Tratamiento de compresión para la prevención del síndrome postrombótico
Resumen
Antecedentes
El síndrome postrombótico (SPT) es una complicación a largo plazo de la trombosis venosa profunda (TVP) que se caracteriza por síntomas crónicos como edema y cambios en la piel, que incluyen ectasia venosa, venas varicosas, enrojecimiento, eccema, hiperpigmentación y, en casos graves, fibrosis de la adiposidad subcutánea en el miembro afectado. Estas molestias crónicas se deben a los efectos de la restricción del flujo venoso que puede causar síntomas como pesadez, prurito, dolor, calambres y parestesia. Entre el 20% y el 50% de los pacientes con TVP desarrollan complicaciones postrombóticas. Se utilizan varias medidas no farmacéuticas para la prevención del síndrome postrombótico durante la fase aguda de la TVP. Éstas incluyen la elevación de las piernas y el tratamiento de compresión. Se han realizado estudios limitados con respecto a la efectividad del tratamiento de compresión para la prevención o el tratamiento del SPT. Como resultado, los médicos y las guías difieren en su evaluación del uso del tratamiento de compresión durante el tratamiento de la TVP y en el tratamiento del SPT. Ésta es una actualización de una revisión publicada por primera vez en 2003.
Objetivos
Evaluar la efectividad del tratamiento de compresión para el tratamiento del síndrome postrombótico, incluidas las medias elásticas de compresión y los dispositivos mecánicos en comparación con ninguna intervención, placebo y entre sí.
Métodos de búsqueda
El Especialista en Información del Grupo Cochrane Vascular realizó búsquedas en las bases de datos del Registro especializado del Grupo Cochrane Vascular, en las bases de datos CENTRAL, MEDLINE, Embase y CINAHL, así como en la World Health Organization International Clinical Trials Registry Platform y en el registro de ensayos de ClinicalTrials.gov hasta el 2 de julio de 2018.
Criterios de selección
Se incluyeron los ensayos que evaluaron el tratamiento de compresión para el SPT. Los resultados primarios fueron la gravedad del SPT y los efectos adversos. No hubo restricciones de fecha ni de idioma. Dos autores de la revisión (SA, DNK) evaluaron de forma independiente si los estudios potencialmente relevantes cumplían con los criterios de inclusión.
Obtención y análisis de los datos
Un autor de la revisión extrajo y resumió los datos y un autor de la revisión (DNK) los verificó. Cualquier desacuerdo se resolvió mediante discusión. La calidad metodológica de los estudios se evaluó con la herramienta Cochrane "Riesgo de sesgo". Se utilizó GRADE para evaluar la calidad general de la evidencia que apoyaba los resultados evaluados en esta revisión.
Resultados principales
Se identificaron cuatro ensayos, con 116 participantes, que investigaron la efectividad del tratamiento de compresión para el tratamiento del SPT. La metodología utilizada por cada ensayo fue demasiado heterogénea para realizar un metanálisis, por lo que los resultados se informaron de manera narrativa.
Dos ensayos estudiaron el efecto de las medias de compresión elásticas graduadas (MCEG) sobre la mejoría de los síntomas del SPT. Un estudio informó efectos hemodinámicos beneficiosos, mientras que el otro no encontró efectos beneficiosos sobre la gravedad del SPT en comparación con placebo (evidencia de certeza muy baja). La evidencia disponible sobre los efectos adversos y la calidad de vida (CdV) fue muy limitada. Los dos estudios no informaron sobre las tasas de cumplimiento durante el período de estudio.
Dos ensayos estudiaron los efectos de los dispositivos de compresión mecánica intermitente. Ambos informaron una mejoría en la gravedad del SPT (evidencia de certeza baja). La mejoría de la gravedad del SPT se definió por el "éxito" o el "fracaso" del tratamiento. Sólo un estudio que comparó los dispositivos de compresión evaluó los efectos adversos y la calidad de vida. Aunque el 9% de los participantes presentaron efectos adversos como infamación de la pierna, irritación, sangrado superficial y prurito en la piel (evidencia de certeza moderada), la CdV mejoró (evidencia de certeza moderada). Los estudios no evaluaron el cumplimiento con el uso de los dispositivos de compresión mecánica intermitente.
Ninguno de los estudios evaluó la satisfacción del paciente.
Conclusiones de los autores
Hay evidencia de certeza muy baja con respecto al uso de las MCEG para el tratamiento del SPT, según lo evaluado por dos estudios pequeños de corta duración. Un estudio informó efectos hemodinámicos beneficiosos, mientras que otro no encontró beneficios sobre la gravedad del SPT en comparación con las medias de control/placebo. Hay evidencia muy limitada de los efectos adversos, la satisfacción de los pacientes, la calidad de vida y las tasas de cumplimiento. Hay evidencia de certeza baja que favorece el uso de dispositivos de compresión neumática intermitente en comparación con un dispositivo de control para el tratamiento de la gravedad, debido a las diferentes medidas utilizadas por los estudios que informan sobre este resultado y a los estudios pequeños de corta duración. Hay evidencia de certeza moderada de una mejoría en la calidad de vida, pero es posible que haya un aumento de los efectos adversos relacionados con el uso de dispositivos de compresión debido a los estudios pequeños de corta duración. Falta evidencia de certeza alta para apoyar el uso del tratamiento de compresión en la prevención del SPT y cualquier conclusión derivada de la evidencia actual se debe interpretar con cuidado. Se necesitan estudios de investigación adicionales para evaluar si la compresión puede dar lugar a la reducción y el alivio a largo plazo de los síntomas causados por el SPT o si puede prevenir el deterioro y la ulceración de la pierna.
PICO
Resumen en términos sencillos
Tratamiento de compresión para la prevención del síndrome postrombótico de gravedad leve a moderada
Antecedentes
La trombosis venosa profunda (TVP) ocurre cuando se forma un coágulo sanguíneo en una vena de la pierna. El coágulo se puede romper y causar una obstrucción potencialmente grave en los vasos sanguíneos. Los pacientes que han tenido una TVP pueden desarrollar el síndrome postrombótico (SPT). Este se caracteriza por problemas como dolor de la pierna, tobillos inflamados y piel decolorada y endurecida. Los síntomas empeoran cuando se está de pie o sentado durante mucho tiempo y pueden limitar el estilo de vida y las actividades cotidianas. En los casos graves, también se desarrollan úlceras venosas, llagas abiertas que no cicatrizan. El uso de vendas de compresión o medias de compresión elásticas graduadas (MCEG) después del tratamiento inicial de dilución de la sangre (anticoagulante) para la TVP se utiliza para reducir la probabilidad de SPT y disminuir los síntomas. En caso de inflamación grave de la pierna (edema), se puede utilizar un dispositivo o máquina para aplicar presión a la pierna con el fin de mejorar la circulación sanguínea (dispositivo de compresión mecánica o dispositivo de compresión neumática intermitente). Se han realizado estudios limitados con respecto a la efectividad del tratamiento de compresión para la prevención o el tratamiento del SPT. Como resultado, los médicos y las guías difieren en su evaluación del uso del tratamiento de compresión durante el tratamiento de la TVP y en el tratamiento del SPT.
Características de los estudios y resultados clave
Los autores de la revisión identificaron cuatro ensayos, con 116 participantes, que investigaron la efectividad de los tratamientos de compresión para el SPT (búsqueda más reciente 2 de julio de 2018). Dos ensayos estudiaron el efecto de las MCEG. Un estudio mostró una mejoría de los síntomas del SPT y otro no mostró efectos beneficiosos. Otros dos ensayos estudiaron el efecto de un dispositivo de compresión neumática intermitente. Ambos informaron una mejoría en la gravedad del SPT. Un estudio evaluó los efectos secundarios y la calidad de vida. Aunque el 9% de los participantes presentaron efectos secundarios como inflamación de la pierna, irritación, hemorragia superficial y prurito en la piel, la calidad de vida tuvo resultados positivos. Ninguno de los estudios evaluó o informó sobre la satisfacción de los pacientes o las tasas de cumplimiento.
Fiabilidad de la evidencia
La evidencia del uso de las MCEG o el dispositivo de compresión neumática intermitente comparado con control para el tratamiento de la gravedad del SPT son de una confiabilidad muy baja y de certeza baja. Esto se debe a resultados contradictorios, estudios pequeños de corta duración y diferencias en la forma en que los estudios midieron los resultados. Se dispuso de evidencia limitada sobre los efectos secundarios, la satisfacción de los pacientes, la calidad de vida y el cumplimiento.
Authors' conclusions
Summary of findings
| Graduated elastic compression stockings compared with placebo stockings or no compression for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: GECSa Comparison: placebo stockings or no compression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo stockings | GECS | |||||
| Severity of post‐thrombotic syndromeb (follow‐up: 0–25.6 months) | Ginsberg 2001 reported 61.1% (11/18) treatment failures in the GECS group and 58.8% (10/17) treatment failure in the placebo group. Lattimer 2013 reported the VFI, VFT90, and VV significantly improved with GECS compared with no compression. VFI: no compression median 4.9 (range 1.7 to 16.3) BKI median 3.7 (range 0–14; 24.5% percentage improvement) AKI median 3.6 (range 0.6–14.5; 26.5%) BKII median 4.0 (range 0.3–16.2; 18.8%) AKII median 3.7 (range 0.5–14.2; 24.5%) | — | 69 (2 RCTs) | ⊕⊝⊝⊝ | 2 small studies of short duration were identified. 1 reported benefit and 1 no benefit from GECS use. | |
| Adverse effects (follow‐up: 25.6 months) | Ginsberg 2001 reported no participants developed ulceration in both groups | — | — | — | Lattimer 2013 did not assess adverse effects. | |
| Patient satisfaction and quality of life | See comments | — | — | — | No study measured quality of life. Lattimer 2013 assessed patient preferences. 52.5% of participants indicated they wanted to change their compression to another type of stocking, 38% of these preferred an AK stocking. | |
| Compliance rate | See comments | — | — | — | Lattimer 2013 and Ginsberg 2001 did not assess compliance rates during the study period. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKI/II: above‐knee thigh length stocking class I or II; BKI/II: below‐knee class I or II; CI: confidence interval; GECS: graduated elastic compression stockings; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; VFI: venous filling index; VFT90: venous filling time; VV: venous volume. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 2001 compared compression stockings (30 mmHg to 40 mmHg) with placebo stockings (one to two sizes too large). Calf length or thigh length compression stockings were administered depending on the localisation of complaints. Lattimer 2013 assessed four different stockings and assessed improvements of compression and length (of their size in random order: class I (18 mmHg to 21 mmHg) and class II (23 mmHg to 32 mmHg), BK and AK. | ||||||
| Intermittent pneumatic compression devices compared with control devices for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: medical compression devicea Comparison: control device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control device | Medical compression device | |||||
| Severity post‐thrombotic syndromeb (follow‐up: 8–20 weeks) | Ginsberg 1999: 80% (12/15) of participants had an improvement in symptom score that was considered a treatment success. The mean difference in scoring at the 2 pressure levels was –2.1 (95% CI –3.6 to –0.7; P = 0.007) O'Donnell 2008: an improvement in Villalta score was reported in the Venowave group (12) compared to the control device group (15) (P = 0.004) | 47 (2 RCTs) | ⊕⊕⊝⊝ | Control devices were used on the same participants that used medical device. | ||
| Adverse effects (follow‐up: 20 weeks) | O'Donnell 2008 reported 9% (3/32) of participants experienced adverse effects, including leg swelling, irritation, superficial bleeding, and skin itching related to Venowave device use | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Patient satisfaction and quality of life (follow‐up: 20 weeks) | O'Donnell 2008 reported the mean VEINES‐QoL score at the end of the study period was significantly higher for Venowave (53) than for the control device (50) (P = 0.004) | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Compliance rate | See comments | — | — | No study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; QoL: quality of life; VEINES‐QOL: VEnous INsufficiency Epidemiological and Economic Study – Quality of Life. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 1999 used an extremity pump twice daily at either 50 mmHg pressure or 15 mmHg pressure. O'Donnell 2008 used Venowave, a lower‐limb venous‐return assist device. | ||||||
Background
Description of the condition
Post‐thrombotic syndrome (PTS) is a long‐term complication of deep vein thrombosis (DVT) that is characterised by chronic complaints such as oedema and skin changes such as venous ectasia, varicose veins, redness, eczema, hyperpigmentation, and in severe cases, fibrosis of the subcutaneous adipose in the affected limb. These chronic complaints are the effects of venous outflow restriction that can cause symptoms such as heaviness, itching, pain, cramps, and paraesthesia. Twenty to fifty percent of people with DVT develop post‐thrombotic sequelae (complications resulting from a disease or condition) (Kahn 2014a). Although there is no gold standard for the diagnosis of PTS, the presence of typical clinical features of venous insufficiency in a person with previous DVT provides strong supporting evidence. Widmer 1985 recognises three stages of chronic venous insufficiency. In the first phase, oedema (swelling of the tissues caused by excessive fluid in the subcutaneous tissue) develops during the day and some skin changes occur (redness, ankle flair). In the second phase, oedema becomes evident, lipodermatosclerosis or white skin atrophy (or both) occurs. Lipodermatosclerosis is the term given to a hardening of the skin, which may gain a red/brown pigmentation, and is accompanied by wasting of the subcutaneous fat. White skin atrophy consists of small depressions of ivory‐coloured hard skin with stippled red spots. In the third stage, venous ulcers develop. Other recognised PTS assessment scales exist. The CEAP (clinical, aetiological, anatomic, pathophysiological) classification also uses clinical grading (Porter 1995). Another assessment tool to diagnose PTS is the Villalta score and this is used to diagnose and grade the severity of PTS (Villalta 1994). It has also been used as a measurement tool for effectiveness of treatments, quality of life (QoL), and follow‐up (Kahn 2003; Kahn 2007; O'Donnell 2008; Prandoni 2004; Soosainathan 2013). The Ginsberg score was designed to diagnose PTS through observation of leg pain and swelling with evidence of valvular incompetence, occurring at least six months after the initial DVT (Soosainathan 2013). The Ginsberg score is used to evaluate compression stockings in prevention and treatment of PTS (Ginsberg 1999; Ginsberg 2001; Kahn 2003; Kahn 2007). Brandjes 1997 developed a score that is similar to the Villalta score. Criteria were chosen from other scoring systems, and points were combined with these subjective and objective criteria (Soosainathan 2013).
Description of the intervention
The likelihood of PTS developing can be reduced by ensuring an adequate duration and intensity of anticoagulation for treatment of the initial DVT, and prescribing appropriate thromboprophylaxis after discontinuation of oral anticoagulants. Daily use of elastic compression stockings after DVT possibly reduces the risk of PTS (Appelen 2017; Aschwanden 2008; Blättler 2003; Brandjes 1997; Ginsberg 2001; Jayaraj 2015; Kahn 2014b; Mol 2016; Prandoni 2004; Prandoni 2012; Roumen‐Klappe 2009). It also prevents worsening of established PTS (Prandoni 1996). Compression therapy, in general, consists of circular pressure on the leg using circular bandages of any type (Partsch 1991). Initially removable bandages or semi‐permanent bandages such as adherent tape and Unna's paste boot are used, while elastic compression stockings are worn for long‐term treatment. In cases of severe oedema, mechanical devices such as intermittent pneumatic compression devices can be used.
How the intervention might work
The mechanism of action of compression therapy of the legs is produced by one or more of the following: reduction of oedema, acceleration of venous blood flow, and improvement in venous pump function (Brakkee 1988; Partsch 1991). Compression can be achieved by using mechanical devices and different types of stockings. Very tight non‐elastic bandages are used in an attempt to reverse venous hypertension (Partsch 1984). Mechanical compression in the form of a venous return‐assisting device utilises a wave‐form upward motion action to enhance circulation through intermittent calf compressions. Although compression therapy is generally harmless, it can lead to complications. Pressure sores may develop if extremely high pressure is given because such pressure will reduce blood supply to the skin (Partsch 1991). Similarly, application of moderate pressure in people with impaired arterial blood supply to the legs may result in exacerbation of arterial insufficiency (Partsch 1984).
Why it is important to do this review
There is no consensus in the assessment of the use of compression therapy for treatment of PTS. While frequently prescribed, it remains unknown whether compression therapies are effective in the treatment of PTS. This systematic review presented the current evidence for compression therapy for treating PTS to help inform patients and healthcare professionals.
Objectives
To assess the effectiveness of compression therapy for treatment of post‐thrombotic syndrome, including elastic compression stockings and mechanical devices compared with no intervention, placebo, and with each other.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), or controlled clinical trials (CCTs), evaluating elastic compression stockings and mechanical devices in the treatment of PTS. We classified trials which used allocation processes that were transparent before assignment, such as open list of random numbers, case record, day of the week, surname, etc, as CCTs.
Types of participants
We included men and women of any age with PTS symptoms or clinical changes (or both) of the legs after a previous DVT that was objectively diagnosed. Methods considered acceptable for diagnosis of DVT included ultrasound, venography, and impedance plethysmography. We excluded people with leg ulcers.
Types of interventions
We included all studies with the primary interventions of medical elastic compression stockings and mechanical devices, including intermittent pneumatic compression.
We intended to compare the following interventions:
-
elastic compression stockings versus control;
-
mechanical compression therapy devices versus control;
-
mechanical compression therapy devices versus compression stockings;
-
compression stockings versus compression stockings;
-
mechanical compression therapy devices versus mechanical compression therapy devices.
We allowed comparison of these with each other, e.g. different brand of stockings, but also different pressure profiles of stockings, different length of stockings, and different heights of pressure for the pneumatic compression units.
Any drug treatment or surgical interventions for PTS were required to be equal in the participant groups in the trials.
Types of outcome measures
Primary outcomes
-
Severity of PTS: as defined in each study provided they included a systematic clinical history and scoring of physical examinations. This is necessary because some trials only report changes in symptoms over time which provides a less valid indicator of effectiveness, as symptoms may vary.
-
Adverse effects (discomfort, skin damage, pain, arterial leg ulceration, and recurrence of thrombosis).
Secondary outcomes
-
Patient satisfaction and quality of life (QoL): generic or specific measures obtained from validated QoL questionnaires.
-
Compliance rate.
Search methods for identification of studies
We had no restrictions on language or publication status.
Electronic searches
The Cochrane Vascular Information Specialist searched the following databases for relevant trials:
-
the Cochrane Vascular Specialised Register (10 March 2017);
-
the Cochrane Central Register of Controlled Trials (CENTRAL (2017, Issue 2)) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Information Specialist also searched the following trial registries for details of ongoing and unpublished studies (10 March 2017):
-
ClinicalTrials.gov (clinicaltrials.gov);
-
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
-
ISRCTN Register (isrctn.com/).
See Appendix 2 for details of the search strategies used.
The Information Specialist subsequently conducted top‐up searches of the following databases for relevant trials on 2 July 2018:
-
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched to 2 July 2018);
-
CENTRAL Cochrane Register of Studies Online (CRSO 2018, issue 6);
-
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched from 1 January 2017 to 2 July 2018);
-
Embase Ovid (searched from 1 January 2017 to 2 July 2018);
-
CINAHL EBSCO (searched from 1 January 2017 to 2 July 2018);
-
AMED Ovid (searched from 1 January 2017 to 2 July 2018).
The Information Specialist modelled search strategies for the listed databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and CCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). See Appendix 3 for search strategies for major databases.
The Information Specialist performed top‐up searches of the following trials registries on 2 July 2018:
-
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
-
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We searched no other resources.
Data collection and analysis
Selection of studies
Two review authors (SA, DNK) independently assessed titles and abstracts of studies identified in terms of their relevance and design, according to the selection criteria. We obtained full‐text versions of articles if they satisfied the inclusion criteria from the initial assessment. Two review authors (SA, DNK) independently assessed whether potentially relevant studies met the inclusion criteria. We resolved any disagreements by discussion. We identified no studies that had been published in duplicate; however, if we identify duplicates in updates of this review we will include them once.
Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references.
Data extraction and management
We extracted and summarised details of the studies using data extraction sheets. One review author (SA) undertook data extracted and a second review author (DNK) checked them for accuracy. When available, we collected the following data:
-
trial setting (country, primary or secondary care);
-
method of randomisation;
-
objective outcome, method of assessment, and whether blinded;
-
length of follow‐up;
-
number of participants (or limbs) randomised;
-
inclusion criteria;
-
exclusion criteria;
-
description of interventions and cointerventions;
-
baseline characteristics of groups for important variables (e.g. first DVT, recurrent DVT);
-
definition of PTS;
-
results;
-
intention‐to‐treat analysis;
-
number and reason for withdrawals;
-
source of funding;
-
use of an a priori sample size/power calculation.
Assessment of risk of bias in included studies
One review author (SA) independently assessed bias in all studies using Cochrane's 'Risk of bias' tool (Higgins 2011). Another review author (DNK) independently repeated this process for verification. We resolved any disagreements by discussion. The 'Risk of bias' tool examined six key sources of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and any other bias. These domains were assessed and classified by low risk of bias or high risk of bias. When insufficient details were available to allow assessment of risk of bias, we reported it as 'unclear' risk. Where there were additional possible bias concerns, we reported them under the domain 'Other bias' and in the Characteristics of included studies table.
Measures of treatment effect
We intended to report dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI) and continuous outcomes as standardised mean difference (SMD) with 95% CI. Due to the different methodologies used in the individual studies we reported the results as presented by each individual study.
Unit of analysis issues
We intended to use the participant as the unit of analysis. However, studies reported both by individual participant and by limb. As we were unable to pool results, we presented data from individual studies separately instead of combining different units of analysis. Should additional data be available in review updates and we are able to combine results, we will deal with this issue by identifying and matching the number of observations in the analysis and the number of 'units' that were randomised in cluster or cross‐over trials; therefore, no special issues with regard to analyses of studies with non‐standard designs would exist (Higgins 2011).
Dealing with missing data
The analyses were based on the intention‐to‐treat data from the individual trials. To maintain intent‐to‐treat analysis, all participants randomised to treatment based on the trial report were used in the analysis of this review. Where necessary, we contacted study authors for missing data.
Assessment of heterogeneity
The studies were visually assessed for clinical heterogeneity. We did not perform further heterogeneity investigations because of the use of different methodologies in the studies. If sufficient trials are available for analysis in review updates, we will assess the degree of heterogeneity among trials by visual inspection of forest plots and performance of Chi2 and I2 tests. We planned to consider I2 values less than 50% as indicative of low heterogeneity, I2 values between 50% and 75% as indicative of moderate heterogeneity, and I2 values greater than 75% as indicative of substantial heterogeneity (Higgins 2011). If we identify substantial clinical, methodological, or statistical heterogeneity, we planned to consider whether pooling of results in a meta‐analysis or using a narrative approach to data synthesis was appropriate.
Assessment of reporting biases
We intended to construct a funnel plot to test for reporting bias in meta‐analyses that included 10 or more studies (Higgins 2011). It was not possible to do this, as we did not identify sufficient studies.
Data synthesis
The comparison of interventions as well as the format of the reported outcomes made the results of these studies unsuitable for the calculation of a summary statistic, hence we performed a narrative overview. In future, if appropriate, we will use a fixed‐effect model to calculate the pooled treatment effect estimate with 95% CIs for dichotomous outcome variables and SMD with 95% CI for continuous outcomes, as detailed under Measures of treatment effect. We will use a random‐effects model if there is substantial heterogeneity (defined as I2 greater than 75%), and we will create a forest plot for each treatment effect.
Subgroup analysis and investigation of heterogeneity
We were unable to carry out any subgroup analysis or investigation of heterogeneity because of the difference in study methodology and reporting of outcomes. If suitable studies are identified in review updates, we will explore heterogeneity by examining factors that may be influential, such as definition of PTS used, care setting, time of follow‐up, and incidence of recurrent DVT.
Sensitivity analysis
We were unable to carry out sensitivity analyses because of the study heterogeneity. If suitable studies had been identified, we intended to test the robustness of our results by analysing RCTs and CCTs separately to access the efficacy of the effect estimation calculated with Mantel Haenszel test (Mantel 1959).
'Summary of findings' tables
We prepared 'Summary of findings' tables to present the evidence for the use of compression therapy in treating PTS among participants with a diagnosis of DVT. We used no external information in generating the 'Assumed risk' column. We used the GRADE approach to evaluate the evidence and to assign one of four levels of certainty: high, moderate, low, or very low (Guyatt 2008; Higgins 2011). We required no departures from standard methods in generating these tables. We included the primary and secondary endpoints described under Types of outcome measures. We chose these endpoints because we deemed them to be most clinically relevant. As we were unable to combine data in a meta‐analysis due to study differences, we presented the evidence narratively. We created a 'Summary of findings' table for the comparisons of 'Graduated elastic compression stockings compared with placebo stockings or no compression for the treatment of PTS' (summary of findings Table for the main comparison), and 'Intermittent pneumatic compression devices compared with control devices for the treatment of PTS' (summary of findings Table 2).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
See Figure 1.
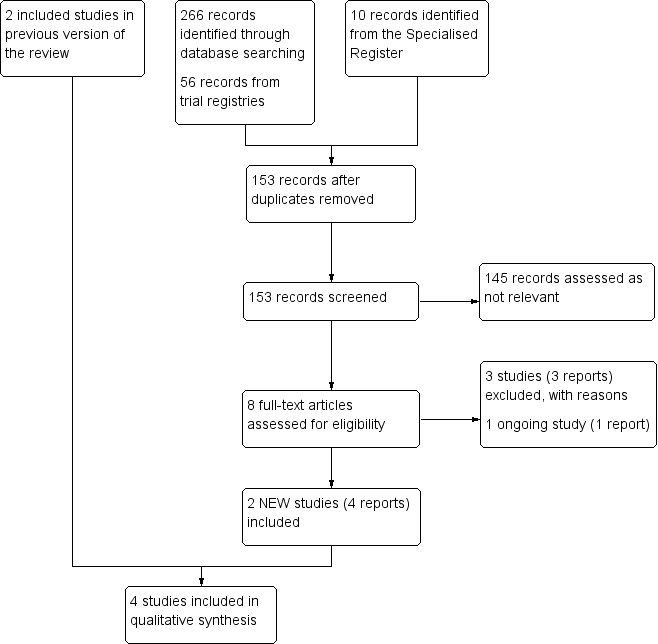
Study flow diagram.
Included studies
The search identified two new studies for this update (Lattimer 2013; O'Donnell 2008), bringing the total to four included studies comprising 116 participants. Two studies evaluated the use of graduated elastic compression stockings (Ginsberg 2001; Lattimer 2013), and two evaluated the use of intermittent compression devices (Ginsberg 1999; O'Donnell 2008).
Two studies, comprising 69 participants, evaluated the ability of graduated elastic compression stockings to reduce symptoms in people with PTS (Ginsberg 2001; Lattimer 2013).
Ginsberg 2001 compared the effectiveness of elastic compression stockings (40 mmHg pressure at the ankle) with placebo stockings (one to two sizes too large) in 35 participants with PTS defined by a symptom list, independent of severity. Participants were screened for PTS one year after diagnosis of DVT. Follow‐up was planned for two years. Participants who had symptomatic DVT (n = 30), and asymptomatic DVT (n = 3) were included. In two participants, it was unclear if they had symptomatic clinical presentation. The primary outcome in this study was treatment failure, defined as pain and swelling that did not improve, or worsened, after the first three months; or worsening of symptoms during further follow‐up; or symptoms that prevented participants from performing their daily activities for five or more days in any three‐month period; or developing a leg ulcer.
Lattimer 2013 conducted a prospective study on the haemodynamic performance of medical elastic compression stockings. They randomly assigned 40 limbs with PTS from 34 consecutive participants to four different stocking types. The median age was 62 years (range 31 to 81 years). Four different stockings were used to determine the best one at reducing reflux in that leg (class I or class II, below knee (BK) or above knee level (AK)). Each participant acted as his or her own control. Compliance rate on the use of stockings in the period prior to the study and stocking preference following the study period were reported as secondary outcomes. The Venous Clinical Severity Score (VCSS), the 'C' part of the CEAP, and the Villalta Scale were used to assess the severity of PTS. Question 10 of the VCSS was used to determine compliance using stockings prior to the study. Air plethysmography was used to measure the venous filling index (VFI), venous volume, and time to fill 90% of the venous volume. There was no follow‐up after the study was conducted.
Two small cross‐over RCTs comprising 47 participants evaluated the use of intermittent compression devices for the treatment of PTS (Ginsberg 1999; O'Donnell 2008).
Ginsberg 1999 compared the effectiveness of intermittent compression units applying a low (15 mmHg) or a higher therapeutic pressure (50 mmHg), in a randomised cross‐over design for one month using the Jobst extremity pump. The Jobst extremity pump consists of an inflatable synthetic sleeve that fits over the extremity and a pump that intermittently inflates and deflates the sleeve. The pump pressure can be adjusted, and pressures of 15 mmHg to 50 mmHg have been used therapeutically to relieve symptoms of leg swelling resulting from lymphoedema. Fifteen participants with severe PTS were included. Treatment was considered successful in participants who preferred the therapeutic pressure, wished to continue to use this pressure, and who demonstrated an improvement on a symptom score list. Scores were derived from answers to a questionnaire assessing symptoms and functional status, completed after each one month segment of the study. Changes in signs and symptoms of PTS were not reported separately.
O'Donnell 2008 evaluated a lightweight, portable, battery‐powered, cuff‐like compression device (Venowave device). This two‐centre, placebo‐controlled, double‐blind, cross‐over RCT of 32 people with severe PTS had a follow‐up of 20 weeks. The mean age was 50 years (range 25 to 80). The primary outcome was defined by clinical success (reporting benefit from the intervention), at least moderate improvement in PTS symptoms, and willingness to continue using the device. Secondary outcomes included each of the component clinical success responses, device preference, PTS severity (Villalta Scale), VEnous INsufficiency Epidemiological and Economic Study – Quality of Life (VEINES‐QOL) and VEnous INsufficiency Epidemiological and Economic Study – Symptoms (VEINES‐Sym).
Excluded studies
We excluded three new studies for this update (Frulla 2005; Kelechi 2011; Stacey 1988). Five studies are excluded in total (Frulla 2005; Kahn 2003; Kakkos 2001; Kelechi 2011; Stacey 1988). Kakkos 2001 was not designed to treat participants but rather to test the effectiveness of a new intermittent pneumatic compression system on 10 participants with PTS and 10 participants with varicose veins. Kahn 2003 was designed to evaluate the effect of graduated elastic compression stockings on leg symptoms and signs during exercise in participants who had a DVT one year previously. In this cross‐over study 40 participants were excluded, of whom 19 were diagnosed as PTS. Frulla 2005 and Stacey 1988 were designed to compare elastic compression stockings with pharmaceuticals and surgery respectively. Kelechi 2011 was designed to asses the effectiveness of cryotherapy gel wrap on microcirculation of skin affected by chronic venous disorders. Summary details of excluded studies are given in the Characteristics of excluded studies.
Ongoing studies
We identified one ongoing study (NCT01637428). See Characteristics of ongoing studies table.
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The included studies had low or unclear overall risk of bias (Ginsberg 1999; Ginsberg 2001; Lattimer 2013; O'Donnell 2008). Overall, the studies were at low risk of performance bias for subjective outcomes because of the way they blinded the participants and personnel. Selection bias was low in three studies (Ginsberg 1999; Lattimer 2013; O'Donnell 2008), because these three studies concealed allocation to treatments, but risk was unclear for Ginsberg 2001 due to the lack of description of their allocation method. Risk of attrition was of low among all four of the included studies. Only one study did not fully describe loss to follow‐up (Ginsberg 2001). Most studies used survival curves to deal with loss to follow‐up, and, therefore, we believe that this approach did not introduce high risk of bias. We found no risk of selective reporting bias in the included studies. Other bias was unclear for all included studies.
Allocation
Three of the four included studies achieved true randomisation with allocation concealment (i.e. the person recruiting the participant into the trial was unaware to which group the participant was assigned). Ginsberg 1999 randomly assigned by means of a computer‐generated randomisation schedule, to use the extremity pump at either 50 mmHg (the therapeutic pressure) or 15 mmHg (the placebo pressure) for the first month. In the second month participants were instructed to use the other pressure, such that those who had been using the therapeutic pressure switched to the placebo pressure and vice versa. Ginsberg 2001 provided unclear information on allocation concealment. Lattimer 2013 applied the stockings (Mediven plus open toe, Bayreuth, Germany) in random order using sealed envelopes. Study personnel were unaware of allocation at the time of outcome assessments. O'Donnell 2008 determined allocation concealment by consecutively numbered participant kits that contained encrypted codes, corresponding to a randomly ordered pair of Venowave or control device.
Blinding
All studies had at least some blinded component. Ginsberg 1999 provided the participants either intervention or control extremity pump. Bias was also reduced by informing the participants that both the efficacy and the optimum pressure for symptom relief were unknown and that they were comparing the relative effects of a high pressure and a low pressure. Ginsberg 2001 and Lattimer 2013 used different stockings in order to blind the participants and assessors. O'Donnell 2008 devices were prepackaged in pairs with each Venowave paired with a control device. Paired devices were labelled corresponding to the order of use, and not related to whether they were active or control. The research nurse provided the participants with the kits without knowing what was in the kit.
Incomplete outcome data
Ginsberg 2001 did not fully describe loss to follow‐up, but indicated that three people in the active stockings group died. All studies reported minimal losses to follow‐up and withdrawals. Overall, we judged the studies to be at low risk of attrition bias.
Selective reporting
All included studies reported on the prespecified outcomes and predictor variables.
Other potential sources of bias
All included studies appeared free of other risk of bias but other bias could not be ruled out. We judged the studies to be at unclear risk of other potential sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Graduated elastic compression stockings for the treatment of PTS; Summary of findings 2 Intermittent pneumatic compression devices for the treatment of PTS
Due to methodological heterogeneity among the included studies, and use of different scoring systems for assessment of severity of PTS, it was not possible to combine the studies in a meta‐analysis. Therefore, we narratively reported our findings by graduated elastic compression stocking (GECS) and intermittent pneumatic compression (medical) devices.
Severity of post‐thrombotic syndrome
Graduated compression stockings
Ginsberg 2001 was conducted as a part of a larger study. The authors used a specially developed scoring system for the participant response to treatment. We evaluated only the part of the study in which they enrolled participants with symptomatic PTS. The definition used in this study was: chronic (duration of more than one month), typical (better after a night's sleep and leg elevation, worse at the end of the day and after prolonged standing or sitting), and pain and swelling of the leg(s) six months or more after a proximal DVT. Participants were categorised as having PTS only if they had both pain and swelling. Of the group with active stockings, 11/18 (61.1%) participants treated were considered to be treatment failures compared with 10/17 (58.8%) participants treated with placebo stockings. Treatment failure was defined as pain and swelling that did not improve, or worsened, after the first three months; or worsening of symptoms during further follow‐up; or symptoms that prevented participants from performing their daily activities for five or more days in any three‐month period; or developing a leg ulcer.
Lattimer 2013 investigated haemodynamic measurements with GECS use and reported that the VFI, venous filling time (VFT90), and the venous volume (VV) significantly improved with all four classes of stockings compared with no compression. The extent of improvement correlated with the initial magnitude of reflux, irrespective of the class or length of stocking. However, only 18% to 30% of the legs showed a normal VFI with stockings. In class I, the VFI improved from a median of 4.9 mL/s (range 1.7 to 16.3 mL/s) without compression; 3.7 mL/s (range 0 to 14 mL/s) with BK (24.5% improvement); and 3.6 mL/s (range 0.6 to 14.5 mL/s) with AK (26.5% improvement). With class II, the corresponding improvement was to 4.0 mL/s (range 0.3 to 16.2 mL/s) with BK (18.8% improvement); and 3.7 mL/s (range 0.5 to 14.2 mL/s) with AK (24.5% improvement).
As one study reported benefit and one reported no benefit from GECS use, we judged this evidence to be very low‐certainty. See summary of findings Table for the main comparison.
Intermittent pneumatic compression devices
Ginsberg 1999 reported that treatment for 12/15 participants (80%, 95% confidence interval (CI) 52% to 96%) was considered successful. However, after discontinuation of the study only nine participants (mean 60%, 95% CI 32% to 100%) continued to use the compression pump successfully, at pressures between 40 mmHg and 50 mmHg; between one and four times a day. Participants reported benefit six to 30 months after their enrolment in the original study. The symptom score at therapeutic pressure levels was 14.3 (standard deviation (SD) 5.4) compared to 16.5 (SD 6.1) at placebo pressure levels. When the authors analysed it as a cross‐over study (paired Student's t‐test), the mean difference in scoring at the two pressure levels was –2.1 (95% CI –3.6 to –0.7; P = 0.007). Appendix 4 shows the individual patient symptom scores.
O'Donnell 2008 used the Venowave device and defined clinical success as reporting benefit from the intervention, at least moderate improvement in PTS symptoms, and willingness to continue using the device. Eight participants reported clinical success with the Venowave device alone, two reported benefit with the control device alone, two reported success with both devices, and 14 reported success with neither device (P = 0.11). The mean Villalta score was significantly decreased for Venowave (12) compared to the control group (15) (P = 0.004).
We judge this evidence to be of low‐certainty. See summary of findings Table 2.
Adverse effects
Graduated compression stockings
In Ginsberg 2001, no participants developed leg ulceration. In addition, no adverse effects after initiation of the compression therapy were mentioned. No information was available on reduction of oedema, pain, recurrence of DVT, and pulmonary embolism. Lattimer 2013 did not assess adverse effects.
Intermittent pneumatic compression devices
Only O'Donnell 2008 measured and reported the adverse effects of treatment. Three of 32 (9%) participants reported adverse effects, including leg swelling, irritation, superficial bleeding, and skin itching, associated with the Venowave device (moderate‐certainty evidence) (O'Donnell 2008). Ginsberg 1999 did not assess adverse effects.
Patient satisfaction and quality of life
Graduated compression stockings
Ginsberg 2001 did not assess patient satisfaction or QoL.
Lattimer 2013 assessed preferences in which way participants preferred the stocking. Results showed a difference favouring wearing of class of stockings with 21 (52.5%) participants indicating they wanted to change their compression to another type of stocking, with 8/21 (38%) of these participants preferring an AK stocking (Lattimer 2013).
Intermittent pneumatic compression devices
In the only study that measured QoL, O'Donnell 2008 reported a mean difference in VEINES‐QOL score of 2.9 points in participants treated with the Venowave versus the placebo device (P = 0.004). The mean VEINES‐QOL score at the end of the study period was significantly higher for Venowave (53) than for the control device (50) (P = 0.004) (moderate‐certainty evidence). Ginsberg 1999 did not asses patient satisfaction or QoL.
Compliance rate
Graduated compression stockings
Only one study assessed and reported on compliance (Lattimer 2013). The participants were questioned on their use of compression, by VCSS, to determine compliance in the period prior to the study. The results were: not used (7/34, 20.6%), intermittent use (3/34, 8.8%), most days (3/34, 8.8%), and full compliance (21/34, 61.8%) (low‐certainty evidence) (Lattimer 2013). Lattimer 2013 did not report on compliance rates during the study period. Ginsberg 2001 did not assess or report compliance rates.
Intermittent pneumatic compression devices
O'Donnell 2008 reported that one participant discontinued wearing the device after an injury that seemed to be unrelated to the study. Ginsberg 1999 did not assess or report compliance rates.
Discussion
Summary of main results
We systematically reviewed the clinical trial literature to assess the effectiveness of compression therapies for the treatment of PTS. Treatments assessed in the reviewed studies included use of graduated elastic compression stocking (GECS) (Ginsberg 2001; Lattimer 2013), and intermittent pneumatic compression devices (Ginsberg 1999; O'Donnell 2008). We were unable to carry out meta‐analyses as the methodologies of the studies were too heterogeneous so we reported and summarised results narratively in 'Summary of findings' tables, one for GECS (summary of findings Table for the main comparison), and one for intermittent compression devices (summary of findings Table 2).
Two studies using GECS had different results for the outcome of PTS severity. The positive haemodynamic effects found in Lattimer 2013 contrasted with the results of Ginsberg 2001, who reported no benefit compared to placebo (very low‐certainty evidence). After three months of follow‐up, there was no substantial benefit of GECS in the reduction of symptoms in Ginsberg 2001, where the assessment of PTS was carried out with a non‐validated scale. Lattimer 2013 and O'Donnell 2008 used the Vallalta scale to assess the severity of PTS.
The two studies using intermittent pneumatic compression found the active devices more effective than the placebo devices in treating PTS (low‐certainty evidence) (Ginsberg 1999; O'Donnell 2008). Both studies assessed a composite outcome measure which was defined as treatment success. The assessments of this definition differed, as did the methods to assess the outcome.
In general, adverse effects of stockings do not often occur. Ginsberg 2001 only reported that there were no ulcerations in the intervention and placebo group. Most frequently reported adverse effects (in one study) during the use of an intermittent compression device were leg swelling, irritation, superficial bleeding, and skin itching. These adverse effects were related to the Venowave device and were relatively low (9%) (moderate‐certainty evidence) (O'Donnell 2008).
Only one included study reported QoL (moderate‐certainty evidence; O'Donnell 2008). O'Donnell 2008 reported a significant improvement in QoL between the Venowave intermittent pneumatic compression device and control group. The mean VEINES‐QOL in the Venowave group was significantly higher (P = 0.004).
None of the studies assessed or reported on patient satisfaction.
Overall completeness and applicability of evidence
Our literature search, which included all languages, yielded four RCTs, demonstrating a scarcity of studies that evaluated compression therapy in PTS. All included trials and results are relevant to our review questions. However, the included studies used different classification systems, including CEAP criteria, the Villalta score, the Ginsberg measure, or the study authors' own classification systems. These scoring systems are used for diagnosis and severity. Although there considerable research on the prevention of PTS, there is less evidence on the diagnosis and management of PTS. The lack of a clear standard definition of PTS creates different outcomes. The lack of standardisation in the assessment of outcomes was a limitation of PTS research in general. Soosainathan and colleagues aimed to assess the most frequently used scoring systems. They illustrated these scoring systems and came to the conclusion that the Villalta score was favourable and should be recommended (Soosainathan 2013).
Ginsberg 2001 concluded that most participants had no PTS one year after proximal DVT and did not require use of elastic compression stockings. However, this study included people with asymptomatic DVT with low risk for PTS. In fact, in a subpopulation of participants with symptomatic DVT, these investigators assessed the prevalence of PTS (using a different, non‐validated, and possibly less‐sensitive scale) as 27%, which was not distinctly different from the other studies. A blinded comparison was enabled by using a stocking that was one to two sizes too large instead of no intervention. This might have diminished the contrast between treatment groups, in that even a stocking that is too large will likely provide some compression. Indeed, it has been shown that stockings that are one or two sizes too large exert considerable pressure when compared with stockings without elastic threads (Partsch 2002). Moreover, the therapeutic stocking used exerted pressure of 20 mmHg to 30 mmHg at the ankle region. This is less than the pressure applied in other studies, which compared a stocking with an ankle pressure of 30 mmHg to 40 mmHg. Thus, it is possible that an effect could have been present had the comparison been done with a true placebo stocking. Additionally, we analysed the data of thigh‐ and knee‐high stockings together because in measurements of venous pressure and foot volume there was no superiority of thigh high stockings (Partsch 1984). More recent studies also found that thigh‐length stockings did not provide better protection than below‐knee stockings, and that lower tolerability of thigh‐length stockings can lead to discontinuation (Lattimer 2013; Prandoni 2012). Although it seemed that these outcomes were similarly defined in Ginsberg 1999 and O'Donnell 2008, the definitions were not identical. Ginsberg 1999 defined treatment as a success if the participant preferred the therapeutic pressure, stated that participants would continue using the intermittent pneumatic compression device, and indicated that the difference in pressure was of at least slight importance. O'Donnell 2008 defined treatment as a success if the participant benefited from the intervention, at least moderate improvement in PTS symptoms, and willingness to continue using the device. A beneficial effect of compression therapy is in line with the observed increased ulcer‐healing rates compared with no compression (Cullum 2003).
As stated before, the methods of outcome assessment differ widely among the included studies. For example, the two studies on intermittent pneumatic compression devices assessed 'treatment success' and although it seemed that these outcomes were similarly defined, the definitions were not identical. Another limitation was that not all outcomes assessed were clinically important. For example, calf and ankle circumference were commonly assessed but it is unclear if changes in these measures translate to improvements in patient‐important outcomes. Only one study assessed QoL. Also, the long‐term benefits or harms of treatments are largely unknown, because of the short duration of treatment in the studies. None of the studies assessed sustained effectiveness. Although we do not expect sustained effectiveness once treatment is discontinued, because PTS is a chronic condition, therapies would be expected to be used for long periods of time and ideally would have sustained benefit and safety with long‐term use. Therefore, it is of importance to evaluate if benefits that are seen in the short term, would continue to be safe if used for longer periods of time.
Only one study specified previous use of graduated compression stockings as an exclusion criteria (Ginsberg 2001). The remaining studies did not report on previous use of compression.
Quality of the evidence
We assessed the overall certainty of the evidence according to the GRADE approach. Our review was limited by considerable heterogeneity among the included studies. For example, Ginsberg 1999 and Ginsberg 2001 assessed methods and outcomes such as PTS severity using a unique scale, whereas O'Donnell 2008 and Lattimer 2013 used the Villalta Scale to grade the clinical severity of PTS. Studies were also small (116 participants in total) and of short duration (one to five months), only one study had a duration of 25.6 months (Ginsberg 2001). Only one study assessed QoL. Compliance to study intervention was not assessed in the included studies.
We judged the certainty of the evidence with GECS use for the severity of PTS as very low. Limited evidence was available for adverse effects, patient satisfaction, QoL, and compliance rate with GECS use. See summary of findings Table for the main comparison for specific reasons for downgrading.
We judged the certainty of the evidence with intermittent compression device use for the severity of PTS as low; and adverse effects, patient satisfaction, and QoL outcomes as moderate. There was no available evidence for compliance with device use. See summary of findings Table 2 for specific reasons for downgrading.
Potential biases in the review process
Methods used in this review such as study selection, data extraction, and risk of bias assessment were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we judged them unlikely to cause any bias. Searching of the literature was extensive, but it remains possible that relevant data were not included in this review because they were not published or were not found during the search.
We were unable to carry out meta‐analyses as the methodologies of the studies were too heterogeneous. Therefore, we reported results narratively.
Agreements and disagreements with other studies or reviews
The results presented here mostly agreed with similar reviews and discussed a need for further research on this topic (Kahn 2016; Pikovsky 2018; Ten Cate‐Hoek 2015). The review by Kahn 2016 described a personal approach on how to treat PTS, suggesting GECS as a treatment starting point and stating that more studies are needed on the effectiveness of GECS and other compression modalities to treat PTS. Ten Cate‐Hoek 2015 and Pikovsky 2018 discussed and summarised the current evidence regarding prevention and treatment of PTS. Their results agreed with our results, that the effectiveness of GECS for PTS treatment has not been conclusively demonstrated.

Study flow diagram.
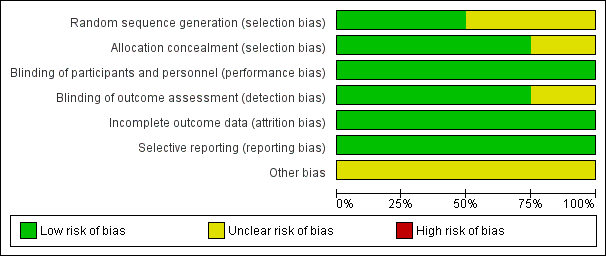
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Graduated elastic compression stockings compared with placebo stockings or no compression for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: GECSa Comparison: placebo stockings or no compression | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo stockings | GECS | |||||
| Severity of post‐thrombotic syndromeb (follow‐up: 0–25.6 months) | Ginsberg 2001 reported 61.1% (11/18) treatment failures in the GECS group and 58.8% (10/17) treatment failure in the placebo group. Lattimer 2013 reported the VFI, VFT90, and VV significantly improved with GECS compared with no compression. VFI: no compression median 4.9 (range 1.7 to 16.3) BKI median 3.7 (range 0–14; 24.5% percentage improvement) AKI median 3.6 (range 0.6–14.5; 26.5%) BKII median 4.0 (range 0.3–16.2; 18.8%) AKII median 3.7 (range 0.5–14.2; 24.5%) | — | 69 (2 RCTs) | ⊕⊝⊝⊝ | 2 small studies of short duration were identified. 1 reported benefit and 1 no benefit from GECS use. | |
| Adverse effects (follow‐up: 25.6 months) | Ginsberg 2001 reported no participants developed ulceration in both groups | — | — | — | Lattimer 2013 did not assess adverse effects. | |
| Patient satisfaction and quality of life | See comments | — | — | — | No study measured quality of life. Lattimer 2013 assessed patient preferences. 52.5% of participants indicated they wanted to change their compression to another type of stocking, 38% of these preferred an AK stocking. | |
| Compliance rate | See comments | — | — | — | Lattimer 2013 and Ginsberg 2001 did not assess compliance rates during the study period. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AKI/II: above‐knee thigh length stocking class I or II; BKI/II: below‐knee class I or II; CI: confidence interval; GECS: graduated elastic compression stockings; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; VFI: venous filling index; VFT90: venous filling time; VV: venous volume. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 2001 compared compression stockings (30 mmHg to 40 mmHg) with placebo stockings (one to two sizes too large). Calf length or thigh length compression stockings were administered depending on the localisation of complaints. Lattimer 2013 assessed four different stockings and assessed improvements of compression and length (of their size in random order: class I (18 mmHg to 21 mmHg) and class II (23 mmHg to 32 mmHg), BK and AK. | ||||||
| Intermittent pneumatic compression devices compared with control devices for the treatment of PTS | ||||||
| Patient or population: adults with PTS Settings: hospitals and clinical centres Intervention: medical compression devicea Comparison: control device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control device | Medical compression device | |||||
| Severity post‐thrombotic syndromeb (follow‐up: 8–20 weeks) | Ginsberg 1999: 80% (12/15) of participants had an improvement in symptom score that was considered a treatment success. The mean difference in scoring at the 2 pressure levels was –2.1 (95% CI –3.6 to –0.7; P = 0.007) O'Donnell 2008: an improvement in Villalta score was reported in the Venowave group (12) compared to the control device group (15) (P = 0.004) | 47 (2 RCTs) | ⊕⊕⊝⊝ | Control devices were used on the same participants that used medical device. | ||
| Adverse effects (follow‐up: 20 weeks) | O'Donnell 2008 reported 9% (3/32) of participants experienced adverse effects, including leg swelling, irritation, superficial bleeding, and skin itching related to Venowave device use | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Patient satisfaction and quality of life (follow‐up: 20 weeks) | O'Donnell 2008 reported the mean VEINES‐QoL score at the end of the study period was significantly higher for Venowave (53) than for the control device (50) (P = 0.004) | 32 (1 RCT) | ⊕⊕⊕⊝ | Not measured by Ginsberg 1999. | ||
| Compliance rate | See comments | — | — | No study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PTS: post‐thrombotic syndrome; RCT: randomised controlled trial; QoL: quality of life; VEINES‐QOL: VEnous INsufficiency Epidemiological and Economic Study – Quality of Life. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aGinsberg 1999 used an extremity pump twice daily at either 50 mmHg pressure or 15 mmHg pressure. O'Donnell 2008 used Venowave, a lower‐limb venous‐return assist device. | ||||||

