Tratamiento de compresión para la prevención del síndrome postrombótico
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, cross‐over design Method of randomisation: computer‐generated randomisation schedule Blinding: independent blinded assessment of the outcome events Exclusions postrandomisation: 0 Losses to follow‐up: 0 Study duration: 2 months | |
| Participants | Country: Canada Setting: Ontario teaching hospital Number of participants: 15 Gender: 12 women and 3 men Age: mean 60 years (range 38 to 81 years) Inclusion criteria: documented DVT and intractable symptoms of PPS causing significant limitation of lifestyle, significant morbidity, or both, as indicated by any of the following: loss of job or absenteeism from work because of PPS; interference with day‐to day activities, e.g. housework, sports; frequent loss of sleep; failure of condition to improve with use of graduated compression stockings; or person's intolerance of, or refusal to use, such stockings Exclusion criteria: DVT within the past 3 months or unable to travel to the clinic | |
| Interventions | Extremity pump twice daily (20 minutes per session) randomly assigned to use either 50 mmHg pressure or 15 mmHg pressure for the first month, the other pressure was used the second month | |
| Outcomes | Successful treatment: defined as preferring the high pressure, and continuing the use of the extremity pump and difference between the 2 pressures of at least slight importance | |
| Notes | Details of funding sources not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned, by means of a computer‐generated randomisation schedule, to use the extremity pump at either 50 mmHg (the therapeutic pressure) or 15 mmHg (the placebo pressure). |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and outcome assessors were blinded. For participants, either intervention or control extremity pump were provided. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and outcome assessors were blinded. For participants, either intervention or control extremity pump were provided. Bias was reduced since we compared the relative effects of a high pressure and a low pressure. |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data were not described. |
| Selective reporting (reporting bias) | Low risk | No selective reporting detected. |
| Other bias | Unclear risk | Study appeared free of other risk of bias. |
| Methods | Study design: randomised, double‐blind clinical trial Method of randomisation: prerandomisation stratification applied, randomisation not stated Concealment of allocation: not stated Blinding: observer and outcome assessor blinded Follow‐up: every 3 months Losses to follow‐up: 3 participants in the treatment group died vs 0 in the control group | |
| Participants | Country: Canada Setting: affiliated hospitals, Hamilton University, ON, Canada Number of participants: 35 18 compression stockings (14 CLCS, 4 TLCS); 17 placebo stockings (13 CLPS, 4 TLPS) Age: mean (range) years:
Gender: (% women):
Inclusion criteria: symptomatic DVT. Evaluation was done 1 year after proximal DVT. PPS was defined as chronic pain and swelling as scored on a standardised questionnaire. Exclusion criteria: previous use of graduated compression stockings, geographic inaccessibility, and failure to provide informed consent | |
| Interventions | Comparison was done with compression stockings (30–40 mmHg) or placebo stockings (1–2 sizes too large). Calf length or thigh length stockings were used depending on the localisation of complaints | |
| Outcomes | Treatment failure: defined as pain and swelling that did not improve, or worsened, after the first 3 months, or if these symptoms worsened during further follow‐up, or if a participant could not perform his daily activities for ≥ 5 days in any 3‐month period, or developed a leg ulcer. Mean follow‐up: treatment group 25.6 months vs placebo group 25.4 months | |
| Notes | Details of funding sources were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and outcome assessors were blinded. For participants, placebo stockings were provided. Participants were informed not to wear stockings to the assessments. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and outcome assessors were blinded. For participants, placebo stockings were provided. Participants were informed not to wear stockings to the assessments. The definition of PTS contained both objective and subjective items. |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data were not described, but study reported 3 participants in the active stocking group died. |
| Selective reporting (reporting bias) | Low risk | Only PTS was a prespecified outcome; this was reported. |
| Other bias | Unclear risk | Study appeared free of other risk of bias. |
| Methods | Prospective study Method of randomisation: different stockings were applied in random order using sealed envelopes Blinding: none Exclusions postrandomisation: none described Losses to follow‐up: 0 Follow‐up: no follow up after the study | |
| Participants | Country: UK Setting: single district general hospital Number of participants: 34 (40 legs) Gender: all men Age: median 62 years (range 31 to 81 years) Inclusion criteria: leg symptoms (ache, heaviness, swelling, cramps, itching, or tingling) and signs (oedema, telangiectasiae, pigmentation, secondary varicose veins, and ulceration) attributable to PTS, previous DVT > 6 months before with duplex evidence of deep venous damage (reflux or obstruction or both) Exclusion criteria: < 3 months recurrent DVT or venous ulceration > 1 cm diameter | |
| Interventions | Assessment of 4 different stockings using improvements of compression and length (of their size in random order: class 1 (18–21 mmHg) and class II (23–32 mmHg), below‐knee, and above‐knee thigh‐length | |
| Outcomes | Primary outcome: reduction in reflux Secondary outcome: participant preferences | |
| Notes | Details of funding sources not provided. Study paper reports on compliance rates of stockings use prior to the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence was not adequately discussed. |
| Allocation concealment (selection bias) | Low risk | Participant could not know what type of stocking they were wearing due to randomly sealed envelopes. Thereby, unpredictable. |
| Blinding of participants and personnel (performance bias) | Low risk | Used randomised sealed envelopes. They could not know which type of stocking they were getting. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of assessors was not adequately discussed. |
| Incomplete outcome data (attrition bias) | Low risk | No description of incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective reporting. |
| Other bias | Unclear risk | Study appeared free of other risk of bias. |
| Methods | Randomised placebo‐controlled trial Method of randomisation: none described Blinding: double‐blind 'cross‐over' Exclusions postrandomisation: 0 Losses to follow‐up: 6 Study duration: 20 weeks | |
| Participants | Country: Canada Setting: 2 clinical centres (Hamilton Health Sciences, Chedoke Division, Hamilton, ON; and the Thrombosis Unit, Sir Mortimer B. Davis Jewish General Hospital, Montreal, QC, Canada) Number of participants: 32 Gender: 16 men and 16 women Age: mean 50 years (range 25–80 years) Inclusion criteria: aged > 18 years with history of objectively documented DVT; daily leg swelling with discomfort (i.e. reported ≥ 1 of the following symptoms: heavy legs, aching legs, or throbbing) for ≤ 6 months that was considered due to PTS; and Villalta Scale score > 14 (i.e. corresponded to severe PTS). Exclusion criteria: unstable symptoms (worsening, improving, or variable over the previous month); chronic lower limb oedema from causes other than DVT; active venous ulceration; baseline calf circumference > 40 cm (cuff was too small); symptomatic PAD; or peripheral neuropathy | |
| Interventions | Participants received either the veno‐device (active or placebo for 8 weeks and crossed over for a further 8 weeks (active or placebo) following a 4 week 'wash out' period. All components of the control and active devices were identical, except for the connection between the motor and the planar sheet was inactive in the control device. | |
| Outcomes | Primary outcome: clinical success defined as fulfilling all of the following criteria: participant reported benefit from the intervention, experienced at least moderate improvement in symptoms of PTS, and was willing to continue to use the device Secondary outcomes: each of the component clinical success responses, device preference, PTS | |
| Notes | Details of funding sources not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated randomly in a 1:1 ratio to follow either Sequence 'A' (Venowave for 8 weeks in period 1 followed by control for 8 weeks in period 2), or Sequence 'B' (control for 8 weeks in period 1 followed by Venowave for 8 weeks in period 2). |
| Allocation concealment (selection bias) | Low risk | Allocation was determined by consecutively numbered participant kits that contained encrypted codes, corresponding to a randomly ordered pair of Venowave or control device. |
| Blinding of participants and personnel (performance bias) | Low risk | The research nurse opened the next participant kit in the sequence and provided the participant with device A. Participants were provided with device B at the end of the washout period. |
| Blinding of outcome assessment (detection bias) | Low risk | Not at risk due to double blinded and controlled randomised approach. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome detected, but 1 participant died and 1 participant withdrew due to injury. |
| Selective reporting (reporting bias) | Low risk | No reporting bias detected. |
| Other bias | Unclear risk | Study appeared free of other risk of bias. |
CLCS: calf length compression stockings; CLPS: calf length placebo stockings; DVT: deep vein thrombosis; GEC: graduated elastic compression; PAD: peripheral arterial disease; PPS: postphlebitic syndrome; TLCS: thigh length compression stockings; TLPS: thigh length placebo stockings; VEINES‐QOL: VEnous INsufficiency Epidemiological and Economic Study – Quality of Life; VEINES‐Sym: VEnous INsufficiency Epidemiological and Economic Study – Symptoms.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Comparison of pharmaceutical intervention vs elastic stocking | |
| Cross‐over study to evaluate the effect of graduated elastic compression stockings on leg symptoms and signs during exercise in people who had had a DVT ≥ 1 year prior to participation in study. Artificial situation with treadmill exercise session with and without stockings. | |
| Study was not a treatment for post‐thrombotic syndrome but designed to test the effectiveness of a new intermittent pneumatic compression system. | |
| Study investigating effectiveness of a cryotherapy gel wrap in chronic venous disorders. | |
| Comparison of surgery vs stocking. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The use of intermittent pneumatic compression device for symptomatic relief in patients with post thrombotic syndrome |
| Methods | Study type: interventional (clinical trial) Endpoint classification: efficacy study Intervention model: cross‐over assignment Masking: none (open label) Primary purpose: supportive care Losses to follow‐up: not available |
| Participants | Country: Israel Setting: Hadassah Ein Karem Medical Center, Jerusalem, Israel No. of participants: 20 Gender: both (specifics unknown yet) Age: 18–80 years Inclusion criteria: people with post‐thrombotic leg symptoms after a deep vein thrombosis event Exclusion criteria: hospitalised people; people with peripheral artery disease, acute deep vein thrombosis, or active leg infection; people who have undergone leg skin transplant; people who were not capable of operating the device |
| Interventions | ActiveCare+S.F.T 3rd generation (an intermittent pneumatic compression device) Compression stockings (current gold standard of care) |
| Outcomes | Primary outcome measures: quality of life (VEINS‐QOL) Secondary outcome measures: Villalta Scale |
| Starting date | 1 July 2012 |
| Contact information | Contact: Galia Spectre, MD; +97226779414; [email protected] Hadas Lemberg, PhD; +97226777572; [email protected] |
| Notes |

Study flow diagram.
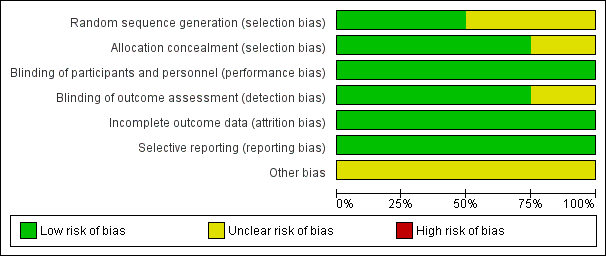
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Graduated elastic compression stockings compared with placebo stockings or no compression for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: GECSa Comparison: placebo stockings or no compression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo stockings | GECS | |||||
| Severity of post‐thrombotic syndromeb (follow‐up: 0–25.6 months) | Ginsberg 2001 reported 61.1% (11/18) treatment failures in the GECS group and 58.8% (10/17) treatment failure in the placebo group. Lattimer 2013 reported the VFI, VFT90, and VV significantly improved with GECS compared with no compression. VFI: no compression median 4.9 (range 1.7 to 16.3) BKI median 3.7 (range 0–14; 24.5% percentage improvement) AKI median 3.6 (range 0.6–14.5; 26.5%) BKII median 4.0 (range 0.3–16.2; 18.8%) AKII median 3.7 (range 0.5–14.2; 24.5%) | — | 69 (2 RCTs) | ⊕⊝⊝⊝ | 2 small studies of short duration were identified. 1 reported benefit and 1 no benefit from GECS use. | |
| Adverse effects (follow‐up: 25.6 months) | Ginsberg 2001 reported no participants developed ulceration in both groups | — | — | — | Lattimer 2013 did not assess adverse effects. | |
| Patient satisfaction and quality of life | See comments | — | — | — | No study measured quality of life. Lattimer 2013 assessed patient preferences. 52.5% of participants indicated they wanted to change their compression to another type of stocking, 38% of these preferred an AK stocking. | |
| Compliance rate | See comments | — | — | — | Lattimer 2013 and Ginsberg 2001 did not assess compliance rates during the study period. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKI/II: above‐knee thigh length stocking class I or II; BKI/II: below‐knee class I or II; CI: confidence interval; GECS: graduated elastic compression stockings; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; VFI: venous filling index; VFT90: venous filling time; VV: venous volume. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 2001 compared compression stockings (30 mmHg to 40 mmHg) with placebo stockings (one to two sizes too large). Calf length or thigh length compression stockings were administered depending on the localisation of complaints. Lattimer 2013 assessed four different stockings and assessed improvements of compression and length (of their size in random order: class I (18 mmHg to 21 mmHg) and class II (23 mmHg to 32 mmHg), BK and AK. | ||||||
| Intermittent pneumatic compression devices compared with control devices for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: medical compression devicea Comparison: control device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control device | Medical compression device | |||||
| Severity post‐thrombotic syndromeb (follow‐up: 8–20 weeks) | Ginsberg 1999: 80% (12/15) of participants had an improvement in symptom score that was considered a treatment success. The mean difference in scoring at the 2 pressure levels was –2.1 (95% CI –3.6 to –0.7; P = 0.007) O'Donnell 2008: an improvement in Villalta score was reported in the Venowave group (12) compared to the control device group (15) (P = 0.004) | 47 (2 RCTs) | ⊕⊕⊝⊝ | Control devices were used on the same participants that used medical device. | ||
| Adverse effects (follow‐up: 20 weeks) | O'Donnell 2008 reported 9% (3/32) of participants experienced adverse effects, including leg swelling, irritation, superficial bleeding, and skin itching related to Venowave device use | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Patient satisfaction and quality of life (follow‐up: 20 weeks) | O'Donnell 2008 reported the mean VEINES‐QoL score at the end of the study period was significantly higher for Venowave (53) than for the control device (50) (P = 0.004) | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Compliance rate | See comments | — | — | No study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; QoL: quality of life; VEINES‐QOL: VEnous INsufficiency Epidemiological and Economic Study – Quality of Life. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 1999 used an extremity pump twice daily at either 50 mmHg pressure or 15 mmHg pressure. O'Donnell 2008 used Venowave, a lower‐limb venous‐return assist device. | ||||||

