Ventilation à haute fréquence versus ventilation conventionnelle pour le traitement d'une lésion pulmonaire aigüe et d'un syndrome de détresse respiratoire aigüe
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Multi‐centre RCT in 5 tertiary care paediatric ICUs in the United States. | |
| Participants | 70 children (weight <35 kg, mean age 2.8) with acute diffuse lung injury and impaired oxygenation. Excluded: if <40 weeks post‐conceptual age or former prematurity with residual chronic lung disease, obstructive airway disease, intractable septic or cardiogenic shock, non‐pulmonary terminal diagnosis. | |
| Interventions | 3100 high‐frequency oscillatory ventilator (SensorMedics). Initial settings of FiO2: 1.0, frequency of 5 to 10 Hz, mPaw of CV+(4 to 8) cm H2O, pressure amplitude of oscillation set for “adequate chest wall movement” or according to transcutaneous PCO2 sensor, bias gas flow 18 L/min. Controls were ventilated with pressure limited conventional mechanical ventilation (Servo 900C, Siemens; Veolar, Hamilton Medical). Target blood gas values were the same as for HFO. Crossover to the alternate ventilator was required if the patient met treatment failure criteria. | |
| Outcomes | Duration of mechanical ventilation, 30‐day mortality, supplemental oxygen at 30 days, neurological events. | |
| Notes | Patients had ARDS (86%) or pulmonary barotrauma requiring chest tube (14%). 21/62 were less than 1 year old. 12 patients excluded from the analysis due to: exclusion from the study within eight hours of enrolment. (n = 6); protocol violations (n = 4); transferred to other institution (n = 2). Open lung approach to achieve oxygenation targets used. No specific use of lung‐volume recruitment manoeuvres. Use of sedation and paralysis was not reported. Use of rescue therapies or co‐interventions for ARDS was not reported. Partial industry support (SensorMedics). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers (e‐mail correspondence, J Arnold, 5 June 2003) |
| Allocation concealment (selection bias) | Low risk | "randomization was based on a serialized form which included the balanced block design, thus the assignment was blinded to the investigator when a patient was selected to be in the study" (A ‐ Adequate) (e‐mail correspondence, J Arnold, 5 June 2003) |
| Incomplete outcome data (attrition bias) | Unclear risk | Data were available for 58 of 70 randomized patients after author contact |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported |
| Other bias | Unclear risk | >10% of patients (30/58) crossed over from assigned ventilator strategy. Lung protective ventilation was not mandated in the control group receiving conventional mechanical ventilation |
| Methods | Multi‐centre RCT in 5 ICUs in 4 European cities. | |
| Participants | 61 adults (mean age 53) with ARDS. Excluded: Patients with a non‐pulmonary terminal disease, severe chronic obstructive pulmonary disease or asthma and grade 3 or 4 air leak. | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Frequency of 5 Hz with inspiratory time of 33%, mPaw of CV+ 5 cm H2O, pressure amplitude of oscillation set according to PaCO2 and to achieve chest wall vibration. Controls were ventilated with time cycled pressure controlled mechanical ventilation with mean tidal volume of 8‐9 mL/kg ideal body weight (calculated from mean tidal volume per kg of ideal body weight on day 1, 2, 3). General physiological targets were provided, including limitation of peak inspiratory pressure to 40 cmH2O, but more detailed ventilation procedures and methods of weaning were according to standard protocols of the investigating centres. Crossover to the alternate ventilator was required if the patient met treatment failure criteria. | |
| Outcomes | Cumulative survival without mechanical ventilation or oxygen dependency at 30 days; mortality at 30 days; therapy failure; crossover rate; and persisting pulmonary problems defined as oxygen dependency or still being on a ventilator at 30 days. Data for ventilator settings and arterial blood gases were also available for the first three days. | |
| Notes | 7/61 patients were reported lost to follow‐up at 30 days; ICU mortality, but not 30‐day mortality, was available for 3/61 patients after author contact. Trial was terminated early for slow recruitment. No specific use of lung‐volume recruitment manoeuvres. Use of sedation and paralysis was not reported. Use of rescue therapies or co‐interventions for ARDS was not reported. Partial industry support (SensorMedics). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A ‐ Adequate) (email correspondence, C. Bollen, June 26, 2009) |
| Incomplete outcome data (attrition bias) | Unclear risk | 30‐day mortality available for 58/61 patients; ICU mortality, but not 30‐day mortality, was available for 3/61 after author contact |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported. |
| Other bias | Unclear risk | >10% of patients (11/61) crossed over from assigned ventilator strategy. Lung protective ventilation was not mandated in the control group receiving conventional mechanical ventilation |
| Methods | Single centre RCT in France. | |
| Participants | 28 adults (mean age 49) with ARDS and PaO2/FiO2 <150 and PEEP ≥5 cm H2O. | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings were FiO2 1.0, frequency of 5 Hz with inspiratory time of 33%, mPaw of CV+ 5 cm H2O (but ≤ plateau pressure), pressure amplitude of oscillation = PaCO2 during conventional mechanical ventilation (max 110). Controls were ventilated with volume‐assist control with tidal volume 6‐7 mL/kg predicted body weight. PEEP was adjusted according to the ARDSNet protocol. | |
| Outcomes | Physiologic data including PaO2/FiO2, OI, venous admixture. | |
| Notes | All patients received conventional mechanical ventilation in the prone position for 12 hours prior to HFO or conventional mechanical ventilation in the supine position. Duration of HFO was limited to 12 hours; therefore we included only physiologic data (PaO2/FiO2 and OI) in pooled analyses Recruitment manoeuvres were performed at HFO initiation, but not during conventional mechanical ventilation. Sedation and paralysis were applied equally to both treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers (e‐mail correspondence, L Papazian, 9 August 2011) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A ‐ Adequate) (e‐mail correspondence, L Papazian, 9 August 2011) |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported; authors provided additional physiologic data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
| Methods | Multi‐centre (13 university‐affiliated medical centres) RCT in the United States and Canada. | |
| Participants | 148 adults (mean age 49) with ARDS and PEEP > 10. | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of FiO2 0.80‐1.0, frequency of 5 Hz, mPaw of CV+5, pressure amplitude of oscillation set for “vibration down to level of mid‐thigh”, bias flow of 40 L/min. Switched back to CV when FiO2 was 0.50 or less and mPaw was weaned to 24 cm H2O or less with an SaO2 of 88% or more. Controls were ventilated using pressure control with an initial tidal volume of 6 to 10 ml/kg actual body weight, RR adjusted for pH greater than 7.15, PEEP of 10, inspiratory time 33%. Subsequent adjustment of PEEP was according to study protocol (range 10‐14). | |
| Outcomes | Survival without need for mechanical ventilation at 30 days from entry to study, 30‐day mortality, six‐month mortality, need for mechanical ventilation at 30 days and six months. Physiologic endpoints and other clinical outcomes were also obtained after author contact. | |
| Notes | Designed as an equivalence trial. Rescue therapies used in 9% of the HFO group (nitric oxide 4/75; prone position 2/75, high‐dose steroids 1/75) and 16% of the CV group (nitric oxide 8/73; prone position 3/73; high‐dose steroids 4/73). Lung‐volume recruitment manoeuvres were permitted, although not protocolized. All patients who received HFO were paralysed; paralysis was not mandatory in patients receiving CV. Partial industry support (SensorMedics). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomization with a balanced block design, balanced with respect to baseline oxygenation index > 40 (e‐mail correspondence, S Derdak, T Bachmann, 20 April 2009) |
| Allocation concealment (selection bias) | Low risk | Computerized randomization program (A ‐ Adequate) (e‐mail correspondence, S Derdak, T Bachmann, 20 April 2009) |
| Incomplete outcome data (attrition bias) | Low risk | Mortality data for withdrawn patients (2/148) obtained after author contact |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Unclear risk | Lung protective ventilation was not mandatory in the control group receiving conventional mechanical ventilation |
| Methods | Single centre RCT in Greece. | |
| Participants | 54 adults (mean age 57) with ARDS; PaO2/FiO2 <150, PEEP 8 cm H2O. | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of frequency of 4 Hz, mPaw of 3 above mean tracheal pressure measured distal to the endotracheal tube, pressure amplitude of oscillation set 30 above baseline PaCO2 during CV. Patients received 6‐24 hr of HFO each day until PaO2/FiO2 ≥150 for >12hr on CV. All patients received tracheal gas insufflation with HFO. Controls were ventilated using volume assist control with an initial tidal volume of 6 to 7 ml/kg predicted body weight. Subsequent adjustment of tidal volume and PEEP was according to the ARDSNet protocol. | |
| Outcomes | Hospital mortality, and other clinical and physiologic outcomes were obtained after author contact. | |
| Notes | Protocols for lung volume recruitment manoeuvres were used for both the HFO and CV group. Steroids for ARDS were used in 20/27 and 21/27 of the HFO and CV groups respectively. Paralysis was administered to 27/27 and 21/27 of the HFO and CV groups respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers (e‐mail correspondence, SD Mentzelopoulus, 9 April 2009) |
| Allocation concealment (selection bias) | Low risk | Telephone (A ‐ Adequate) (e‐mail correspondence, SD Mentzelopoulus, 9 April 2009) |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | |
| Methods | Single centre RCT in France. | |
| Participants | 26 adults (mean age 51) with ARDS; PaO2/FiO2≤150, PEEP ≥5 cm H2O. | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of FiO2 1.0, frequency of 5 Hz with inspiratory time of 33%, bias flow of 20 L/min, mPaw of CV+ 5 cm H2O, pressure amplitude of oscillation = PaCO2 during conventional mechanical ventilation (max 110). All patients were ventilated in the prone position. Controls were ventilated with volume‐assist control with tidal volume 6 mL/kg predicted body weight. PEEP was set to 2 cm H2O above the lower inflection point of the pressure volume curve. All patients were ventilated in the prone position. | |
| Outcomes | Physiologic data (including PaO2/FiO2 and OI), haemodynamics, and inflammatory mediators in BAL fluid and blood. | |
| Notes | All patients were ventilated in the prone position. Recruitment manoeuvres (45 cm H2O x 40 seconds) were performed at HFO initiation, but not during conventional mechanical ventilation. Duration of HFO was limited to 12 hours; therefore we included only physiologic data (PaO2/FiO2 and OI) in pooled analyses. Sedation and paralysis were applied equally to both treatment groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers (e‐mail correspondence, L Papazian, 9 August 2011) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A ‐ Adequate) (e‐mail correspondence, L Papazian, 9 August 2011) |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
| Methods | Single centre RCT in Bangkok, Thailand. | |
| Participants | 16 children (weight <35 kg; mean age 5) with ARDS, PEEP >5 cm H2O; FiO2 >0.6 for 12 hr to keep SaO2 >92%; OI >15 for ≥4 hr. | |
| Interventions | SensorMedics 3100 high‐frequency oscillatory ventilator. Initial settings of frequency of 4‐10 Hz, mPaw of CV+(2 or 3), pressure amplitude of oscillation set for 10 above peak inspiratory pressure during CV. Switched back to CV when mPaw was weaned to approximately 18 cm H2O and patients were tolerating suctioning. Controls were ventilated using pressure control with an initial tidal volume of 6 to 10 mL/kg actual body weight, RR adjusted for pH greater than 7.15, PEEP of 10, inspiratory time 33%. PEEP was adjusted according to the ARDS Network protocol. | |
| Outcomes | Plasma sICAM‐1 measured by enzyme linked immunosorbent assay on days 1, 3, 5 and 7 of ARDS. Authors also reported duration of mechanical ventilation, daily OI and PaO2/FiO2, and hospital mortality. | |
| Notes | No specific use of lung‐volume recruitment manoeuvres. 1/7 and 0/9 children received inhaled nitric oxide in the HFO and CV groups respectively. All patients were sedated and paralysed. Not analysed by intention to treat. (One patient who crossed over from CV to HFO shortly after randomization and was analysed as treated; authors provided data which allowed analysis of this patient according to assigned group for clinical outcomes such as mortality and treatment failure, but not for physiologic outcomes such as OI and PaO2/FiO2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers (e‐mail correspondence, R Samransamjuajkit, 3 March 2009) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A ‐ Adequate) (e‐mail correspondence, R Samransamjuajkit, 13 March 2009) |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | |
| Methods | Single centre RCT in the United Kingdom. | |
| Participants | 28 adults (mean age 49) with ARDS. | |
| Interventions | 3100B high‐frequency oscillatory ventilator (SensorMedics). Initial settings of frequency of 5 Hz, mPaw of CV+5, pressure amplitude of oscillation set for “vibration down to level of mid‐thigh”. No specific criteria for transitioning to CV were reported but HFO was continued until "resolution of ARDS". Controls were ventilated with time cycled pressure controlled mechanical ventilation with mean tidal volume of 7‐8 mL/kg ideal body weight (calculated from mean tidal volume per kg of ideal body weight on day 1, 2, 3). Tidal volume and PEEP were adjusted according to the ARDS Network low tidal volume protocol. | |
| Outcomes | Changes in ventilatory parameters (PaO2/FiO2 and FiO2) over the first 72 hours of HFO or CV. Data for 30‐day mortality and other outcomes were also available after author contact. | |
| Notes | No specific use of lung‐volume recruitment manoeuvres. Protocols for sedation and paralysis were applied equally to HFO and CV groups. No use of rescue therapies or co‐interventions for ARDS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random draw ("sealed opaque envelopes which were drawn in a random manner by physician independent of the research team") (e‐mail correspondence, S Shah, 30 Nov 2007) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A‐ Adequate) (e‐mail correspondence, S Shah, 30 Nov 2007) |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported; authors provided additional clinical and physiologic outcome data for this review after being contacted |
| Other bias | Low risk | No other source of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Patient population had “acute respiratory failure” from a variety of reasons and included many patients requiring mechanical ventilation who would not necessarily fit modern criteria for ALI or ARDS. | |
| Randomized on inhaled nitric oxide, not HFO. | |
| Randomized on frequency of oscillation, not HFO. | |
| Patients served as their own controls. Total of nine patients randomized. | |
| Patients in the study who received HFOV were only “at risk” of developing ALI/ARDS. | |
| Randomized on tracheal gas insufflation, not HFO. Crossover design. | |
| Randomized on tracheal gas insufflation, not HFO. Crossover design. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The Oscillation for ARDS Treated Early (OSCILLATE) Trial |
| Methods | Multi‐centre RCT |
| Participants | Patients of either sex, 16 years and older; Acute onset of respiratory failure, with fewer than 2 weeks of new pulmonary symptoms; Endotracheal intubation or tracheostomy; Hypoxaemia ‐ defined as PaO2/FiO2 < 200 mmHg on FiO2 ≥ 0.5, regardless of PEEP; Bilateral alveolar consolidation (airspace disease) seen on frontal chest radiograph. |
| Interventions | Intervention group: high frequency oscillatory (HFO) ventilation using a lung‐open approach and an explicit protocol. |
| Outcomes | Hospital mortality; also 6 month mortality, quality of life at 6 months |
| Starting date | June 1, 2009 |
| Contact information | |
| Notes | Planned enrolment of 1200 patients |
| Trial name or title | OSCAR: High Frequency OSCillation in ARDS |
| Methods | Multi‐centre RCT |
| Participants | Patients age ≥16 years; Weight ≥35 kg; Endotracheal intubation or tracheostomy; Hypoxaemia defined as PaO2/FiO2 ratio ≤26.7kPa (200 mmHg), with PEEP ≥ 5 cm H2O, determined on two arterial blood samples 12 hours apart; Bilateral infiltrates on chest radiograph; One or more risk factors for ARDS (including pneumonia, aspiration of gastric contents, inhalation injury, sepsis, major trauma, multiple transfusions, drug overdose, burn injury, acute pancreatitis, or shock); Predicted to require at least 48 hours of artificial ventilation from the time of randomization. |
| Interventions | Intervention: High Frequency Oscillatory Ventilation (HFOV) Control: Conventional positive pressure ventilation |
| Outcomes | 30‐day mortality. An economic analysis is also being carried out. |
| Starting date | June 1, 2007 |
| Contact information | |
| Notes | Planned enrolment of 1006 patients. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital or 30‐day Mortality Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| Analysis 1.1  Comparison 1 Mortality, Outcome 1 Hospital or 30‐day Mortality. | ||||
| 2 Hospital or 30‐day Mortality (Bollen 2005 patients lost to follow‐up excluded) Show forest plot | 6 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| Analysis 1.2 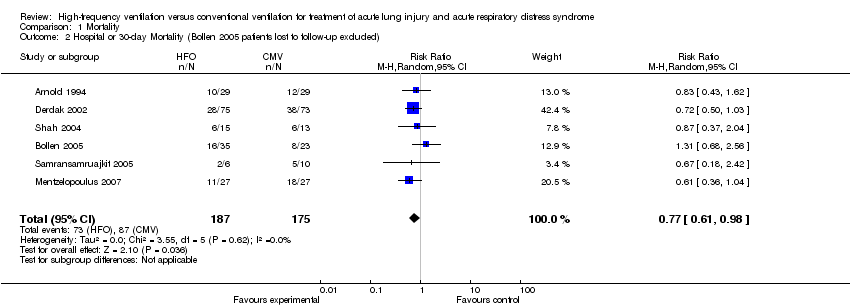 Comparison 1 Mortality, Outcome 2 Hospital or 30‐day Mortality (Bollen 2005 patients lost to follow‐up excluded). | ||||
| 3 Hospital or 30‐day mortality: Adult versus paediatric trials Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| Analysis 1.3  Comparison 1 Mortality, Outcome 3 Hospital or 30‐day mortality: Adult versus paediatric trials. | ||||
| 3.1 Adult Trials | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 3.2 Paediatric Trials | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.44, 1.43] |
| 4 Hospital or 30‐day Mortality: Low risk of bias versus unclear risk of bias Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| Analysis 1.4 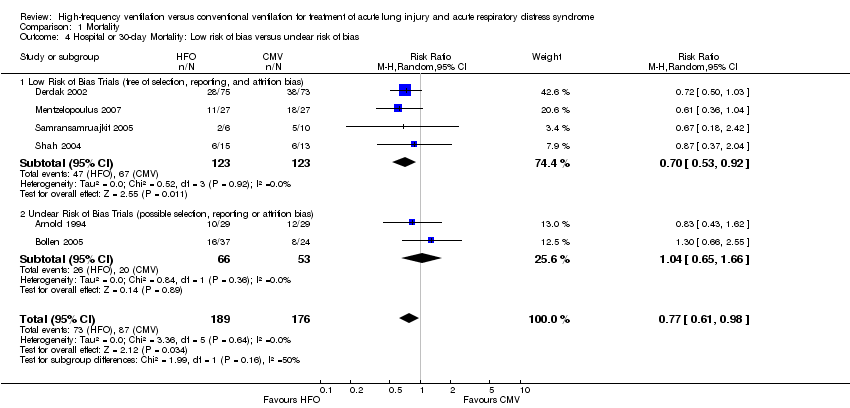 Comparison 1 Mortality, Outcome 4 Hospital or 30‐day Mortality: Low risk of bias versus unclear risk of bias. | ||||
| 4.1 Low Risk of Bias Trials (free of selection, reporting, and attrition bias) | 4 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.53, 0.92] |
| 4.2 Unclear Risk of Bias Trials (possible selection, reporting or attrition bias) | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.65, 1.66] |
| 5 Hospital or 30‐day Mortality: Lung protective ventilation mandatory vs. not mandatory Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| Analysis 1.5  Comparison 1 Mortality, Outcome 5 Hospital or 30‐day Mortality: Lung protective ventilation mandatory vs. not mandatory. | ||||
| 5.1 Lung Protective Ventilation Not Mandatory | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| 5.2 Lung Protective Ventilation Mandatory | 3 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.44, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment Failure (Intractable Hypoxia, Hypotension, Acidosis, Hypercapnea requiring discontinuation of study intervention) Show forest plot | 5 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.99] |
| Analysis 2.1  Comparison 2 Adverse events, Outcome 1 Treatment Failure (Intractable Hypoxia, Hypotension, Acidosis, Hypercapnea requiring discontinuation of study intervention). | ||||
| 2 Barotrauma Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.37, 1.22] |
| Analysis 2.2  Comparison 2 Adverse events, Outcome 2 Barotrauma. | ||||
| 3 Hypotension Show forest plot | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.34, 7.02] |
| Analysis 2.3 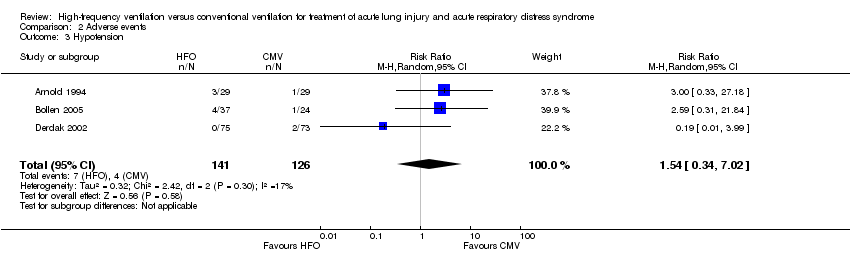 Comparison 2 Adverse events, Outcome 3 Hypotension. | ||||
| 4 Hypotension (Shah and Mentzelopoulos included) Show forest plot | 5 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.77, 2.76] |
| Analysis 2.4  Comparison 2 Adverse events, Outcome 4 Hypotension (Shah and Mentzelopoulos included). | ||||
| 5 ETT Obstruction Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Adverse events, Outcome 5 ETT Obstruction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of Mechanical Ventilation Show forest plot | 4 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐5.36, 3.85] |
| Analysis 3.1 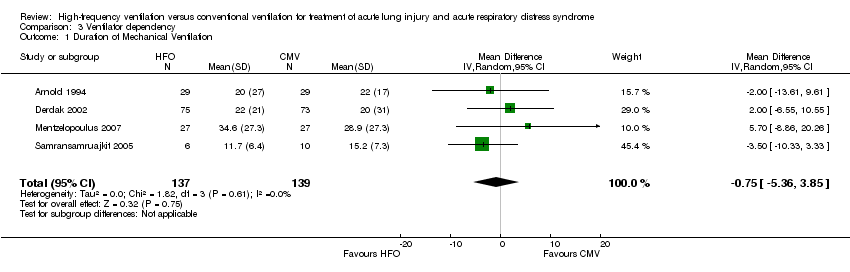 Comparison 3 Ventilator dependency, Outcome 1 Duration of Mechanical Ventilation. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PaO2/FiO2 (Ratio of Means) Show forest plot | 7 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| Analysis 4.1 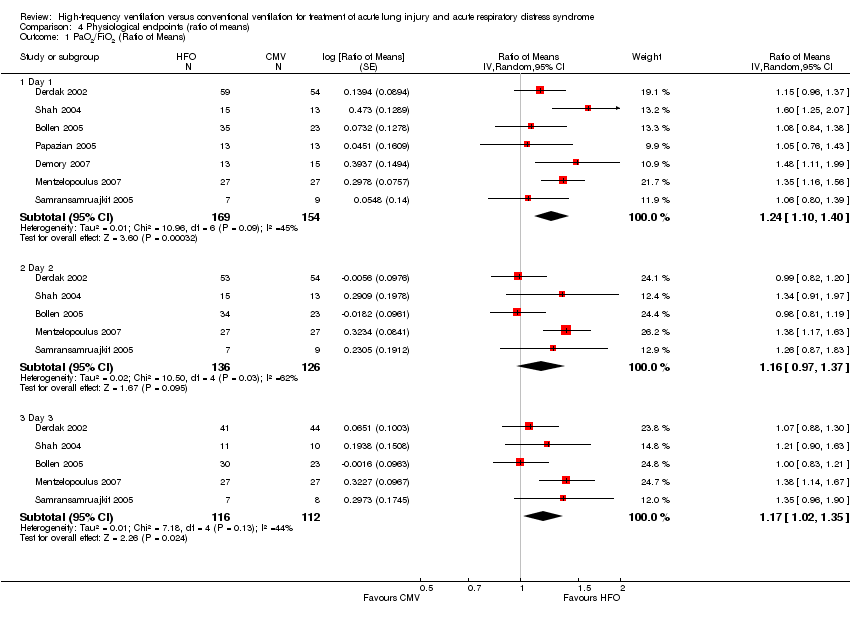 Comparison 4 Physiological endpoints (ratio of means), Outcome 1 PaO2/FiO2 (Ratio of Means). | ||||
| 1.1 Day 1 | 7 | 323 | Ratio of Means (Random, 95% CI) | 1.24 [1.10, 1.40] |
| 1.2 Day 2 | 5 | 262 | Ratio of Means (Random, 95% CI) | 1.16 [0.97, 1.37] |
| 1.3 Day 3 | 5 | 228 | Ratio of Means (Random, 95% CI) | 1.17 [1.02, 1.35] |
| 2 Oxygenation Index (Ratio of Means) Show forest plot | 7 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Physiological endpoints (ratio of means), Outcome 2 Oxygenation Index (Ratio of Means). | ||||
| 2.1 Day 1 | 7 | 352 | Ratio of Means (Random, 95% CI) | 1.11 [0.97, 1.26] |
| 2.2 Day 2 | 6 | 306 | Ratio of Means (Random, 95% CI) | 1.07 [0.92, 1.24] |
| 2.3 Day 3 | 6 | 266 | Ratio of Means (Random, 95% CI) | 1.07 [0.88, 1.29] |
| 3 PaCO2 (Ratio of Means) Show forest plot | 8 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Physiological endpoints (ratio of means), Outcome 3 PaCO2 (Ratio of Means). | ||||
| 3.1 Day 1 | 8 | 386 | Ratio of Means (Random, 95% CI) | 0.91 [0.78, 1.07] |
| 3.2 Day 2 | 6 | 310 | Ratio of Means (Random, 95% CI) | 0.87 [0.72, 1.06] |
| 3.3 Day 3 | 6 | 267 | Ratio of Means (Random, 95% CI) | 0.98 [0.84, 1.14] |
| 4 Mean Airway Pressure (Ratio of Means) Show forest plot | 8 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Physiological endpoints (ratio of means), Outcome 4 Mean Airway Pressure (Ratio of Means). | ||||
| 4.1 Day 1 | 8 | 389 | Ratio of Means (Random, 95% CI) | 1.33 [1.27, 1.40] |
| 4.2 Day 2 | 6 | 309 | Ratio of Means (Random, 95% CI) | 1.26 [1.16, 1.37] |
| 4.3 Day 3 | 6 | 274 | Ratio of Means (Random, 95% CI) | 1.22 [1.07, 1.39] |
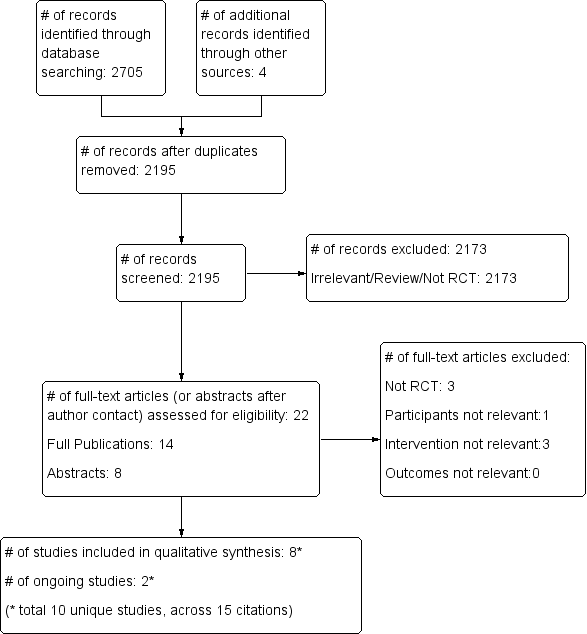
Study flow diagram.
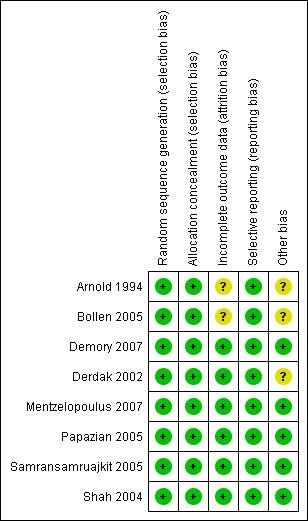
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
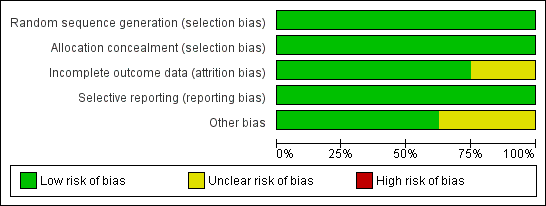
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
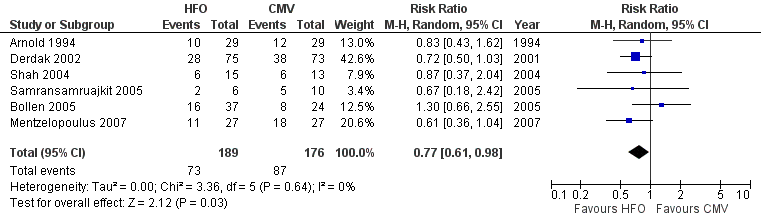
Forest plot of comparison: 1 Mortality, outcome: 1.1 Hospital or 30‐day mortality.

Forest plot of comparison: 2 Adverse events, outcome: 2.1 Treatment failure (intractable hypoxia, hypotension, acidosis, hypercapnoea requiring discontinuation of study intervention).

Comparison 1 Mortality, Outcome 1 Hospital or 30‐day Mortality.

Comparison 1 Mortality, Outcome 2 Hospital or 30‐day Mortality (Bollen 2005 patients lost to follow‐up excluded).

Comparison 1 Mortality, Outcome 3 Hospital or 30‐day mortality: Adult versus paediatric trials.

Comparison 1 Mortality, Outcome 4 Hospital or 30‐day Mortality: Low risk of bias versus unclear risk of bias.
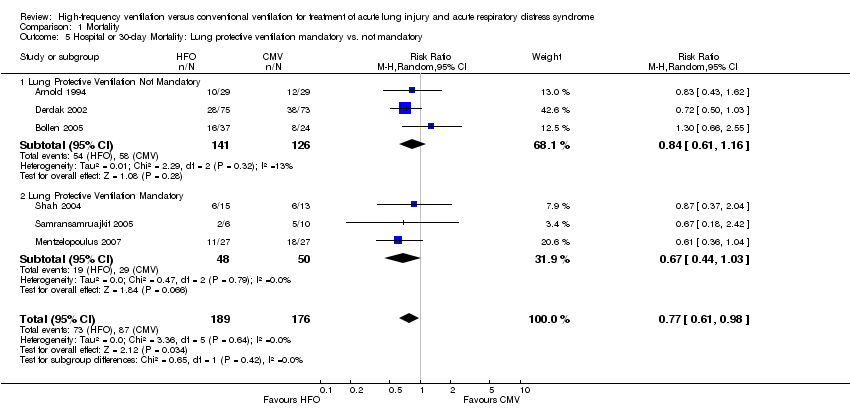
Comparison 1 Mortality, Outcome 5 Hospital or 30‐day Mortality: Lung protective ventilation mandatory vs. not mandatory.
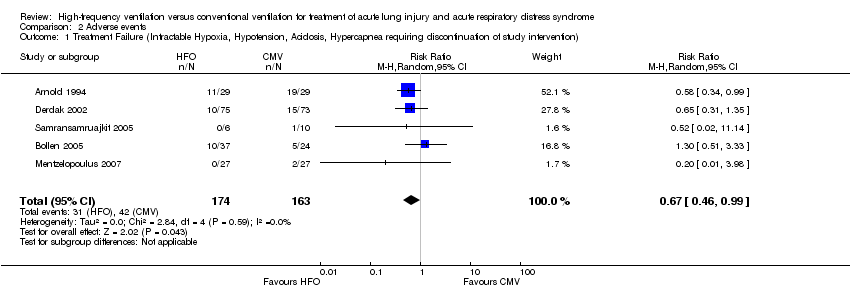
Comparison 2 Adverse events, Outcome 1 Treatment Failure (Intractable Hypoxia, Hypotension, Acidosis, Hypercapnea requiring discontinuation of study intervention).

Comparison 2 Adverse events, Outcome 2 Barotrauma.
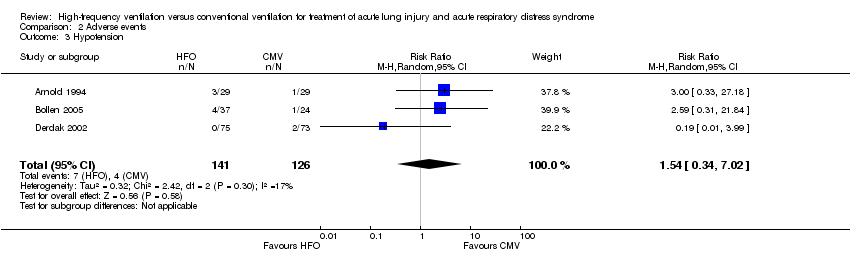
Comparison 2 Adverse events, Outcome 3 Hypotension.

Comparison 2 Adverse events, Outcome 4 Hypotension (Shah and Mentzelopoulos included).

Comparison 2 Adverse events, Outcome 5 ETT Obstruction.
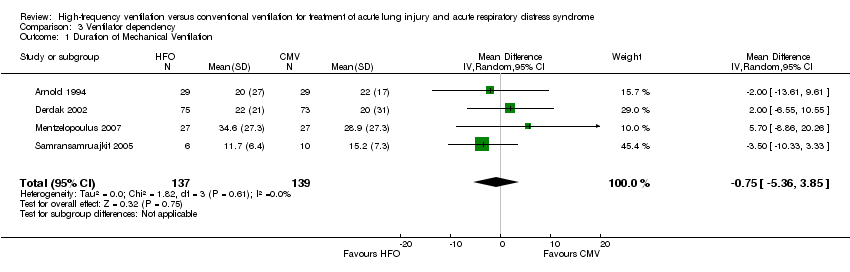
Comparison 3 Ventilator dependency, Outcome 1 Duration of Mechanical Ventilation.
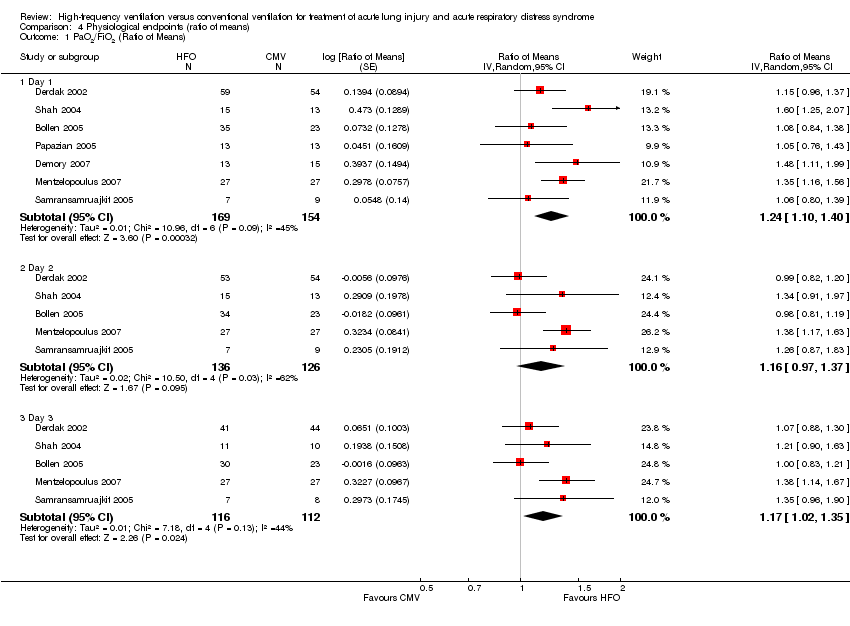
Comparison 4 Physiological endpoints (ratio of means), Outcome 1 PaO2/FiO2 (Ratio of Means).

Comparison 4 Physiological endpoints (ratio of means), Outcome 2 Oxygenation Index (Ratio of Means).

Comparison 4 Physiological endpoints (ratio of means), Outcome 3 PaCO2 (Ratio of Means).

Comparison 4 Physiological endpoints (ratio of means), Outcome 4 Mean Airway Pressure (Ratio of Means).
| HFO compared to conventional mechanical ventilation for ALI and ARDS | ||||||
| Patient or population: patients with ALI and ARDS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional mechanical ventilation | High Frequency Oscillation | |||||
| Hospital (or 30 day) mortality | Typical risk1 | RR 0.77 | 365 | ⊕⊕⊕⊝ | ||
| 443 per 1000 | 341 per 1000 | |||||
| 6 month mortality | 589 per 10004 | 465 per 1000 | RR 0.79 | 148 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The basis of the assumed risk is a systematic review and meta‐analysis of the mortality in patients with ARDS (Phua 2009). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospital or 30‐day Mortality Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 2 Hospital or 30‐day Mortality (Bollen 2005 patients lost to follow‐up excluded) Show forest plot | 6 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 3 Hospital or 30‐day mortality: Adult versus paediatric trials Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 3.1 Adult Trials | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 3.2 Paediatric Trials | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.44, 1.43] |
| 4 Hospital or 30‐day Mortality: Low risk of bias versus unclear risk of bias Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 4.1 Low Risk of Bias Trials (free of selection, reporting, and attrition bias) | 4 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.53, 0.92] |
| 4.2 Unclear Risk of Bias Trials (possible selection, reporting or attrition bias) | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.65, 1.66] |
| 5 Hospital or 30‐day Mortality: Lung protective ventilation mandatory vs. not mandatory Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.98] |
| 5.1 Lung Protective Ventilation Not Mandatory | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.61, 1.16] |
| 5.2 Lung Protective Ventilation Mandatory | 3 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.44, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment Failure (Intractable Hypoxia, Hypotension, Acidosis, Hypercapnea requiring discontinuation of study intervention) Show forest plot | 5 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.99] |
| 2 Barotrauma Show forest plot | 6 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.37, 1.22] |
| 3 Hypotension Show forest plot | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.34, 7.02] |
| 4 Hypotension (Shah and Mentzelopoulos included) Show forest plot | 5 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.77, 2.76] |
| 5 ETT Obstruction Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of Mechanical Ventilation Show forest plot | 4 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐5.36, 3.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PaO2/FiO2 (Ratio of Means) Show forest plot | 7 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 1.1 Day 1 | 7 | 323 | Ratio of Means (Random, 95% CI) | 1.24 [1.10, 1.40] |
| 1.2 Day 2 | 5 | 262 | Ratio of Means (Random, 95% CI) | 1.16 [0.97, 1.37] |
| 1.3 Day 3 | 5 | 228 | Ratio of Means (Random, 95% CI) | 1.17 [1.02, 1.35] |
| 2 Oxygenation Index (Ratio of Means) Show forest plot | 7 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 2.1 Day 1 | 7 | 352 | Ratio of Means (Random, 95% CI) | 1.11 [0.97, 1.26] |
| 2.2 Day 2 | 6 | 306 | Ratio of Means (Random, 95% CI) | 1.07 [0.92, 1.24] |
| 2.3 Day 3 | 6 | 266 | Ratio of Means (Random, 95% CI) | 1.07 [0.88, 1.29] |
| 3 PaCO2 (Ratio of Means) Show forest plot | 8 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 3.1 Day 1 | 8 | 386 | Ratio of Means (Random, 95% CI) | 0.91 [0.78, 1.07] |
| 3.2 Day 2 | 6 | 310 | Ratio of Means (Random, 95% CI) | 0.87 [0.72, 1.06] |
| 3.3 Day 3 | 6 | 267 | Ratio of Means (Random, 95% CI) | 0.98 [0.84, 1.14] |
| 4 Mean Airway Pressure (Ratio of Means) Show forest plot | 8 | Ratio of Means (Random, 95% CI) | Subtotals only | |
| 4.1 Day 1 | 8 | 389 | Ratio of Means (Random, 95% CI) | 1.33 [1.27, 1.40] |
| 4.2 Day 2 | 6 | 309 | Ratio of Means (Random, 95% CI) | 1.26 [1.16, 1.37] |
| 4.3 Day 3 | 6 | 274 | Ratio of Means (Random, 95% CI) | 1.22 [1.07, 1.39] |

