Quimioterapia para el cáncer gástrico avanzado
Appendices
Appendix 1. CENTRAL update January 2013
1. exp stomach neoplasms/
2. (stomach adj5 neoplas$).ti,ab.
3. (stomach adj5 cancer$).ti,ab.
4. (stomach adj5 carcin$).ti,ab.
5. (stomach adj5 tumo$).ti,ab.
6. (stomach adj5 metasta$).ti,ab.
7. (stomach adj5 malig$).ti,ab.
8. (gastric adj5 neoplas$).ti,ab.
9. (gastric adj5 cancer$).ti,ab.
10. (gastric adj5 carcin$).ti,ab.
11. (gastric adj5 tumo$).ti,ab.
12. (gastric adj5 metasta$).ti,ab.
13. (gastric adj5 malig$).ti,ab.
14. or/1‐13
15. exp drug therapy/
16. chemothera$.ti,ab.
17. drug therap$.ti,ab.
18. antineoplastic$.ti,ab.
19. or/15‐18
20. exp palliative care/
21. palliat$.ti,ab.
22. unresect$.ti,ab.
23. inopera$.ti,ab.
24. advanc$.ti,ab.
25. (best adj5 support$ adj5 care).ti,ab.
26. unopera$.ti,ab.
27. (non adj5 resect$).ti,ab.
28. nonresect$.ti,ab.
29. or/20‐28
30. 14 and 19
31. 29 and 30
32. limit 31 to yr="2009 ‐ 2013"
Appendix 2. MEDLINE update March 2009‐Jan 2013
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. or/1‐8
10. exp animals/ not humans.sh.
11. 9 not 10
12. exp stomach neoplasms/
13. (stomach adj5 neoplas$).tw.
14. (stomach adj5 cancer$).tw.
15. (stomach adj5 carcin$).tw.
16. (stomach adj5 tumo$).tw.
17. (stomach adj5 metasta$).tw.
18. (stomach adj5 malig$).tw.
19. (gastric adj5 neoplas$).tw.
20. (gastric adj5 cancer$).tw.
21. (gastric adj5 carcin$).tw.
22. (gastric adj5 tumo$).tw.
23. (gastric adj5 metasta$).tw.
24. (gastric adj5 malig$).tw.
25. or/12‐24
26. exp drug therapy/
27. chemothera$.tw.
28. drug therap$.tw.
29. antineoplastic$.tw.
30. or/26‐29
31. exp palliative care/
32. palliat$.tw.
33. unresect$.tw.
34. inopera$.tw.
35. advanc$.tw.
36. (best adj5 support$ adj5 care).tw.
37. unopera$.tw.
38. (non adj5 resect$).tw.
39. nonresect$.tw.
40. or/31‐39
41. 25 and 30
42. 40 and 41
43. 42 and 11
44. limit 43 to ed=20090309‐20130131
Appendix 3. Embase update March 2009‐Jan 2013
1. exp randomized controlled trial/
2. randomi?ed controlled trial$.tw.
3. exp randomization/
4. exp single blind procedure/
5. exp double blind procedure/
6. or/1‐5
7. animal.hw.
8. human.hw.
9. 7 not (7 and 8)
10. 6 not 9
11. exp clinical trial/
12. (clin$ adj3 stud$).ti,ab,tw.
13. (clin$ adj3 trial$).ti,ab,tw.
14. ((singl$ or doubl$ or treb$ or tripl$) adj3 (blind$ or mask$)).ti,ab,tw.
15. exp placebo/
16. placebo$.ti,ab,tw.
17. random.ti,ab,tw.
18. (crossover$ or cross‐over$).ti,ab,tw.
19. or/11‐18
20. 19 not 9
21. 20 not 10
22. exp comparative study/
23. exp evaluation/
24. exp prospective study/
25. exp controlled study/
26. (control$ or prospective$ or volunteer$).ti,ab,tw.
27. or/22‐26
28. 27 not 9
29. 10 or 21 or 28
30. exp stomach tumor/
31. (stomach adj5 neoplas$).tw.
32. (stomach adj5 cancer$).tw.
33. (stomach adj5 carcin$).tw.
34. (stomach adj5 tumo$).tw.
35. (stomach adj5 metasta$).tw.
36. (stomach adj5 malig$).tw.
37. (gastric adj5 neoplas$).tw.
38. (gastric adj5 cancer$).tw.
39. (gastric adj5 carcin$).tw.
40. (gastric adj5 tumo$).tw.
41. (gastric adj5 metasta$).tw.
42. (gastric adj5 malig$).tw.
43. or/30‐42
44. exp drug therapy/
45. chemothera$.tw.
46. drug therap$.tw.
47. antineoplastic$.tw.
48. or/44‐47
49. exp palliative therapy/
50. palliat$.tw.
51. unresect$.tw.
52. inopera$.tw.
53. advanc$.tw.
54. (best adj5 support$ adj5 care).tw.
55. unopera$.tw.
56. (non adj5 resect$).tw.
57. nonresect$.tw.
58. or/49‐57
59. 43 and 48
60. 58 and 59
61. 29 and 60
62. limit 61 to em=200910‐201306

Study flow diagram: review update

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Chemotherapy versus best supportive care, Outcome 1 Overall survival.

Comparison 1 Chemotherapy versus best supportive care, Outcome 2 Time to progression.

Comparison 2 Combination versus single‐agent chemotherapy, Outcome 1 Overall survival.

Comparison 2 Combination versus single‐agent chemotherapy, Outcome 2 Tumour response.
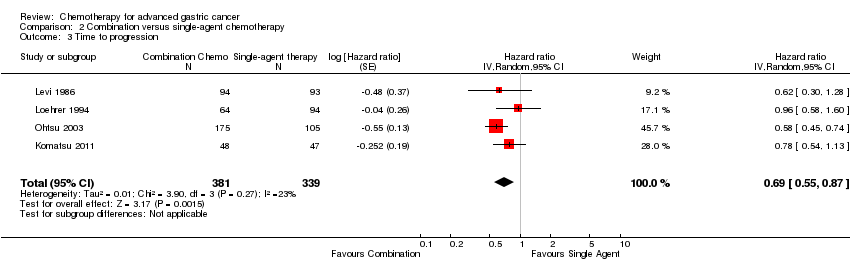
Comparison 2 Combination versus single‐agent chemotherapy, Outcome 3 Time to progression.

Comparison 2 Combination versus single‐agent chemotherapy, Outcome 4 Treatment‐related death.
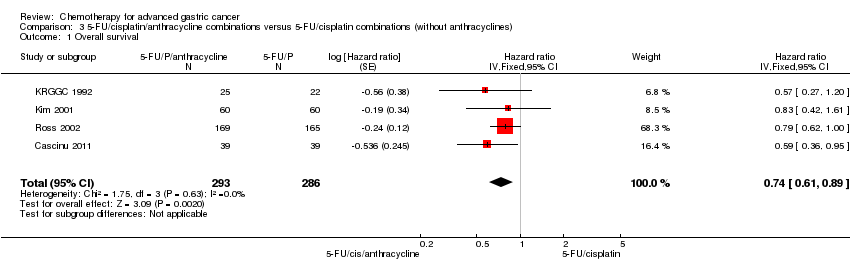
Comparison 3 5‐FU/cisplatin/anthracycline combinations versus 5‐FU/cisplatin combinations (without anthracyclines), Outcome 1 Overall survival.

Comparison 3 5‐FU/cisplatin/anthracycline combinations versus 5‐FU/cisplatin combinations (without anthracyclines), Outcome 2 Tumour response.

Comparison 3 5‐FU/cisplatin/anthracycline combinations versus 5‐FU/cisplatin combinations (without anthracyclines), Outcome 3 Time to progression.
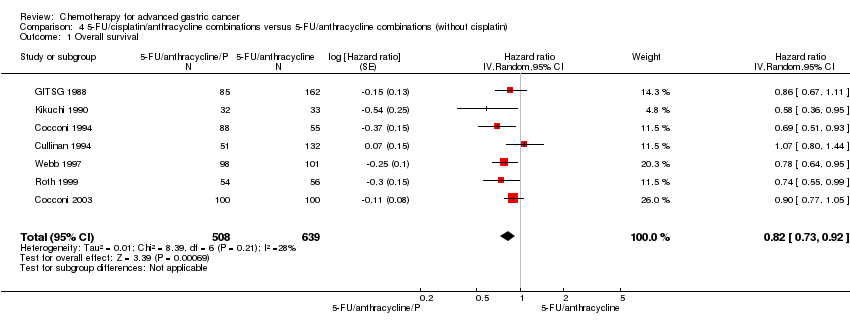
Comparison 4 5‐FU/cisplatin/anthracycline combinations versus 5‐FU/anthracycline combinations (without cisplatin), Outcome 1 Overall survival.

Comparison 5 Chemotherapy with irinotecan versus non‐irinotecan‐containing regimes, Outcome 1 Overall survival.

Comparison 5 Chemotherapy with irinotecan versus non‐irinotecan‐containing regimes, Outcome 2 Tumour response.

Comparison 5 Chemotherapy with irinotecan versus non‐irinotecan‐containing regimes, Outcome 3 Progression‐free survival.
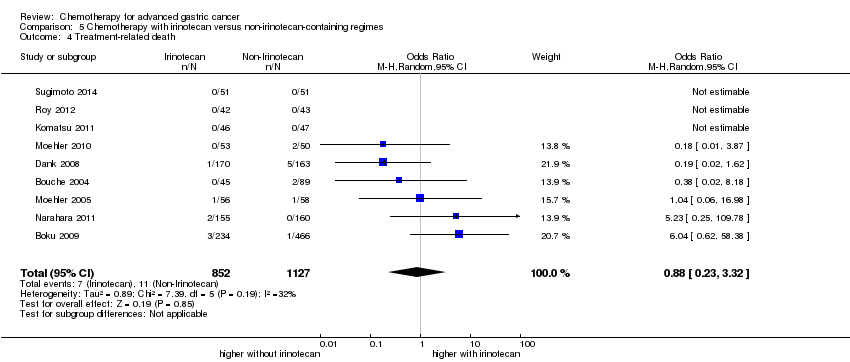
Comparison 5 Chemotherapy with irinotecan versus non‐irinotecan‐containing regimes, Outcome 4 Treatment‐related death.

Comparison 5 Chemotherapy with irinotecan versus non‐irinotecan‐containing regimes, Outcome 5 Treatment discontinuation due to toxicity.

Comparison 6 Chemotherapy with docetaxel versus non‐docetaxel‐containing regimes, Outcome 1 Overall survival.

Comparison 6 Chemotherapy with docetaxel versus non‐docetaxel‐containing regimes, Outcome 2 Tumour response.

Comparison 6 Chemotherapy with docetaxel versus non‐docetaxel‐containing regimes, Outcome 3 Time to progression.

Comparison 6 Chemotherapy with docetaxel versus non‐docetaxel‐containing regimes, Outcome 4 Progression‐free survival.

Comparison 6 Chemotherapy with docetaxel versus non‐docetaxel‐containing regimes, Outcome 5 Treatment‐related death.
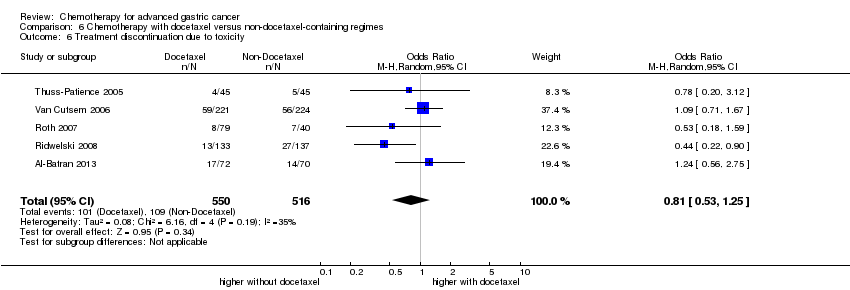
Comparison 6 Chemotherapy with docetaxel versus non‐docetaxel‐containing regimes, Outcome 6 Treatment discontinuation due to toxicity.

Comparison 7 Chemotherapy with capecitabine versus 5‐FU‐containing regimes, Outcome 1 Overall Survival.
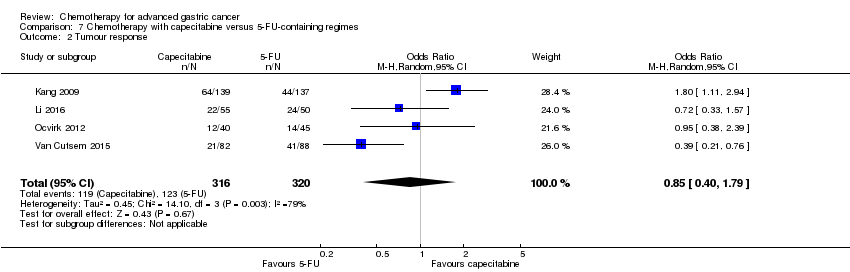
Comparison 7 Chemotherapy with capecitabine versus 5‐FU‐containing regimes, Outcome 2 Tumour response.

Comparison 7 Chemotherapy with capecitabine versus 5‐FU‐containing regimes, Outcome 3 Time to progression.

Comparison 7 Chemotherapy with capecitabine versus 5‐FU‐containing regimes, Outcome 4 Progression‐free survival.

Comparison 7 Chemotherapy with capecitabine versus 5‐FU‐containing regimes, Outcome 5 Treatment‐related death.

Comparison 7 Chemotherapy with capecitabine versus 5‐FU‐containing regimes, Outcome 6 Treatment discontinuation due to toxicity.
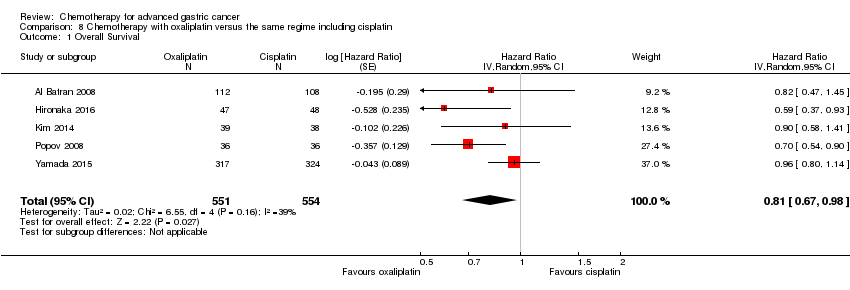
Comparison 8 Chemotherapy with oxaliplatin versus the same regime including cisplatin, Outcome 1 Overall Survival.
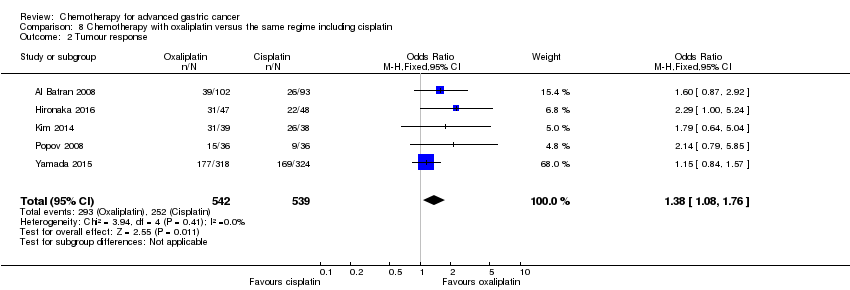
Comparison 8 Chemotherapy with oxaliplatin versus the same regime including cisplatin, Outcome 2 Tumour response.

Comparison 8 Chemotherapy with oxaliplatin versus the same regime including cisplatin, Outcome 3 Progression‐free survival.

Comparison 8 Chemotherapy with oxaliplatin versus the same regime including cisplatin, Outcome 4 Treatment‐related death.

Comparison 8 Chemotherapy with oxaliplatin versus the same regime including cisplatin, Outcome 5 Treatment discontinuation due to toxicity.

Comparison 9 Taxane‐platinum‐fluoropyrimidine combinations versus taxane‐platinum (without fluoropyrimidine), Outcome 1 Overall survival.

Comparison 9 Taxane‐platinum‐fluoropyrimidine combinations versus taxane‐platinum (without fluoropyrimidine), Outcome 2 Tumour response.
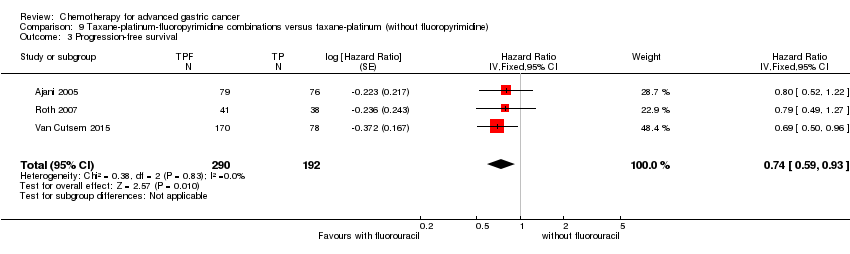
Comparison 9 Taxane‐platinum‐fluoropyrimidine combinations versus taxane‐platinum (without fluoropyrimidine), Outcome 3 Progression‐free survival.
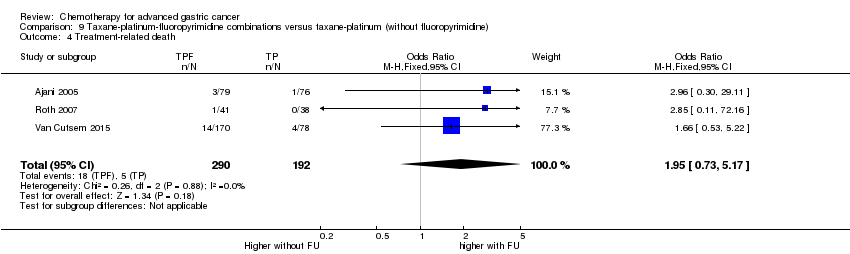
Comparison 9 Taxane‐platinum‐fluoropyrimidine combinations versus taxane‐platinum (without fluoropyrimidine), Outcome 4 Treatment‐related death.
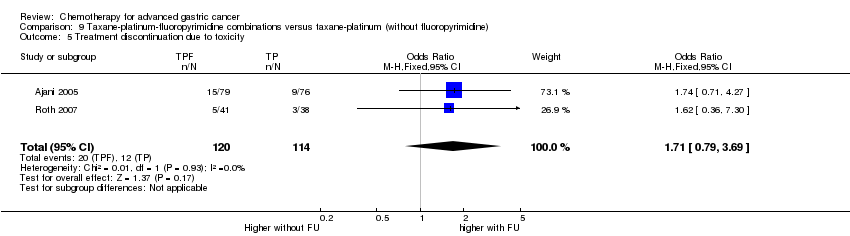
Comparison 9 Taxane‐platinum‐fluoropyrimidine combinations versus taxane‐platinum (without fluoropyrimidine), Outcome 5 Treatment discontinuation due to toxicity.

Comparison 10 S‐1 versus 5‐FU‐containing regimes, Outcome 1 Overall Survival.

Comparison 10 S‐1 versus 5‐FU‐containing regimes, Outcome 2 Tumour response.

Comparison 10 S‐1 versus 5‐FU‐containing regimes, Outcome 3 Progression‐free survival.

Comparison 10 S‐1 versus 5‐FU‐containing regimes, Outcome 4 Time‐to treatment failure.
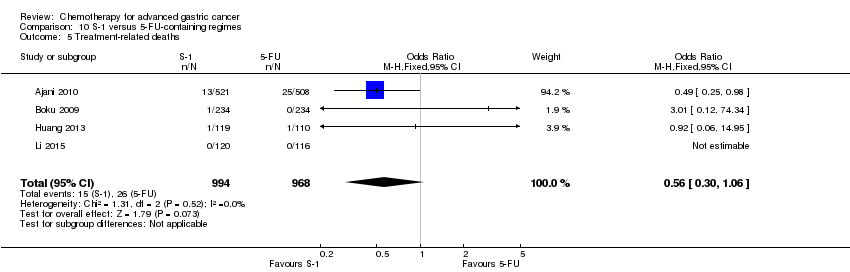
Comparison 10 S‐1 versus 5‐FU‐containing regimes, Outcome 5 Treatment‐related deaths.
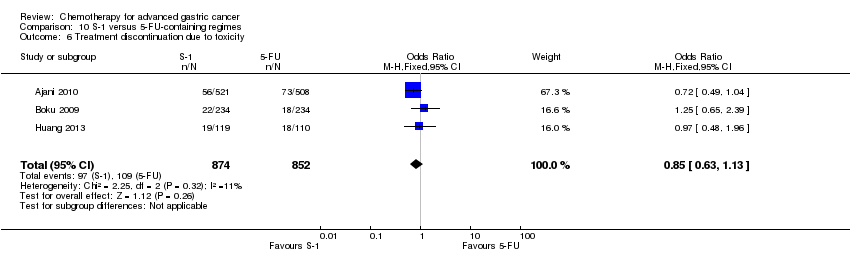
Comparison 10 S‐1 versus 5‐FU‐containing regimes, Outcome 6 Treatment discontinuation due to toxicity.
| Chemotherapy versus best supportive care for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: best supportive care alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Best supportive care | Chemotherapy | |||||
| Overall survival | Study population | HR 0.37 | 184 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 4.3 months | 11.0 months | |||||
| Time to progression | Study population | HR 0.31 | 144 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 2.5 months | 7.4 months | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Early termination of Pyrhönen 1995; downgraded by one level for risk of bias. Outcomes shown include those which were measured in the studies, or reported in a consistent fashion across included studies. Several critical outcomes (e.g. tumour response, treatment‐related death, and discontinuation due to toxicity) were not evaluated or reported in a consistent fashion in these studies, as they were mainly conducted before year 2000. | ||||||
| Combination versus single‐agent chemotherapy for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: single‐agent chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Single‐agent chemotherapy | Combination | |||||
| Overall survival | Study population | HR 0.84 | 4447 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
|
|
| |||||
| Tumour response | Study population | OR 2.30 | 2833 | ⊕⊕⊕⊕ | ||
| 226 per 1000 | 402 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 409 per 1000 | |||||
| Time to progression | Study population | HR 0.69 | 720 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 2.8 months | 4.1 months | |||||
| Treatment‐related death | Study population | OR 1.64 | 3876 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. | ||||||
| 5‐FU/cisplatin/anthracycline combinations versus 5‐FU/cisplatin combinations (without anthracyclines) for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: 5‐FU/cisplatin combinations (without anthracyclines) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU/cisplatin combinations (without anthracyclines) | 5‐FU/cisplatin/anthracycline combinations | |||||
| Overall survival | Study population | HR 0.74 | 579 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 8.6 months | 9.9 months | |||||
| Tumour response | Study population | OR 2.86 | 78 | ⊕⊕⊝⊝ | ||
| 385 per 1000 | 641 per 1000 | |||||
| Moderate | ||||||
| 385 per 1000 | 642 per 1000 | |||||
| Time to progression | Study population | HR 0.62 | 78 | ⊕⊕⊝⊝ | Median survival durations from the only included study | |
| 7.9 months | 12.1 months | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. Outcomes shown include those which were measured in the studies, or reported in a consistent fashion across included studies. Several critical outcomes (e.g. treatment‐related death and discontinuation due to toxicity) were not evaluated or reported in a consistent fashion in these studies, as they were mainly conducted before year 2000. | ||||||
| 5‐FU/cisplatin/anthracycline combinations versus 5‐FU/anthracycline combinations (without cisplatin) for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: 5‐FU/cisplatin combinations (without anthracyclines) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU/anthracycline combinations (without cisplatin) | 5‐FU/cisplatin/anthracycline combinations | |||||
| Overall survival | Study population | HR 0.82 | 1147 | ⊕⊕⊝⊝ | Weighted average of median survival durations from included studies | |
| 6.2 months | 8.4 months | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. Several critical outcomes (i.e. tumour response, progression‐free survival, treatment‐related death and discontinuation due to toxicity) were not evaluated or reported in a consistent fashion in these studies, most of which were conducted before year 2000. | ||||||
| Irinotecan versus non‐irinotecan‐containing regimens for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: non‐irinotecan‐containing regimens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐irinotecan‐containing regimens | Chemotherapy with Irinotecan | |||||
| Overall survival | Study population | HR 0.87 | 2135 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 9.7 months | 11.3 months | |||||
| Overall survival ‐ Substitutive comparisons | Study population | HR 0.87 (0.75 to 1.00) | 826 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 9.1 months | 9.9 months | |||||
| Overall survival ‐ Additive comparisons | Study population | HR 0.88 | 500 | ⊕⊕⊝⊝ | Weighted average of median survival durations from included studies | |
| 10.9 months | 11.9 months | |||||
| Overall survival ‐ Other comparisons | Study population | HR 0.87 | 809 | ⊕⊝⊝⊝ | Weighted average of median survival durations from included studies | |
| 11.4 months | 12.6 months | |||||
| Tumour response | Study population | OR 1.72 (1.24 to 2.40) | 1266 | ⊕⊕⊝⊝ | ||
| 288 per 1000 | 410 per 1000 | |||||
| Moderate | ||||||
| 275 per 1000 | 395 per 1000 | |||||
| Tumour response ‐ Substitutive comparisons | Study population | OR 1.53 (0.93 to 2.50) | 756 | ⊕⊕⊝⊝ | ||
| 297 per 1000 | 393 per 1000 | |||||
| Moderate | ||||||
| 294 per 1000 | 389 per 1000 | |||||
| Tumour response ‐ Additive comparisons | Study population | OR 2.18 (1.25 to 3.80) | 345 | ⊕⊕⊝⊝ | ||
| 224 per 1000 | 386 per 1000 | |||||
| Moderate | ||||||
| 219 per 1000 | 379 per 1000 | |||||
| Tumour response ‐ Other comparisons | Study population | OR 1.87 | 165 | ⊕⊝⊝⊝ | ||
| 376 per 1000 | 530 per 1000 | |||||
| Moderate | ||||||
| 367 per 1000 | 520 per 1000 | |||||
| Progression‐free survival | Study population | HR 0.76 (0.69 to 0.84) | 1640 | ⊕⊕⊕⊕ | Weighted average of median survival durations from included studies | |
| 4.4 months | 5.9 months | |||||
| Progression‐free survival ‐ Substitutive comparison | Study population | HR 0.85 (0.72 to 1.00) | 741 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 4.2 months | 5.3 months | |||||
| Progression‐free survival ‐ Additive comparisons | Study population | HR 0.51 | 90 | ⊕⊕⊕⊝ | Median survival durations from the only included study | |
| 3.2 months | 6.9 months | |||||
| Progression‐free survival ‐ Other comparisons | Study population | HR 0.74 (0.66 to 0.84) | 809 | ⊕⊕⊕⊕ | Weighted average of median survival durations from included studies | |
| 5.4 months | 6.6 months | |||||
| Treatment‐related death | Study population | OR 0.88 (0.23 to 3.32) | 1979 | ⊕⊕⊝⊝ | ||
| 10 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 2 per 1000 | 2 per 1000 | |||||
| Treatment discontinuation due to toxicity | Study population | OR 1.00 (0.46 to 2.20) | 1979 | ⊕⊝⊝⊝ | ||
| 137 per 1000 | 137 per 1000 | |||||
| Moderate | ||||||
| 215 per 1000 | 215 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. | ||||||
| Docetaxel versus non‐docetaxel‐containing regimens for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: non‐docetaxel‐containing regimens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐docetaxel‐containing regimens | Chemotherapy with docetaxel | |||||
| Overall survival | Study population | HR 0.86 (0.78 to 0.95) | 2001 | ⊕⊕⊕⊕ | Weighted average of median survival durations from included studies | |
| 9.9 months | 11.2 months | |||||
| Overall survival ‐ Substitutive comparisons | Study population | HR 1.05 (0.87 to 1.27) | 479 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 9.4 months | 9.2 months | |||||
| Overall survival ‐ Additive comparisons | Study population | HR 0.80 (0.71 to 0.91) | 1466 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 10.6 months | 12.3 months | |||||
| Overall survival ‐ Other comparisons | Study population | HR 0.80 (0.46 to 1.39) | 56 | ⊕⊝⊝⊝ | Median survival durations from the only included study | |
| 9.5 months | 11.9 months | |||||
| Tumour response | Study population | OR 1.37 (1.03 to 1.83) | 1820 | ⊕⊕⊕⊝ | ||
| 311 per 1000 | 382 per 1000 | |||||
| Moderate | ||||||
| 310 per 1000 | 381 per 1000 | |||||
| Tumour response ‐ Substitutive comparison | Study population | OR 1.03 (0.71 to 1.50) | 525 | ⊕⊕⊕⊝ | ||
| 314 per 1000 | 320 per 1000 | |||||
| Moderate | ||||||
| 327 per 1000 | 334 per 1000 | |||||
| Tumour response ‐ Additive comparison | Study population | OR 1.83 (1.45 to 2.32) | 1235 | ⊕⊕⊕⊕ | ||
| 295 per 1000 | 434 per 1000 | |||||
| Moderate | ||||||
| 296 per 1000 | 435 per 1000 | |||||
| Tumour response ‐ Other comparison | Study population | OR 0.33 (0.12 to 0.96) | 60 | ⊕⊝⊝⊝ | ||
| 600 per 1000 | 331 per 1000 | |||||
| Moderate | ||||||
| 600 per 1000 | 331 per 1000 | |||||
| Time to progression | Study population | HR 1.06 (0.85 to 1.32) | 360 | ⊕⊝⊝⊝ | Weighted average of median survival durations from included studies | |
| 6.0 months | 5.9 months | |||||
| Progression‐free survival | Study population | HR 0.76 (0.63 to 0.91) | 1498 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 4.8 months | 6.0 months | |||||
| Progression‐free survival ‐ Substitutive comparisons | Study population | HR 1.15 (0.77 to 1.72) | 119 | ⊕⊝⊝⊝ | Median survival durations from the only included study | |
| 4.9 months | 4.6 months | |||||
| Progression‐free survival ‐ Additive comparison | Study population | HR 0.70 (0.61 to 0.81) | 1323 | ⊕⊕⊕⊕ | Weighted average of median survival durations from included studies | |
| 4.3 months | 6.0 months | |||||
| Progression‐free survival ‐ Other comparison | Study population | HR 0.94 (0.55 to 1.60) | 56 | ⊕⊝⊝⊝ | Median survival durations from the only included study | |
| 6.4 months | 6.8 months | |||||
| Treatment‐related death | Study population | OR 1.10 | 2113 | ⊕⊕⊕⊝ | ||
| 12 per 1000 | 14 per 1000 | |||||
| Moderate | ||||||
| 5 per 1000 | 5 per 1000 | |||||
| Treatment discontinuation due to toxicity | Study population | OR 0.81 (0.53 to 1.25) | 1066 | ⊕⊕⊝⊝ | ||
| 211 per 1000 | 178 per 1000 | |||||
| Moderate | ||||||
| 197 per 1000 | 166 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for imprecision. | ||||||
| Capecitabine versus 5‐FU‐containing regimens for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: 5‐FU‐containing regimens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU‐containing regimens | Capecitabine‐containing regimens | |||||
| Overall Survival | Study population | HR 0.94 (0.79 to 1.11) | 732 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 10.9 months | 10.8 months | |||||
| Tumour response | Study population | OR 0.85 (0.40 to 1.79) | 636 | ⊕⊝⊝⊝ | ||
| 384 per 1000 | 347 per 1000 | |||||
| Moderate | ||||||
| 394 per 1000 | 356 per 1000 | |||||
| Time to progression | Study population | HR 0.72 (0.47 to 1.12) | 85 | ⊕⊝⊝⊝ | Median survival durations from the only included study | |
| 5.5 months | 6.8 months | |||||
| Progression‐free survival | Study population | HR 0.98 (0.77 to 1.23) | 647 | ⊕⊝⊝⊝ | Weighted average of median survival durations from included studies | |
| 6.7 months | 6.5 months | |||||
| Treatment‐related death | Study population | OR 1.88 (0.23 to 15.15) | 481 | ⊕⊝⊝⊝ | ||
| 21 per 1000 | 38 per 1000 | |||||
| Moderate | ||||||
| 24 per 1000 | 44 per 1000 | |||||
| Treatment discontinuation due to toxicity | Study population | OR 0.99 (0.56 to 1.77) | 311 | ⊕⊕⊝⊝ | ||
| 181 per 1000 | 179 per 1000 | |||||
| Moderate | ||||||
| 181 per 1000 | 180 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. | ||||||
| Oxaliplatin versus the same regimen including cisplatin for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: the same regimen including cisplatin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cisplatin‐containing regimen | Oxaliplatin‐containing regimen | |||||
| Overall Survival | Study population | HR 0.81 (0.67 to 0.98) | 1105 | ⊕⊕⊝⊝ | Weighted average of median survival durations from included studies | |
| 11.3 months | 14.0 months | |||||
| Tumour response | Study population | OR 1.38 (1.08 to 1.76) | 1081 | ⊕⊕⊕⊝ | ||
| 468 per 1000 | 548 per 1000 | |||||
| Moderate | ||||||
| 458 per 1000 | 538 per 1000 | |||||
| Progression‐free survival | Study population | HR 0.88 (0.66 to 1.19) | 1034 | ⊕⊕⊝⊝ | Weighted average of median survival durations from included studies | |
| 4.9 months | 6.0 months | |||||
| Treatment‐related death | Study population | OR 0.47 (0.17 to 1.30) | 1132 | ⊕⊕⊝⊝ | ||
| 20 per 1000 | 9 per 1000 | |||||
| Moderate | ||||||
| 24 per 1000 | 11 per 1000 | |||||
| Treatment discontinuation due to toxicity | Study population | OR 0.97 (0.44 to 2.13) | 970 | ⊕⊝⊝⊝ | ||
| 95 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 102 per 1000 | 99 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. | ||||||
| Taxane‐platinum‐fluoropyrimidine combinations versus taxane‐platinum (without fluoropyrimidine) for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: taxane‐platinum (without fluoropyrimidine) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Taxane‐platinum (without fluoropyrimidine) | Taxane‐platinum‐fluoropyrimidine combination | |||||
| Overall survival | Study population | OR 0.86 | 482 | ⊕⊝⊝⊝ | Weighted average of median survival durations from included studies | |
| 10.0 months | 11.7 months | |||||
| Tumour response | Study population | OR 2.08 | 482 | ⊕⊕⊝⊝ | ||
| 234 per 1000 | 389 per 1000 | |||||
| Moderate | ||||||
| 231 per 1000 | 385 per 1000 | |||||
| Progression‐free survival | Study population | OR 0.74 | 482 | ⊕⊕⊕⊝ | Weighted average of median survival durations from included studies | |
| 4.4 months | 5.7 months | |||||
| Treatment‐related death | Study population | OR 1.95 | 482 | ⊕⊝⊝⊝ | ||
| 26 per 1000 | 50 per 1000 | |||||
| Moderate | ||||||
| 13 per 1000 | 25 per 1000 | |||||
| Treatment discontinuation due to toxicity | Study population | OR 1.71 | 234 | ⊕⊝⊝⊝ | ||
| 105 per 1000 | 167 per 1000 | |||||
| Moderate | ||||||
| 99 per 1000 | 158 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias. | ||||||
| S‐1 versus 5‐FU‐containing regimens for advanced gastric cancer | ||||||
| Patient or population: people with advanced gastric cancer Control: 5‐FU‐containing regimens | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐FU‐containing regimens | S‐1 containing regimens | |||||
| Overall Survival | Study population | HR 0.91 | 1793 | ⊕⊕⊕⊕ | Weighted average of median survival durations from included studies | |
| 9.1 months | 9.6 months | |||||
| Tumour response | Study population | OR 1.73 | 1753 | ⊕⊝⊝⊝ | ||
| 256 per 1000 | 374 per 1000 | |||||
| Moderate | ||||||
| 320 per 1000 | 449 per 1000 | |||||
| Progression‐free survival | Study population | HR 0.85 | 1942 | ⊕⊕⊝⊝ | Weighted average of median survival durations from included studies | |
| 4.3 months | 5.0 months | |||||
| Time‐to treatment failure | Study population | HR 0.88 | 1818 | ⊕⊕⊝⊝ | Weighted average of median survival durations from included studies | |
| 3.1 months | 3.9 months | |||||
| Treatment‐related deaths | Study population | OR 0.56 | 1962 | ⊕⊕⊕⊝ | ||
| 27 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 5 per 1000 | 3 per 1000 | |||||
| Treatment discontinuation due to toxicity | Study population | OR 0.85 | 1726 | ⊕⊕⊕⊕ | ||
| 128 per 1000 | 111 per 1000 | |||||
| Moderate | ||||||
| 144 per 1000 | 125 per 1000 | |||||
| *For time‐to‐event outcomes, e.g. overall survival, the assumed and corresponding risks were obtained by calculating the weighted average of the median survival durations reported in included studies. For dichotomous outcomes, the assumed and corresponding risks (and their 95% confidence interval) are based on proportions of events in the control and intervention groups respectively. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by two levels for severe statistical heterogeneity. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | 184 | Hazard ratio (Random, 95% CI) | 0.37 [0.24, 0.55] |
| 2 Time to progression Show forest plot | 2 | 144 | Hazard ratio (Fixed, 95% CI) | 0.31 [0.22, 0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 23 | 4447 | Hazard ratio (Fixed, 95% CI) | 0.84 [0.79, 0.89] |
| 2 Tumour response Show forest plot | 18 | 2833 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.94, 2.72] |
| 3 Time to progression Show forest plot | 4 | 720 | Hazard ratio (Random, 95% CI) | 0.69 [0.55, 0.87] |
| 4 Treatment‐related death Show forest plot | 18 | 3876 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.83, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 4 | 579 | Hazard ratio (Fixed, 95% CI) | 0.74 [0.61, 0.89] |
| 2 Tumour response Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Time to progression Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 7 | 1147 | Hazard ratio (Random, 95% CI) | 0.82 [0.73, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 10 | 2135 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 1.1 Substitutive comparisons | 6 | 826 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.75, 1.00] |
| 1.2 Additive comparisons | 3 | 500 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.76, 1.03] |
| 1.3 Other comparisons | 2 | 809 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 2 Tumour response Show forest plot | 10 | 1266 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [1.24, 2.40] |
| 2.1 Substitutive comparisons | 6 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.93, 2.50] |
| 2.2 Additive comparisons | 3 | 345 | Odds Ratio (M‐H, Random, 95% CI) | 2.18 [1.25, 3.80] |
| 2.3 Other Comparisons | 2 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [0.89, 3.91] |
| 3 Progression‐free survival Show forest plot | 7 | 1640 | Hazard Ratio (Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 3.1 Substitutive comparison | 5 | 741 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.72, 1.00] |
| 3.2 Additive comparisons | 1 | 90 | Hazard Ratio (Fixed, 95% CI) | 0.51 [0.33, 0.77] |
| 3.3 Other comparisons | 2 | 809 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.66, 0.84] |
| 4 Treatment‐related death Show forest plot | 9 | 1979 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.23, 3.32] |
| 5 Treatment discontinuation due to toxicity Show forest plot | 9 | 1979 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.46, 2.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 8 | 2001 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.78, 0.95] |
| 1.1 Substitutive comparisons | 3 | 479 | Hazard Ratio (Fixed, 95% CI) | 1.05 [0.87, 1.27] |
| 1.2 Additive comparisons | 4 | 1466 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.71, 0.91] |
| 1.3 Other comparisons | 1 | 56 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.46, 1.39] |
| 2 Tumour response Show forest plot | 9 | 1820 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [1.03, 1.83] |
| 2.1 Substitutive comparison | 4 | 525 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.71, 1.50] |
| 2.2 Additive comparison | 4 | 1235 | Odds Ratio (M‐H, Random, 95% CI) | 1.83 [1.45, 2.32] |
| 2.3 Other comparisons | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.96] |
| 3 Time to progression Show forest plot | 2 | 360 | Hazard Ratio (Random, 95% CI) | 1.06 [0.85, 1.32] |
| 4 Progression‐free survival Show forest plot | 5 | 1498 | Hazard Ratio (Random, 95% CI) | 0.76 [0.63, 0.91] |
| 4.1 Substitutive comparisons | 1 | 119 | Hazard Ratio (Random, 95% CI) | 1.15 [0.77, 1.72] |
| 4.2 Additive comparison (PFS) | 3 | 1323 | Hazard Ratio (Random, 95% CI) | 0.70 [0.61, 0.81] |
| 4.3 Other comparisons | 1 | 56 | Hazard Ratio (Random, 95% CI) | 0.94 [0.55, 1.60] |
| 5 Treatment‐related death Show forest plot | 7 | 2113 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.55, 2.20] |
| 6 Treatment discontinuation due to toxicity Show forest plot | 5 | 1066 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.53, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall Survival Show forest plot | 5 | 732 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 2 Tumour response Show forest plot | 4 | 636 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.40, 1.79] |
| 3 Time to progression Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 4 Progression‐free survival Show forest plot | 4 | 647 | Hazard Ratio (Random, 95% CI) | 0.98 [0.77, 1.23] |
| 5 Treatment‐related death Show forest plot | 2 | 481 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.23, 15.15] |
| 6 Treatment discontinuation due to toxicity Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall Survival Show forest plot | 5 | 1105 | Hazard Ratio (Random, 95% CI) | 0.81 [0.67, 0.98] |
| 2 Tumour response Show forest plot | 5 | 1081 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.08, 1.76] |
| 3 Progression‐free survival Show forest plot | 4 | 1034 | Hazard Ratio (Random, 95% CI) | 0.88 [0.66, 1.19] |
| 4 Treatment‐related death Show forest plot | 5 | 1132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.30] |
| 5 Treatment discontinuation due to toxicity Show forest plot | 3 | 970 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.44, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 3 | 482 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.71, 1.06] |
| 2 Tumour response Show forest plot | 3 | 482 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.37, 3.15] |
| 3 Progression‐free survival Show forest plot | 3 | 482 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.59, 0.93] |
| 4 Treatment‐related death Show forest plot | 3 | 482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.73, 5.17] |
| 5 Treatment discontinuation due to toxicity Show forest plot | 2 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.79, 3.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall Survival Show forest plot | 4 | 1793 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 2 Tumour response Show forest plot | 7 | 1753 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [1.01, 2.94] |
| 3 Progression‐free survival Show forest plot | 4 | 1942 | Hazard Ratio (Random, 95% CI) | 0.85 [0.70, 1.04] |
| 4 Time‐to treatment failure Show forest plot | 5 | 1818 | Hazard Ratio (Random, 95% CI) | 0.88 [0.76, 1.01] |
| 5 Treatment‐related deaths Show forest plot | 4 | 1962 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.06] |
| 6 Treatment discontinuation due to toxicity Show forest plot | 3 | 1726 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.13] |

