Risperidona de depósito para la esquizofrenia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Allocation: randomised, stratified by PANSS, ESRS, depot in last 6/12, dose of risperidone at end of run in. | |
| Participants | Diagnosis: schizophrenia (DSM IV). | |
| Interventions | 1. Risperidone depot: 25, 50 or 75mg, 2 weekly + daily placebo tablets. N=319. | |
| Outcomes | Mental state: PANSS. Unable to use ‐ | |
| Notes | * blindness was maintained with different doses by using the same volume of diluent. ** Numbers randomised not consistent in presentations (426 vs 640). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised, not described. | |
| Participants | Diagnosis: schizophrenia (DSM IV). | |
| Interventions | 1. Risperidone depot: 25mg 2 weekly + 2mg/day oral risperidone for 3/52. N=99. | |
| Outcomes | Global state: CGI. Unable to use ‐ | |
| Notes | * Unclear how blindness was maintained as it was necessary to inject different doses and therefore number of millilitres. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
DSM IV: Diagnostic and Statistical Mannual version 1V.
ESRS: Extrapyramidal Symptom Rating Scale.
N: Number.
PANSS: Positive And Negative Symptom Scale.
SD: Standard Deviation.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Allocation: randomised. | |
| Allocation: not randomised, open label. | |
| Allocation: not randomised, review. | |
| Allocation: randomised. | |
| Allocation: non‐randomised trial. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: Exacerbation of specific symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 1 Mental state: Exacerbation of specific symptoms. | ||||
| 1.1 anxiety | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.05] |
| 1.2 agitation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 1.3 hallucinations | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.22] |
| 1.4 nervousness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.25] |
| 1.5 psychosis | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.83] |
| 2 Leaving the study early: 1. Any reason (by time period) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 2 Leaving the study early: 1. Any reason (by time period). | ||||
| 2.1 very early on (<1 injection) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.55, 3.08] |
| 2.2 by 12 weeks | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.88] |
| 3 Leaving the study early: 2. Any reason (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 3 Leaving the study early: 2. Any reason (by doses). | ||||
| 3.1 all doses risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.88] |
| 3.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.94] |
| 3.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 3.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 4 Leaving the study early: 3. Because of insufficient response (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 4 Leaving the study early: 3. Because of insufficient response (by doses). | ||||
| 4.1 all three doses | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.79] |
| 4.2 25mg depot risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.17] |
| 4.3 50mg depot risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.83] |
| 4.4 75mg depot risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 5 Adverse events: 1. General: 1. Death by 12 weeks Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.65] |
| Analysis 1.5  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 5 Adverse events: 1. General: 1. Death by 12 weeks. | ||||
| 6 Adverse events: 1. General: 2. Severe adverse event (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 6 Adverse events: 1. General: 2. Severe adverse event (by doses). | ||||
| 6.1 any dose risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| 6.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 6.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.06] |
| 6.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.15] |
| 7 Adverse events: 1. General: 3. Adverse event necessitating withdrawal from study (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 7 Adverse events: 1. General: 3. Adverse event necessitating withdrawal from study (by doses). | ||||
| 7.1 any dose risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.84] |
| 7.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.96] |
| 7.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |
| 7.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.35] |
| 8 Adverse events: 2. Specific: 1. Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 8 Adverse events: 2. Specific: 1. Cardiovascular. | ||||
| 8.1 dizziness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.62, 3.43] |
| 8.2 tachycardia | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.98] |
| 9 Adverse events: 2. Specific: 2. Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 9 Adverse events: 2. Specific: 2. Gastrointestinal. | ||||
| 9.1 constipation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.17 [0.84, 45.46] |
| 9.2 diarrhoea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.23, 3.20] |
| 9.3 nausea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.76] |
| 9.4 vomiting | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.23, 1.57] |
| 10 Adverse events: 2. Specific: 3. Movement disorders: a. Extrapyramidal disorder ‐ spontaneously reported (by do Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 10 Adverse events: 2. Specific: 3. Movement disorders: a. Extrapyramidal disorder ‐ spontaneously reported (by do. | ||||
| 10.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.73, 7.78] |
| 10.2 25mg risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.74] |
| 10.3 50mg risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.69, 9.29] |
| 10.4 75mg risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.93, 11.51] |
| 11 Adverse events: 2. Specific: 4. Movement disorders: b. Hyperkinesia (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 11 Adverse events: 2. Specific: 4. Movement disorders: b. Hyperkinesia (by doses). | ||||
| 11.1 all doses of risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.60, 4.84] |
| 11.2 25mg risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.64] |
| 11.3 50mg risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.68, 6.73] |
| 11.4 75mg of risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.79, 7.55] |
| 12 Adverse events: 2. Specific: 5. Movement disorders: c. Hypertonia (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 12 Adverse events: 2. Specific: 5. Movement disorders: c. Hypertonia (by doses). | ||||
| 12.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.22] |
| 12.2 25mg risperidone | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.22, 2.86] |
| 12.3 50mg risperidone | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.19] |
| 12.4 75mg risperidone | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.70, 5.53] |
| 13 Adverse events: 2. Specific: 6. Pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 13 Adverse events: 2. Specific: 6. Pain. | ||||
| 13.1 headache | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.88, 2.80] |
| 13.2 pain ‐ unspecified | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.48, 4.00] |
| 14 Adverse events: 2. Specific: 7. Salivation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 14 Adverse events: 2. Specific: 7. Salivation. | ||||
| 14.1 decreased | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.37, 22.76] |
| 14.2 increased | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.37, 22.76] |
| 15 Adverse events: 2. Specific: 8. Sleep disturbances Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 15 Adverse events: 2. Specific: 8. Sleep disturbances. | ||||
| 15.1 insomnia | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.60, 1.82] |
| 15.2 somnolence | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.69, 7.45] |
| 16 Adverse events: 2. Specific: 9. Weight gain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 16 Adverse events: 2. Specific: 9. Weight gain. | ||||
| 16.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.48, 9.18] |
| 16.2 25mg risperidone | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.49, 12.45] |
| 16.3 50mg risperidone | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.36, 10.16] |
| 16.4 75mg risperidone | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.37, 10.46] |
| 17 Adverse events: 2. Specific: 10. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 17 Adverse events: 2. Specific: 10. Others. | ||||
| 17.1 coughing | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.32, 2.95] |
| 17.2 fatigue | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.82 [0.53, 147.05] |
| 17.3 injury | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.10] |
| 17.4 rhinitis | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.47, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Moderate to severly ill at end of study period (CGI rating) Show forest plot | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| Analysis 2.1  Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 1 Global state: Moderate to severly ill at end of study period (CGI rating). | ||||
| 2 Mental state: 1. Average endpoint scores (PANSS total, non ITT data, high score = poor) Show forest plot | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.91, 2.91] |
| Analysis 2.2  Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 2 Mental state: 1. Average endpoint scores (PANSS total, non ITT data, high score = poor). | ||||
| 3 Mental state: 2. Average change scores (PANSS, non ITT data, high score = good) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 3 Mental state: 2. Average change scores (PANSS, non ITT data, high score = good). | ||||
| 3.1 PANSS total | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.84, 1.04] |
| 3.2 PANSS positive | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.86, 0.26] |
| 3.3 PANSS negative | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.93, 0.73] |
| 4 Poor compliance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 4 Poor compliance. | ||||
| 4.1 <4 injections or "major protocol violation" | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.81, 1.67] |
| 4.2 leaving the study early for any reason | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.90, 1.78] |
| 4.3 withdrew consent or "non‐compliance" or lost to follow up | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.68, 2.19] |
| 5 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 5 Adverse events. | ||||
| 5.1 death | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.20] |
| 5.2 adverse event leading to withdrawal from study | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.35] |
| 5.3 any adverse event reported | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 1 Mental state: Exacerbation of specific symptoms.
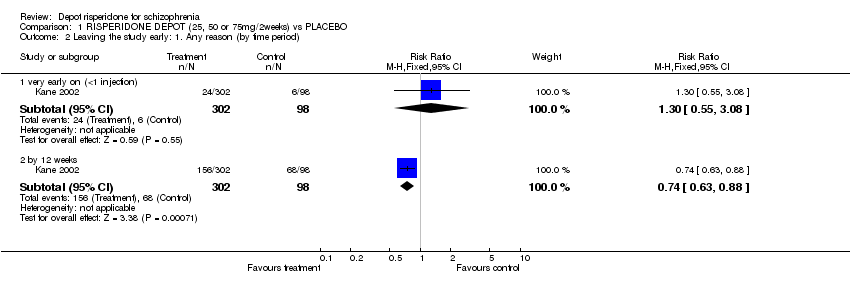
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 2 Leaving the study early: 1. Any reason (by time period).

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 3 Leaving the study early: 2. Any reason (by doses).
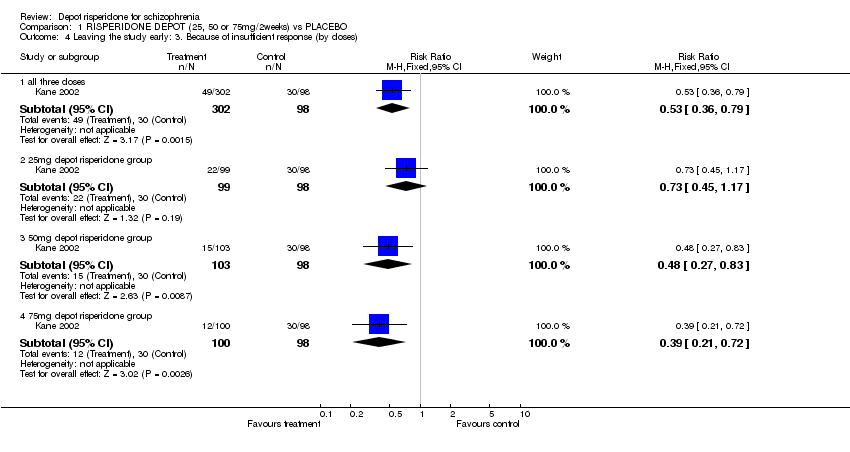
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 4 Leaving the study early: 3. Because of insufficient response (by doses).

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 5 Adverse events: 1. General: 1. Death by 12 weeks.
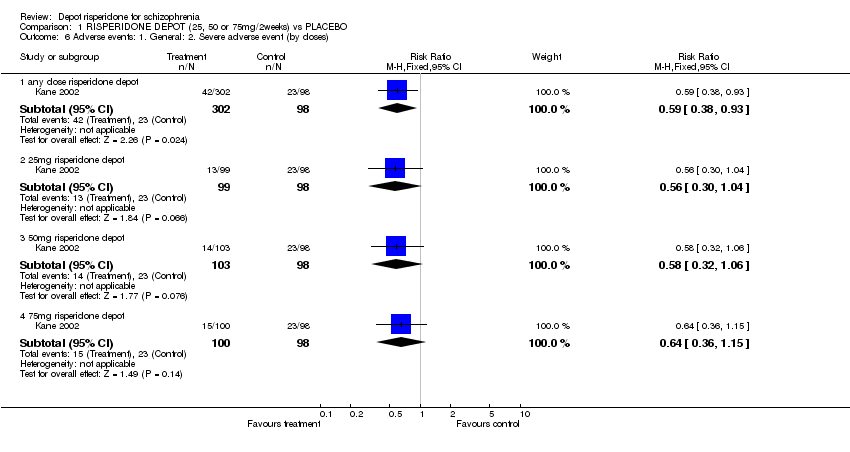
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 6 Adverse events: 1. General: 2. Severe adverse event (by doses).
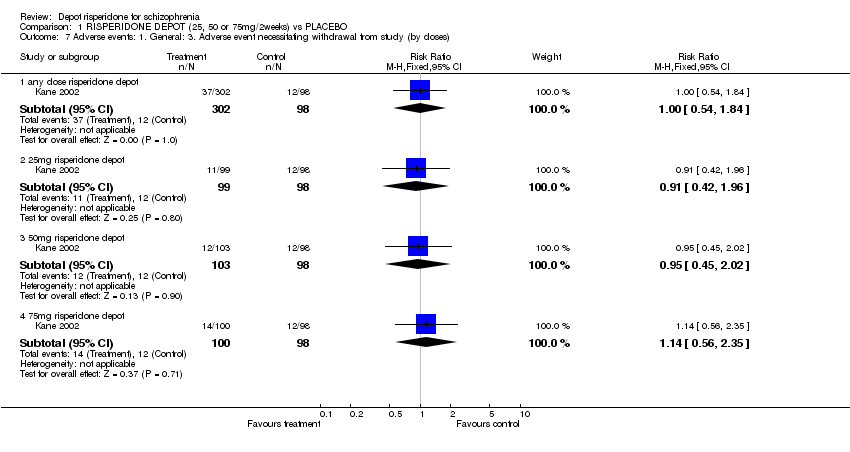
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 7 Adverse events: 1. General: 3. Adverse event necessitating withdrawal from study (by doses).

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 8 Adverse events: 2. Specific: 1. Cardiovascular.

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 9 Adverse events: 2. Specific: 2. Gastrointestinal.

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 10 Adverse events: 2. Specific: 3. Movement disorders: a. Extrapyramidal disorder ‐ spontaneously reported (by do.

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 11 Adverse events: 2. Specific: 4. Movement disorders: b. Hyperkinesia (by doses).

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 12 Adverse events: 2. Specific: 5. Movement disorders: c. Hypertonia (by doses).

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 13 Adverse events: 2. Specific: 6. Pain.

Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 14 Adverse events: 2. Specific: 7. Salivation.
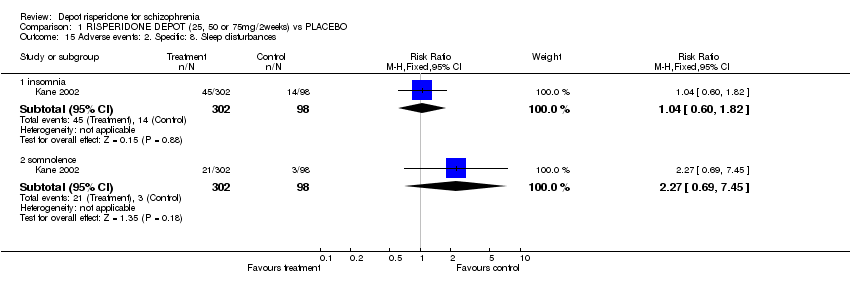
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 15 Adverse events: 2. Specific: 8. Sleep disturbances.
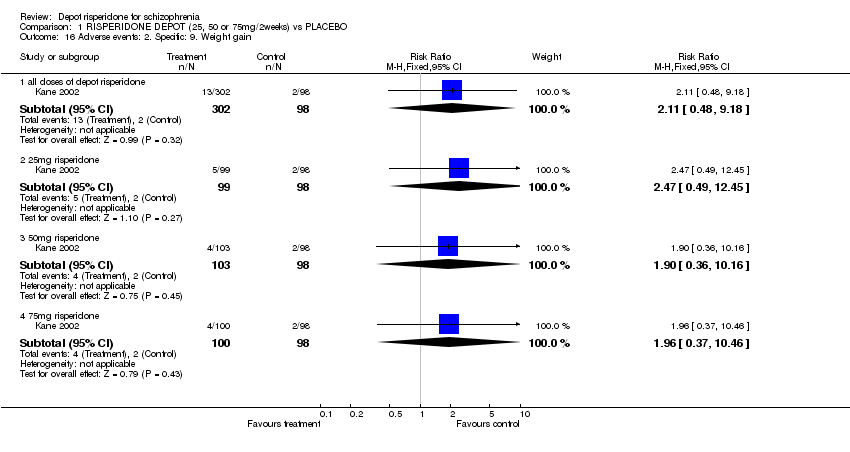
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 16 Adverse events: 2. Specific: 9. Weight gain.
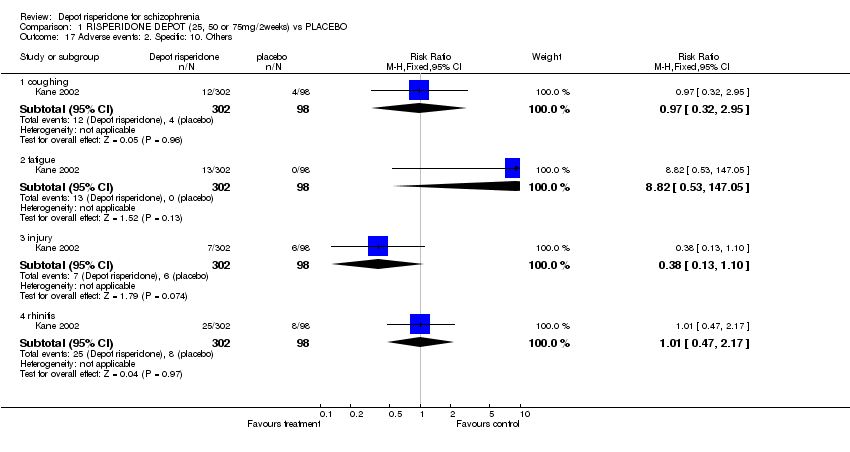
Comparison 1 RISPERIDONE DEPOT (25, 50 or 75mg/2weeks) vs PLACEBO, Outcome 17 Adverse events: 2. Specific: 10. Others.
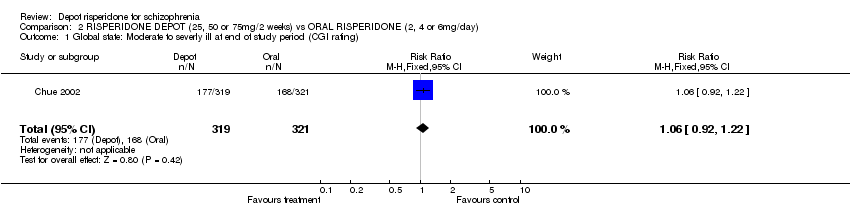
Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 1 Global state: Moderate to severly ill at end of study period (CGI rating).
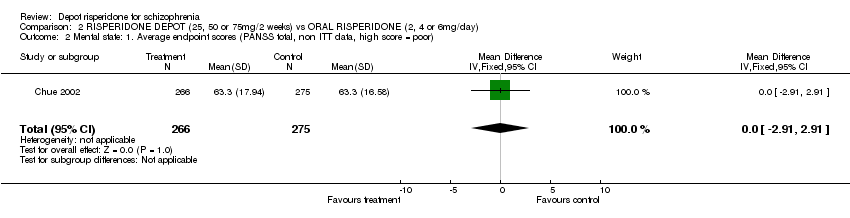
Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 2 Mental state: 1. Average endpoint scores (PANSS total, non ITT data, high score = poor).

Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 3 Mental state: 2. Average change scores (PANSS, non ITT data, high score = good).
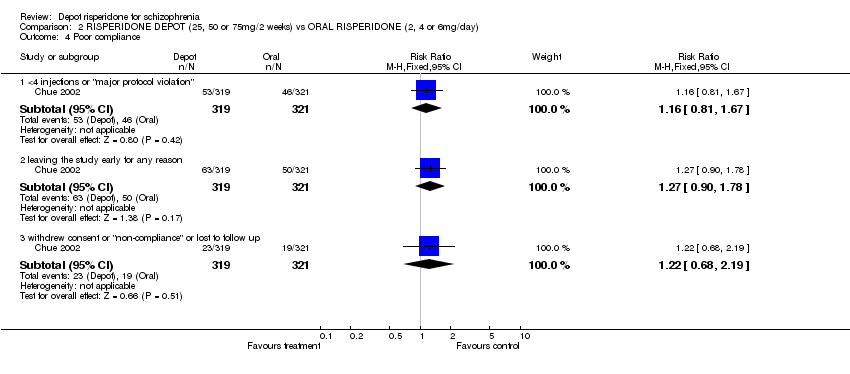
Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 4 Poor compliance.

Comparison 2 RISPERIDONE DEPOT (25, 50 or 75mg/2 weeks) vs ORAL RISPERIDONE (2, 4 or 6mg/day), Outcome 5 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: Exacerbation of specific symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 anxiety | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.05] |
| 1.2 agitation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 1.3 hallucinations | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.22] |
| 1.4 nervousness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.25] |
| 1.5 psychosis | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.83] |
| 2 Leaving the study early: 1. Any reason (by time period) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 very early on (<1 injection) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.55, 3.08] |
| 2.2 by 12 weeks | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.88] |
| 3 Leaving the study early: 2. Any reason (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 all doses risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.88] |
| 3.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.94] |
| 3.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 3.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 4 Leaving the study early: 3. Because of insufficient response (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 all three doses | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.79] |
| 4.2 25mg depot risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.45, 1.17] |
| 4.3 50mg depot risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.83] |
| 4.4 75mg depot risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 5 Adverse events: 1. General: 1. Death by 12 weeks Show forest plot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.65] |
| 6 Adverse events: 1. General: 2. Severe adverse event (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 any dose risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| 6.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.04] |
| 6.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.06] |
| 6.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.36, 1.15] |
| 7 Adverse events: 1. General: 3. Adverse event necessitating withdrawal from study (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 any dose risperidone depot | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.84] |
| 7.2 25mg risperidone depot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.96] |
| 7.3 50mg risperidone depot | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |
| 7.4 75mg risperidone depot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.35] |
| 8 Adverse events: 2. Specific: 1. Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 dizziness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.62, 3.43] |
| 8.2 tachycardia | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.11, 0.98] |
| 9 Adverse events: 2. Specific: 2. Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 constipation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.17 [0.84, 45.46] |
| 9.2 diarrhoea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.23, 3.20] |
| 9.3 nausea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.39, 2.76] |
| 9.4 vomiting | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.23, 1.57] |
| 10 Adverse events: 2. Specific: 3. Movement disorders: a. Extrapyramidal disorder ‐ spontaneously reported (by do Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.73, 7.78] |
| 10.2 25mg risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.74] |
| 10.3 50mg risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.69, 9.29] |
| 10.4 75mg risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.93, 11.51] |
| 11 Adverse events: 2. Specific: 4. Movement disorders: b. Hyperkinesia (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 all doses of risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.60, 4.84] |
| 11.2 25mg risperidone group | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.64] |
| 11.3 50mg risperidone group | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.68, 6.73] |
| 11.4 75mg of risperidone group | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.79, 7.55] |
| 12 Adverse events: 2. Specific: 5. Movement disorders: c. Hypertonia (by doses) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.47, 3.22] |
| 12.2 25mg risperidone | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.22, 2.86] |
| 12.3 50mg risperidone | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.28, 3.19] |
| 12.4 75mg risperidone | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.70, 5.53] |
| 13 Adverse events: 2. Specific: 6. Pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 headache | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.88, 2.80] |
| 13.2 pain ‐ unspecified | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.48, 4.00] |
| 14 Adverse events: 2. Specific: 7. Salivation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 decreased | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.37, 22.76] |
| 14.2 increased | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.37, 22.76] |
| 15 Adverse events: 2. Specific: 8. Sleep disturbances Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 insomnia | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.60, 1.82] |
| 15.2 somnolence | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.69, 7.45] |
| 16 Adverse events: 2. Specific: 9. Weight gain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 all doses of depot risperidone | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.48, 9.18] |
| 16.2 25mg risperidone | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.49, 12.45] |
| 16.3 50mg risperidone | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.36, 10.16] |
| 16.4 75mg risperidone | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.37, 10.46] |
| 17 Adverse events: 2. Specific: 10. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 coughing | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.32, 2.95] |
| 17.2 fatigue | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.82 [0.53, 147.05] |
| 17.3 injury | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.10] |
| 17.4 rhinitis | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.47, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Moderate to severly ill at end of study period (CGI rating) Show forest plot | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| 2 Mental state: 1. Average endpoint scores (PANSS total, non ITT data, high score = poor) Show forest plot | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.91, 2.91] |
| 3 Mental state: 2. Average change scores (PANSS, non ITT data, high score = good) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 PANSS total | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.84, 1.04] |
| 3.2 PANSS positive | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.86, 0.26] |
| 3.3 PANSS negative | 1 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.93, 0.73] |
| 4 Poor compliance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 <4 injections or "major protocol violation" | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.81, 1.67] |
| 4.2 leaving the study early for any reason | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.90, 1.78] |
| 4.3 withdrew consent or "non‐compliance" or lost to follow up | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.68, 2.19] |
| 5 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 death | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.20] |
| 5.2 adverse event leading to withdrawal from study | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.35] |
| 5.3 any adverse event reported | 1 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |

