Tratamiento de compresión para la prevención del síndrome postrombótico
Appendices
Appendix 1. CENTRAL search strategy March 2017
| Search run on Fri Mar 10 2017 | ||
| #1 | MESH DESCRIPTOR Postthrombotic Syndrome EXPLODE ALL TREES | 28 |
| #2 | postthrombotic:TI,AB,KY | 68 |
| #3 | (post near3 thrombot*):TI,AB,KY | 146 |
| #4 | PTS*:TI,AB,KY | 8745 |
| #5 | MESH DESCRIPTOR Postphlebitic Syndrome EXPLODE ALL TREES | 24 |
| #6 | postphlebit*:TI,AB,KY | 36 |
| #7 | (post near3 phlebit*):TI,AB,KY | 15 |
| #8 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 | 8905 |
| #9 | MESH DESCRIPTOR Bandages | 1409 |
| #10 | MESH DESCRIPTOR Compression Bandages EXPLODE ALL TREES | 254 |
| #11 | (stocking* or hosiery or tights or sock*):TI,AB,KY | 1442 |
| #12 | compress*:TI,AB,KY | 5957 |
| #13 | #9 OR #10 OR #11 OR #12 | 7796 |
| #14 | #8 AND #13 | 125 |
| #15 | * NOT SR‐PVD:CC | 1014031 |
| #16 | #14 AND #15 | 72 |
Appendix 2. Trial registries searches March 2017
ClinicalTrials.gov
28 studies found for: (postthrombotic OR post‐thrombotic) AND (compression OR stocking OR bandage)
World Health Organization International Clinical Trials Registry Platform
10 reports of 10 trials for
(postthrombotic OR post‐thrombotic) in Title or Condition
and (compression OR stocking OR bandage) in Intervention
ISRCTN Register
10 results postthrombotic OR post‐thrombotic
Appendix 3. Database searches July 2018
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Postthrombotic Syndrome EXPLODE ALL TREES 73 #2 postthrombotic:TI,AB,KY 121 #3 (post near3 thrombot*):TI,AB,KY 193 #4 PTS*:TI,AB,KY 13458 #5 MESH DESCRIPTOR Postphlebitic Syndrome EXPLODE ALL TREES 59 #6 postphlebit*:TI,AB,KY 71 #7 (post near3 phlebit*):TI,AB,KY 16 #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 13677 #9 MESH DESCRIPTOR Bandages 1505 #10 MESH DESCRIPTOR Compression Bandages EXPLODE ALL TREES 307 #11 (stocking* or hosiery or tights or sock*):TI,AB,KY 1827 #12 compress*:TI,AB,KY 7559 #13 #9 OR #10 OR #11 OR #12 9757 #14 #8 AND #13 158 #15 01/01/2017 TO 02/07/2018:CD 292648 #16 #14 AND #15 42 | 42 |
| Clinicaltrials.gov | post‐thrombotic OR Postphlebitic OR Postthrombotic | stocking* OR hosiery OR tights OR sock* OR Bandages OR Compression | Start date on or after 01/01/2017 | Last update posted on or before 07/02/2018 | 7 |
| ICTRP Search Portal | 1 | |
| MEDLINE | 1 exp Postthrombotic Syndrome/ 582 2 postthrombotic.ti,ab. 822 3 (post adj3 thrombot*).ti,ab. 1422 4 PTS*.ti,ab. 31964 5 exp Postphlebitic Syndrome/ 625 6 postphlebit*.ti,ab. 455 7 (post adj3 phlebit*).ti,ab. 318 8 or/1‐7 34763 9 BANDAGES/ 15813 10 exp Compression Bandages/ 2077 11 (stocking* or hosiery or tights or sock*).ti,ab. 13552 12 compress*.ti,ab. 138887 13 or/9‐12 164825 14 8 and 13 708 15 (2017* or 2018*).ed. 1414704 16 14 and 15 52 | 52 |
| Embase | 1 exp postthrombosis syndrome/ 2534 2 postthrombotic.ti,ab. 1223 3 (post adj3 thrombot*).ti,ab. 2193 4 PTS*.ti,ab. 117282 5 exp chronic vein insufficiency/ 3520 6 postphlebit*.ti,ab. 470 7 (post adj3 phlebit*).ti,ab. 332 8 or/1‐7 124321 9 bandage/ 10647 10 exp compression bandage/ 2145 11 (stocking* or hosiery or tights or sock*).ti,ab. 16365 12 compress*.ti,ab. 171688 13 or/9‐12 195287 14 8 and 13 2015 15 randomized controlled trial/ 507666 16 controlled clinical trial/ 460068 17 random$.ti,ab. 1314352 18 randomization/ 78496 19 intermethod comparison/ 236587 20 placebo.ti,ab. 274310 21 (compare or compared or comparison).ti. 470900 22 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1760878 23 (open adj label).ti,ab. 64733 24 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 209628 25 double blind procedure/ 151259 26 parallel group$1.ti,ab. 21867 27 (crossover or cross over).ti,ab. 93244 28 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 284035 29 (assigned or allocated).ti,ab. 333229 30 (controlled adj7 (study or design or trial)).ti,ab. 296091 31 (volunteer or volunteers).ti,ab. 224978 32 trial.ti. 251985 33 or/15‐32 4052435 34 14 and 33 644 35 (2017* or 2018*).em. 3645726 36 34 and 35 97 37 from 36 keep 1‐97 97 | 97 |
| CINAHL | S31 S29 AND S30 3 S30 EM 2017 OR EM 2018 371,243 S29 S13 AND S28 70 S28 S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 342,293 S27 MH "Random Assignment" 38,732 S26 MH "Triple‐Blind Studies" 85 S25 MH "Double‐Blind Studies" 24,826 S24 MH "Single‐Blind Studies" 7,986 S23 MH "Crossover Design" 11,215 S22 MH "Factorial Design" 919 S21 MH "Placebos" 8,355 S20 MH "Clinical Trials" 93,004 S19 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,486 S18 TX crossover OR "cross‐over" 14,567 S17 AB placebo* 28,322 S16 TX random* 219,135 S15 TX trial* 250,574 S14 TX "latin square" 142 S13 S7 AND S12 155 S12 S8 OR S9 OR S10 OR S11 27,971 S11 TX compress* 15,914 S10 TX stocking* or hosiery or tights or sock* 5,717 S9 (MH "Elastic Bandages") 115 S8 (MH "Bandages and Dressings") 7,898 S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 9,103 S6 TX post n3 phlebit* 15 S5 TX postphlebit* 19 S4 TX PTS* 8,928 S3 TX post n3 thrombot* 96 S2 TX postthrombotic 110 S1 (MH "Postthrombotic Syndrome") 62 | 3 |
| AMED | 1 postthrombotic.ti,ab. 3 2 (post adj3 thrombot*).ti,ab. 5 3 PTS*.ti,ab. 634 4 postphlebit*.ti,ab. 0 5 (post adj3 phlebit*).mp. [mp=abstract, heading words, title] 0 6 or/1‐5 640 7 exp Bandages/ 604 8 (stocking* or hosiery or tights or sock*).ti,ab. 442 9 compress*.ti,ab. 2149 10 or/7‐9 3051 11 6 and 10 4 12 ("2017" or "2018").yr. 2075 13 11 and 12 0 | 0 |
Appendix 4. Symptom/functionality scores (Ginsberg 1999)
| Participant | Therapeutic (50 mmHg) | Placebo (15 mmHg) |
| 1 | 27 | 24 |
| 2 | 19 | 17 |
| 3 | 22 | 17 |
| 4 | 22 | 18 |
| 5 | 10 | 9 |
| 6 | 9 | 5 |
| 7 | 25 | 24 |
| 8 | 21 | 18 |
| 9 | 15 | 13 |
| 10 | 15 | 13 |
| 11 | 15 | 10 |
| 12 | 15 | 10 |
| 13 | 15 | 15 |
| 14 | 7 | 12 |
| 15 | 10 | 10 |

Study flow diagram.
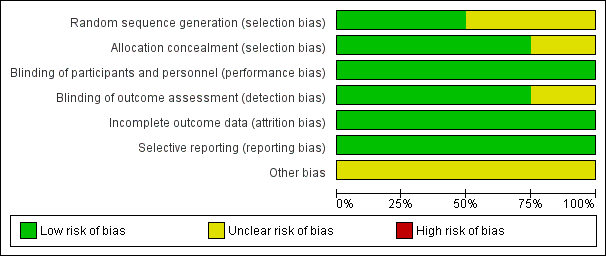
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Graduated elastic compression stockings compared with placebo stockings or no compression for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: GECSa Comparison: placebo stockings or no compression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo stockings | GECS | |||||
| Severity of post‐thrombotic syndromeb (follow‐up: 0–25.6 months) | Ginsberg 2001 reported 61.1% (11/18) treatment failures in the GECS group and 58.8% (10/17) treatment failure in the placebo group. Lattimer 2013 reported the VFI, VFT90, and VV significantly improved with GECS compared with no compression. VFI: no compression median 4.9 (range 1.7 to 16.3) BKI median 3.7 (range 0–14; 24.5% percentage improvement) AKI median 3.6 (range 0.6–14.5; 26.5%) BKII median 4.0 (range 0.3–16.2; 18.8%) AKII median 3.7 (range 0.5–14.2; 24.5%) | — | 69 (2 RCTs) | ⊕⊝⊝⊝ | 2 small studies of short duration were identified. 1 reported benefit and 1 no benefit from GECS use. | |
| Adverse effects (follow‐up: 25.6 months) | Ginsberg 2001 reported no participants developed ulceration in both groups | — | — | — | Lattimer 2013 did not assess adverse effects. | |
| Patient satisfaction and quality of life | See comments | — | — | — | No study measured quality of life. Lattimer 2013 assessed patient preferences. 52.5% of participants indicated they wanted to change their compression to another type of stocking, 38% of these preferred an AK stocking. | |
| Compliance rate | See comments | — | — | — | Lattimer 2013 and Ginsberg 2001 did not assess compliance rates during the study period. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKI/II: above‐knee thigh length stocking class I or II; BKI/II: below‐knee class I or II; CI: confidence interval; GECS: graduated elastic compression stockings; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; VFI: venous filling index; VFT90: venous filling time; VV: venous volume. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 2001 compared compression stockings (30 mmHg to 40 mmHg) with placebo stockings (one to two sizes too large). Calf length or thigh length compression stockings were administered depending on the localisation of complaints. Lattimer 2013 assessed four different stockings and assessed improvements of compression and length (of their size in random order: class I (18 mmHg to 21 mmHg) and class II (23 mmHg to 32 mmHg), BK and AK. | ||||||
| Intermittent pneumatic compression devices compared with control devices for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: medical compression devicea Comparison: control device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control device | Medical compression device | |||||
| Severity post‐thrombotic syndromeb (follow‐up: 8–20 weeks) | Ginsberg 1999: 80% (12/15) of participants had an improvement in symptom score that was considered a treatment success. The mean difference in scoring at the 2 pressure levels was –2.1 (95% CI –3.6 to –0.7; P = 0.007) O'Donnell 2008: an improvement in Villalta score was reported in the Venowave group (12) compared to the control device group (15) (P = 0.004) | 47 (2 RCTs) | ⊕⊕⊝⊝ | Control devices were used on the same participants that used medical device. | ||
| Adverse effects (follow‐up: 20 weeks) | O'Donnell 2008 reported 9% (3/32) of participants experienced adverse effects, including leg swelling, irritation, superficial bleeding, and skin itching related to Venowave device use | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Patient satisfaction and quality of life (follow‐up: 20 weeks) | O'Donnell 2008 reported the mean VEINES‐QoL score at the end of the study period was significantly higher for Venowave (53) than for the control device (50) (P = 0.004) | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Compliance rate | See comments | — | — | No study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; QoL: quality of life; VEINES‐QOL: VEnous INsufficiency Epidemiological and Economic Study – Quality of Life. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 1999 used an extremity pump twice daily at either 50 mmHg pressure or 15 mmHg pressure. O'Donnell 2008 used Venowave, a lower‐limb venous‐return assist device. | ||||||

