Tratamiento antibiótico oral versus intravenoso para la neutropenia febril en pacientes con cáncer
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomisation: list of random numbers | |
| Participants | 62 episodes in 52 patients, during 2004 to 2007, in Switzerland and Germany Type of malignancy: 50% solid tumour, 50% acute lymphoblastic leukaemia (not during induction) | |
| Interventions | Sequential Oral: ciprofloxacin (25–30 mg/kg/day, top dose 1500 mg/day, given in 2 daily doses) plus amoxicillin (65–80 mg/kg/day, top dose 2250 mg/day, given in 2 daily doses) IV: intravenous antimicrobial therapy | |
| Outcomes | All cause mortality Length of therapy, and hospitalisation | |
| Notes | Early termination of the trial "Because of insufficient accrual, the study was closed early, before reaching the number of 90 randomized low‐risk FN episodes of the first interim monitoring" Power calculation: as for a non‐inferiority study: "Both efficacy and safety of experimental treatment were tested for non‐inferiority compared to standard treatment using an unconditional exact non‐inferiority test for binomial difference based on the standardized (score) test statistic [26– 28]. The 95% upper confidence bound (UCB) corresponding to this one‐sided test was calculated. The acceptable non‐inferiority margins of difference were set at 3.5% for safety and 10% for efficacy" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "list of random numbers" |
| Allocation concealment (selection bias) | Low risk | "At randomization, one of a set of numbered, sealed randomization envelopes containing the randomization allocation was opened by the treating oncologist" |
| Blinding of participants and personnel (performance bias) | High risk | No blinding procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 7/69 episodes were excluded. 6 episodes due to protocol violation their allocation was not specified , 1 due to hypersensitivity/adverse event, 1 lost to follow‐up |
| Methods | Randomisation: not reported | |
| Participants | episodes, 2002‐2005, Brazil | |
| Interventions | Oral ciprofloxacin amoxycillin‐clavulanate IV: cefepime | |
| Outcomes | Successful versus unsuccessful: unsuccessful if one or more of the following conditions indicative of invasive bacterial infection was observed: 1) haemodynamic instability unrelated to lost volume; 2) fever that had not abated 72 hours after starting antibiotics; 3) repeat episode of fever lasting at least 24 hours and occurring after the 48‐hour period with no fever; 4) death during infection; 5) grade III and IV vomiting; and 6) infections that demanded the addition of antibiotics not included in the study protocol Adverse events | |
| Notes | Early termination of the trial: no Power calculation: not as a non‐inferiority study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Randomization consisted of distributing patients into blocks of 10, with selection made by a pharmacist who drew lots before patients were recruited. Patients were allocated to either group A or group B, where patients in group A were given oral antimicrobial treatment and those in group B were given intravenous treatment" |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | All randomised episodes were included in analysis |
| Methods | Randomisation: not reported | |
| Participants | 34 episodes, during 2000‐2002, Germany | |
| Interventions | Initial oral IV: tazocin 4.5 g q8h | |
| Outcomes | All cause mortality | |
| Notes | Randomisation of episodes Failure: no success, no fever 72h after randomisation with at least 7 subsequent afebrile days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | By phone |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | All randomised episodes/patients were analysed |
| Methods | Randomisation: no information | |
| Participants | USA, 1988‐1989 | |
| Interventions | Sequential Oral2: ceftazidime 2g q8h +amikacin 7.5mg/kg q12h for at least 72 hours, if favourable response change to ciprofluoxacin 750 mg q12h as inpatients | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Unclear risk | 7/86 episodes, of unknown treatment assignment |
| Methods | Randomisation: no information Follow‐up period: end of fever and neutropenia | |
| Participants | USA, 1992‐1997 | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: no information | |
| Participants | Greece, 1992‐1995 | |
| Interventions | Sequential | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Central |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: "computer spreadsheet program" | |
| Participants | 123 episodes in 88 patients, 2006‐2007, India Age: the median age was 8.25 years (oral) and 7.75 years (intravenous) Type of malignancy: most frequent: acute lymphoblastic leukaemia in maintenance phase of therapy (33%), primitive neuro‐ectodermal tumour (21%) and rhabdomyosarcoma (20%) | |
| Interventions | Oral: ofloxacin 7.5mg/kg/dose every 12 hours + amoxycillin‐clavulanate 12.5mg/kg (amoxycillin) every 8 hours IV: ceftriaxone 75 mg/kg/day + amikacin 15 mg/kg once daily | |
| Outcomes | Treatment success (primary outcome variable) was defined as resolution of the febrile episode and neutropenia without change of regimen or hospitalisation. Non‐resolution of fever or any other serious medical complications (with or without resolution of fever) requiring change in therapy or hospitalisation were classified as treatment failures. Addition of acyclovir and/or fluconazole to antibiotic therapy was regarded as treatment modification rather than treatment failure Adverse events | |
| Notes | Early termination: no Power calculation: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer spreadsheet program" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: random table | |
| Participants | Spain | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: no information | |
| Participants | UK, 1997‐2000 | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Published and unpublished data. Definitions of outcomes: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: computer program | |
| Participants | Greece, Spain, Slovak Republic, Turkey, Italy, Luxembourg, Germany, France, Switzerland, Belgium, UK, Czech Republic, Canada, Israel, 1995‐1997 | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Central |
| Blinding of participants and personnel (performance bias) | High risk | |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: no information | |
| Participants | Pakistan, 1989‐1991 | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: computer program | |
| Participants | USA, 1995‐1997 | |
| Interventions | Sequential | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | |
| Methods | Randomisation: random number table | |
| Participants | 36 neutropenic patients with 41 febrile episodes, during 1995‐2001, in Japan Age: range 51‐76 yrs Type of malignancy: all solid tumour | |
| Interventions | Initial oral | |
| Outcomes | Treatment failure | |
| Notes | Randomisation of episodes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | All randomised patients were analysed |
| Methods | Randomisation: computer program | |
| Participants | Argentina, 1997‐1998 | |
| Interventions | Sequential | |
| Outcomes | All cause mortality | |
| Notes | Randomisation of episodes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program |
| Allocation concealment (selection bias) | Low risk | Central |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Methods | Randomisation: computer program | |
| Participants | Argentina, during 2000‐2002 | |
| Interventions | Sequential | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | |
| Methods | Randomisation generation: no information | |
| Participants | Brazil, 1993‐1995 Type of malignancy: solid tumour (91%), lymphoma (4.3%) not receiving high dose chemotherapy | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons of attrition and allocation were reported |
| Methods | Randomisation: no information | |
| Participants | USA | |
| Interventions | Initial oral | |
| Outcomes | Infectious related mortality | |
| Notes | Conference proceedings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Unclear risk | No data on exclusions were reported |
| Methods | Randomisation: no information, stratified according to leukaemia | |
| Participants | USA, 1989‐1990 | |
| Interventions | Initial oral | |
| Outcomes | Infection‐related mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to allocation |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: no information | |
| Participants | Greece, 1994‐1996 | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Conference proceedings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Allocation of patients excluded after randomisation was reported |
| Methods | Randomisation: no information | |
| Participants | 96 episodes in 90 patients, during 2003‐2005, multi‐centre Age: mean 52 years, median 54 years Type of malignancy: 30% lymphoma; 35% solid tumour no metastasis; 34% solid tumour with metastasis | |
| Interventions | Oral: moxifloxacin 400 mg once daily ceftriaxone 2 gr intravenously as a single daily dose | |
| Outcomes | Global success of the at‐home antibiotic therapy Effectiveness of the antibiotic monotherapy Quality of life Toxicity | |
| Notes | Early termination due to recruitment problems Power calculation reported, a non‐inferiority study Definitions of outcomes: Global success of the at‐home antibiotic therapy. The overall strategy (antibiotics and early hospital discharge) was considered a success not only when the effectiveness of the antibiotic therapy was achieved (as defined by the resolution of fever and of the possible clinical or microbiological manifestations of the infection) but also in the presence of the following criteria: early hospital discharge (within 24 or 48 h), no death from any cause, no sign or symptom of clinical deterioration requiring hospital readmission, no initial infection by a pathogen resistant in vitro to the antibiotics tested, no modification of initial anti‐biotherapy, no relapse or new infection during antibiotic treatment, no toxic reaction to the antibiotic requiring discontinuation of treatment, and no re‐hospitalisation of the patient for any cause. Effectiveness of the antibiotic monotherapy (as evidenced by the lack of need for any additional antibiotics besides ceftriaxone or moxifloxacin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Randomization was centralized and was stratified according to the participating center" |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition and allocation were reported |
| Methods | Randomisation: computer program, stratified by hierarchical rules according to absolute neutrophil count<100 cells/m3, expected duration of neutropenia ≥5days, non‐standard initial empiric antibiotic regimen, presence of indwelling venous catheter, diagnosis of acute myeloid leukaemia, persistent fever at randomisation Allocation concealment: central pharmacy | |
| Participants | USA, 1991‐1995 | |
| Interventions | Sequential | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | All included in analysis |
| Methods | Randomisation: random number table Blinding: no | |
| Participants | Brasil, 1991‐1992 | |
| Interventions | Initial oral | |
| Outcomes | All cause mortality | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | |
| Incomplete outcome data (attrition bias) | Low risk | Allocation of excluded patients was reported |
General exclusion criteria:
haemodynamic instability, hypotension; altered mental status; respiratory failure; poor clinical condition, renal failure, abnormal liver function tests, no ability to swallow or take oral medication (vomiting, severe mucositis); hypersensitivity; pregnancy, lactation
yrs = years
vs = versus
FUO = fever of unknown origin
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| IV antibiotics in both groups | |
| Not a randomised controlled trial, correspondence to Paganini 2003 | |
| A retrospective trial | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| A review | |
| A review, not a randomised controlled trial | |
| Prospective, not randomised clinical trial | |
| Prospective clinical trial, not randomised; step‐down to oral antibiotics | |
| Prospective clinical trial, not randomised | |
| Intervention: pegfilgrastim, not antibiotics | |
| A review | |
| Not a randomised controlled trial | |
| Editorial, not a randomised controlled trial | |
| Prospective, not randomised clinical trial | |
| No fever for inclusion in trial | |
| Home intravenous antibiotic treatment compared to continued inpatient care | |
| Prospective, not randomised clinical trial | |
| No fever for inclusion in trial | |
| An observational study | |
| A review | |
| Prospective, not randomised clinical trial | |
| Intervention: oral antibiotic was compared to placebo (no treatment) | |
| An observational, non‐randomised study | |
| Prospective, not randomised clinical trial | |
| A review | |
| An observational study of children with febrile neutropenia, non‐randomised study | |
| Prospective, not randomised clinical trial | |
| Intervention: oral ofloxacin as inpatients versus oral ofloxacin as outpatients | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| No oral treatment arm | |
| Included cancer patients with fever non‐neutropenic and neutropenic | |
| Prospective, not randomised clinical trial | |
| A review | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| Intervention: sequential IV to oral antibiotics was given for both trial arms | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial | |
| Prospective, non‐randomised clinical trial | |
| Not a randomised controlled trial | |
| All patients received IV antibiotics | |
| Prospective, non‐randomised clinical trial | |
| A prospective study with no control group, not a randomised controlled trial | |
| Intervention: oral antibiotics was compared to no treatment (discontinuation of IV antibiotics before recovery of neutrophil count, no oral antibiotics were given after stopping IV therapy) | |
| Outpatient versus inpatient treatment. All patients received IV ceftriaxone | |
| No oral antibiotic treatment group | |
| All patients received IV antibiotics RCT | |
| Same antibiotic in the 2 groups | |
| A review | |
| Prophylactic antibiotics (no patients with fever) | |
| A review; not RCT | |
| Prospective, not randomised clinical trial | |
| Prospective, not randomised clinical trial |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 9 | 1392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.68] |
| Analysis 1.1  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 1 Mortality. | ||||
| 1.1 Initially oral | 6 | 961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.62] |
| 1.2 Sequential | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.45, 4.22] |
| 2 Treatment failure Show forest plot | 22 | 3142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
| Analysis 1.2  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 2 Treatment failure. | ||||
| 2.1 Initially oral treatment | 16 | 2196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.03] |
| 2.2 Sequential IV to oral treatment | 6 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.27] |
| 3 Treatment failure ‐ per protocol analysis Show forest plot | 22 | 2912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| Analysis 1.3  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 3 Treatment failure ‐ per protocol analysis. | ||||
| 3.1 Initially oral treatment | 16 | 1991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
| 3.2 Sequential IV to oral treatment | 6 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.29] |
| 4 Adverse events requiring discontinuation of antibiotics Show forest plot | 15 | 1823 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.61, 3.46] |
| Analysis 1.4  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 4 Adverse events requiring discontinuation of antibiotics. | ||||
| 4.1 Initially oral treatment | 10 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.14, 6.75] |
| 4.2 Sequential IV to oral treatment | 5 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.25] |
| 5 Gastrointestinal adverse events ('post‐protocol' analysis) Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 5 Gastrointestinal adverse events ('post‐protocol' analysis). | ||||
| 5.1 Initially oral treatment | 11 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [2.87, 7.04] |
| 5.2 Sequential IV to oral treatment | 4 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.03, 7.66] |
| 6 Lost to follow‐up Show forest plot | 19 | 2810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.10] |
| Analysis 1.6  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 6 Lost to follow‐up. | ||||
| 7 Treatment failure not dt modification in update Show forest plot | 21 | 3041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| Analysis 1.7  Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 7 Treatment failure not dt modification in update. | ||||
| 7.1 Initially oral treatment | 15 | 2095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 7.2 Sequential IV to oral treatment | 6 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure ‐ age Show forest plot | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 1 Treatment failure ‐ age. | ||||
| 1.1 Children | 8 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 1.2 Adults | 12 | 1652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.12] |
| 2 Treatment failure ‐ source of infection Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 2 Treatment failure ‐ source of infection. | ||||
| 2.1 Unexplained fever | 10 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.33] |
| 2.2 Documented infection | 10 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 3 Treatment failure ‐ severity of neutropenia Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 3 Treatment failure ‐ severity of neutropenia. | ||||
| 3.1 Absolute neutrophil count >=10^9/L | 3 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| 3.2 Absolute neutrophil count <10^9/L | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.49] |
| 4 Treatment failure ‐ type of malignancy Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 4 Treatment failure ‐ type of malignancy. | ||||
| 4.1 Solid tumour | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 4.2 Haemetologic malignancy | 4 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Allocation concealment Show forest plot | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Methodological quality of studies, Outcome 1 Allocation concealment. | ||||
| 1.1 Adequate (A) | 12 | 1651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 1.2 Unclear (B) | 10 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Setting Show forest plot | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Post hoc subgroup analyses, Outcome 1 Setting. | ||||
| 1.1 Oral‐outpatient, IV‐inpatients | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.43] |
| 1.2 Inpatients | 6 | 1128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 1.3 Outpatients | 7 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 1.4 Only first dose in | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.34] |
| 2 Type of oral antibiotics Show forest plot | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Post hoc subgroup analyses, Outcome 2 Type of oral antibiotics. | ||||
| 2.1 Quinolones only | 7 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 2.2 Quinolones in combination with augmentin, ampicillin‐sulbactam, penicillin V or clindamycin | 11 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 2.3 Cefixime | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.64, 1.56] |
| 2.4 New quinolones | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.86] |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
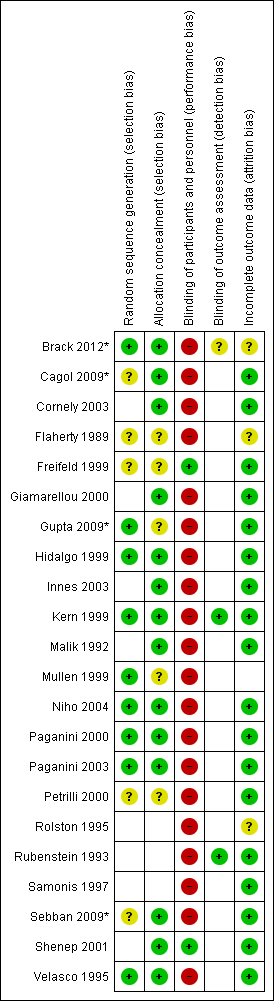
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Oral versus intravenous antibiotic therapy, outcome: 1.1 Mortality.
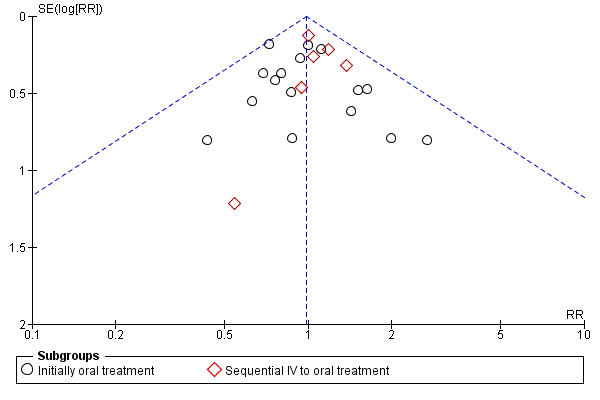
Funnel plot of comparison: 1 Oral versus intravenous antibiotic therapy, outcome: 1.3 Treatment failure ‐ per protocol analysis.
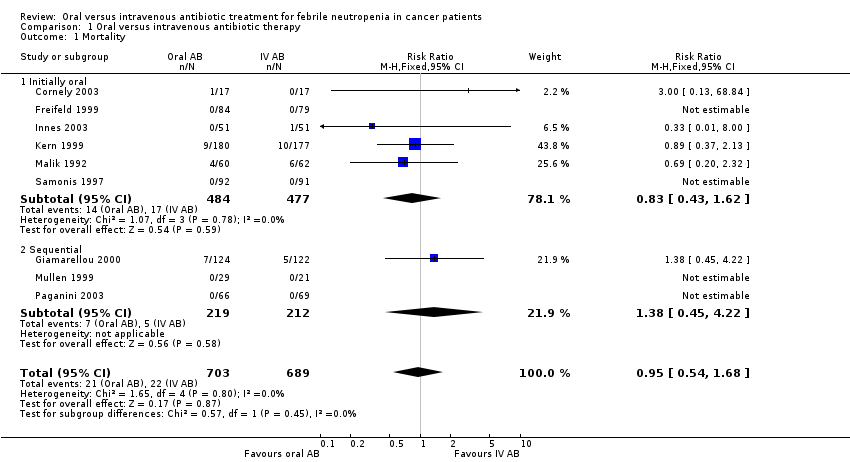
Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 1 Mortality.
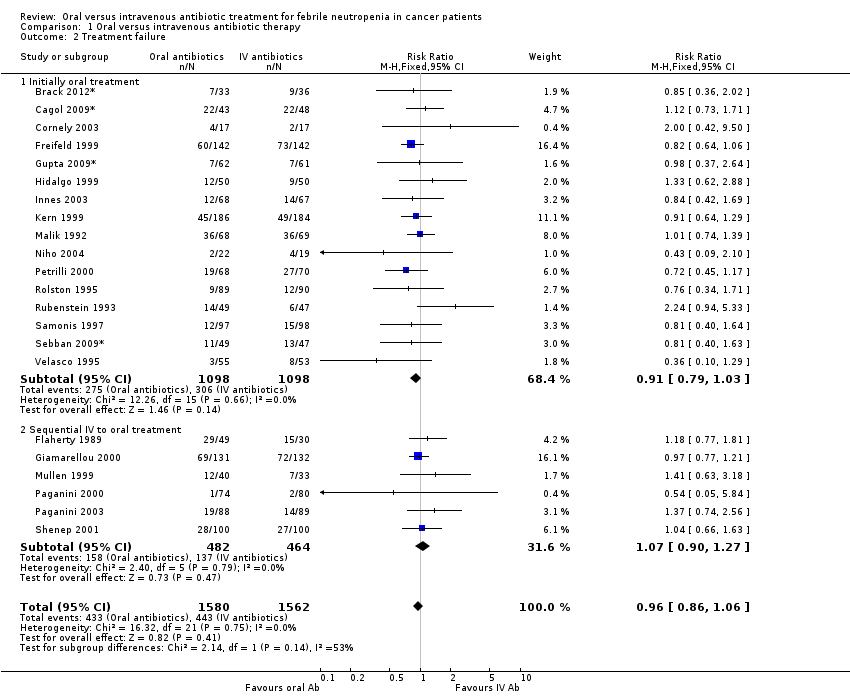
Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 2 Treatment failure.
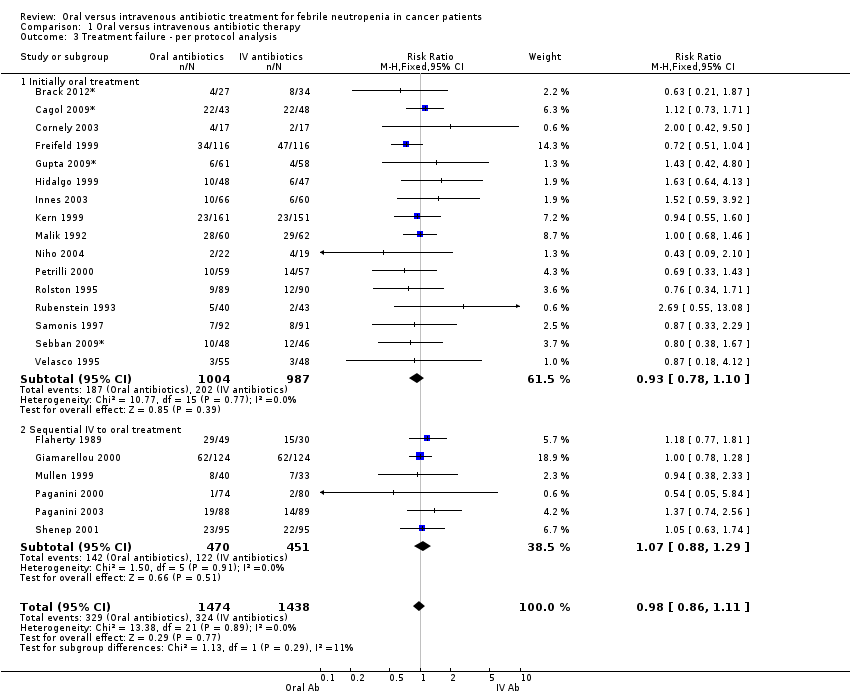
Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 3 Treatment failure ‐ per protocol analysis.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 4 Adverse events requiring discontinuation of antibiotics.
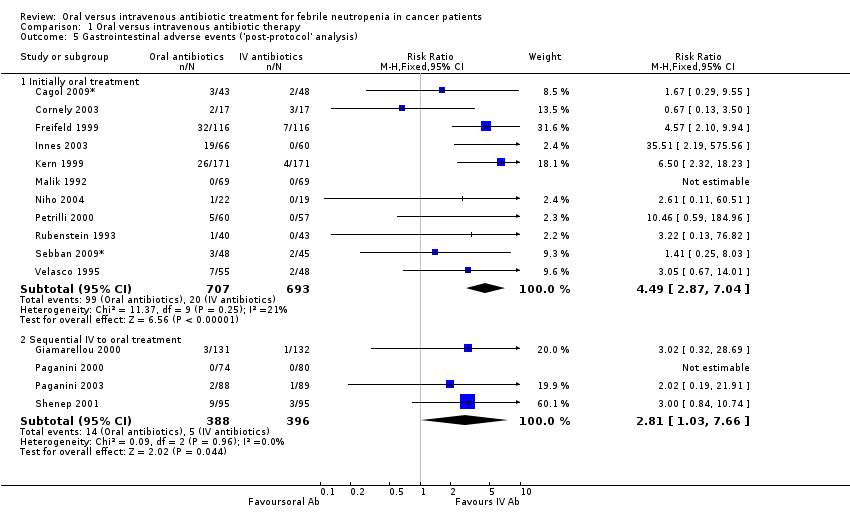
Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 5 Gastrointestinal adverse events ('post‐protocol' analysis).
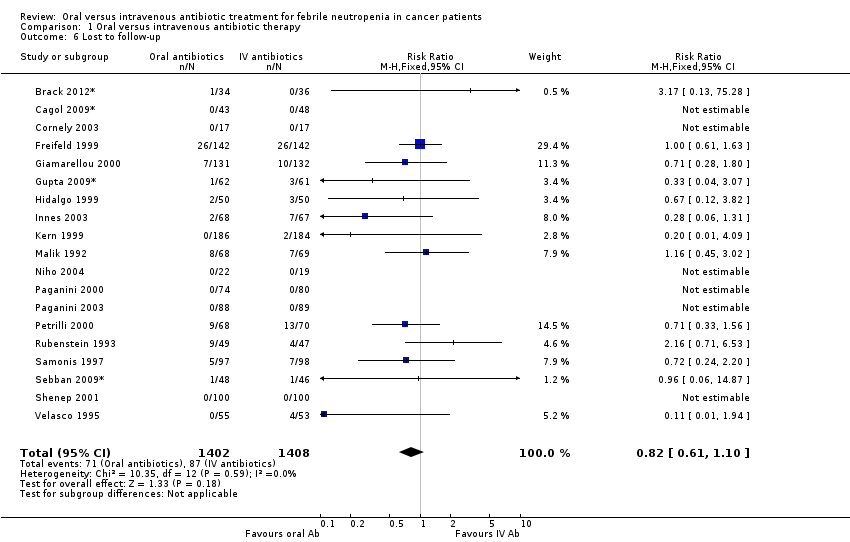
Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 6 Lost to follow‐up.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 7 Treatment failure not dt modification in update.
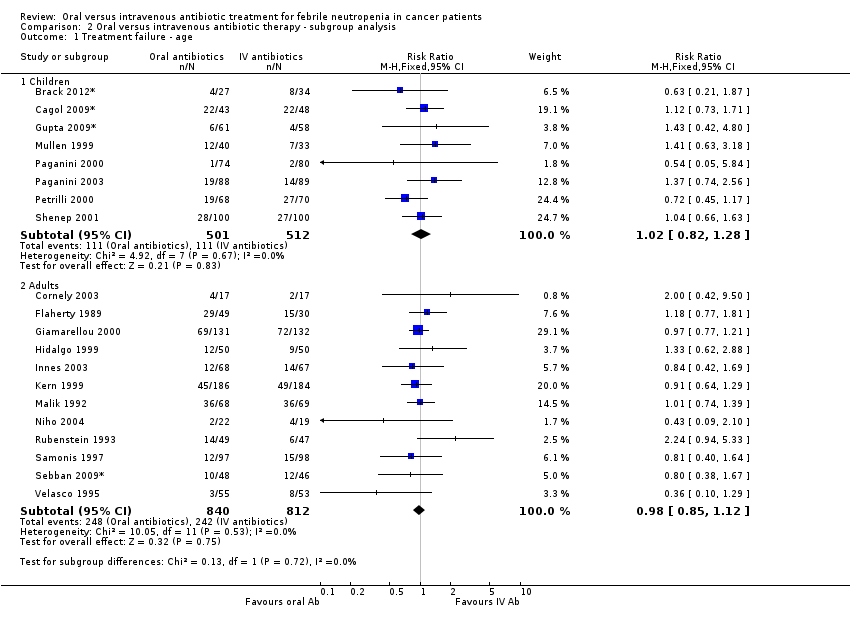
Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 1 Treatment failure ‐ age.
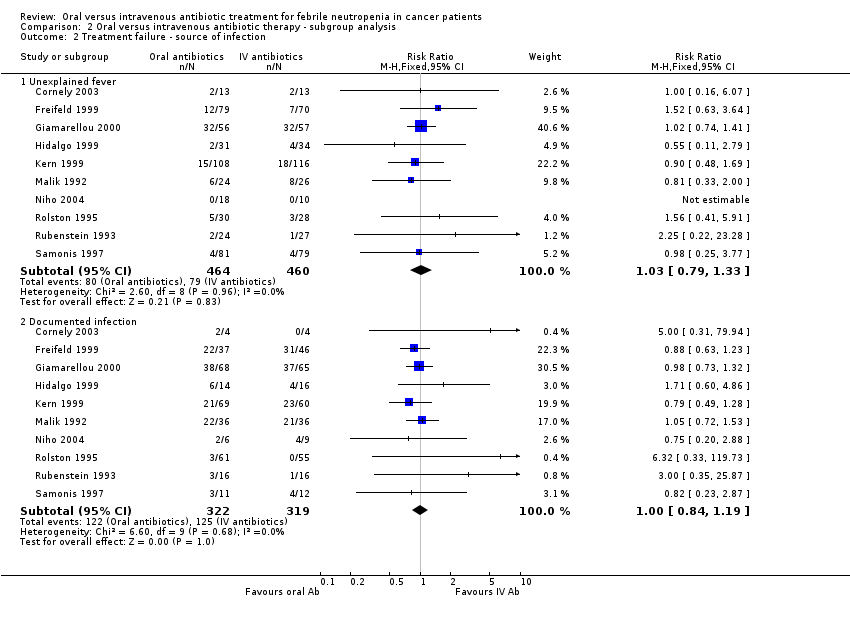
Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 2 Treatment failure ‐ source of infection.
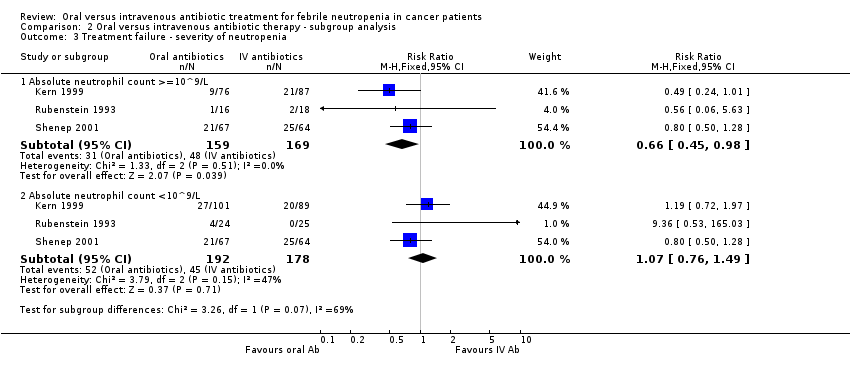
Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 3 Treatment failure ‐ severity of neutropenia.

Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 4 Treatment failure ‐ type of malignancy.
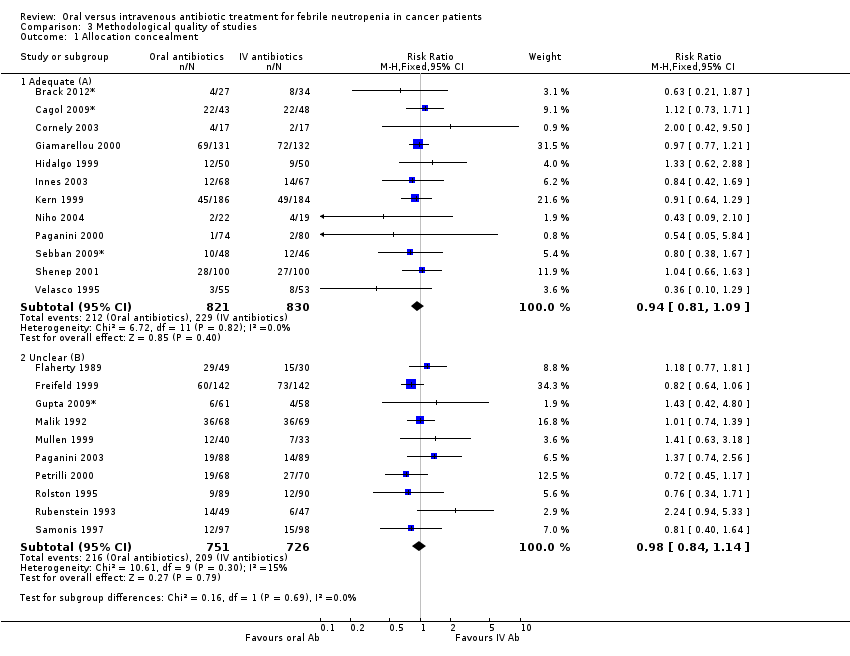
Comparison 3 Methodological quality of studies, Outcome 1 Allocation concealment.

Comparison 4 Post hoc subgroup analyses, Outcome 1 Setting.

Comparison 4 Post hoc subgroup analyses, Outcome 2 Type of oral antibiotics.
| Oral compared to intravenous antibiotic therapy for febrile neutropenia in cancer patients | ||||||
| Patient or population: patients with febrile neutropenia in cancer patients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| intravenous antibiotic therapy | Oral | |||||
| Mortality | Study population | RR 0.95 | 1392 | ⊕⊕⊕⊝ | ||
| 32 per 1000 | 30 per 1000 | |||||
| Low risk | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment failure | Study population | RR 0.96 | 3142 | ⊕⊕⊕⊝ moderate1 | ||
| 284 per 1000 | 272 per 1000 | |||||
| Moderate | ||||||
| 211 per 1000 | 203 per 1000 | |||||
| Treatment failure ‐ per protocol analysis | Study population | RR 0.98 | 2912 | ⊕⊕⊕⊝ | ||
| 225 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 184 per 1000 | 180 per 1000 | |||||
| Adverse events requiring discontinuation of antibiotics | Study population | RR 1.45 | 1823 | ⊕⊕⊝⊝ low1, 2 | ||
| 21 per 1000 | 31 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment failure not dt modification in update | Study population | RR 0.95 | 3041 | ⊕⊕⊕⊝ | ||
| 267 per 1000 | 254 per 1000 | |||||
| Moderate | ||||||
| 180 per 1000 | 171 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High risk of detection bias in most of the trials 2 A wide CI | ||||||
| Common criteria |
| Haemodynamic stablity |
| No organ failure |
| Ability to take oral medications |
| No pneumonia |
| No infection of a central line |
| No severe soft‐tissue infection |
| No acute leukaemia as the background malignancy |
| No known drug allergy |
| Not pregnant or lactating women |
| Study ID | Evident infection | Previous AB | Prolonged neutropeni | Performance status | Active malignancy | BMT/PSCT | Other |
| Kern 1999 | Infected catheter or CNS infection, known bacterial /viral/fungal infection | yes | yes | no | no | yes | Need of IV supportive therapy, expected to die within 48 hours, HIV, fever unrelated to infection and protocol violation |
| Mullen 1999 | A source of infection that required hospitalisation as: tunnelitis, pneumonia, perirectal cellulitis, typhlitis, resistant microorganism to one of the study's drugs | no | no | no | yes | yes | >10% dehydration, bleeding requiring platelet transfusion, |
| Paganini 2000 | Infected catheter, perineal/ facial cellulitis, uncontrolled local infection, positive blood cultures at 72 hours | no | no | no | yes | yes | Persistance of fever >48 hours, incorrectable bleeding; refractory hypoglycemia or hypocalcemia |
| Rubenstein 1993 | Known resistant microorganism | no | no | no | no | no | Na<128, uncontrolled hypercalcemia, more than 30 miles away |
| Samonis 1997 | Pneumonia, deep organ infection | yes | yes | no | yes | no* | Prior hospitalisation |
| Shenep 2001 | Pneumonia, clinical or radiographic evidence of focal bacterial infection, severe mucositis, positive blood cultures at 48 hours | no | no | no | no | no | MRSA or P.Aeroginosa in any culture obtained in preceding 12weeks |
| Velasco1995 | Meningitis, pyelonephritis | yes | no | yes | no | no* | Long term central vein catheter |
| Petrilli 1999 | no | no | no | no | no | no* | |
| Flaherty 1989 | no | yes | no | no | no | no | |
| Freifeld 1999 | Intravascular infection, tunnelitis, pneumonia, neurologic symtoms, | no | yes | no | no | yes | Treatment with Ca‐Mg or probenecid or alluporinol or theophylline, HIV |
| Giamarelou 2000 | Suspected anaerobes | no | no | yes | no | no | Moribund and high probability of dying within 48 hours |
| Hidalgo 1999 | Pneumonia, extensive cellulitis, meningitis, pyelonephritis | no | no | yes | yes | no | Clotting abnormalities, acidosis, hypercalcaemia, uncontrolled bleeding, live >2h apart from hospital; Hx of tumour fever, other severe extra hematologic chemotherapy induced toxicity, no 24 hours home companion |
| Innes 2003 | Tunnelitis, cellulitis, abcess, clinically documented infection likely to require prolonged antibiotic therapy | yes | yes | no | no | yes | Need for the use of G/GM‐CSF and cytokines; no responsible adult living with them (carer); |
| Malik 1992 | no | yes | no | no | no | no | Recurrent FUO |
| Cornely 2003 | not excluded | excluded (except cotrimoxazole prophylaxis) | yes | yes | excluded | excluded | potential compromised absorption; inability to take oral medication; tenopathy, epilepsy; aplastic anaemia, acute leukaemia; septic shock or signs of sever infection; HIV carrier; serious concomitant disease, liver transaminase> x5 of norm. |
| Niho 2004 | not excluded | excluded | no | not excluded | no | yes | Recurrent FUO; renal insufficiency; hepatic insufficiency; hypotension or peripheral circulatory failure; uncontrolled hypercalcaemia; altered sensorium; respiratory rate >30 breaths/min; serum sodium <128 mg/dl; inability to take oral medications; intestinal malabsorption |
| Paganini 2003 | Fascial, perineal, or catheter‐associated cellulites; uncontrolled local infection; positive blood cultures within the first 48 hours; infection with microorganisms known as resistant to ceftriaxone or ciprofloxacin | included | yes | not excluded | not excluded | excluded | severe comorbidity factors; respiratory failure |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 9 | 1392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.68] |
| 1.1 Initially oral | 6 | 961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.62] |
| 1.2 Sequential | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.45, 4.22] |
| 2 Treatment failure Show forest plot | 22 | 3142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
| 2.1 Initially oral treatment | 16 | 2196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.03] |
| 2.2 Sequential IV to oral treatment | 6 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.27] |
| 3 Treatment failure ‐ per protocol analysis Show forest plot | 22 | 2912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 3.1 Initially oral treatment | 16 | 1991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
| 3.2 Sequential IV to oral treatment | 6 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.29] |
| 4 Adverse events requiring discontinuation of antibiotics Show forest plot | 15 | 1823 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.61, 3.46] |
| 4.1 Initially oral treatment | 10 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.14, 6.75] |
| 4.2 Sequential IV to oral treatment | 5 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.25] |
| 5 Gastrointestinal adverse events ('post‐protocol' analysis) Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Initially oral treatment | 11 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [2.87, 7.04] |
| 5.2 Sequential IV to oral treatment | 4 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.03, 7.66] |
| 6 Lost to follow‐up Show forest plot | 19 | 2810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.10] |
| 7 Treatment failure not dt modification in update Show forest plot | 21 | 3041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| 7.1 Initially oral treatment | 15 | 2095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 7.2 Sequential IV to oral treatment | 6 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure ‐ age Show forest plot | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 8 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 1.2 Adults | 12 | 1652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.12] |
| 2 Treatment failure ‐ source of infection Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Unexplained fever | 10 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.33] |
| 2.2 Documented infection | 10 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 3 Treatment failure ‐ severity of neutropenia Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Absolute neutrophil count >=10^9/L | 3 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| 3.2 Absolute neutrophil count <10^9/L | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.49] |
| 4 Treatment failure ‐ type of malignancy Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Solid tumour | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 4.2 Haemetologic malignancy | 4 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Allocation concealment Show forest plot | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adequate (A) | 12 | 1651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 1.2 Unclear (B) | 10 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Setting Show forest plot | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oral‐outpatient, IV‐inpatients | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.43] |
| 1.2 Inpatients | 6 | 1128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 1.3 Outpatients | 7 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 1.4 Only first dose in | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.34] |
| 2 Type of oral antibiotics Show forest plot | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quinolones only | 7 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 2.2 Quinolones in combination with augmentin, ampicillin‐sulbactam, penicillin V or clindamycin | 11 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 2.3 Cefixime | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.64, 1.56] |
| 2.4 New quinolones | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.86] |

