Fluticasona más salmeterol versus dosis fijas de budesonida y formoterol para el asma crónica en adultos y niños
Resumen
Antecedentes
Los beta‐agonistas de acción prolongada son un tratamiento de segunda línea frecuente en los pacientes con asma que nos e controla de manera adecuada con los corticosteroides inhalados. Los inhaladores de dispositivo único combinan un beta‐agonista de acción prolongada con un esteroide inhalado y administra ambos fármacos como un régimen de tratamiento de mantenimiento. Esta revisión actualizada compara dos opciones de dosis fija, fluticasona/salmeterol y budesonida/formoterol, ya que esta comparación representa una opción terapéutica frecuente.
Objetivos
Evaluar los efectos relativos de fluticasona/salmeterol y budesonida/formoterol en pacientes con asma.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Especializado de Ensayos Controlados del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group), mediante términos preespecificados. Adicionalmente se realizó una búsqueda manual en los sitios web de los fabricantes y en los registros de ensayos en línea. Los resultados de la búsqueda están actualizados hasta junio de 2011.
Criterios de selección
Se incluyeron los estudios aleatorizados que compararon dosis fijas de fluticasona/salmeterol y budesonida/formoterol en adultos o niños con diagnóstico de asma. El tratamiento en los estudios tenía que durar como mínimo 12 semanas.
Obtención y análisis de los datos
Dos autores de forma independiente evaluaron los estudios para su inclusión en la revisión. los resultados de los datos continuos se combinaron mediante la diferencia de medias (DM), y los resultados de los datos dicotómicos mediante el odds ratio (OR). La calidad de la evidencia se evaluó mediante los criterios Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Resultados principales
Cinco estudios cumplieron los criterios de inclusión en la revisión (5537 participantes). Las poblaciones de estudio ingresaron a los estudios después de haber recibido tratamiento previo con esteroides inhalados y tenían obstrucción moderada o leve de las vías respiratorias (FEV1 medio previsto entre el 65% y el 84% al inicio del estudio). La mayoría de los estudios evaluaron el tratamiento durante un período de seis meses. Los estudios tuvieron riesgo bajo de sesgo de selección y se sesgo de realización/detección, aunque no fue posible determinar si los datos faltantes tuvieron repercusión sobre los resultados. La disponibilidad de los datos de los resultados fue satisfactoria.
Resultados primarios
El odds‐ratio para las exacerbaciones que requirieron esteroides orales fue menor con fluticasona/salmeterol, pero no alcanzó la significación estadística (OR 0,89; intervalo de confianza [IC] del 95%: 0,74 a 1,07; cuatro estudios; n = 4949). Con un riesgo supuesto con budesonida/formoterol de 106/1000 participantes que requieren esteroides orales, el tratamiento con fluticasona/salmeterol daría lugar a que entre 25 personas menos y siete más por cada 1000 recibieran un ciclo de esteroides orales. Aunque las probabilidades de ingreso hospitalario fueron mayores con fluticasona/salmeterol, no se alcanzó la significación estadística (OR 1,29; IC del 95%: 0,68 a 2,47; cuatro estudios, 4879 participantes). Con un riesgo supuesto con budesonida/formoterol de 7/1000, entre tres personas menos y nueve más por 1000 serían hospitalizadas con fluticasona/salmeterol. Las probabilidades de un evento adverso grave relacionado con el asma fueron mayores con fluticasona/salmeterol, pero no difirieron de manera significativa entre los tratamientos (OR 1,47; IC del 95%: 0,75 a 2,86; tres estudios, 4054 participantes). Con un riesgo supuesto con budesonida/formoterol de 7/1000, entre dos pacientes menos y 13 más por 1000 experimentarían un evento adverso grave con fluticasona/salmeterol.
Resultados secundarios
Los resultados de la función pulmonar, los síntomas, la medicación de rescate, el resultado compuesto de exacerbaciones que dieron lugar a una consulta o a un ingreso hospitalario, los retiros y los eventos adversos no difirieron estadísticamente entre los tratamientos. La evaluación de la calidad de vida se limitó a dos estudios que proporcionaron resultados que no alcanzaron significación estadística. Un estudio informó una muerte por cada 1000 participantes con fluticasona/salmeterol y ninguna muerte en un número similar de participantes tratados con budesonida/formoterol. Los otros estudios no informaron muertes.
Conclusiones de los autores
La imprecisión estadística en las estimaciones del efecto para las exacerbaciones y los eventos adversos graves no permite concluir que cualquiera de los dos tratamientos sea superior. La incertidumbre en torno a las estimaciones del efecto justifica ensayos adicionales para establecer conclusiones más definitivas; la calidad general de las evidencia basada en las recomendaciones GRADE para los tres resultados primarios y los retiros debido a eventos adversos graves fue moderada. La calidad de la evidencia para al mortalidad se calificó como baja. Los resultados de la función pulmonar mostraron que los fármacos fueron suficientemente similares como para que los estudios de investigación adicionales probablemente no cambien estos efectos. No se identificaron ensayos en menores de 12 años y los estudios de investigación en esta población tienen una alta prioridad. La evaluación de la calidad de vida es una prioridad para los estudios de investigación futuros.
PICO
Resumen en términos sencillos
Diferentes combinaciones de esteroides inhalados y beta‐agonistas de acción prolongada para el asma crónica (fluticasona/salmeterol versus budesonida/formoterol)
Los pacientes con asma persistente a menudo requieren un tratamiento adicional a los esteroides inhalados habituales. Algunas preparaciones de beta‐agonistas de acción prolongada se administran en el mismo dispositivo de inhalación que los corticosteroides inhalados. Los esteroides inhalados ayudan a tratar la inflamación de las vías respiratorias y los beta‐agonistas de acción prolongada ayudan a que las vías respiratorias se relajen, lo que mejora los síntomas y la función pulmonar. Esta revisión sistemática examinó los ensayos controlados aleatorizados que compararon dos combinaciones frecuentemente disponibles administradas a dosis fija con un solo inhalador, fluticasona/salmeterol (FP/SAL) y budesonida/formoterol (BUD/F). Se incluyeron cinco estudios que reclutaron 5537 pacientes. En general los ensayos estuvieron bien diseñados, pero solo reclutaron adultos y adolescentes, y no niños. Los participantes ya tomaban esteroides inhalados regularmente antes de que comenzaran los estudios y tenían asma leve o moderada según sus pruebas respiratorias. Se encontró que el número de personas que requirieron tratamiento con esteroides orales e ingreso hospitalario fue similar entre los tratamientos, pero debido a la incertidumbre estadística de este resultado no fue posible descartar diferencias importantes a favor de cualquiera de las combinaciones de fármacos. Los ensayos adicionales permitirían establecer conclusiones más confiables acerca de qué tan bien funcionan estos fármacos comparados entre sí. También se examinaron los eventos adversos graves. Nuevamente, los resultados no indicaron que una combinación fuera claramente mejor que la otra, pero estos resultados también fueron imprecisos, por lo que no es posible tener certeza sobre este hallazgo. Sin embargo, la función pulmonar y la administración de fármacos de rescate fueron similares entre los tratamientos. No fue posible evaluar los efectos relativos de estos fármacos sobre la mortalidad debido a que las pocas muertes que ocurrieron provocaron incertidumbre estadística; un paciente murió en los cinco estudios. La calidad de vida se midió de diferentes maneras en dos estudios y no fue posible determinar cómo se compararon los tratamientos con respecto a esta medida de resultado. Se necesitan estudios adicionales para fortalecer y explicar mejor estos hallazgos. En particular, son prioritarios los estudios que evalúan los efectos de estas terapias en los niños y los estudios que miden la calidad de vida.
Authors' conclusions
Summary of findings
| Combination fluticasone/salmeterol versus budesonide/formoterol for chronic asthma in adults and children | ||||||
| Patient or population: Patients with chronic asthma in adults and children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combination budesonide/formoterol | Combination fluticasone/salmeterol | |||||
| Participants experiencing exacerbations requiring oral steroid treatment | 106 per 10001 | 95 per 1000 | OR 0.89 | 4949 | ⊕⊕⊕⊝ | |
| Participants experiencing exacerbations requiring admission to hospital | 7 per 10001 | 8 per 1000 | OR 1.29 | 4879 | ⊕⊕⊕⊝ | |
| Asthma‐related serious adverse event | 7 per 10001 | 10 per 1000 | OR 1.47 | 4054 | ⊕⊕⊕⊝ | |
| Mortality | See comment | See comment | Not estimable | 4819 | ⊕⊕⊝⊝ | The data did not generate a pooled effect estimate as no deaths occurred in four out of the five studies. |
| Withdrawals (adverse events) | 16 per 10001 | 15 per 1000 | OR 0.94 | 5082 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The mean rate of exacerbations across the BUD/F arms of the trials was used to calculate the assumed risk. | ||||||
Background
Description of the condition
Asthma is a chronic inflammatory disease of the airways, and anti‐inflammatory treatment is a cornerstone of asthma therapy. Treatment with inhaled corticosteroids (ICS) improves lung function and reduces asthma symptoms in asthmatic patients (Adams 2008).
Description of the intervention
Many patients remain symptomatic despite using optimal doses of ICS. There is strong evidence to support the use of long‐acting beta‐agonists (LABAs) as a means of reducing the requirement for short burst oral steroid therapy improving lung function (Ducharme 2010a; Ducharme 2010b).
The two interventions being assessed in this review combine a LABA and ICS as a fixed‐dose maintenance regimen, namely fluticasone and salmeterol (marketed by GSK as 'Seretide', 'Advair' or 'Viani'), and budesonide and formoterol (marketed by AstraZeneca as 'Symbicort').
How the intervention might work
The principal advantage of combining ICS and LABA in one inhaler is the simultaneous delivery of two effective inhaled therapies with complementary anti‐inflammatory and bronchodilatory properties. This may facilitate better adherence to fixed dosing regimens, especially given concerns over the use of LABA therapy without a regular background steroid (Walters 2007).
Why it is important to do this review
There is some uncertainty as to which particular combination may be suitable. Previous assessments have considered the addition of any LABA to any ICS when the dose of ICS is increased or when the study drugs are titrated according to symptoms (Ducharme 2010b; Gibson 2005). Although the LABAs commonly used in combination preparations have a similar duration of effect of around 12 hours or more, salmeterol and formoterol also have differing pharmacological properties. The onset of action of formoterol is faster than that of salmeterol (Palmqvist 1997; Van Noord 1996) and has as rapid an onset of action as salbutamol in asthma (Cazzola 2002). Some differences exist also between fluticasone and budesonide despite the shared anti‐inflammatory effect (Adams 2007), and so a systematic exploration of the relative efficacy of these different drug combinations is justified.
Objectives
To compare the combinations of salmeterol/fluticasone and budesonide/formoterol in single inhaler devices in chronic asthma in terms of asthma control, safety and lung function.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a parallel design, as the minimum wash‐out period of inhaled steroids has not been adequately established which precludes the inclusion of crossover trials.
Types of participants
We included trials involving adults and children with a diagnosis of chronic asthma. We accepted trialist‐defined asthma. We accepted any severity of asthma and patients on any co‐intervention (as long as the co‐interventions were not part of the randomised treatment) but we excluded studies on acute asthma.
Types of interventions
The preparations considered by this review were:
-
the combination of the inhaled steroid fluticasone (FP) and long‐acting beta‐agonist salmeterol (SAL); and
-
the inhaled steroid budesonide (BUD) and long‐acting beta‐agonist formoterol (F).
We only included studies where both preparations were delivered in one inhaler device. We included studies which assessed the combination of drugs in either metered dose inhalers (MDI) or dry powder inhaler (DPI). We considered fixed‐dose comparisons between these preparations only and we have excluded studies evaluating different dosing strategies of budesonide/formoterol ('single inhaler therapy' or 'adjustable maintenance dosing') with fixed dose fluticasone/salmeterol.
We only included trials with a minimum treatment duration of 12 weeks.
Types of outcome measures
Primary outcomes
-
Exacerbations of asthma requiring oral steroids.
-
Exacerbations of asthma requiring hospital admission.
-
Asthma‐related serious adverse events (including asthma‐related death and intubation).
Secondary outcomes
-
Exacerbations leading to emergency department (ED) visit/admission to hospital.
-
Mortality.
-
Quality of life.
-
Diary card morning and evening peak expiratory flow (PEF).
-
Clinic spirometry (FEV1, clinic PEF, FVC).
-
Rescue medication use.
-
Symptoms.
-
Adverse events.
-
Study withdrawal.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the Specialised Register coded as 'asthma' using the following terms:
("single inhaler" or symbicort or seretide or advair or viani) or ((steroid* or corticosteroid* or ICS or fluticasone or FP or Flixotide or budesonide or BUD or Pulmicort) and ("long acting beta agonist*" or "*beta‐agonist*" or LABA* or salmeterol or serevent or formoterol or eformoterol or oxis or foradil))
Searches are current to June 2011.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references. We contacted authors of identified randomised trials to ask about knowledge of other published and unpublished studies. We also contacted manufacturers of combination single inhaler devices regarding other published and unpublished studies.
We contacted trialists and manufacturers in order to obtain unreported data and to establish whether other unpublished or ongoing studies are available for assessment. We undertook additional handsearching of clinical trial web sites (www.clinicalstudyresults.org; www.clinicaltrials.gov; www.fda.gov) and the clinical trial web sites of manufacturers (www.ctr.gsk.co.uk; www.astrazenecaclinicaltrials.com).
Data collection and analysis
Selection of studies
Following electronic literature searches, two review authors (TJL and GF) independently selected articles on the basis of title and/or abstract for full text scrutiny. The authors agreed a list of articles which were retrieved, and they subsequently assessed each reference to determine whether it met the review eligibility criteria.
Data extraction and management
One author (TJL) extracted information from each study for the following characteristics.
-
Design (description of randomisation, blinding, number of study centres and location, number of withdrawals).
-
Participants (numbers, mean age, age range of the study, gender ratio, baseline lung function, % on maintenance ICS or ICS/LABA combination and average daily dose of steroid (beclomethasone (BDP) equivalent), entry criteria).
-
Intervention (type and dose of component ICS and LABA, dosing schedule, inhaler device, study duration and run‐in).
-
Outcomes (type of outcome analysis, outcomes analysed, numerical data).
A second author double‐checked and agreed this information (GF).
Assessment of risk of bias in included studies
We judged the risk of bias (high, low or unclear) for each included study in relation to the following criteria in accordance with recommendations described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
-
Selection bias (allocation sequence generation).
-
Selection bias (concealment of allocation sequence).
-
Performance bias (blinding of study participants and personnel).
-
Detection bias (blinding of outcome assessors).
-
Attrition bias (frequency and nature of withdrawals). This was considered in relation to the outcomes of oral steroid requirement and hospital admission.
-
Publication bias (selective reporting of outcome measures).
-
Other bias (other type of bias).
We noted funding source, but did not consider it to be a source of bias.
Dealing with missing data
We contacted study sponsors for additional data which we required for our primary outcomes of oral steroid‐treated exacerbations, and exacerbations leading to hospital admission.
Assessment of heterogeneity
We measured statistical variation between studies by the I2 statistic (Higgins 2003). We considered possible causes of any statistical variation (see Subgroup analysis and investigation of heterogeneity).
Data synthesis
We combined data with RevMan 2011, using a fixed‐effect odds ratio (OR) for dichotomous variables, and a fixed‐effect mean difference (MD) (calculated as either a mean difference or a mean difference weighted by generic inverse variance) for continuous data variables.
We presented a Summary of Findings table for the primary outcomes in the review (exacerbations requiring oral steroids, exacerbations leading to hospital admission, and serious adverse events) based on recommendations described in Chapter 11 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We intended to subgroup on age and asthma severity.
We considered adult studies as those which recruited participants from aged 18 upwards. We considered adult and adolescent studies as those which recruited participants from aged 12 upwards. We considered participants in studies where the upper age limit was 12 years as children, and in studies where the upper age limit was 18 years as children and adolescents.
We performed subgroup analyses based on the severity of asthma as assessed according to international guidelines (GINA 2006: controlled, partly controlled, uncontrolled), and we considered trials on patients using oral steroid treatment separately. We restricted subgroup analysis to our primary outcomes.
Sensitivity analysis
We conducted sensitivity analysis on the risk of bias, whereby we removed studies at a high risk of bias based on the assessment of randomisation, blinding, withdrawal or other sources of bias. We also considered the impact of dosing and inhaler devices for both interventions. We produced and inspected funnel plots to assess the presence of publication bias.
Results
Description of studies
Results of the search
We have provided details of the literature search and study assessment processes up to June 2011 in Figure 1.
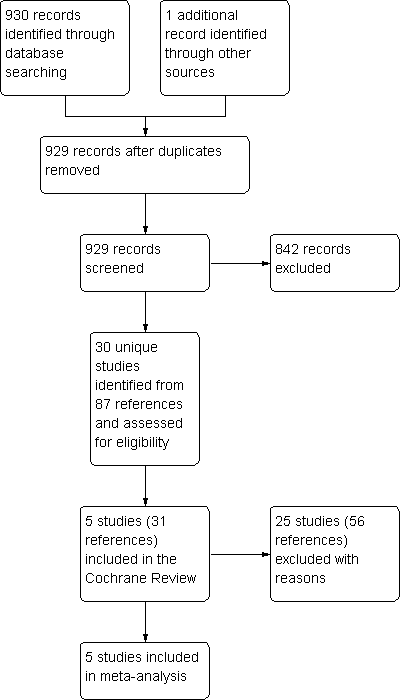
Study flow diagram. This represents the results of all literature searches up to June 2011.
The 2011 update search yielded 184 citations. When added to search results from previous years we have identified a total of 931 references up to June 2011. Five studies (reported in 31 articles) met the inclusion criteria of the review. For further details please refer to Characteristics of included studies. Of the five included studies, four are full‐text publications, and one is available as a download from a manufacturer's online clinical trial results registry (SAM40048). All of the included studies were industry‐sponsored: GlaxoSmithKline (manufacturers of FP/SAL) sponsored EXCEL; SAM40048 and AstraZeneca (manufacturers of BUD/F) sponsored Aalbers 2004; Busse 2008; COMPASS.
Included studies
Population
A total of 5537 adult and adolescent participants were recruited to the studies. The studies required participants to have a history of chronic asthma, treated with maintenance inhaled corticosteroids at moderate to high doses prior to study entry.
In the five studies, participants had to be stable for one month before the run‐in period. Once in the run‐in phase, participants were further required to demonstrate the need for frequent reliever inhaler use. On the basis of these characteristics we adjudged the trial populations to be partly controlled, since the requirement for relief medication was in addition to chronically applied inhaled steroids (GINA 2006).
The severity of airway obstruction varied between the trials, with the participants with the lowest percentage predicted of FEV1 recruited to SAM40048 (65%), Busse 2008, COMPASS and EXCEL recruiting participants with moderate airway obstruction (79%, 73% and 79%, respectively), and participants with milder obstruction represented in Aalbers 2004 (84%).
Interventions & comparisons
Converting the inhaled steroid load to BDP equivalent indicated that the trials assessed high doses of inhaled steroids in both FP/SAL and BUD/F groups, although FP/SAL was higher in BDP equivalence terms than BUD/F (1000 versus 400 to 800 mcg/day). All doses were given twice daily via different inhalers (Diskus and Turbohaler for FP/SAL and BUD/F respectively). Two studies were open label (Aalbers 2004; Busse 2008). In all studies the dose of FP/SAL was 500/100 µg/day, and that of BUD/F was 400/12 to 800/24 µg/day.
Concomitant use of reliever medication was permitted in four studies; terbutaline in COMPASS, salbutamol in Busse 2008 and EXCEL, and terbutaline or salbutamol as preferred in Aalbers 2004. In SAM40048 the reliever medication was not reported.
Outcomes
Four trials measured exacerbations as those treated with oral steroids and hospital admission (Aalbers 2004; Busse 2008; COMPASS; EXCEL). They also gave numerical data for serious adverse events. All studies reported lung function measurements. Data on admission to hospital were made available to the review authors on request from GSK and AZ (Aalbers 2004; COMPASS; EXCEL). We were informed verbally by the study sponsors of SAM40048 that exacerbation outcome data were not collected in a way that was suitable for us to use in our review.
Excluded studies
A total of 25 studies failed to meet the review eligibility criteria. We have provided the reasons for their exclusion in Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias. We have provided additional details in Characteristics of included studies. In general, the studies were well‐designed.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
The four studies available as full‐text articles reported computer‐generated randomisation sequences, with adequate concealment of treatment group allocation. Demographic characteristics of all four of the studies indicated that treatment groups were well balanced at baseline. Details on SAM40048 were not adequately reported for us to establish the appropriateness of the concealment of allocation.
Blinding
We used outcome data from an open label phase in two studies (Aalbers 2004; Busse 2008). We did not consider this aspect of the design to have an important impact on the direction of the effect for the primary outcomes. The remaining studies used a double‐dummy design to control for awareness of treatment group allocation. Blinding of outcome assessors was not reported across the studies.
Incomplete outcome data
The intention to treat principle was described in all of the studies, but explicit description of the handling of missing data were not provided. Based on the information provided, we were unable to verify whether participants who withdrew contributed data to numerators for the co‐primary outcomes.
Selective reporting
Primary outcome data were either reported in the studies or made available to the authors on request. We needed to contact the study sponsors (AstraZeneca) of Aalbers 2004 and COMPASS for data pertaining to our primary outcomes of exacerbations. The sponsors of EXCEL confirmed data on the primary outcomes, and made data for exacerbations leading to ED visits and admission to hospital available to us (see Published notes). A subsequent trial report associated with Busse 2008 indicated that quality of life (Asthma Quality of Life Questionnaire (AQLQ)) was measured in the original study, but was not available until two years after the initial2008 publication.
Effects of interventions
Using published data and unpublished data obtained through correspondence with study sponsors, we included four of the five eligible trials in the three co‐primary outcomes representing 88% of randomised participants.
FP/SAL 500/100 µg/d versus BUD/F 400‐800/12‐24 µg/d
The comparisons are presented such that FP/SAL is the 'intervention' group and BUD/F is the 'control' group.
Primary outcomes
Exacerbations leading to treatment with oral steroids
Treatment with FP/SAL led to a slightly lower odds of experiencing an exacerbation requiring OCS treatment compared with BUD/F, although the wide confidence interval meant that this could have been a chance finding (OR 0.89, 95% CI 0.74 to 1.07, P = 0.22). This result was based on data from four studies conducted over six months in 4949 adults (Figure 3). With an assumed control group rate of 106 per 1000 over six months in the BUD/F group, between 81 and 113 per 1000 participants given FP/SAL would experience an exacerbation (summary of findings Table for the main comparison).

Forest plot of comparison: 1 Combination fluticasone/salmeterol versus budesonide/formoterol, outcome: 1.1 Participants experiencing exacerbations requiring oral steroid treatment.
Hospital admission
Treatment with FP/SAL The odds of an exacerbation resulting in admission to hospital were higher with FP/SAL but the difference was not significantly different (four studies, N = 4053; OR 1.29, 95% CI 0.68 to 2.47, P = 0.43; Figure 4). Based on assumed control group rate of seven per 1000 over six months in the BUD/F groups, between four and 16 per 1000 participants would experience hospital admission in the FP/SAL group (summary of findings Table for the main comparison).
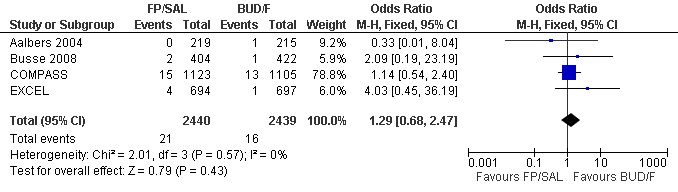
Forest plot of comparison: 1 Combination fluticasone/salmeterol versus budesonide/formoterol, outcome: 1.2 Participants experiencing exacerbations requiring admission to hospital.
Asthma‐related serious adverse events
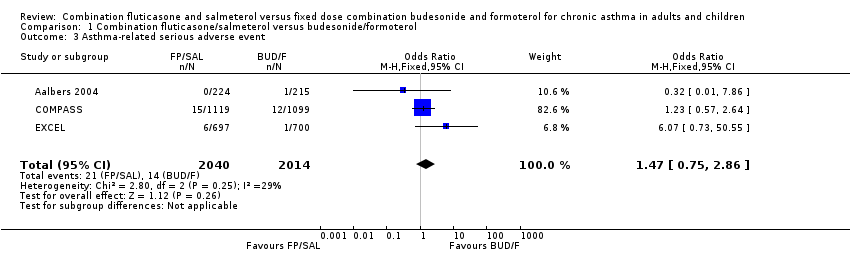
The risk of an asthma‐related serious adverse event was higher with FP/SAL but the confidence interval was wide and the overall result was not statistically significant (three studies, N = 4879; OR 1.47, 95% CI 0.75 to 2.86, P = 0.26; Figure 5). Based on an assumed control group rate of seven per 1000 over six months in the BUD/F groups, between five and 20 people per 1000 given FP/SAL would experience a serious adverse event (summary of findings Table for the main comparison).

Forest plot of comparison: 1 Combination fluticasone/salmeterol versus budesonide/formoterol, outcome: 1.3 Asthma‐related serious adverse event.
Secondary outcomes
Exacerbations requiring ED visit/hospital admission (composite)
There was no statistically significant difference in the odds of ED visit/admission to hospital between the treatments (four studies, N = 4861; OR 1.3, 95% CI 0.94 to 1.8; Analysis 1.4).
Mortality
COMPASS reported one death in the FP/SAL group and no deaths in the BUD/F group, and there were no other deaths in any of the studies (Analysis 1.5).
Quality of life
Two studies assessed quality of life withAQLQ. However, differences in the way that this outcome was measured in the studies (mean differences in COMPASS, and the number of participants achieving clinically meaningful change in Busse 2008) precluded us from combining their data. No statistically significant differences between the treatments were reported in either study.
Diary card peak flow
There was no significant difference between treatments in mean change in morning (five studies, N = 5101; 2.24 L/min, 95% CI ‐0.24 to 4.73; Analysis 1.8) or evening peak flow (four studies, N = 4299; 0.25 L/min, 95% CI ‐0.80 to 1.30; Analysis 1.9).
FEV1
There was no significant difference in the change from baseline between treatments (three studies, N = 4845; 0 L, 95% CI ‐0.02 to 0.02; Analysis 1.10).
Rescue medication use
There was no significant difference between treatments in mean change from baseline in rescue medication use (three studies, N = 3469; ‐0.06 puffs per day, 95% CI ‐0.13 to 0.02; Analysis 1.12).
Symptoms
There was no significant difference between treatments in the mean change in symptom scores (three studies, N = 3464; ‐0.02, 95% CI ‐0.6 to 0.03; Analysis 1.13), and also in the mean change in symptom‐free days (two studies, N = 3027; 1.25 days, 95% CI ‐1.18 to 3.67; Analysis 1.14).
Adverse events
The odds of experiencing any adverse event were similar between FP/SAL and BUD/F (three studies, N = 3547; OR 1.00, 95% CI 0.88 to 1.15; Analysis 1.16).
Differences between treatments in the odds of headache (OR 1.08, 95% CI 0.82 to 1.43; Analysis 1.17), candidiasis (OR 1.64, 95% CI 0.68 to 4.00; Analysis 1.18), upper respiratory tract infection (OR 1.09, 95% CI 0.81 to 1.47; Analysis 1.19), dysphonia (OR 1.45, 95% CI 0.87 to 2.43; Analysis 1.20) and throat irritation (Analysis 1.22) did not differ significantly between treatments.
Study withdrawals were not significantly more frequent with either treatment in terms of overall discontinuations (Analysis 1.25). We added withdrawals due to adverse events to summary of findings Table for the main comparison. The pooled result gave an OR of the withdrawals due to adverse events of 0.94 (95% CI 0.60 to 1.46). With an assumed rate of withdrawal due to adverse events of 16 per 1000 over six months in the BUD/F group the corresponding risk of withdrawal in the FP/SAL group is between 10 and 23 per 1000 participants.
Discussion
Summary of main results
This review summarises evidence from five well‐designed industry‐sponsored studies randomising over 5000 adults and adolescent patients. We identified three co‐primary outcomes, two pertaining to different severities of asthma exacerbation (those requiring oral steroid treatment, and those leading to hospitalisation), and one relating to asthma‐related serious adverse events. None of the results for our primary outcomes reached conventional thresholds of statistical significance. Based on our analyses, there remains some uncertainty as to the superiority of either drug combination.
Neither lung function parameters, symptom scores nor rescue medication use identified statistically significant differences between treatments. The estimates for FEV1 and peak flow are sufficiently close and statistically precise that further research is unlikely to change the similar effect of these drugs on these outcomes. One subsequent trial report for COMPASS reported quality of life on the AQLQ scale. Although the trial was large, the wide confidence intervals for this result make the small difference observed uncertain and further evaluation of this important outcome is required. Outcome data relating to harms indicated that our analyses were underpowered to detect equivalent or increased risk of candidiasis, loss of voice, respiratory tract infections or headache, with either therapy.
Overall completeness and applicability of evidence
The studies assessed the two drug combinations in predominantly adult populations with mild or moderate airway obstruction partly controlled regular inhaled steroids at baseline. Follow‐up was adequate for the key outcomes of interest to the review. The greatest limitation of the evidence base in this area is the absence of data in children under the age of 12 years. However, there is an overriding need to establish the additive benefit of long‐acting beta‐agonists in children more generally (Ducharme 2011; Ni Chroinin 2009).
The dose comparisons across the studies were similar, except for SAM40048 where the dose of budesonide was half of that in the other studies. Based on UK recommendations, the BUD/F dose was the maximum licensed dosing for asthma in the UK, and the FP/SAL dose is the medium dose recommended in the UK for asthma (BNF 2007).
Quality of the evidence
Five outcomes populate the summary of findings Table for the main comparison: oral steroid‐treated exacerbations, hospital admission and asthma‐related serious adverse events; and for the 2011 update we also included withdrawal due to adverse events and mortality. Based on the GRADE recommendations we did not consider the risk of bias, inconsistency, indirectness or biases of publication/reporting to affect our confidence in the results. However, we downgraded the quality of evidence to 'moderate' for exacerbations and SAEs, and to 'low' for mortality. For all five outcomes we downgraded the quality of the evidence due to statistical imprecision. The low event rate and lack of a pooled estimate for mortality prompted us to downgrade two points rather than one.
Requirement for a course of oral steroid treatment is a treatment‐driven rather than a symptom‐driven definition, but gives some indication as to whether maintenance therapy reduces inflammation sufficiently to prevent requirement for additional steroid. The ratio of such events was close to one in the four studies, and the BUD/F event rate was similar between the trials (Aalbers 2004: 15%; Busse 2008: 9%; COMPASS: 10%; EXCEL: 11%). Whilst the absence of a statistically significant difference may reflect the effectiveness of adding long‐acting beta‐agonists to ICS in reducing exacerbations (Ducharme 2010b), the low frequency of this outcome across the studies underpowered our analyses to determine either no important difference between the treatments or superiority of either therapy.
The morbidity associated with hospital admission is considerable, and may indicate severe uncontrolled disease as well as predict future hospitalisation and mortality (Suissa 2001). Superiority of FP over BUD in dose ratio comparisons of 1:1 and 1:2 has been demonstrated for lung function endpoints, but not exacerbation rate data (Adams 2007). In this review the risk of hospitalisation did not differ significantly between therapies, although the confidence interval was wide and further evidence is necessary before a conclusion of equivalence could be drawn reliably.
The relative effects of these treatments on serious harms including death remain to be fully elucidated. Monotherapeutic use of LABAs is discouraged since the bronchodilatory effects of LABAs possibly masks deterioration in underlying airway inflammation (Cates 2008a; Cates 2008b; Walters 2007). However, recent reviews assessing LABAs as additive treatment have also failed to identify an abolition of risk with ICS (Cates 2009a; Cates 2009b). Our analyses lack statistical precision since serious adverse events did not occur very frequently in the studies.
Potential biases in the review process
We limited our analyses to parallel studies on the assumption that optimum washout in steroid trials is uncertain, and that our primary outcome of exacerbations was best measured in long‐term studies with a between‐patient design. We assumed that requirement for oral steroids and admission to hospital are independent, and that participants who experienced hospital admission after a course of oral steroids would feature in both outcomes. This may not be the case, although it is reasonable to expect that poor asthma control when associated with poor adherence to maintenance inhaled steroids is a useful predictor for the requirement of rescue oral steroid therapy and hospital admission (Williams 2004). Assessment of patient severity was confined to GINA defined control status, and this categorisation may not be sensitive enough to discern between severities of asthma.
Agreements and disagreements with other studies or reviews
A related Cochrane Review on safety issues comparing different long‐acting beta‐agonists in addition to regular inhaled steroid treatment provides similarly equivocal results on the outcomes of serious harms and mortality (Cates 2010). Our composite analysis of ED visit/hospitalisation gave an effect estimate that was similar in terms of size and direction to that reported by Edwards 2007. However, the confidence interval was narrower in the Edwards 2007 meta‐analysis giving a result favouring BUD/F that was statistically significant. The data for that analysis were in part based upon hospitalisation data from EXCEL and did not include additional ED visits.
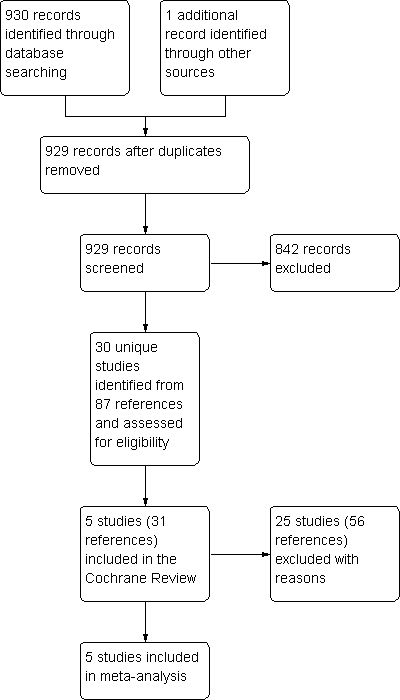
Study flow diagram. This represents the results of all literature searches up to June 2011.
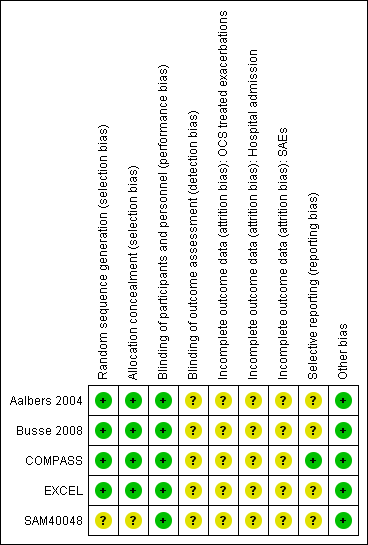
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 Combination fluticasone/salmeterol versus budesonide/formoterol, outcome: 1.1 Participants experiencing exacerbations requiring oral steroid treatment.

Forest plot of comparison: 1 Combination fluticasone/salmeterol versus budesonide/formoterol, outcome: 1.2 Participants experiencing exacerbations requiring admission to hospital.

Forest plot of comparison: 1 Combination fluticasone/salmeterol versus budesonide/formoterol, outcome: 1.3 Asthma‐related serious adverse event.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 1 Participants experiencing exacerbations requiring oral steroid treatment.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 2 Participants experiencing exacerbations requiring admission to hospital.
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 3 Asthma‐related serious adverse event.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 4 Participants experiencing exacerbations requiring ED visit/hospitalisation.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 5 Mortality.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 6 Asthma Quality of Life Questionnaire.
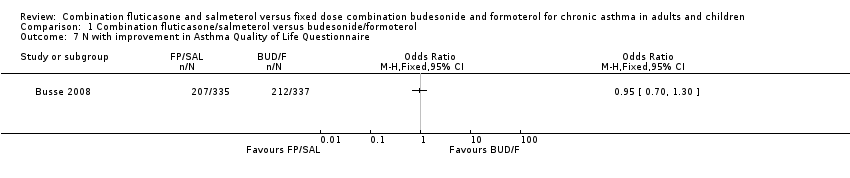
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 7 N with improvement in Asthma Quality of Life Questionnaire.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 8 Change in am PEF.
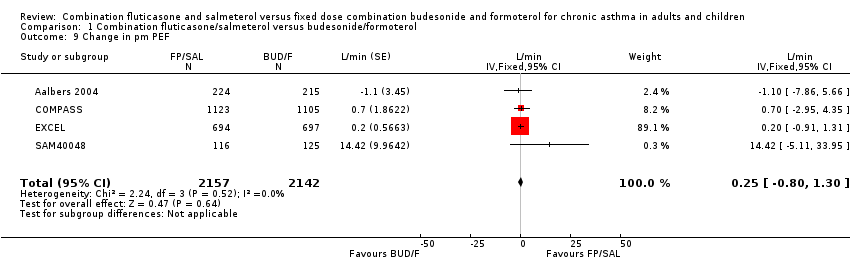
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 9 Change in pm PEF.
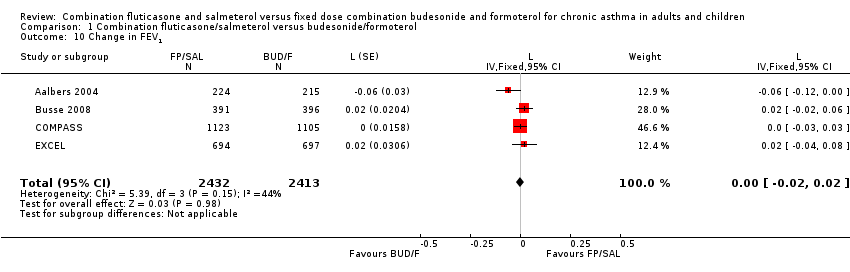
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 10 Change in FEV1.
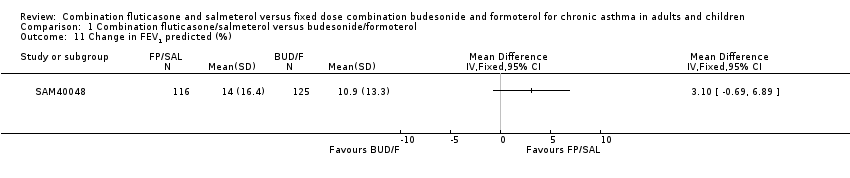
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 11 Change in FEV1 predicted (%).

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 12 Change in rescue medication use.
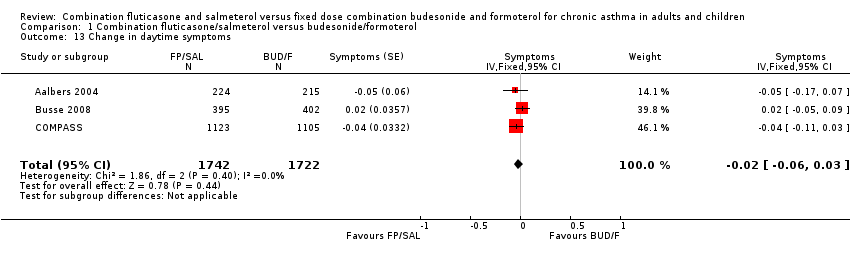
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 13 Change in daytime symptoms.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 14 Change in symptom‐free days.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 15 Change in nocturnal awakenings.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 16 Adverse events.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 17 Headache.
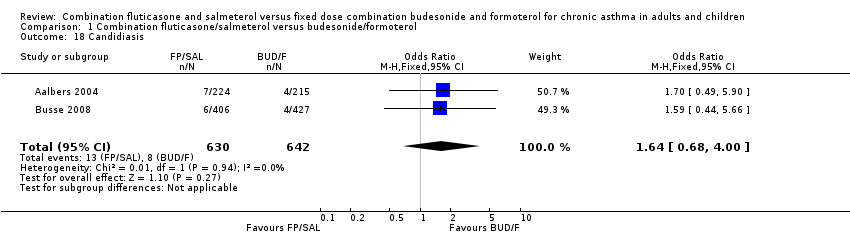
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 18 Candidiasis.
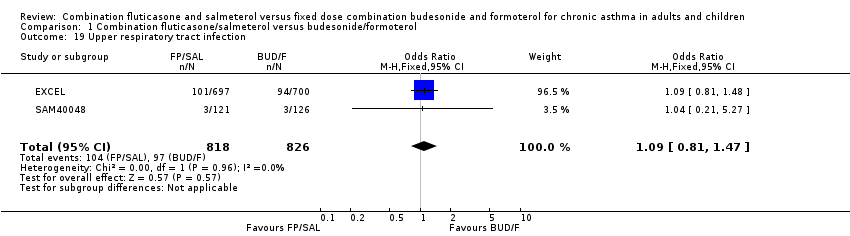
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 19 Upper respiratory tract infection.
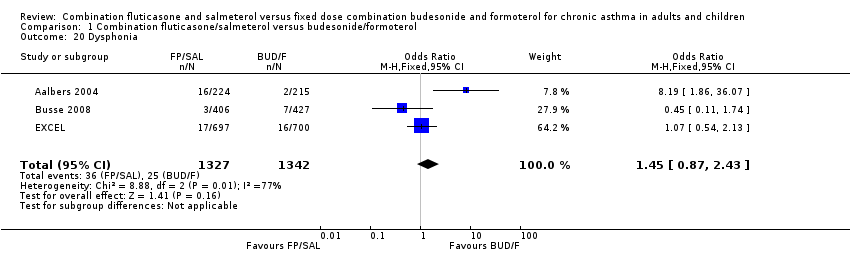
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 20 Dysphonia.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 21 Rhinitis.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 22 Throat irritation.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 23 Cough.

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 24 Tremor.
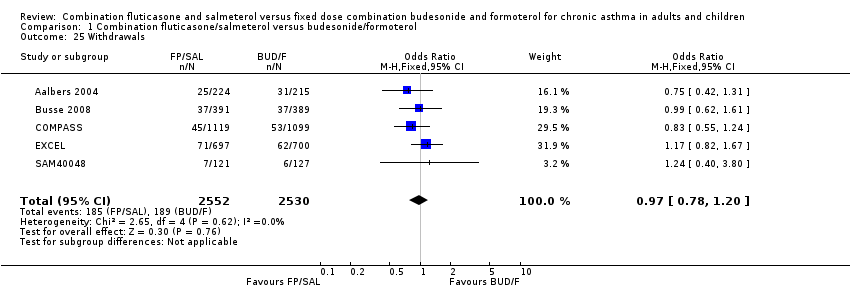
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 25 Withdrawals.
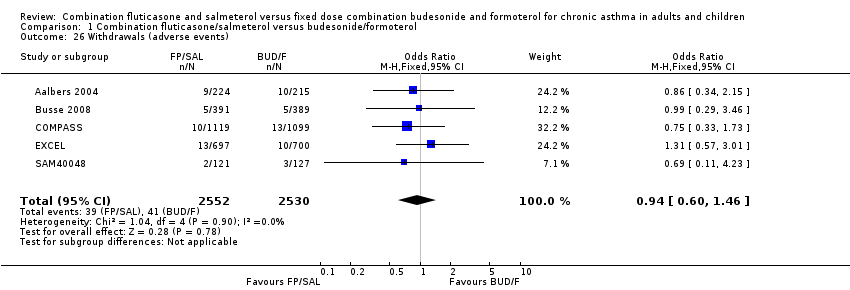
Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 26 Withdrawals (adverse events).

Comparison 1 Combination fluticasone/salmeterol versus budesonide/formoterol, Outcome 27 Withdrawals (lack of efficacy).
| Combination fluticasone/salmeterol versus budesonide/formoterol for chronic asthma in adults and children | ||||||
| Patient or population: Patients with chronic asthma in adults and children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combination budesonide/formoterol | Combination fluticasone/salmeterol | |||||
| Participants experiencing exacerbations requiring oral steroid treatment | 106 per 10001 | 95 per 1000 | OR 0.89 | 4949 | ⊕⊕⊕⊝ | |
| Participants experiencing exacerbations requiring admission to hospital | 7 per 10001 | 8 per 1000 | OR 1.29 | 4879 | ⊕⊕⊕⊝ | |
| Asthma‐related serious adverse event | 7 per 10001 | 10 per 1000 | OR 1.47 | 4054 | ⊕⊕⊕⊝ | |
| Mortality | See comment | See comment | Not estimable | 4819 | ⊕⊕⊝⊝ | The data did not generate a pooled effect estimate as no deaths occurred in four out of the five studies. |
| Withdrawals (adverse events) | 16 per 10001 | 15 per 1000 | OR 0.94 | 5082 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The mean rate of exacerbations across the BUD/F arms of the trials was used to calculate the assumed risk. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants experiencing exacerbations requiring oral steroid treatment Show forest plot | 4 | 4949 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
| 2 Participants experiencing exacerbations requiring admission to hospital Show forest plot | 4 | 4879 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.68, 2.47] |
| 3 Asthma‐related serious adverse event Show forest plot | 3 | 4054 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.75, 2.86] |
| 4 Participants experiencing exacerbations requiring ED visit/hospitalisation Show forest plot | 4 | 4861 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.94, 1.80] |
| 5 Mortality Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Asthma Quality of Life Questionnaire Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 N with improvement in Asthma Quality of Life Questionnaire Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Change in am PEF Show forest plot | 5 | 5101 | L/min (Fixed, 95% CI) | 2.24 [‐0.24, 4.73] |
| 9 Change in pm PEF Show forest plot | 4 | 4299 | L/min (Fixed, 95% CI) | 0.25 [‐0.80, 1.30] |
| 10 Change in FEV1 Show forest plot | 4 | 4845 | L (Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 11 Change in FEV1 predicted (%) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Change in rescue medication use Show forest plot | 3 | 3469 | Puffs/d (Fixed, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 13 Change in daytime symptoms Show forest plot | 3 | 3464 | Symptoms (Fixed, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 14 Change in symptom‐free days Show forest plot | 2 | 3027 | Symptoms (Fixed, 95% CI) | 1.25 [‐1.18, 3.67] |
| 15 Change in nocturnal awakenings Show forest plot | 1 | Symptoms (Fixed, 95% CI) | Totals not selected | |
| 16 Adverse events Show forest plot | 3 | 3547 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.15] |
| 17 Headache Show forest plot | 4 | 2916 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 18 Candidiasis Show forest plot | 2 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.68, 4.00] |
| 19 Upper respiratory tract infection Show forest plot | 2 | 1644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.47] |
| 20 Dysphonia Show forest plot | 3 | 2669 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.87, 2.43] |
| 21 Rhinitis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22 Throat irritation Show forest plot | 2 | 1644 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.82, 2.35] |
| 23 Cough Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24 Tremor Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25 Withdrawals Show forest plot | 5 | 5082 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.20] |
| 26 Withdrawals (adverse events) Show forest plot | 5 | 5082 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.46] |
| 27 Withdrawals (lack of efficacy) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

