Thermothérapie par micro‐ondes dans les cas d'hyperplasie bénigne de la prostate
Résumé scientifique
Contexte
La résection transurétrale de la prostate (RTUP) est le traitement de référence pour soulager les symptômes urinaires et améliorer le débit urinaire chez les hommes souffrant d'une hyperplasie bénigne de la prostate (HBP). Cependant, la morbidité de la RTUP avoisine les 20 % et des techniques moins invasives ont été développées pour traiter la HBP. Les données préliminaires suggèrent que la thermothérapie par micro‐ondes, qui fournit une énergie par micro‐ondes pour produire une nécrose par coagulation des tissus de la prostate, est un traitement sûr, efficace, pour la HBP.
Objectifs
Évaluer l'efficacité thérapeutique et l'innocuité des techniques de thermothérapie par micro‐ondes pour traiter les hommes souffrant d'une obstruction bénigne de la prostate symptomatique.
Stratégie de recherche documentaire
Des essais contrôlés randomisés ont été identifiés à partir de recherches dans The Cochrane Library, MEDLINE, EMBASE, les bibliographies d'articles trouvés, les revues, les rapports techniques, et en contactant des investigateurs experts concernés et des fabricants de micro‐ondes.
Critères de sélection
Tous les essais contrôlés randomisés évaluant la thermothérapie par micro‐ondes transurétrales (TMTU) pour les hommes souffrant d'une HBP symptomatique ont été éligibles pour cette revue. Les groupes de comparaison pouvaient comprendre la résection transurétrale de la prostate, des techniques de prostatectomie mini‐invasives, de fausses procédures de thermothérapie et des médicaments. Les critères de jugement comprenaient les symptômes urinaires, la fonction urinaire, le volume de la prostate, la mortalité, la morbidité et la reprise du traitement. Deux auteurs de la revue ont identifié de façon indépendante des résumés potentiellement pertinents, puis ont évalué les articles complets en vue de leur inclusion.
Recueil et analyse des données
Deux auteurs de la revue ont extrait de façon indépendante le plan d'étude, les caractéristiques initiales et les données relatives aux critères de jugement, puis ont évalué la qualité méthodologique au moyen d'un formulaire standard. Nous avons tenté d'obtenir les données manquantes auprès des auteurs ou des commanditaires, ou des deux.
Résultats principaux
Dans cette mise à jour, nous n'avons découvert aucune nouvelle comparaison randomisée de la TMTU qui fournissait des données évaluables sur l'efficacité. Quinze études, portant sur 1 585 patients, ont répondu aux critères d'inclusion, à savoir six comparaisons de la thermothérapie par micro‐ondes à la RTUP, huit comparaisons à de fausses procédures de thermothérapie et une comparaison à un alpha‐bloquant. La durée des études allait de 3 à 60 mois. L'âge moyen des participants était de 66,8 ans et les scores des symptômes et les débits urinaires initiaux, qui n'étaient pas différents selon les groupes de traitement, ont démontré des symptômes des voies urinaires inférieures modérément graves. Les scores de symptômes urinaires moyens combinés ont diminué de 65 % avec la TMTU et de 77 % avec la RTUP. La différence moyenne pondérée (DMP) avec intervalle de confiance (IC) à 95 % pour le score international symptomatique de la prostate (IPSS) a été de ‐1,00 (IC à 95 % ‐2,03 à ‐0,03), en faveur de la RTUP. Le débit urinaire maximal moyen combiné a augmenté de 70 % avec la TMTU et de 119 % avec la RTUP. La DMP pour le débit urinaire maximal a été de 5,08 mL/s (IC à 95 % 3,88 à 6,28 mL/s), en faveur de la RTUP. Comparé à la RTUP, la TMTU a été associée à une diminution des risques d'éjaculation rétrograde, de traitement de strictions, d'hématurie, de transfusions sanguines et de syndrome de résection transurétrale, mais à des risques accrus de dysurie, de rétention urinaire et de reprise du traitement en raison de symptômes de HBP. La thermothérapie par micro‐ondes a amélioré les scores IPSS des symptômes (DMP ‐5,15, IC à 95 % ‐4,26 à ‐6,04) et le débit urinaire maximal (DMP 2,01 mL/s, IC à 95 % 0,85 à 3,16) comparé aux fausses procédures. La thermothérapie par micro‐ondes a également amélioré les scores IPSS des symptômes (DMP ‐4,20, IC à 95 % ‐3,15 à ‐5,25) et le débit urinaire maximal (DMP 2,30 mL/s, IC à 95 % 1,47 à 3,13) dans la comparaison avec les alpha‐bloquants. Aucune étude n'a évalué les effets de la durée des symptômes, des caractéristiques des patients, des taux d'antigène prostatique spécifique ou du volume de la prostate sur la réponse au traitement.
Conclusions des auteurs
Les techniques de thermothérapie par micro‐ondes sont des alternatives efficaces à la RTUP et aux alpha‐bloquants pour traiter la HBP symptomatique chez les hommes sans antécédents de rétention urinaire ou n'ayant pas subi de procédures prostatiques antérieures et ayant un volume de prostate de 30 à 100 mL. Cependant, la RTUP a donné des plus grandes améliorations du score de symptômes et du débit urinaire et a réduit la nécessité de traitements ultérieurs de la HBP comparé à la TMTU. La petite taille des échantillons et les différences de plan d'étude limitent les comparaisons entre les dispositifs de conception et de niveaux d'énergie différents. Les effets de la durée des symptômes, des caractéristiques des patients ou du volume de la prostate sur la réponse au traitement sont inconnus.
PICO
Résumé simplifié
La thermothérapie est un traitement efficace pour soulager les symptômes urinaires et les problèmes de débit urinaire provoqués par une prostate volumineuse
L'hyperplasie bénigne de la prostate (HBP) peut conduire à des troubles urinaires gênants, en particulier chez les hommes âgés. La résection transurétrale de la prostate (RTUP) est considérée comme le traitement définitif de la HBP. Cependant, la RTUP est associée à des complications. Par conséquent, des techniques moins invasives ont été développées. Celles‐ci comprennent la thermothérapie par micro‐ondes qui applique de l'énergie (chaleur) pour réduire les tissus volumineux de la prostate. Nous avons découvert que la thermothérapie par micro‐ondes était une option thérapeutique relativement sûre et efficace. La thermothérapie par micro‐ondes peut être pratiquée en ambulatoire et présente des effets secondaires moins nombreux et moins graves que la RTUP. Cependant, la RTUP a donné de plus grandes améliorations des symptômes urinaires et du débit urinaire et moins d'hommes ont nécessité une reprise du traitement. Des études supplémentaires doivent être réalisées pour déterminer les critères d'évaluation à long terme de la thermothérapie par micro‐ondes et identifier les dispositifs de thermothérapie par micro‐ondes et les réglages d'énergie le plus efficaces.
Authors' conclusions
Summary of findings
| Transurethral microwave thermotherapy (TUMT) compared with transurethral resection of the prostate (TURP) for symptomatic benign prostatic hyperplasia | ||||
| Patient or population: men with symptomatic benign prostatic hyperplasia Settings: office (TUMT) or hospital (TURP) Intervention: Transurethral microwave thermotherapy (TUMT) Comparison: Transurethral resection of the prostate (TURP) | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| TURP | TUMT | |||
| International Prostate Symptom Score (IPSS). Range: 0 to 35 points. Follow‐up: 6 mos ‐ 1 year | The mean IPSS scores ranged across control groups from 3.4 to 7.1 points | The mean IPSS in the intervention group was 1 point higher (95% CI, 0.03 lower to 2.03 higher) | 306 | ⊕⊕⊕⊝ |
| Madsen‐Iversen Symptom Score Range 0 to 27 points Follow‐up: 6 mos ‐ 1 year | The mean Madsen‐Iversen Symptom scores ranged across control groups from 0.62 to 2.7 points | The mean Madsen‐Iversen Symptom score in the intervention group was 1.59 points higher (95% CI, 0.69 lower to 2.48 higher) | 338 | ⊕⊕⊕⊝ |
| Peak Flow Rate (Qmax): Measurement: mL per second Follow‐up: 6 mos ‐ 1 year | The mean Peak Flow Rate ranged across control groups from 14.6 to 20.6 mL/s | The mean urinary peak flow rate in the intervention group was 5.08 mL/s lower (95% CI, 3.88 to 6.28 lower) | 108 | ⊕⊕⊕⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1 <400 subjects evaluated for this outcome which is a non‐optimal information size | ||||
Background
Description of the condition
Benign prostatic hyperplasia (BPH) is a common condition in older men who can result in bothersome lower urinary tract symptoms (LUTS). The histological changes of hyperplasia are found in over half of 60‐year‐old men and nearly 90% of 85‐year‐old men (McConnell 1994). Over 30% of men 65 years and older have irritative (urgency, frequency, nocturia) and/or obstructive (weak stream, hesitancy, intermittency, and incomplete emptying)urinary symptoms attributed to BPH (Chapple 2001). In 2000, LUTS accounted for nearly 8 million physician visits in the United States with nonpharmacologic BPH treatment costs estimated to be USD 1.1 billion (Wei 2004). Nearly 90,000 prostatectomies were performed for BPH that year (Wei 2004), and an estimated one in four men will have undergone treatment to relieve BPH symptoms by age 80 (Barry 1990). Medicare data from 2005 documented the performance of 127,786 BPH procedures (Yu 2008).
For many years the transurethral resection of the prostate (TURP) has been the definitive treatment for BPH. Although TURP has been proven to reduce symptoms of BPH and increase urinary flow compared with watchful waiting (Wasson 1995), the procedure has some limitations. The perioperative and postoperative (early and late) morbidity of TURP exceeds 20%, including blood loss requiring transfusion, infections, strictures, sexual dysfunction, urinary incontinence, urinary retention, and the development of the transurethral resection syndrome (Borboroglu 1999; Mebust 1989). Transurethral resection syndrome is a dilutional hyponatremia caused by absorbing large volumes of irrigation fluid during the procedure. Presentation of this potentially lethal condition includes nausea, vomiting, abdominal pain, confusion, seizures, and coma. However, over time, the risk for transfusion has decreased from 2.5% to 0.4% and the risk of the transurethral resection syndrome has decreased from 2% to 0.8%. About 10% of patients undergoing TURP will still have persistent bothersome lower urinary tract symptoms by three years after surgery (Wasson 1995), and reoperation rates are about 1% to 2% annually (Roos 1998; Wasson 1995).
Description of the intervention
In recent years the number of men undergoing TURP has steadily declined due to increasing utilization of pharmacologic treatments (alpha‐blockers and 5‐alpha‐reductase inhibitors) and of minimally invasive surgical techniques, such as transurethral needle ablation, laser coagulation and vaporization techniques, and microwave thermotherapy (Yu 2008). Microwave techniques vary by the degree of prostatic heating and include hyperthermia (heating prostate tissue to 42 ºC to 44 ºC), thermotherapy (45 ºC to 60 ºC), and thermoablation (60 ºC to 75 ºC). Preliminary data suggest that microwave prostatectomies are effective and safe treatments for BPH (Walmsley 2004). Transurethral thermotherapy has become the preferred microwave technique because treatment can be provided in a single session (Jepsen 1998). Transurethral thermotherapy techniques have evolved over the past decade. Initial systems worked at lower energy or heat settings and treatment would take around an hour with minimal discomfort; however, results were disappointing. Subsequent systems incorporated catheters that provided urethral cooling thus allowing higher energy delivery. These advancements reduced the procedure time to around 30 minutes and improved outcomes. However, the higher energy leads to greater discomfort during the procedure, where patients often require sedation and analgesia, and continued risk for urinary retention (EAU 2002; Walmsley 2004).
Compared to TURP, transurethral thermotherapy procedures have fewer major complications and can be performed on an outpatient basis (Walmsley 2004). However, direct comparisons of TUMT with TURP and with other minimally invasive procedures are limited, the effect of using different microwave thermotherapy systems has not been systematically evaluated, and few long‐term follow‐up data are available. We conducted and updated a systematic review, first published in 2008, to search for and combine all evidence from randomized controlled trials evaluating the efficacy and safety of microwave thermotherapy in treating men with symptomatic BPH.
Objectives
-
To quantify the therapeutic efficacy of microwave thermotherapy techniques for men with symptomatic benign prostatic hyperplasia or obstruction
-
To quantify the adverse effects of microwave thermotherapy techniques for men with symptomatic benign prostatic hyperplasia or obstruction
-
To assess whether effects are dependent on specific thermotherapy systems or techniques or on clinical characteristics of trial participants
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials, with or without blinding, of at least three months duration and a minimum of 10 participants in each treatment arm.
Types of participants
Men with symptomatic BPH as determined by elevated urinary symptom scores with or without documented decreased urinary flow rates.
Types of interventions
Microwave thermotherapy techniques that were reviewed included transurethral thermotherapy and transrectal thermotherapy. Control interventions could have included sham thermotherapy, transurethral resection of the prostate (TURP), open prostatectomy, laser prostatectomy, transurethral incision of the prostate, pharmacologic therapy, watchful waiting, electrovaporization of the prostate, prostate stents, radiofrequency transurethral needle ablation, or high‐intensity focused ultrasound.
Types of outcome measures
The primary outcome was the efficacy of microwave thermotherapy in improving urinary tract symptoms based on changes in urologic symptom scale scores (American Urological Association (AUA) Symptom Index, International Prostate Symptom Score (IPSS), Madsen‐Iversen, Boyarsky). Secondary outcomes included mean and peak urinary flow, post‐void residual, prostate volume, and quality of life. Measures of mortality and morbidity included perioperative death, bleeding requiring transfusion, urinary tract infections, epididymitis or orchitis, dysuria, clot retention, urinary retention, erectile dysfunction, retrograde ejaculation, urethral and bladder neck strictures, urinary incontinence, transurethral resection syndrome, and the need for retreatment either surgical or pharmacologic. Hospital length‐of‐stay and catheter duration were also evaluated. Baseline covariates included age, race or ethnicity, prostate size, residual volume, and prostate‐specific antigen (PSA) levels.
Search methods for identification of studies
The search began with The Cochrane Library of randomized trials. MEDLINE and EMBASE were searched from 1989 through July 2011 using validated Cochrane Collaboration strategies for identifying randomized controlled trials. Search terms included prostatectomy, prostatic hyperplasia/surgery, and microwave thermotherapy. Additional studies were identified from bibliographies of retrieved articles and reviews, Science Citation Index, expert trialists, microwave manufacturers, handsearching of the Journal of Urology and also Urology, systematic reviews, and technical reviews.
Data collection and analysis
Selection of studies
Two independent review authors evaluated titles and abstracts of the electronic search results. From the results of the electronic searches, bibliography searches, handsearches, and contact with experts and manufacturers, two review authors independently selected trials that met previously defined inclusion criteria. Trials selected by at least one review author were retrieved.
Data extraction and management
Two review authors then independently abstracted study characteristics and outcomes, including information on study design, participant characteristics, interventions, follow‐up, treatment outcomes, and adverse events. Differences were resolved by discussion among the review authors or using an independent arbitrator. Reasons for study exclusion were documented.
Assessment of risk of bias in included studies
As a measure of overall methodologic study quality, and bias, we assessed scales and criteria developed by Schulz and The Cochrane Collaboration (Cochrane Handbook 2011; Schulz 1995). The seven criteria addressed were:
-
selection bias I (Was there an articulated rule for allocating interventions based on chance?);
-
selection bias II (Was there any foreknowledge of the allocation of interventions by anyone?);
-
blinding bias I (During the course of the trial were study participants and personnel blinded to the knowledge of who received which intervention?);
-
blinding bias II (Were the outcome assessors blinded to who received the intervention and who did not?);
-
attrition bias (Did the trial assess all patients, or account for those not assessed?);
-
reporting bias (Were outcomes selectively reported?);
-
other bias (Were arms assessed differently?).
Each criterion was answered by 'low risk', 'unclear risk', and 'high risk', and summarized here (Figure 1). For the main therapeutic efficacy outcomes, we also assessed the quality of evidence in the 'Summary of findings' table using GRADEpro (GRADEPro 2008).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We calculated relative risks (RR) and absolute risk differences (RD) with associated 95% confidence intervals (CI) for dichotomous data using an intention‐to‐treat principle (we assumed that people who dropped out had negative outcomes, with the exception of death). Weighted mean differences (WMD) with 95% CI were calculated for continuous data.
Assessment of heterogeneity
We used a fixed‐effect model unless heterogeneity was present. Heterogeneity was defined as I2 > 50%.
Results
Description of studies
The combined search strategies identified 43 reports of trials, with 15 distinct randomized trials meeting our inclusion criteria. Among the eligible reports, we evaluated six comparisons of transurethral microwave thermotherapy (TUMT) with TURP (Ahmed 1997; Dahlstrand 1995; D'Ancona 1998; Floratos 2001; Norby 2002a; Wagrell 2002), eight comparisons of TUMT with sham treatment (Abbou 1995 (complication data only); Albala 2002 (complication data only); Bdesha 1994; Blute 1996; De Wildt 1996; Larson 1998; Nawrocki 1997; Roehrborn 1998), and one comparison of TUMT with alpha‐blockers (Djavan 1999). We identified 11 duplicate or serial reports of trials (Bdesha 1993; Dahlstrand 1993; Dahlstrand 1994; D'Ancona 1997; D'Ancona 1997b; de la Rosette 1994; Francisca 1997; Francisca 2000; Ogden 1993; Trachtenberg 1998, Trock 2004). Some of these studies provided supplemental information on baseline patient characteristics and perioperative complications. Three studies (two reports on Wagrell 2002) provided long‐term follow‐up beyond one year, but results from these reports were not systematically evaluated due to poor follow‐up rates and different control groups (Djavan 2001; Mattiasson 2007; Tan 2005; Wagrell 2004).
We excluded studies that provided primarily economic outcome data (de la Rosette 2003; Kobelt 2004; Norby 2002b; Walden 1998) or that provided no evaluable outcomes or complications data (Brehmer 1999; Venn 1995). We also excluded one comparison of TUMT with periurethral TUMT (Albala 2000) and another study where all participants underwent TUMT but were randomized to receive neo‐adjuvant alpha‐blocker therapy or placebo (Djavan 1999b). We excluded two comparisons that utilized hyperthermia rather than thermotherapy (Baert 1994; Zerbib 1992; Zerbib 1994). We also excluded one study comparing TUMT with TURP for men with persistent urinary retention (Schelin 2006) and another study comparing two high‐energy TUMT systems that differed only by one system having an adjunct balloon dilator (Shore 2010).
Risk of bias in included studies
Two studies (comparisons of TUMT with sham controls) met criteria for adequate treatment allocation concealment (central randomization by telephone, opaque envelopes, on‐site computer, etc.) (Blute 1996; Nawrocki 1997). None of the TUMT versus TURP or alpha‐blocker trials blinded patients or providers; two studies indicated that outcomes assessors were blinded (Abbou 1995; Wagrell 2004). However, all studies of TUMT versus sham controls blinded patients and outcomes assessors. Participants randomized to sham procedures underwent insertion of a treatment catheter under local anesthesia and then a simulated treatment program, which could have included circulating coolant and a displayed treatment profile but without the application of microwave energy. Although information was not consistently reported, three studies explicitly described administering prophylactic oral antibiotics to all participants (Blute 1996; Larson 1998; Roehrborn 1998) and three studies reported administering periprocedural oral or parenteral, or both, analgesics (Albala 2002; Blute 1996; Roehrborn 1998) or sedation (Roehrborn 1998). However, parental medications were used infrequently and less often in sham participants. A number of studies evaluated whether blinding was effective. Immediately after undergoing a procedure, Nawrocki et al (Nawrocki 1997) reported that 63% of all participants were uncertain whether they underwent TUMT and Ogden et al reported that over 90% of all participants thought that they had undergone TUMT (Ogden 1993). Differences emerged at three‐month follow‐up; approximately 90% of TUMT participants but only 40% to 50% of sham participants thought that they had undergone TUMT (Bdesha 1994; Larson 1998; Ogden 1993).
All studies provided information on study inclusion and exclusion criteria. Almost all studies reported excluding patients in urinary retention (variably defined by a post‐void residual ranging from 250 mL to 300 mL, catheterization requirement, or history of being unable to void). Most studies also excluded men with prostatic urethral lengths less than 25 mm, prostate volumes less than 30 mL or greater than 100 mL, prominent median lobes, prostate cancer, or history of prostate surgery. Follow‐up duration for TURP studies ranged from six months to three years. One sham‐controlled study provided one‐year follow‐up data (Abbou 1995), three provided six‐month follow‐up data (Larson 1998; Nawrocki 1997; Roehrborn 1998), and four sham‐control studies provided three‐month follow‐up data, at which time participants were unblinded and followed out to one year (Albala 2002; Bdesha 1994; Blute 1996; De Wildt 1996). The study comparing TUMT with alpha‐blockers followed participants for 18 months. The proportion of participants available for follow‐up ranged from 85% to 93% in the studies with six to 12 months of follow‐up (Djavan 1999).
Effects of interventions
See: Summary of findings for the main comparison
TUMT versus TURP
Study participants and characteristics
Overall, 540 participants were randomized in the six trials, including 322 to TUMT and 218 to TURP. Studies in general enrolled men aged 45 years and older with symptom scores above 7, peak urinary flow less than 15 mL/s, and prostate gland volumes between 30 mL and 100 mL. The pooled mean values with the corresponding range of individual mean study values were the following: age, 67.8 years (65 to 70 years); baseline symptom score, 19.5 (15.7 to 21.3); baseline peak urinary flow, 8.6 mL/s (7.9 to 10.1 mL/s); PSA level, 3.2 ng/mL (2.9 to 3.4 ng/mL); and prostate volume, 44.5 mL (33.9 to 52.7). These values did not differ by treatment group.
Clinical outcomes
High‐energy TUMT systems (Prostatron with Prostasoft 2.5 software, ProstaLund Feedback Treatment) were used in five studies; the sixth study used the Prostatron device with Prostasoft 2.0 software (Dahlstrand 1995). TUMT was routinely performed as an outpatient procedure while TURP was an inpatient procedure with a median hospital length‐of‐stay of five days. Catheter duration was consistently longer following TUMT, though usually protocol driven, ranging from 7 to 15 days compared to 2 to 4 days following TURP. Five studies found significant decreases in urinary symptoms and significant increases in peak urinary flow between baseline and follow‐up for both TURP and TUMT. Ahmed and colleagues found that TUMT did not improve peak urinary flow (Ahmed 1997).
TUMT was less effective than TURP in reducing lower urinary tract symptoms. The pooled mean symptom score for men undergoing TUMT decreased 65% in 12 months (19.4 to 6.7) versus 77% (19.6 to 4.5) in the men undergoing TURP. The WMD for the four studies reporting IPSS at follow‐up was ‐1.00 points (95% CI ‐2.03 to 0.03), favoring TURP. Three studies used the Madsen‐Iversen symptom score and the WMD for symptom score at follow‐up was ‐1.59 points (95% CI ‐2.48 to ‐0.69), favoring TURP. Except for one TUMT treatment group (Ahmed 1997), both procedures consistently reduced mean urinary symptom scores from moderate and severe ranges to the mildly symptomatic range. In secondary outcomes, two studies (Ahmed 1997; Dahlstrand 1995) also reported the proportion of participants whose symptom scores improved by > 50% following treatment. Overall, 72% of TUMT participants achieved this level of improvement compared to 98% of TURP participants, RR of 0.74 (95% CI 0.50 to 1.09).
TURP led to greater improvement in peak urinary flow than TUMT. The pooled mean peak urinary flow for men undergoing TUMT increased 70% (from 7.9 mL/s to 13.5 mL/s) versus 119% (from 8.6 mL/s to 18.7 mL/s) in men undergoing TURP. The WMD for peak urinary flow at follow‐up was 5.08 mL/s (95% CI 3.88 to 6.28), favoring TURP. Only two studies reported a mean peak urinary flow following TUMT that exceeded 15 mL/s compared to five studies that reported a mean peak urinary flow exceeding 15 mL/s following TURP. The five studies using Prostatron systems found that TURP consistently improved peak urinary flow compared to TUMT, by 2.20 mL/s to 7.40 mL/s. The one CoreTherm device study (Wagrell 2004) found that TURP was not significantly better than TUMT (WMD 1.90 mL/s, 95% CI ‐1.17 to 4.97).
We were not able to statistically detect any treatment differences between low‐energy and high‐energy systems or between treatment devices for improving urinary symptom scores or peak urinary flow. We found substantial overlap between the pooled point estimates and 95% confidence intervals when comparing low‐ and high‐energy systems with TURP. Although one study using both low‐energy (Prostasoft 2.0) and high‐energy (Prostasoft 2.5) systems found greater improvement in symptom scores (mean difference 2.4) and peak urinary flow (mean flow increase 3.1 mL/s) with the high‐energy system, the differences were not statistically significant (Norby 2002a).
Two studies reported long‐term follow‐up data. Overall, only 71% of participants enrolled by Wagrell and colleagues were available for follow‐up at 36 months (76% TURP, 69% TUMT) (Wagrell 2004). The mean IPSS symptom score was significantly lower among TURP participants compared to TUMT participants (5.0 versus 8.2, P = 0.02). Mean peak urinary flow rates were similar for TURP (13.5 mL/s) and TUMT (11.9 mL/s) participants (P = 0.58). Sixty‐six per cent of these participants completed 60 months of follow‐up (Mattiasson 2007). Symptom scores (6.0 versus 7.4) and peak urinary flows (13.6 mL/s versus 11.4 mL/s) still favored TURP, but differences were not significant. Less than half of the participants enrolled by Floratos and colleagues were available for follow‐up at 36 months (Floratos 2001). The mean IPSS scores were significantly lower following TURP (3 versus 12, P < 0.01) and mean peak urinary flow rates were significantly higher following TURP (24.7 mL/s versus 11.9 mL/s, P < 0.01).
Three studies (Floratos 2001; Norby 2002a; Wagrell 2002) evaluated the effect of treatment on quality of life (QOL) using the IPSS question, "If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?: delighted (1 point), pleased (2 points), mostly satisfied (3 points), mixed (4 points), mostly dissatisfied (5 points), or unhappy (6 points)". The pooled mean QOL score (reported data were insufficient for estimating WMD) for men undergoing TUMT decreased 58.5% (4.1 to 1.7) versus 63.4% (4.1 to 1.2) in men undergoing TURP. QOL significantly improved following both TUMT and TURP and there were no significant differences between treatments.
In the four studies reporting this outcome, TUMT participants (7.54/100 person‐years) were more likely than TURP participants (1.05/100 person‐years) to require retreatment for BPH symptoms (P < 0.001), relative hazard of 10.0 (95% CI 2.44 to 50). Most retreatments were surgical; only two studies reported whether medications were added and two studies failed to characterize the retreatment. In the five studies reporting this outcome, TURP participants (5.85/100 person‐years) were more likely than TUMT participants (0.63/100 person‐years) to require surgical treatment for strictures (meatal, urethral, or bladder neck) (P <0.001), relative hazard of 9.76, (95% CI 2.22 to 42.96).
Adverse events
Most studies did not comprehensively report perioperative and postoperative adverse events. Compared to participants undergoing TURP, significantly fewer TUMT participants required blood transfusion (RR 0.11, 95% CI 0.01 to 0.86; RD ‐0.11, 95% CI ‐0.21 to 0.02), or developed clinically important hematuria (RR 0.25, 95% CI 0.07 to 0.85; RD ‐0.06, 95% CI ‐0.12 to 0.0) or the transurethral resection (TUR) syndrome (RR 0.13, 95% CI 0.02 to 0.81; RD ‐0.05, 95% CI ‐0.11 to 0.0). However, more participants developed dysuria, urgency and urinary retention (defined as the prolonged postprocedure requirement for an indwelling catheter due to failing a voiding trial) following TUMT than TURP. Sexually active participants undergoing TUMT were significantly less likely to experience retrograde ejaculation (RR 0.39, 95% CI 0.21 to 0.75; RD ‐0.34, 95% CI ‐0.55 to ‐0.13). Few studies provided any data on the duration of urinary retention and these could not be statistically compared because mean values were not reported. However, the range or median durations of urinary retention were consistently higher following TUMT (Ahmed 1997; Dahlstrand 1995; Norby 2002a; Wagrell 2002). There were no deaths during the study period following a procedure.
TUMT versus sham control
Study participants and characteristics
Overall, 1045 participants were randomized in the eight trials, including 653 to TUMT and 392 to sham controls. The mean age (66.0 years, range 63 to 70 years), mean baseline symptom score (21.3, range 18.0 to 23.7 for AUA scores; 13.1, range 11.9 to 14.2 for Madsen scores), mean baseline peak urinary flow (8.6 mL/s, range 7.3 to 11.6), and mean prostate volume (43.4 mL, range 39.8 to 45.1) did not differ by treatment group.
Clinical outcomes
Only two of the eight studies used high‐energy systems (Dornier Urowave TUMT device (Roehrborn 1998), Urologix Targis thermo ablation system (Larson 1998)), while the remaining studies used low‐energy systems. All procedures were performed in an outpatient setting. Studies did not report on the duration of urinary catheterization. Participants treated with TUMT had a greater improvement in symptom scores than sham control participants, whether measured by the IPSS symptom score (WMD ‐5.15, 95% CI ‐4.26 to 6.04) or the Madsen symptom score (WMD ‐5.10, 95% CI ‐3.79 to ‐6.42). The pooled mean symptom score for men undergoing TUMT decreased 50% in 3 to 6 months (21.4 to 10.8) versus 41% (21.3 to 12.6) in the men undergoing sham treatment. Peak urinary flow rates increased slightly more following TUMT compared to sham treatment with a WMD of 2.015 mL/s (95% CI 0.85 to 3.16). The pooled mean peak urinary flow for men undergoing TUMT increased 43% (from 8.1 mL/s to 11.6 mL/s) versus 11% (from 8.7 mL/s to 9.7 mL/s) in men undergoing sham treatment. Retreatment for BPH symptoms with either procedures or medications occurred significantly less often following TUMT (1.5/100 person‐years) than with sham treatment (13.5/100 person‐years), relative hazard of 0.12 (95% CI 0.03 to 0.48).
Two studies evaluated QOL (Larson 1998; Roehrborn 1998). The WMD was ‐0.95 (95% CI ‐0.77 to ‐1.14), favoring TUMT. The pooled mean quality of life score decreased 63% (from 4.3 to 1.6) following TUMT versus 26% (from 4.2 to 3.1) in men undergoing sham treatment.
Adverse events
Most studies did not comprehensively report perioperative or postoperative adverse events. Men undergoing TUMT were significantly more likely than those undergoing sham treatment to develop urinary retention (RR 6.04, 95% CI 2.51 to 14.5; RD 0.10, 95% CI 0.06 to 0.13), dysuria (RR 2.06, 95% CI 1.03 to 4.13; RD 0.07, 95% CI 0. 01 to 0.13), and postprocedure hematuria (RR 1.34, 95% CI 1.03 to 1.74; RD 0.07, 95% CI 0.01 to 0.13). Men undergoing TUMT were more likely than those undergoing sham procedures to develop strictures, urinary tract infections, urinary incontinence, and ejaculatory disorders, though the differences were not statistically significant. No participants died or required blood transfusion.
TUMT versus alpha‐blocker
Study participants and characteristics
One study (Djavan 1999) compared high‐energy TUMT using the Targis microwave system (n = 51) versus the alpha‐blocker terazosin, titrated up to a maximal dose of 10 mg daily (n = 52). Exclusion criteria included being on an alpha‐blocker within the preceding three months, previous finasteride use, prostate surgery, or other prostate procedures. The mean age (66.2 versus 64.0 years), mean baseline IPSS symptom score (19.4 versus 18.9), mean baseline peak urinary flow (8.3 mL/s versus 8.9 mL/s), prostate‐specific antigen (2.8 ng/mL versus 2.2 ng/mL), prostate volume (39.6 cm versus 39.1 cm), and mean QOL score (3.9 versus 3.8) did not differ by treatment group.
Clinical outcomes
At six‐month follow‐up, men treated with TUMT had significantly greater symptom relief as measured by the IPSS (WMD ‐4.20, 95% CI ‐5.25 to ‐3.15) and improvement in peak urinary flow (WMD 2.30 mL/s, 95% CI 1.47 to 3.13). By 18 months, the symptom and urinary flow outcomes still favored TUMT and a significantly lower proportion of men assigned to TUMT had undergone retreatment (RR 0.12, 95% CI 0.04 to 0.38; RD ‐0.43, 95% CI ‐0.27 to ‐0.59).
Adverse events
Fewer patients receiving alpha‐blocker therapy, compared to TUMT, suffered adverse events such as urinary tract infection, urinary retention, or retrograde ejaculation though the differences were not significant. However, the proportions of participants experiencing dizziness (RR 0.07, 95% CI 0.0 to 1.16; RD ‐0.13, 95% CI ‐0.04 to ‐0.23) or asthenia (RR 0.11, 95% CI 0.01 to 2.05; RD ‐0.08, 95% CI 0.0 to ‐0.16) were lower among men treated with TUMT.
Discussion
Our systematic review identified six randomized comparisons of transurethral microwave thermotherapy (TUMT) with TURP, eight comparisons with sham thermotherapy treatment, and one comparison with an oral alpha‐blocker. We found that TUMT effectively reduced urinary symptoms attributable to BPH and improved peak urinary flow for 12 months following the procedure. Overall, TUMT reduced symptom scores by 65% and increased peak urinary flow by 70%. However, TUMT was less effective than TURP in improving urinary symptom scores and peak urinary flows. An advantage for TUMT, though, was that procedures were performed in the outpatient setting, while TURP patients were hospitalized for a median of five days. However, none of these studies were performed in the United States, where the standard of care is usually to discharge the patient within a day after surgery. Adverse events generally occurred less frequently following TUMT, including retreatment for strictures, transfusions, retrograde ejaculation, and the TUR syndrome. TUMT, though, was not compared with newer techniques such as bipolar TURP or laser ablation of the prostate that have lower complication rates than traditional TURP. Urinary retention and dysuria occurred more frequently following TUMT. Additionally, the BPH retreatment rate was significantly higher following TUMT than TURP. Study inclusion criteria (moderately severe symptoms (AUA/IPSS scores > 7), peak urinary flow < 15 mL/s, and age above 45 years) and exclusion criteria (very large prostates, large median lobes, urinary retention, prostate cancer, and previous prostate surgery) were similar to those used in other surgical studies of BPH treatment, suggesting that our results are generalizable.
TUMT consistently produced greater improvement in symptom score (5 points) and peak urinary flow (1.7 mL/s) than sham treatments. However, study follow‐up durations for the sham comparison studies were limited, and patients may have become aware that they were randomized to active treatment based on receiving perioperative analgesics and sedatives, particularly parental medications, and prolonged catheterization. The one comparison of TUMT with an alpha‐blocker showed improved symptom relief and peak urinary flow and less retreatment following TUMT. We did not find any randomized comparisons of TUMT with other minimally invasive or pharmacologic BPH treatments.
Although we limited our analyses to randomized controlled trials, the majority of the studies had important potential methodological flaws. Only two studies clearly had adequate concealment of randomization while six studies reported blinding outcomes assessors. Two of the studies comparing TUMT with TURP had only six months of follow‐up data, though few participants were lost to follow‐up. Even though several of these studies followed participants for at least two years, the attrition rate was substantial. Four of the studies comparing TUMT with sham procedures followed participants for only three months before offering active treatment to the sham group. We analyzed only the three‐month TUMT versus sham intention‐to‐treat results. None of the studies provided data on the mean changes in symptom scores or peak urinary flow so that we could compare only follow‐up values. These analyses, which are unable to adjust for baseline function, are less representative of an individual's experience. We were unable to detect any statistically significant outcome differences between high‐ or low‐energy TUMT systems or between different devices. Bolmsjo and colleagues have reported substantial differences in heating profiles between devices with different microwave antenna designs (Bolmsjo 1996). However, these authors considered the reported amount of microwave energy delivered by the TUMT equipment to be an unreliable measure of the microwave energy actually absorbed by the prostate.
Studies did not consistently report on or define perioperative adverse events, particularly dysuria, hematuria, and sexual dysfunction, and our estimates for these complications may be unreliable. Few studies evaluated QOL outcomes. Although studies usually reported the occurrence of urinary retention, they did not consistently or uniformly indicate its duration. One important complication that was not reported in the clinical trial literature was thermal injury. On October 11, 2000, the United States Food and Drug Administration (FDA) published a Public Health Notification because they had received 16 reports of severe thermal injury associated with TUMT, including 10 resulting in fistula formation and six resulting in tissue damage to the penis or urethra (FDA 2000). The FDA noted that the injuries could take hours or days to develop. Although the FDA recommended several corrective measures for physicians, they considered TUMT to be safe and effective based on performance of over 25,000 procedures.
Recent guidelines considered TUMT to be an appropriate alternative for treating men with lower urinary tract symptoms (AUA 2011;CUA 2010). The AUA recommended TUMT for men with moderate to severe symptoms, noting that TUMT is effective in partially relieving symptoms due to BPH, though evidence is insufficient to determine whether one device is superior to another. The Canadian guidelines considered TUMT an optional treatment for men with moderate symptoms, and small‐ to moderate‐size prostate glands, who wish to avoid more invasive therapy. TUMT has become the most widely utilized procedure for minimally invasive surgical therapies among the Medicare population (Yu 2008). Overall use increased from just 4874 procedures in 1999 to 28,969 (rate of 262 per 100,000 men) by 2005.
Our results may help guide patients and providers in weighing the short‐term relative risks and benefits of TUMT versus TURP or an alpha‐blocker. The best available clinical data suggested that TUMT was an effective BPH treatment, could be performed as an outpatient procedure, and had fewer adverse events than TURP. However, TURP produced greater improvements in symptom scores and peak urinary flow and fewer patients required retreatment for symptomatic BPH. Further research is needed to evaluate the long‐term effectiveness and safety of TUMT and to determine which devices and energy settings are most effective.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
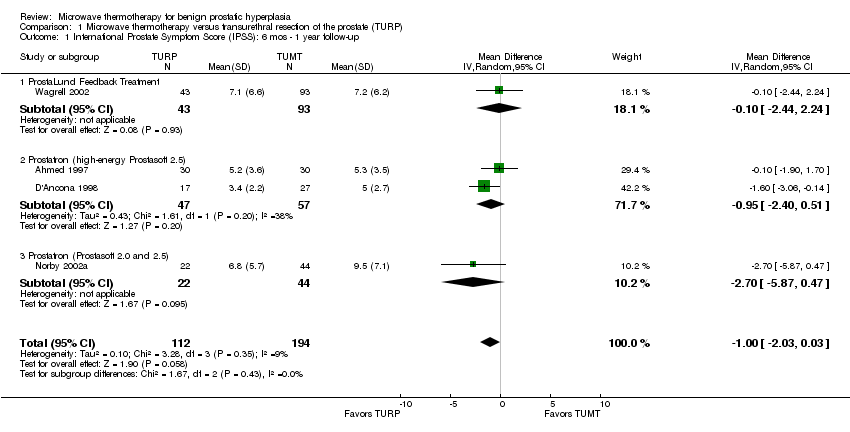
Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 1 International Prostate Symptom Score (IPSS): 6 mos ‐ 1 year follow‐up.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 2 Madsen‐Iversen Symptom Score (range 0 to 27): 6 mos ‐ 1 year follow‐up.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 3 50% Improvement in AUA Symptom Score: # subjects.
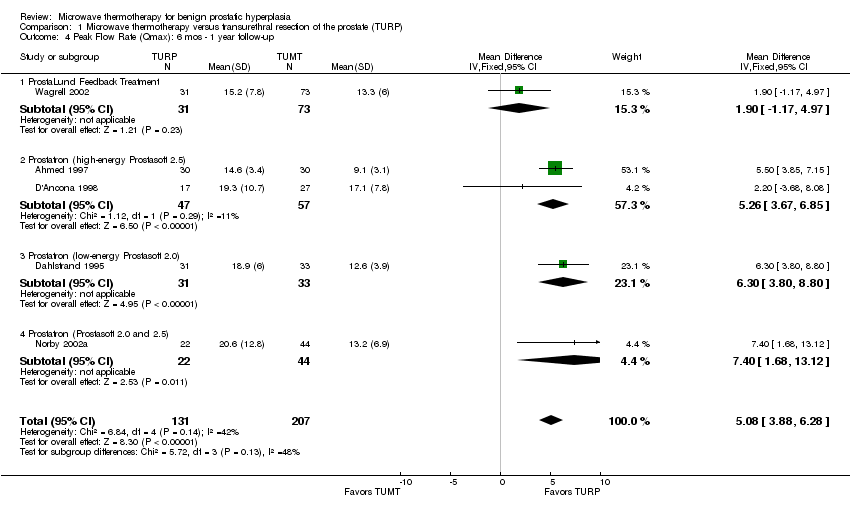
Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 4 Peak Flow Rate (Qmax): 6 mos ‐ 1 year follow‐up.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 5 Hematuria (requiring additional treatment/judged to be serious).

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 6 Urinary retention.
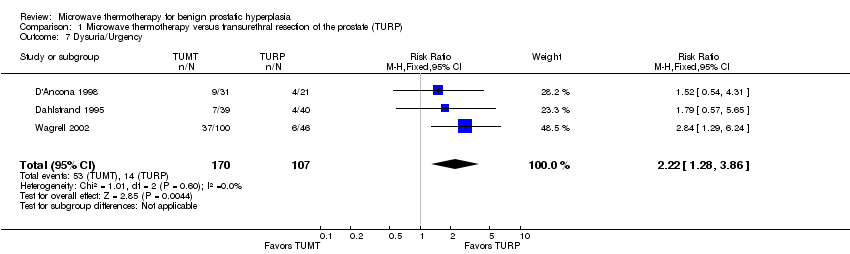
Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 7 Dysuria/Urgency.
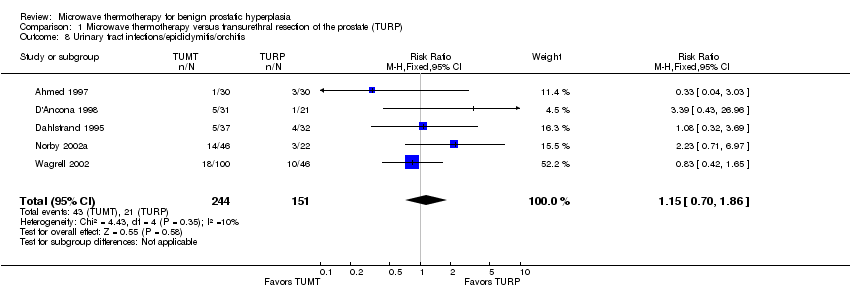
Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 8 Urinary tract infections/epididymitis/orchitis.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 9 Retrograde ejaculation (sexually active men only).

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 10 Erectile dysfunction.
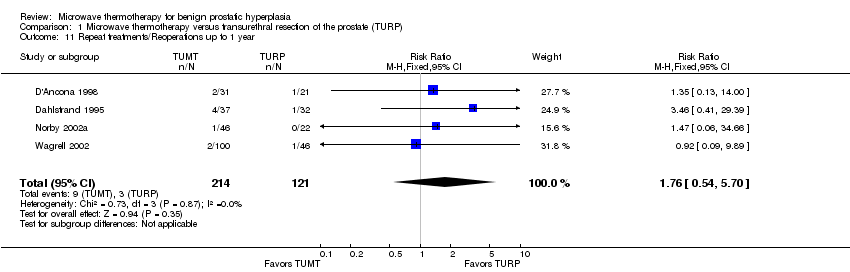
Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 11 Repeat treatments/Reoperations up to 1 year.
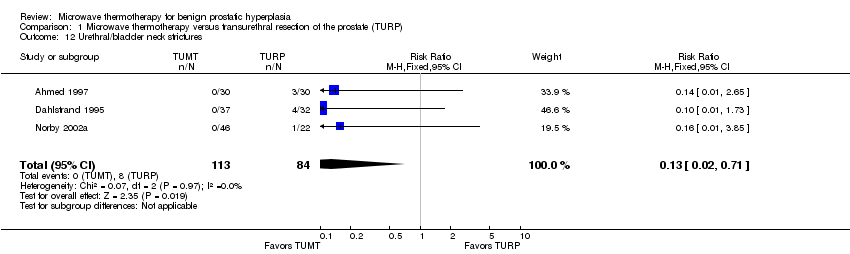
Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 12 Urethral/bladder neck strictures.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 13 Transfusions.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 14 Clot retention.

Comparison 1 Microwave thermotherapy versus transurethral resection of the prostate (TURP), Outcome 15 TURP Syndrome.

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 1 International Prostate Symptom Score (IPSS).
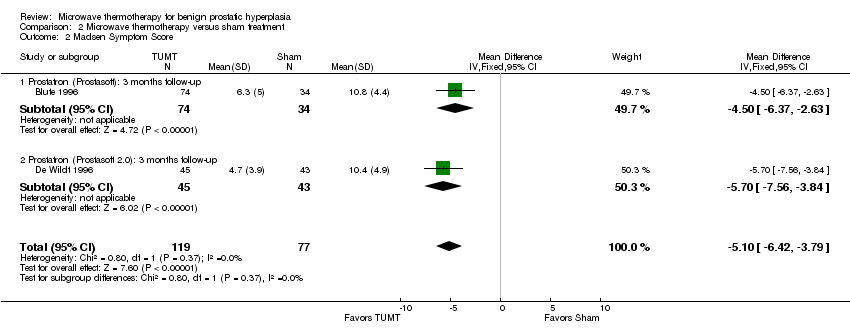
Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 2 Madsen Symptom Score.
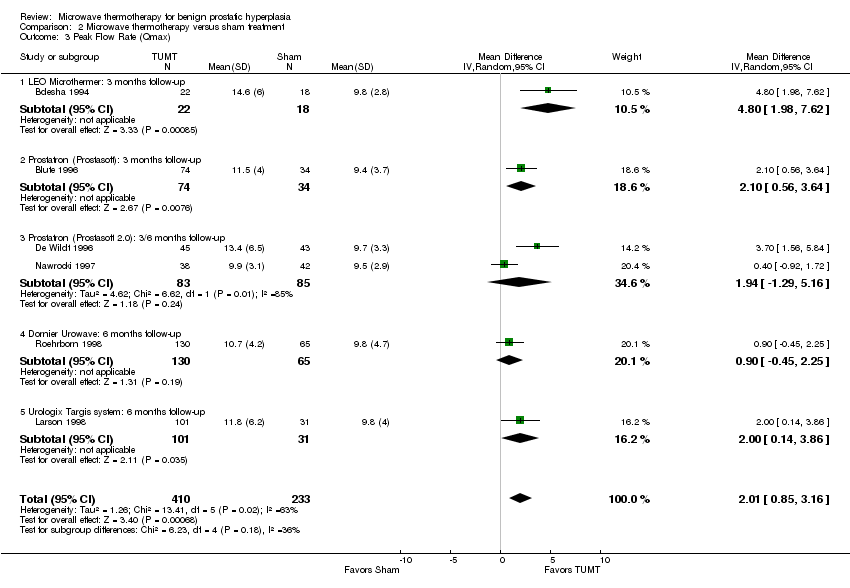
Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 3 Peak Flow Rate (Qmax).

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 4 Hematuria (post‐procedure).

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 5 Urinary retention.

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 6 Dysuria (self‐limited).

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 7 UTI/epididymitis.

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 8 Ejaculatory disorders.

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 9 Retreatment.

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 10 Quality of Life.

Comparison 2 Microwave thermotherapy versus sham treatment, Outcome 11 Bladder spasm.
| Transurethral microwave thermotherapy (TUMT) compared with transurethral resection of the prostate (TURP) for symptomatic benign prostatic hyperplasia | ||||
| Patient or population: men with symptomatic benign prostatic hyperplasia Settings: office (TUMT) or hospital (TURP) Intervention: Transurethral microwave thermotherapy (TUMT) Comparison: Transurethral resection of the prostate (TURP) | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | |||
| TURP | TUMT | |||
| International Prostate Symptom Score (IPSS). Range: 0 to 35 points. Follow‐up: 6 mos ‐ 1 year | The mean IPSS scores ranged across control groups from 3.4 to 7.1 points | The mean IPSS in the intervention group was 1 point higher (95% CI, 0.03 lower to 2.03 higher) | 306 | ⊕⊕⊕⊝ |
| Madsen‐Iversen Symptom Score Range 0 to 27 points Follow‐up: 6 mos ‐ 1 year | The mean Madsen‐Iversen Symptom scores ranged across control groups from 0.62 to 2.7 points | The mean Madsen‐Iversen Symptom score in the intervention group was 1.59 points higher (95% CI, 0.69 lower to 2.48 higher) | 338 | ⊕⊕⊕⊝ |
| Peak Flow Rate (Qmax): Measurement: mL per second Follow‐up: 6 mos ‐ 1 year | The mean Peak Flow Rate ranged across control groups from 14.6 to 20.6 mL/s | The mean urinary peak flow rate in the intervention group was 5.08 mL/s lower (95% CI, 3.88 to 6.28 lower) | 108 | ⊕⊕⊕⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1 <400 subjects evaluated for this outcome which is a non‐optimal information size | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 International Prostate Symptom Score (IPSS): 6 mos ‐ 1 year follow‐up Show forest plot | 4 | 306 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.03, 0.03] |
| 1.1 ProstaLund Feedback Treatment | 1 | 136 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.44, 2.24] |
| 1.2 Prostatron (high‐energy Prostasoft 2.5) | 2 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐2.40, 0.51] |
| 1.3 Prostatron (Prostasoft 2.0 and 2.5) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | ‐2.7 [‐5.87, 0.47] |
| 2 Madsen‐Iversen Symptom Score (range 0 to 27): 6 mos ‐ 1 year follow‐up Show forest plot | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [0.69, 2.48] |
| 2.1 Prostatron (high‐energy Prostasoft 2.5) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐1.07, 4.07] |
| 2.2 Prostatron (low‐energy Prostasoft 2.0) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [0.64, 2.56] |
| 3 50% Improvement in AUA Symptom Score: # subjects Show forest plot | 2 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.09] |
| 4 Peak Flow Rate (Qmax): 6 mos ‐ 1 year follow‐up Show forest plot | 5 | 338 | Mean Difference (IV, Fixed, 95% CI) | 5.08 [3.88, 6.28] |
| 4.1 ProstaLund Feedback Treatment | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐1.17, 4.97] |
| 4.2 Prostatron (high‐energy Prostasoft 2.5) | 2 | 104 | Mean Difference (IV, Fixed, 95% CI) | 5.26 [3.67, 6.85] |
| 4.3 Prostatron (low‐energy Prostasoft 2.0) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 6.30 [3.80, 8.80] |
| 4.4 Prostatron (Prostasoft 2.0 and 2.5) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [1.68, 13.12] |
| 5 Hematuria (requiring additional treatment/judged to be serious) Show forest plot | 3 | 258 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.15, 0.05] |
| 6 Urinary retention Show forest plot | 4 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [1.52, 5.70] |
| 7 Dysuria/Urgency Show forest plot | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.28, 3.86] |
| 8 Urinary tract infections/epididymitis/orchitis Show forest plot | 5 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.70, 1.86] |
| 9 Retrograde ejaculation (sexually active men only) Show forest plot | 2 | 78 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.34 [‐0.55, ‐0.13] |
| 10 Erectile dysfunction Show forest plot | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.16, 1.05] |
| 11 Repeat treatments/Reoperations up to 1 year Show forest plot | 4 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.54, 5.70] |
| 12 Urethral/bladder neck strictures Show forest plot | 3 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.71] |
| 13 Transfusions Show forest plot | 2 | 128 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.21, ‐0.02] |
| 14 Clot retention Show forest plot | 3 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.06, 1.31] |
| 15 TURP Syndrome Show forest plot | 3 | 274 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.11, ‐0.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 International Prostate Symptom Score (IPSS) Show forest plot | 4 | 482 | Mean Difference (IV, Fixed, 95% CI) | ‐5.15 [‐6.04, ‐4.26] |
| 1.1 LEO Microthermer: 3 months follow‐up | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐9.1 [‐15.64, ‐2.56] |
| 1.2 Prostatron (Prostasoft 2.0): 3/6 months follow‐up | 1 | 94 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐8.09, ‐1.91] |
| 1.3 Dornier Urowave: 6 months follow‐up | 1 | 193 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐6.32, ‐4.28] |
| 1.4 Urologix Targis system: 6 months follow‐up | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐3.80 [‐6.26, ‐1.34] |
| 2 Madsen Symptom Score Show forest plot | 2 | 196 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐6.42, ‐3.79] |
| 2.1 Prostatron (Prostasoft): 3 months follow‐up | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐6.37, ‐2.63] |
| 2.2 Prostatron (Prostasoft 2.0): 3 months follow‐up | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐7.56, ‐3.84] |
| 3 Peak Flow Rate (Qmax) Show forest plot | 6 | 643 | Mean Difference (IV, Random, 95% CI) | 2.01 [0.85, 3.16] |
| 3.1 LEO Microthermer: 3 months follow‐up | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 4.80 [1.98, 7.62] |
| 3.2 Prostatron (Prostasoft): 3 months follow‐up | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 2.10 [0.56, 3.64] |
| 3.3 Prostatron (Prostasoft 2.0): 3/6 months follow‐up | 2 | 168 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐1.29, 5.16] |
| 3.4 Dornier Urowave: 6 months follow‐up | 1 | 195 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.45, 2.25] |
| 3.5 Urologix Targis system: 6 months follow‐up | 1 | 132 | Mean Difference (IV, Random, 95% CI) | 2.0 [0.14, 3.86] |
| 4 Hematuria (post‐procedure) Show forest plot | 4 | 684 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.13] |
| 5 Urinary retention Show forest plot | 7 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.04 [2.51, 14.52] |
| 6 Dysuria (self‐limited) Show forest plot | 2 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.03, 4.13] |
| 7 UTI/epididymitis Show forest plot | 3 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.70, 2.39] |
| 8 Ejaculatory disorders Show forest plot | 2 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.10 [0.83, 45.08] |
| 9 Retreatment Show forest plot | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.48] |
| 10 Quality of Life Show forest plot | 2 | 347 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.14, ‐0.77] |
| 11 Bladder spasm Show forest plot | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.47] |

