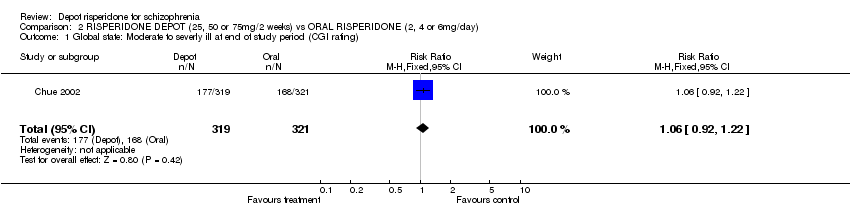
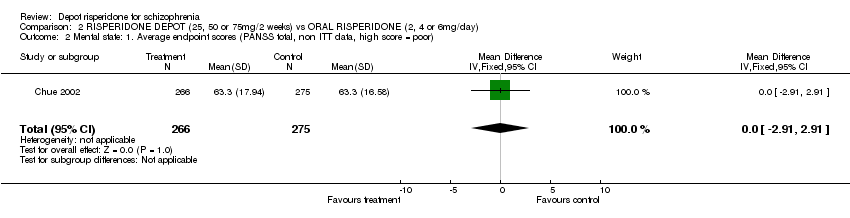
| 1 Mental state: Exacerbation of specific symptoms Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 1.1 anxiety | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.05] |
| 1.2 agitation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 1.3 hallucinations | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.22] |
| 1.4 nervousness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.25] |
| 1.5 psychosis | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.83] |
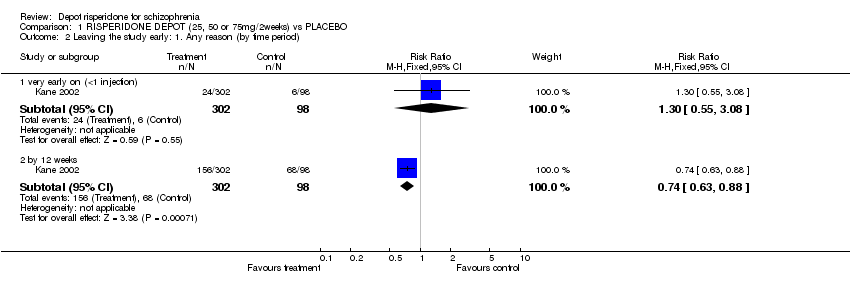
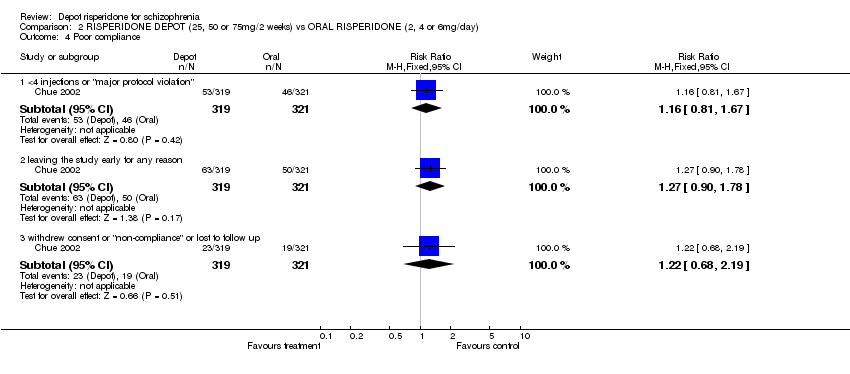
| 2 Leaving the study early: 1. Any reason (by time period) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 2.1 very early on (<1 injection) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.55, 3.08] |
| 2.2 by 12 weeks | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.88] |
| 3 Leaving the study early: 2. Any reason (by doses) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 3.1 all doses risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.88] |
| 3.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.94] |
| 3.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 3.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
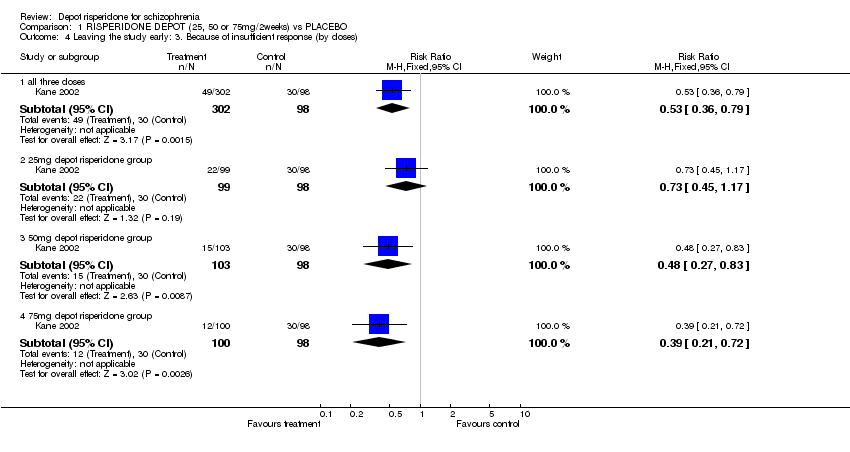
| 4 Leaving the study early: 3. Because of insufficient response (by doses) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 4.1 all three doses | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.79] |
| 4.2 25mg depot risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.17] |
| 4.3 50mg depot risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.83] |
| 4.4 75mg depot risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 5 Adverse events: 1. General: 1. Death by 12 weeks Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.65] |
|
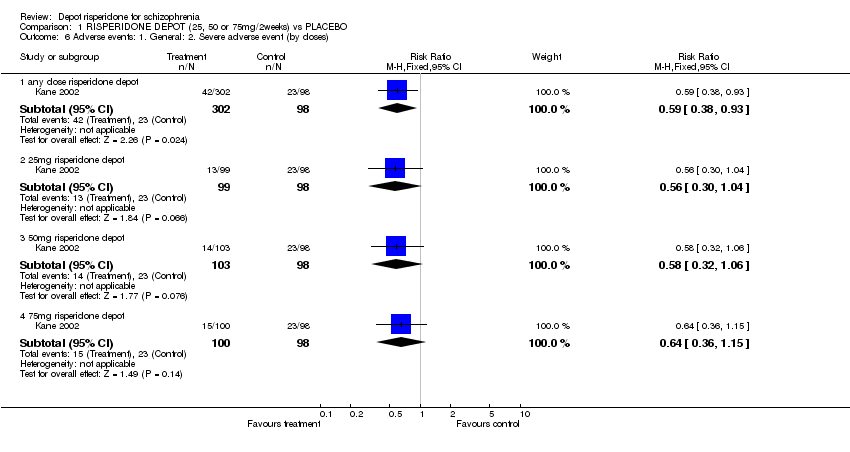
| 6 Adverse events: 1. General: 2. Severe adverse event (by doses) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 6.1 any dose risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| 6.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 6.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.06] |
| 6.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.15] |
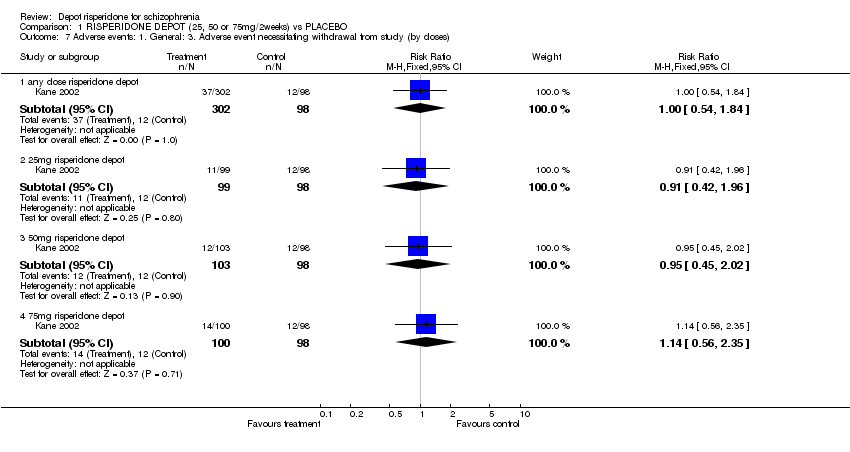
| 7 Adverse events: 1. General: 3. Adverse event necessitating withdrawal from study (by doses) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 7.1 any dose risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.84] |
| 7.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.96] |
| 7.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |
| 7.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.35] |
| 8 Adverse events: 2. Specific: 1. Cardiovascular Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 8.1 dizziness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.62, 3.43] |
| 8.2 tachycardia | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.98] |
| 9 Adverse events: 2. Specific: 2. Gastrointestinal Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 9.1 constipation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.17 [0.84, 45.46] |
| 9.2 diarrhoea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.23, 3.20] |
| 9.3 nausea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.76] |
| 9.4 vomiting | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.23, 1.57] |
| 10 Adverse events: 2. Specific: 3. Movement disorders: a. Extrapyramidal disorder ‐ spontaneously reported (by do Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 10.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.73, 7.78] |
| 10.2 25mg risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.74] |
| 10.3 50mg risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.69, 9.29] |
| 10.4 75mg risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.93, 11.51] |
| 11 Adverse events: 2. Specific: 4. Movement disorders: b. Hyperkinesia (by doses) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 11.1 all doses of risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.60, 4.84] |
| 11.2 25mg risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.64] |
| 11.3 50mg risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.68, 6.73] |
| 11.4 75mg of risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.79, 7.55] |
| 12 Adverse events: 2. Specific: 5. Movement disorders: c. Hypertonia (by doses) Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 12.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.22] |
| 12.2 25mg risperidone | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.22, 2.86] |
| 12.3 50mg risperidone | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.19] |
| 12.4 75mg risperidone | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.70, 5.53] |
| 13 Adverse events: 2. Specific: 6. Pain Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 13.1 headache | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.88, 2.80] |
| 13.2 pain ‐ unspecified | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.48, 4.00] |
| 14 Adverse events: 2. Specific: 7. Salivation Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 14.1 decreased | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.37, 22.76] |
| 14.2 increased | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.37, 22.76] |
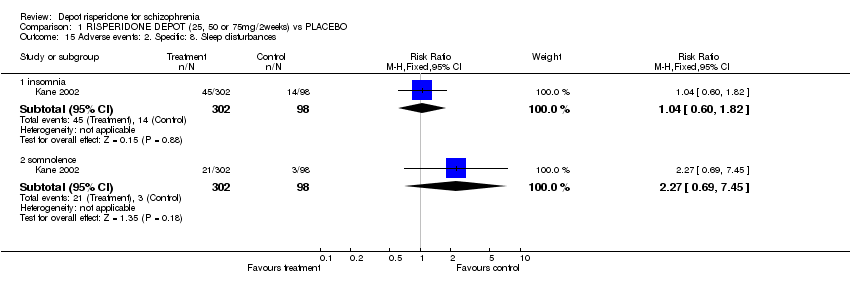
| 15 Adverse events: 2. Specific: 8. Sleep disturbances Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 15.1 insomnia | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.60, 1.82] |
| 15.2 somnolence | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.69, 7.45] |
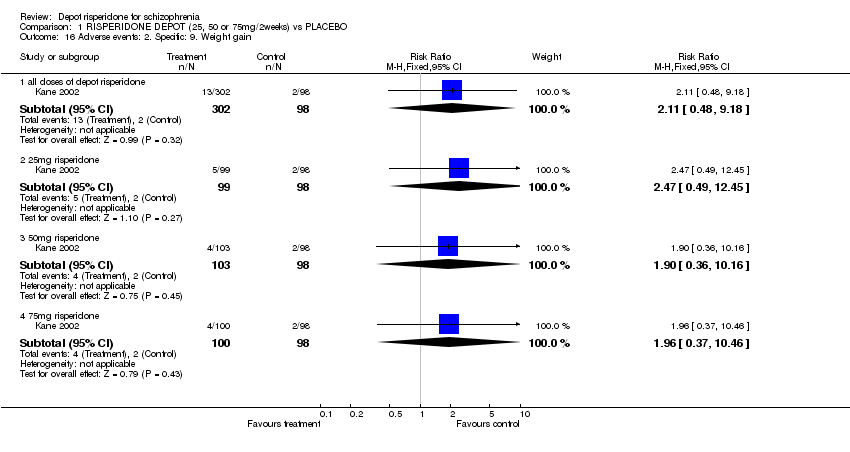
| 16 Adverse events: 2. Specific: 9. Weight gain Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 16.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.48, 9.18] |
| 16.2 25mg risperidone | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.49, 12.45] |
| 16.3 50mg risperidone | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.36, 10.16] |
| 16.4 75mg risperidone | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.37, 10.46] |
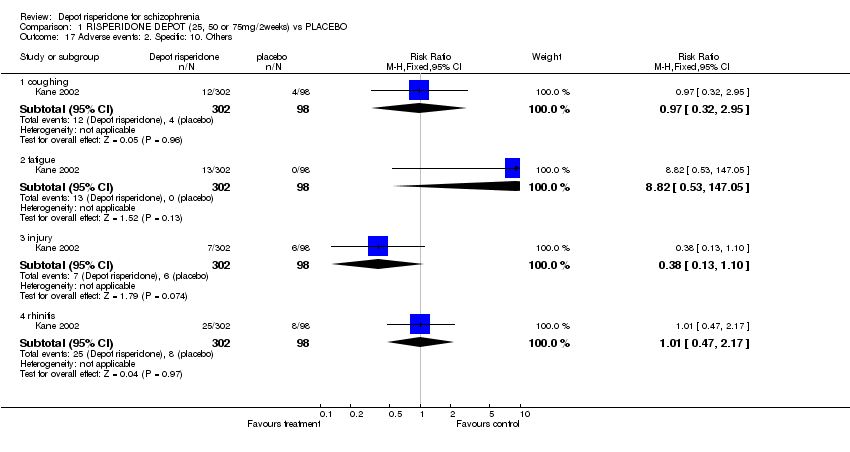
| 17 Adverse events: 2. Specific: 10. Others Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 17.1 coughing | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.32, 2.95] |
| 17.2 fatigue | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.82 [0.53, 147.05] |
| 17.3 injury | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.10] |
| 17.4 rhinitis | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.47, 2.17] |