1.6 Fluoxetine vs Imipramine | 5 | 761 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.41, 1.35] |
| 1.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.55, 1.78] |
| 1.8 Fluoxetine vs Trimipramine | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.56, 7.45] |
| 2 End‐point score on rating scale Show forest plot | 50 | 3393 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.07, 0.14] |
| 2.1 Fluoxetine vs Amiptriptyline | 19 | 1023 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.09, 0.29] |
| 2.2 Fluoxetine vs Clomipramine | 5 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.10] |
| 2.3 Fluoxetine vs Desipramine | 4 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.32, 0.86] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 4 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.27, 0.59] |
| 2.5 Fluoxetine vs Imipramine | 12 | 1063 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.16, 0.42] |
| 2.7 Fluoxetine vs Nomifensine | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.12, 0.38] |
| 2.8 Fluoxetine vs Nortriptyline | 2 | 251 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.20, 0.24] |
| 2.9 Fluoxetine vs Trimipramine | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.10, 0.92] |
| 3 Failure to complete ‐ Total Show forest plot | 49 | 4194 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.65, 0.96] |
| 3.1 Fluoxetine vs Amitriptyline | 18 | 1089 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.85] |
| 3.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.14] |
| 3.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 3.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.92, 2.69] |
| 3.5 Fluoxetine vs Doxepine | 4 | 323 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.49, 1.32] |
| 3.6 Fluoxetine vs Imipramine | 12 | 1225 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.21] |
| 3.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 3.8 Fluoxetine vs Nomifensine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 6.33 [0.67, 60.16] |
| 3.9 Fluoxetine vs Nortriptyline | 3 | 448 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.36, 1.47] |
| 3.10 Fluoxetine vs Trimipramine | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 2.0 [0.41, 9.78] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 33 | 2911 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.96, 1.72] |
| 4.1 Fluoxetine vs Amitriptyline | 13 | 835 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.44, 1.88] |
| 4.2 Fluoxetine vs Clomipramine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [0.37, 145.75] |
| 4.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.35] |
| 4.4 Fluoxetine vs Dothiepin/dosulepin | 3 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.49, 3.66] |
| 4.5 Fluoxetine vs Doxepine | 3 | 283 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.60, 4.92] |
| 4.6 Fluoxetine vs Imipramine | 10 | 1093 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.97, 2.05] |
| 4.7 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.03] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 40 | 3647 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.40, 0.75] |
| 5.1 Fluoxetine vs Amitriptyline | 16 | 1038 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.71] |
| 5.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.79] |
| 5.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.68] |
| 5.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.59, 7.16] |
| 5.5 Fluoxetine vs Doxepine | 3 | 283 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.44, 1.53] |
| 5.6 Fluoxetine vs Imipramine | 10 | 1093 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.86] |
| 5.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.38] |
| 5.8 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 1.95 [0.91, 4.18] |
| 1.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.27, 2.38] |
| 2 End‐point score on rating scale Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Maprotiline | 5 | 433 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.15, 0.23] |
| 2.2 Fluoxetine vs Mianserin | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.38, 1.23] |
| 3 Failure to complete ‐ Total Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Maprotiline | 4 | 351 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [0.90, 3.41] |
| 3.2 Fluoxetine vs Mianserin | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.18, 2.25] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Maprotiline | 3 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 2.54 [0.33, 19.19] |
| 4.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 2.27 [0.38, 13.63] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Maprotiline | 3 | 209 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.15, 1.93] |
| 5.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.20, 1.79] |
| 1.2 Fluoxetine vs Escitalopram | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.56, 1.85] |
| 1.3 Fluoxetine vs Fluvoxamine | 1 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.74] |
| 1.4 Fluoxetine vs Paroxetine | 9 | 1574 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.93, 1.65] |
| 1.5 Fluoxetine vs Sertraline | 6 | 1188 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [1.08, 1.74] |
| 2 End‐point score on rating scales Show forest plot | 21 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Citalopram | 3 | 661 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.10, 0.21] |
| 2.2 Fluoxetine vs Escitalopram | 1 | 231 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.19, 0.33] |
| 2.3 Fluoxetine vs Paroxetine | 11 | 2061 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.26, 0.24] |
| 2.4 Fluoxetine vs Sertraline | 7 | 1160 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.20] |
| 3 Failure to complete ‐ Total Show forest plot | 25 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Citalopram | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.27] |
| 3.2 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.00, 2.37] |
| 3.3 Fluoxetine vs Fluvoxamine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.37] |
| 3.4 Fluoxetine vs Paroxetine | 10 | 1848 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.20] |
| 3.5 Fluoxetine vs Sertraline | 9 | 1591 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.93, 1.49] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 13 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Citalopram | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.48, 1.56] |
| 4.2 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.46, 6.53] |
| 4.3 Fluoxetine vs Paroxetine | 4 | 1005 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 4.4 Fluoxetine vs Sertraline | 5 | 1056 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.68, 1.77] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Citalopram | 3 | 732 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.20] |
| 5.2 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.12] |
| 5.3 Fluoxetine vs Fluvoxamine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 7.71] |
| 5.4 Fluoxetine vs Paroxetine | 9 | 1509 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.16] |
| 5.5 Fluoxetine vs Sertraline | 9 | 1591 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.92, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Duloxetine | 1 | 103 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.61, 3.25] |
| 1.2 Fluoxetine vs Milnacipran | 2 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.84] |
| 1.3 Fluoxetine vs Venlafaxine | 12 | 3387 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [1.10, 1.51] |
| 2 End‐point score on rating scale Show forest plot | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Milnacipran | 2 | 213 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.63, ‐0.08] |
| 2.2 Fluoxetine vs Venlafaxine | 13 | 3097 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [0.00, 0.19] |
| 3 Failure to complete ‐ Total Show forest plot | 19 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Duloxetine | 2 | 532 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.53, 1.52] |
| 3.2 Fluoxetine vs Milnacipran | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.68, 1.42] |
| 3.3 Fluoxetine vs Venlafaxine | 14 | 2683 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.74, 1.06] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Duloxetine | 2 | 432 | Odds Ratio (M‐H, Random, 95% CI) | 3.33 [0.92, 12.11] |
| 4.2 Fluoxetine vs Milnacipran | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.68, 2.30] |
| 4.3 Fluoxetine vs Venlafaxine | 13 | 2640 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.91, 1.89] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 18 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Duloxetine | 2 | 532 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.23] |
| 5.2 Fluoxetine vs Milnacipran | 3 | 560 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.81, 2.76] |
| 5.3 Fluoxetine vs Venlafaxine | 13 | 2640 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.56, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Agomelatine | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.99, 2.09] |
| 1.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.04] |
| 1.3 Fluoxetine vs Moclobemide | 7 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.92, 1.75] |
| 1.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.34] |
| 1.5 Fluoxetine vs Reboxetine | 3 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.10] |
| 2 End‐point score on rating scales Show forest plot | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Agomelatine | 3 | 1213 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.18, 0.23] |
| 2.2 Fluoxetine vs Mirtazapine | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.15, 1.29] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 540 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.30] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.67, 0.57] |
| 2.5 Fluoxetine vs Reboxetine | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.40] |
| 3 Failure to complete ‐ Total Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.49] |
| 3.2 Fluoxetine vs Mirtazapine | 3 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.68] |
| 3.3 Fluoxetine vs Moclobemide | 7 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.70, 1.47] |
| 3.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.01] |
| 3.5 Fluoxetine vs Reboxetine | 4 | 764 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.88] |
| 4.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 2.96] |
| 4.3 Fluoxetine vs Moclobemide | 6 | 679 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.32, 1.56] |
| 4.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Fluoxetine vs Reboxetine | 3 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.47, 1.77] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 16 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.73, 3.08] |
| 5.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.66] |
| 5.3 Fluoxetine vs Moclobemide | 7 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 5.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 5.5 Fluoxetine vs Reboxetine | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.10, 1.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Amineptine | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.04] |
| 1.2 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
| 1.3 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.24, 1.26] |
| 1.4 Fluoxetine vs Tianeptine | 1 | 387 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.75, 1.67] |
| 1.5 Fluoxetine vs Trazodone | 3 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.13, 1.86] |
| 2 End‐point score on rating scales Show forest plot | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs ABT‐200 | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐2.25, ‐1.45] |
| 2.2 Fluoxetine vs Amisulpride | 1 | 268 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.07, 0.41] |
| 2.3 Fluoxetine vs Nefazodone | 4 | 271 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.30, 0.18] |
| 2.4 Fluoxetine vs Tianeptine | 3 | 730 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 2.5 Fluoxetine vs Trazodone | 4 | 203 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.76, 0.26] |
| 3 Failure to complete ‐ Total Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs ABT‐200 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.08, 0.39] |
| 3.2 Fluoxetine vs Amineptine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.17, 2.21] |
| 3.3 Fluoxetine vs Amisulpride | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.38] |
| 3.4 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.67, 1.48] |
| 3.5 Fluoxetine vs Nefazodone | 3 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.31] |
| 3.6 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.42] |
| 3.7 Fluoxetine vs Tianeptine | 3 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.69, 1.33] |
| 3.8 Fluoxetine vs Trazodone | 4 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.13] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs ABT‐200 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 2.20] |
| 4.2 Fluoxetine vs Amineptine | 1 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.19, 5.57] |
| 4.3 Fluoxetine vs Amisulpride | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.10] |
| 4.4 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.33, 4.10] |
| 4.5 Fluoxetine vs Nefazodone | 3 | 161 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.05, 10.71] |
| 4.6 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.05, 4.51] |
| 4.7 Fluoxetine vs Tianeptine | 3 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.27, 2.53] |
| 4.8 Fluoxetine vs Trazodone | 2 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.51] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 17 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs ABT‐200 | 1 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.27] |
| 5.2 Fluoxetine vs Amineptine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.03, 7.82] |
| 5.3 Fluoxetine vs Amisulpride | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.33, 1.82] |
| 5.4 Fluoxetine vs Bupropion | 2 | 436 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.25] |
| 5.5 Fluoxetine vs Nefazodone | 4 | 286 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.81] |
| 5.6 Fluoxetine vs Pramipexole | 1 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.50] |
| 5.7 Fluoxetine vs Tianeptine | 3 | 830 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.71, 1.80] |
| 5.8 Fluoxetine vs Trazodone | 3 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.20, 2.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Failure to respond ‐ HDRS (‐50%) Show forest plot | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Crocus Sativus | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.60] |
| 1.2 Fluoxetine vs Hypericum | 6 | 717 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.55, 1.73] |
| 2 End‐point score on rating scales Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Hypericum | 5 | 648 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.02, 0.29] |
| 3 Failure to complete ‐ Total Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Crocus Sativus | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 17.18] |
| 3.2 Fluoxetine vs Hypericum | 5 | 679 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.58, 1.85] |
| 4 Failure to complete ‐ Inefficacy Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Fluoxetine vs Hypericum | 2 | 401 | Odds Ratio (M‐H, Random, 95% CI) | 4.70 [0.22, 99.39] |
| 5 Failure to complete ‐ Side Effects Show forest plot | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Fluoxetine vs Hypericum | 5 | 679 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.56, 2.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 5 | 341 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.35, 1.27] |
| 1.1 Fluoxetine vs Amitriptyline | 3 | 207 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.65] |
| 1.2 Fluoxetine vs Clomipramine | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.45] |
| 1.3 Fluoxetine vs Imipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.34] |
| 2 follow‐up 6‐16 weeks Show forest plot | 18 | 1742 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.31] |
| 2.1 Fluoxetine vs Amitriptyline | 8 | 570 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.50] |
| 2.2 Fluoxetine vs Desipramine | 2 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.56, 5.15] |
| 2.3 Fluoxetine vs Dothiepin/dosulepin | 2 | 144 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [1.08, 4.20] |
| 2.4 Fluoxetine vs Doxepine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| 2.5 Fluoxetine vs Imipramine | 4 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.34] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.55, 1.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 12 | 541 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.15, 0.50] |
| 1.1 Fluoxetine vs Amiptriptyline | 6 | 290 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.25, 1.02] |
| 1.2 Fluoxetine vs Clomipramine | 2 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.36, 0.35] |
| 1.3 Fluoxetine vs Desipramine | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.72, 0.82] |
| 1.4 Fluoxetine vs Imipramine | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐1.31, 1.22] |
| 1.5 Fluoxetine vs Trimipramine | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.24, 1.00] |
| 2 follow‐up 6‐16 weeks Show forest plot | 36 | 2727 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 2.1 Fluoxetine vs Amiptriptyline | 13 | 733 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.07, 0.22] |
| 2.2 Fluoxetine vs Clomipramine | 3 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 2.3 Fluoxetine vs Desipramine | 3 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.47, 1.12] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 4 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.27, 0.59] |
| 2.5 Fluoxetine vs Imipramine | 10 | 1003 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
| 2.6 Fluoxetine vs Lofepramine | 1 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.16, 0.42] |
| 2.7 Fluoxetine vs Nortriptyline | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.44, 0.20] |
| 2.8 Fluoxetine vs Trimipramine | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.44, 1.39] |
| 3 follow‐up >16 weeks Show forest plot | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.27, ‐0.44] |
| 3.1 Fluoxetine vs Nortriptyline | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.27, ‐0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 11 | 663 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.05] |
| 1.1 Fluoxetine vs Amitriptyline | 6 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.13] |
| 1.2 Fluoxetine vs Doxepine | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.29, 5.31] |
| 1.3 Fluoxetine vs Imipramine | 3 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.10, 3.13] |
| 1.4 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.44, 1.35] |
| 2 follow‐up 6‐16 weeks Show forest plot | 36 | 3450 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 2.1 Fluoxetine vs Amitriptyline | 12 | 780 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.44, 0.90] |
| 2.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.14] |
| 2.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.24] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.92, 2.69] |
| 2.5 Fluoxetine vs Doxepine | 3 | 272 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.40] |
| 2.6 Fluoxetine vs Imipramine | 9 | 1127 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.51, 1.27] |
| 2.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.24, 1.04] |
| 2.8 Fluoxetine vs Nortriptyline | 2 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 3.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 5 | 401 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.16, 1.50] |
| 1.1 Fluoxetine vs Amitriptyline | 2 | 105 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.09] |
| 1.2 Fluoxetine vs Doxepine | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Fluoxetine vs Imipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 3.15 [0.12, 82.16] |
| 1.4 Fluoxetine vs Nortriptyiline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.07, 2.03] |
| 2 follow‐up 6‐16 weeks Show forest plot | 28 | 2510 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [1.02, 1.87] |
| 2.1 Fluoxetine vs Amitriptyline | 11 | 730 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.50, 2.47] |
| 2.2 Fluoxetine vs Clomipramine | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 7.37 [0.37, 145.75] |
| 2.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.20, 5.35] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 3 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.49, 3.66] |
| 2.5 Fluoxetine vs Doxepine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.60, 4.92] |
| 2.6 Fluoxetine vs Imipramine | 9 | 1053 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.96, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 7 | 554 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.46, 1.43] |
| 1.1 Fluoxetine vs Amitriptyline | 4 | 258 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.21, 1.82] |
| 1.2 Fluoxetine vs Doxepine | 1 | 51 | Odds Ratio (M‐H, Random, 95% CI) | 3.13 [0.30, 32.31] |
| 1.3 Fluoxetine vs Imipramine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.09, 4.24] |
| 1.4 Fluoxetine vs Nortriptyline | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.77] |
| 2 follow‐up 6‐16 weeks Show forest plot | 33 | 3093 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.72] |
| 2.1 Fluoxetine vs Amitriptyline | 12 | 780 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.61] |
| 2.2 Fluoxetine vs Clomipramine | 2 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.79] |
| 2.3 Fluoxetine vs Desipramine | 2 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.04, 1.68] |
| 2.4 Fluoxetine vs Dothiepin/dosulepin | 5 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.59, 7.16] |
| 2.5 Fluoxetine vs Doxepine | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.42, 1.28] |
| 2.6 Fluoxetine vs Imipramine | 9 | 1053 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.25, 0.87] |
| 2.7 Fluoxetine vs Lofepramine | 1 | 183 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.34, 0.46] |
| 2 follow‐up 6‐16 weeks Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Maprotiline | 3 | 252 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| 2.2 Fluoxetine vs Mianserin | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.38, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 2 | 188 | Odds Ratio (M‐H, Random, 95% CI) | 1.54 [0.63, 3.75] |
| 2 follow‐up 6‐16 weeks Show forest plot | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 2.06 [0.75, 5.63] |
| 2.2 Fluoxetine vs Mianserin | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.18, 2.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 follow‐up 6‐16 weeks Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 2.54 [0.33, 19.19] |
| 2.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 2.27 [0.38, 13.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Maprotiline | 1 | 46 | Odds Ratio (M‐H, Random, 95% CI) | 0.41 [0.07, 2.49] |
| 2 follow‐up 6‐16 weeks Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Maprotiline | 2 | 163 | Odds Ratio (M‐H, Random, 95% CI) | 0.69 [0.11, 4.38] |
| 2.2 Fluoxetine vs Mianserin | 1 | 53 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.20, 1.79] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Fluvoxamine | 1 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.52, 1.74] |
| 2.2 Fluoxetine vs Paroxetine | 9 | 1574 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.93, 1.65] |
| 2.3 Fluoxetine vs Sertraline | 5 | 950 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [1.00, 1.71] |
| 2.4 Fluoxetine vs Escitalopram | 1 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.56, 1.85] |
| 3 follow‐up >16 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Sertraline | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.97, 2.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Citalopram | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.48, 0.61] |
| 2 follow‐up >16 weeks Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Paroxetine | 1 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.01, 0.49] |
| 2.2 Fluoxetine vs Sertraline | 1 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.08, 0.53] |
| 3 follow‐up 6‐16 weeks Show forest plot | 18 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Citalopram | 2 | 610 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.10, 0.21] |
| 3.2 Fluoxetine vs Paroxetine | 10 | 1819 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.31, 0.24] |
| 3.3 Fluoxetine vs Sertraline | 6 | 992 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.19] |
| 3.4 Fluoxetine vs Escitalopram | 1 | 231 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.19, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.22, 4.27] |
| 2 follow‐up 6‐16 weeks Show forest plot | 21 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Citalopram | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.59, 1.27] |
| 2.2 Fluoxetine vs Fluvoxamine | 2 | 284 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.37] |
| 2.3 Fluoxetine vs Paroxetine | 9 | 1606 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.25] |
| 2.4 Fluoxetine vs Sertraline | 7 | 1111 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.85, 1.48] |
| 2.5 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [1.00, 2.37] |
| 3 follow‐up >16 weeks Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Paroxetine | 1 | 242 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.49] |
| 3.2 Fluoxetine vs Sertraline | 2 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.84, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.43] |
| 2 follow‐up >16 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Sertraline | 1 | 238 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.47, 2.07] |
| 3 follow‐up 6‐16 weeks Show forest plot | 11 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Citalopram | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.49, 1.65] |
| 3.2 Fluoxetine vs Paroxetine | 4 | 1005 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 3.3 Fluoxetine vs Sertraline | 4 | 818 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.61, 2.29] |
| 3.4 Fluoxetine vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.46, 6.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Citalopram | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 1.5 [0.23, 9.70] |
| 2 follow‐up 6‐16 weeks Show forest plot | 20 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Citalopram | 2 | 673 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.12] |
| 2.2 Fluoxetine vs Fluvoxamine | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.14, 7.71] |
| 2.3 Fluoxetine vs Paroxetine | 9 | 1509 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.16] |
| 2.4 Fluoxetine vs Sertraline | 7 | 1111 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.71] |
| 2.5 Fluoxetina vs Escitalopram | 2 | 578 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.12] |
| 3 follow‐up >16 weeks Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Fluoxetine vs Sertraline | 2 | 480 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.72, 2.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.39, 2.60] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Agomelatine | 1 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.99, 2.09] |
| 2.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.04] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.90, 1.89] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.34] |
| 2.5 Fluoxetine vs Reboxetine | 3 | 721 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Moclobemide | 1 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.66, 0.42] |
| 2 follow‐up 6‐16 weeks Show forest plot | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Agomelatine | 3 | 1213 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.18, 0.23] |
| 2.2 Fluoxetine vs Mirtazapine | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.15, 1.29] |
| 2.3 Fluoxetine vs Moclobemide | 5 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.02, 0.33] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.67, 0.57] |
| 2.5 Fluoxetine vs Reboxetine | 2 | 205 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.31, 2.76] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetina vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.49] |
| 2.2 Fluoxetine vs Mirtazapine | 3 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.51, 1.68] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.53] |
| 2.4 Fluoxetine vs Reboxetine | 4 | 764 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.44, 0.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.20, 5.68] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.88] |
| 2.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.71, 2.96] |
| 2.3 Fluoxetine vs Moclobemide | 5 | 609 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.23, 1.65] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Fluoxetine vs Reboxetine | 3 | 464 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.47, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 follow‐up <6 weeks Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Fluoxetine vs Moclobemide | 1 | 70 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 follow‐up 6‐16 weeks Show forest plot | 15 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Fluoxetine vs Agomelatine | 2 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [0.73, 3.08] |
| 2.2 Fluoxetine vs Mirtazapine | 4 | 600 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.66] |
| 2.3 Fluoxetine vs Moclobemide | 6 | 651 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 2.01] |
| 2.4 Fluoxetine vs Phenelzine | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.26] |
| 2.5 Fluoxetine vs Reboxetine | 2 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.10, 1.61] |

