Traitement antibiotique oral ou intraveineux pour la neutropénie fébrile chez les patients atteints de cancer
Résumé scientifique
Contexte
La fièvre survenant chez un patient neutropénique reste une complication engageant le pronostic vital de la chimiothérapie pour des cancers. La prise en charge habituelle est l'hospitalisation et le traitement les malades de manière empirique avec des antibiotiques à large spectre par voie intraveineuse. Un traitement oral pourrait être une alternative pour les patients sélectionnés.
Objectifs
Comparer l'efficacité des antibiotiques oraux versus le traitement antibiotique intraveineux (IV) chez les patients cancéreux atteints de neutropénie fébrile.
Stratégie de recherche documentaire
Le registre Cochrane des essais contrôlés (CENTRAL) (2013, numéro 1) dans la bibliothèque Cochrane, MEDLINE (de 1966 à la 4ème semaine de janvier 2013), EMBASE (de 1980 à 2013 4ème semaine) et LILACS (de 1982 à 2007). Nous avons effectué des recherches dans plusieurs bases de données pour les essais en cours. Nous avons examiné les actes de la conférence inter scientifique d'antimicrobiens et de Chimiothérapie (ICAAC) (de 1995 à 2007), et toutes les références bibliographiques des études incluses ainsi que des principales revues.
Critères de sélection
Des essais contrôlés randomisés (ECR) comparent des antibiotiques oraux aux antibiotiques intraveineux pour le traitement des patients cancéreux avec une neutropénie et de la fièvre. La comparaison entre les deux a pu être faite au début (traitement oral initial) ou après un traitement initial antibiotique intraveineux (séquentiel).
Recueil et analyse des données
Deux auteurs de la revue ont indépendamment évalué l'éligibilité et la qualité méthodologique et extrait les données de manière indépendante. Les données concernant la mortalité, les échecs du traitement et les événements indésirables pour les mesures des résultats ont été extraits des études incluses supposant une 'intention de traiter' lorsque cela a été possible. Les risques relatifs (RR) avec des intervalles de confiance (IC) à 95% ont été estimés pour les données dichotomiques. L'évaluation du risque de biais était également dans le cadre de la méthodologie de la collaboration Cochrane.
Résultats principaux
Vingt‐deux essais (3142 épisodes de 2372 patients) ont été inclus dans les analyses. Le taux de mortalité était similaire dans la comparaison de la voie orale à un traitement antibiotique intraveineux (RR 0,95, IC à 95%, entre 0,54 et 1,68, 9 essais, 1392 patients, avec une moyenne de 0 de mortalité, allant de 0 à 8,8%). Les taux d'échec du traitement étaient également similaires (RR 0,96, IC à 95%, de 0,86 à 1,06, dans tous les essais). Aucune hétérogénéité significative n’a été démontrée dans toutes les comparaisons, mais dans les événements indésirables. L'effet était stable chez un large éventail de patients. Les quinolones seules ou combinées à un autre antibiotique ont été utilisées avec des résultats comparables. Les réactions indésirables, principalement gastro‐intestinales, étaient plus fréquentes avec les antibiotiques oraux.
Conclusions des auteurs
D'après les données actuelles, le traitement par voie orale est une alternative acceptable à un traitement antibiotique intraveineux chez les patients cancéreux atteints de neutropénie fébrile (à l'exclusion des patients atteints de leucémie aiguë) qui ont une stabilité hémodynamique, sans défaillance d'organes, et n'ont pas de pneumonie, d’infection d'une voie centrale ou une infection sévère des tissus mous. Les larges IC pour la mortalité admet utilisation du traitement oral dans les groupes de patients présentant un faible risque prévu de mortalité, d'autres recherches doivent être effectués à clarifier la définition de patients à faible risque.
PICOs
Résumé simplifié
Des antibiotiques oraux pour le traitement de la neutropénie fébrile chez les patients cancéreux présentant de faibles risques de complications
La neutropénie (faible nombre de globules blancs) est une complication de la chimiothérapie anticancéreuse qui expose les patients à des infections à un risque vital. La prise en charge habituelle des patients neutropéniques avec fièvre est l'hospitalisation et le traitement avec des antibiotiques intraveineux. La neutropénie fébrile englobe un éventail de la maladie et les patients à faible risque peuvent être traités moins agressivement. Cette revue des essais contrôlés randomisés a montré des résultats comparables de décès et des taux d'échec d'antibiotiques par voie orale et intraveineuse pour les patients à faible risque, les patients atteints de tumeurs solides ou leucémies chroniques ou de lymphome, indépendamment de leur âge, la source de l'infection et la gravité de la neutropénie.
Authors' conclusions
Summary of findings
| Oral compared to intravenous antibiotic therapy for febrile neutropenia in cancer patients | ||||||
| Patient or population: patients with febrile neutropenia in cancer patients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| intravenous antibiotic therapy | Oral | |||||
| Mortality | Study population | RR 0.95 | 1392 | ⊕⊕⊕⊝ | ||
| 32 per 1000 | 30 per 1000 | |||||
| Low risk | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment failure | Study population | RR 0.96 | 3142 | ⊕⊕⊕⊝ moderate1 | ||
| 284 per 1000 | 272 per 1000 | |||||
| Moderate | ||||||
| 211 per 1000 | 203 per 1000 | |||||
| Treatment failure ‐ per protocol analysis | Study population | RR 0.98 | 2912 | ⊕⊕⊕⊝ | ||
| 225 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 184 per 1000 | 180 per 1000 | |||||
| Adverse events requiring discontinuation of antibiotics | Study population | RR 1.45 | 1823 | ⊕⊕⊝⊝ low1, 2 | ||
| 21 per 1000 | 31 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment failure not dt modification in update | Study population | RR 0.95 | 3041 | ⊕⊕⊕⊝ | ||
| 267 per 1000 | 254 per 1000 | |||||
| Moderate | ||||||
| 180 per 1000 | 171 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High risk of detection bias in most of the trials 2 A wide CI | ||||||
Background
Patients with cancer who experience fever while being neutropenic are at risk of serious infections (Bodey 1966; Klaassen 2000a; Lucas 1996; Pizzo 1982; Rackoff 1996). Empirical use of antibiotics, based on previous practice without knowledge of the cause of infection, has lowered the incidence of death and serious complications (Schimpff 1971; Schimpff 1986, Talcott 1988; Viscoli 2002). Traditionally the practice is to admit and treat neutropenic patients empirically with intravenous (IV) broad‐spectrum antibiotics at the emergence of a fever (Hughes 2002).
The empirical selection of an appropriate antibiotic is based on the patient's immune status (that is being neutropenic) as well as the suspected invading organism and its susceptibility to antibiotics. Neutropenic cancer patients form a heterogeneous group. A retrospective study indicated the existence of a patient subpopulation responding promptly to antibiotic therapy, thus raising the possibility of using different treatment strategies, such as oral therapy and outpatient treatment (Talcott 1988). In parallel, the pattern of pathogens (that is bacteria) in neutropenic patients with fever has changed, with a declining incidence of Gram‐negative bacteraemia and increasing incidence of Gram‐positive infections (EORTC 1990; Hann 1997; Hughes 2002), resulting in a change in antibiotic practice. Oral treatment became a viable option with the advent of new extended spectrum oral antibiotics. The potential of oral treatment and the deleterious effects of hospitalisation (with need for an intravenous access line, exposure to multi‐drug resistant organisms) and greater awareness of the importance of quality of life and patient satisfaction, especially among cancer patients (Talcott 1994), has led to a re‐evaluation of the approach to neutropenic febrile patients.
Research has therefore focused on methods to prospectively identify neutropenic patients with fever who are at low risk of complications. Several clinical prediction rules were developed and validated for children and adult populations at low risk (Klaassen 2000a; Klastersky 2000; Rackoff 1996; Talcott 1988; Talcott 1992). Talcott et al constructed four groups out of which one included patients with an expected low complications rate. The patients at low risk developed fever out of hospital, had controlled cancer and had no co‐morbidity. The rule was validated prospectively and later tested in a pilot study for its ability to select patients for early switch from IV to oral treatment (Talcott 1992). A consensus panel, the Multinational Association for Supportive Care in Cancer (MASCC), developed a set of criteria that are predictors of good prognosis in 'low risk' adult patients: acquisition of fever out of hospital, age younger then 60 years, absent to moderate symptoms, no hypotension, no chronic bronchitis, and a background of either a solid tumour or haematological malignancy with no history of fungal infection; in a validated scoring system. While receiving conventional therapy patients with a score of equal to or greater than 21 had a low rate of serious medical complication (6%) and only 1% mortality (Klastersky 2000).
Simultaneously, clinical trials (Malik 1992; Rubenstein 1993) have examined the safety and feasibility of oral antibiotic treatment in selected patients. In the absence of an accepted definition there was no consistency in the selection of patients defined as a 'low risk' group. Most of these trials were small, single centre trials. Thus, although reporting similar rates of success for oral and intravenous therapy, the superiority of an intravenous regimen cannot be ruled out.
The most recent guidelines for antibiotic treatment in neutropenic patients with cancer (Hughes 2002) are very cautious regarding the use of oral antibiotics alone. They recommend careful selection of low risk patients and limit this approach to adults, of whom only some may receive the treatment at home.
In the present systematic review we intended to provide better evidence regarding the safety and efficacy of oral treatment as opposed to intravenous treatment. We tried to clarify the definitions of the low risk subgroup and the appropriate antibiotics for oral treatment as well as the limits of present knowledge.
Objectives
To compare the efficacy of oral antibiotics versus intravenous antibiotic therapy in febrile neutropenic cancer patients.
In addition, we compared the efficacy of these treatment modalities in the following subgroups.
-
Patients with unexplained fever (versus documented infection).
-
Patients with an absolute neutrophil count of more than 0.1 cells x 10^9/L (versus those with a lower neutrophil count).
-
Patients with a solid tumour (versus those with a haematological malignancy).
-
Patients 60 years old and under (versus those above 60 years).
-
Children (according to trial definition) (versus adults).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing any oral antibiotics to any intravenous (IV) antibiotics for the treatment of febrile neutropenia in cancer patients. The oral antibiotics could be started at presentation in patients allocated to oral treatment (viz, initial oral) or as part of a sequential IV to oral strategy where all patients were initially treated intravenously and those allocated to oral treatment were switched to oral therapy after a predefined time independent of the neutropenic episode (viz., sequential).
Types of participants
Patients with cancer and chemotherapy‐induced neutropenia or patients with cancer who underwent bone marrow transplantation who presented with fever.
Types of interventions
A comparison of the following.
(1) Any oral antibiotics, administered as a single drug or as a combination of orally administered antibiotics.
(2) Any antibiotics administered intravenously, either as monotherapy or combination therapy.
Studies in which patients were allocated to these regimens initially, before administration of any other antibiotics for the specific febrile episode (initial oral), were analysed separately from studies in which intravenous antibiotics had been first given to all patients (that is sequential intravenous to oral therapy).
Types of outcome measures
Primary outcomes
-
All cause mortality at 30 days follow‐up
-
Mortality caused by the infectious episode at end of follow‐up (restricted to 30 days)
-
Treatment failure (restricted to 30 days)
For the purpose of this review, treatment failure was defined as a composite endpoint comprising one or more of the following: death; persistence, recurrence or worsening of clinical signs or symptoms of presenting infection; any addition to or modification of the assigned intervention (Consensus panel 1990; Feld 1998).
Secondary outcomes
-
Treatment failure not due to modification of the primary intervention
-
Lost to follow‐up before end of study (dropouts)
Adverse effects
-
Life‐threatening or associated with permanent disability
-
Requiring discontinuation of therapy
Search methods for identification of studies
Electronic searches
Relevant trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 1) (Appendix 1), MEDLINE (1966 to January week 4, 2013) (Appendix 2), EMBASE (1980 to 2013 week 4) (Appendix 3) and LILACS (1982 to 2007) (Appendix 4).
Searching other resources
References of all identified studies as well as major reviews were inspected for more studies. We searched the following conference proceedings for unpublished trials: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (1995 to 2006); The American Society of Hematology (2001 to 2002) (available at http://www.hematology.org/). We searched the following trial databases for ongoing and unpublished trials: Current Controlled Trials in the metaRegister of controlled clinical trials (http://www.controlled‐trials.com/); UKCCCR Register of Cancer Trials (www.ctu.mrc.ac.uk/ukcccr); PDQ (Physician Data Query) database of the National Cancer Institute (http://www.cancer.gov/search/clinical_trials/); and the National Institutes of Health database (http://clinicaltrials‐nccs.nlm.nih.gov/).
Additionally, the first or corresponding author of each included study and pharmaceutical companies were contacted for complementary information or information regarding unpublished trials. Letters, abstracts and unpublished trials were accepted in order to reduce the influence of publication bias.
Data collection and analysis
Selection of studies
One review author inspected the abstract of each reference identified by the search and applied the inclusion criteria. For possibly relevant articles the full article was obtained and inspected by two review authors independently.
Data extraction and management
Two review authors independently extracted data from the included trials. In addition the third review author extracted data from 10% of the studies, selected randomly. Data extractions were discussed, decisions documented, and all authors of included studies were contacted for clarification. Justifications for excluding studies from the review were also documented. Differences in the data extracted were resolved by discussion. In the case of disagreement between the two review authors, a third review author extracted the data. All data were collected on an intention‐to‐treat (ITT) basis whenever possible.
Trials were identified by the name of the first author and year in which the trial was first published, and ordered chronologically. The following data were extracted, checked and recorded.
Characteristics of trials
-
Date, location and setting of trial (inpatients or ambulatory patients)
-
Publication status
-
Case definitions used (inclusion and exclusion criteria)
-
Design (intention to treat, method of randomisation and allocation)
-
Unit of randomisation
-
Sponsor of trial
Characteristics of patients
-
Number of participants and number of episodes in each group
-
Age (mean and SD, or median plus range)
-
Underlying malignancy and malignancy status
-
Site of infection (three most common)
-
Disease severity measure: septic shock, confusion, respiratory, liver and renal impairment
-
Percentage of patients with neutrophil count more than 0.1 cells x 10^9/L in each group
-
Percentage of patients with a solid tumour or lymphoma and chronic leukaemia in each group
-
Number of patients with unexplained fever in each group
-
The three most common pathogens (Gram‐negative bacteria, Gram‐positive bacteria)
-
Number of patients developing resistant superinfection or colonisation, or both
-
Number of patients 60 years of age and younger
-
Number of children (according to trial's definition)
Characteristics of interventions
-
Antibiotic type, mode of administration, dose and interval
-
'Initially oral' or 'sequential intravenous to oral' study (see: 'Types of studies')
-
Duration of therapy (median)
Characteristics of outcome measures
-
Number of deaths at 30 days or during the study duration
-
Mortality caused by the infectious episode
-
Number of treatment failures (as defined)
-
Number of treatment failures not due to addition of intravenous antibiotic for the primary infection
-
Adverse reactions (causing death or permanent disability; requiring discontinuation; any other)
-
Number of patients excluded after randomisation
-
Lost to follow‐up (dropouts) before the end of study
Assessment of risk of bias in included studies
Two review authors working independently assessed the trials fulfilling the review inclusion criteria for methodological quality. This was done using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which are based on the evidence of a strong association between poor allocation concealment and overestimation of effect (Schulz 1995), as defined below.
A. Low risk of bias (adequate allocation concealment).
B. Moderate risk of bias (uncertainty regarding allocation concealment).
C. High risk of bias (inadequate allocation concealment).
In addition to the adequacy of allocation concealment, methods of allocation generation, blinding, intention‐to‐treat analysis, exclusions after randomisation, randomisation unit and publication status were recorded independently by the two review authors. Measures of quality were used for sensitivity analysis.
Assessment of heterogeneity
Heterogeneity (degree of difference between the results of different trials) in the results of the trials was initially graphically inspected and assessed by a test of heterogeneity (Chi2 test and I2 statistic). We had anticipated between‐trial variation in estimation of morbidity and mortality for studies comparing broad‐spectrum oral treatment versus narrow‐spectrum treatment and for studies comparing patients at different risk levels. Heterogeneity was explored through stratifying the above defined patient subgroups (in the 'Objectives'), separating patients with low risk criteria (Table 1).
| Common criteria |
| Haemodynamic stablity |
| No organ failure |
| Ability to take oral medications |
| No pneumonia |
| No infection of a central line |
| No severe soft‐tissue infection |
| No acute leukaemia as the background malignancy |
| No known drug allergy |
| Not pregnant or lactating women |
A funnel plot estimating the treatment effect against the precision of the trials (plots of the log of the relative risk for efficacy against the standard error) was examined in order to estimate potential asymmetry that may indicate selection bias (the selective publication of trials with positive findings) or methodological flaw in the small studies.
Data synthesis
Pooled risk ratios (RR) with 95% confidence intervals (CIs) were calculated for dichotomous data. Exclusions after randomisation were reported. A fixed‐effect model (Mantel‐Haenszel method) was used unless significant heterogeneity (P less than 0.10) was detected, in which case a random‐effects model (DerSimonian and Laird method) was used.
Subgroup analyses were performed to investigate the effects of age (children versus adults), source of infection (unexplained fever versus documented infection), severity of neutropenia (absolute neutrophil count equal to or greater than 0.1 cells x 10^9/L versus absolute neutrophil count less than 0.1 cells x 10^9/L), and type of malignancy (solid tumour versus haematological malignancy).
Results
Description of studies
Results of the search
Potentially relevant references were identified through the electronic databases. These references were screened for RCTs in cancer patients with neutropenia, fever and antibiotic regimens according to the protocol. Eighty trials were considered potentially eligible for this review, including six articles identified through references cited in the included studies and in major review articles in the field (Freifeld 1997; Hughes 2002; Kern 2001a; Klaassen 2000a; Paesmans 2000; Rolston 1999; Viscoli 2002).
Included studies
Twenty‐two published RCTs were included in the review. Additionally two conference proceedings, identified through the ICAAC search, were included (Rolston 1995; Samonis 1997). Further information was provided by Dr Anaissie (Samonis 1997).
The studies were performed between the years 1989 and 2007, and included 965 randomised patients and an additional 2177 randomised episodes in 1407 patients. The age of the patients ranged from nine months to 85 years. Oral antibiotics were compared to intravenous antibiotics, both given empirically and as the initial empirical treatment ('initial oral') in 16 trials (Brack 2012*; Cagol 2009*; Cornely 2003; Freifeld 1999; Gupta 2009*; Hidalgo 1999; Innes 2003; Kern 1999; Malik 1992; Niho 2004; Petrilli 2000; Rolston 1995; Rubenstein 1993; Samonis 1997; Sebban 2009*; Velasco 1995). In the other five trials (Flaherty 1989; Giamarellou 2000; Mullen 1999; Paganini 2000; Paganini 2003; Shenep 2001) the patients randomised to 'oral treatment' received intravenous antibiotics prior to oral therapy ('sequential'). In two sequential trials (Flaherty 1989; Giamarellou 2000) the randomisation procedure was carried out at presentation but the patients were switched to oral therapy only 72 hours later.
Some exclusion criteria were similar among trials: haemodynamic instability, hypotension, altered mental status, respiratory failure, poor clinical condition, renal failure, abnormal liver function tests, no ability to swallow or take oral medication, allergy to study drugs, pregnancy and lactation. Additional case definitions varied between the trials despite the attempts of all trials to identify patients at low risk for mortality and complications (Table 2). Patients with haematological malignancy were excluded in three studies (Hidalgo 1999; Niho 2004; Samonis 1997). Patients with acute leukaemia were included in Brack 2012* (after maintenance treatment only); Freifeld 1999 (excluding patients with "neutropenia expected to last greater than 10 days"); Gupta 2009* (after maintenance treatment only); Giamarellou 2000; Malik 1992; Paganini 2003; Rolston 1995; Rubenstein 1993. Patients undergoing bone marrow or allogeneic stem cell transplantation were specifically excluded in eight trials (Brack 2012*; Cornely 2003; Freifeld 1999; Gupta 2009*; Innes 2003; Kern 1999; Mullen 1999; Paganini 2000).
| Study ID | Evident infection | Previous AB | Prolonged neutropeni | Performance status | Active malignancy | BMT/PSCT | Other |
| Kern 1999 | Infected catheter or CNS infection, known bacterial /viral/fungal infection | yes | yes | no | no | yes | Need of IV supportive therapy, expected to die within 48 hours, HIV, fever unrelated to infection and protocol violation |
| Mullen 1999 | A source of infection that required hospitalisation as: tunnelitis, pneumonia, perirectal cellulitis, typhlitis, resistant microorganism to one of the study's drugs | no | no | no | yes | yes | >10% dehydration, bleeding requiring platelet transfusion, |
| Paganini 2000 | Infected catheter, perineal/ facial cellulitis, uncontrolled local infection, positive blood cultures at 72 hours | no | no | no | yes | yes | Persistance of fever >48 hours, incorrectable bleeding; refractory hypoglycemia or hypocalcemia |
| Rubenstein 1993 | Known resistant microorganism | no | no | no | no | no | Na<128, uncontrolled hypercalcemia, more than 30 miles away |
| Samonis 1997 | Pneumonia, deep organ infection | yes | yes | no | yes | no* | Prior hospitalisation |
| Shenep 2001 | Pneumonia, clinical or radiographic evidence of focal bacterial infection, severe mucositis, positive blood cultures at 48 hours | no | no | no | no | no | MRSA or P.Aeroginosa in any culture obtained in preceding 12weeks |
| Velasco1995 | Meningitis, pyelonephritis | yes | no | yes | no | no* | Long term central vein catheter |
| Petrilli 1999 | no | no | no | no | no | no* | |
| Flaherty 1989 | no | yes | no | no | no | no | |
| Freifeld 1999 | Intravascular infection, tunnelitis, pneumonia, neurologic symtoms, | no | yes | no | no | yes | Treatment with Ca‐Mg or probenecid or alluporinol or theophylline, HIV |
| Giamarelou 2000 | Suspected anaerobes | no | no | yes | no | no | Moribund and high probability of dying within 48 hours |
| Hidalgo 1999 | Pneumonia, extensive cellulitis, meningitis, pyelonephritis | no | no | yes | yes | no | Clotting abnormalities, acidosis, hypercalcaemia, uncontrolled bleeding, live >2h apart from hospital; Hx of tumour fever, other severe extra hematologic chemotherapy induced toxicity, no 24 hours home companion |
| Innes 2003 | Tunnelitis, cellulitis, abcess, clinically documented infection likely to require prolonged antibiotic therapy | yes | yes | no | no | yes | Need for the use of G/GM‐CSF and cytokines; no responsible adult living with them (carer); |
| Malik 1992 | no | yes | no | no | no | no | Recurrent FUO |
| Cornely 2003 | not excluded | excluded (except cotrimoxazole prophylaxis) | yes | yes | excluded | excluded | potential compromised absorption; inability to take oral medication; tenopathy, epilepsy; aplastic anaemia, acute leukaemia; septic shock or signs of sever infection; HIV carrier; serious concomitant disease, liver transaminase> x5 of norm. |
| Niho 2004 | not excluded | excluded | no | not excluded | no | yes | Recurrent FUO; renal insufficiency; hepatic insufficiency; hypotension or peripheral circulatory failure; uncontrolled hypercalcaemia; altered sensorium; respiratory rate >30 breaths/min; serum sodium <128 mg/dl; inability to take oral medications; intestinal malabsorption |
| Paganini 2003 | Fascial, perineal, or catheter‐associated cellulites; uncontrolled local infection; positive blood cultures within the first 48 hours; infection with microorganisms known as resistant to ceftriaxone or ciprofloxacin | included | yes | not excluded | not excluded | excluded | severe comorbidity factors; respiratory failure |
Patients with any source of infection at presentation were included in six of the 'initial oral' trials (Cornely 2003; Malik 1992; Niho 2004; Petrilli 2000; Rubenstein 1993; Velasco 1995) and the 'sequential' studies. In the other studies patients with a specific source of infection were excluded: pneumonia was an exclusion criterion in six trials (Freifeld 1999; Hidalgo 1999; Innes 2003; Mullen 1999; Samonis 1997; Shenep 2001); patients with infected intravascular catheters or tunnelitis were excluded in seven trials (Freifeld 1999; Innes 2003; Kern 1999; Mullen 1999; Paganini 2000; Paganini 2003; Shenep 2001); perirectal or severe cellulitis was an exclusion criterion in six trials (Hidalgo 1999; Innes 2003; Mullen 1999; Paganini 2000; Paganini 2003; Shenep 2001); Kern 1999 excluded known bacterial, viral or fungal infection; and Paganini 2000 excluded uncontrolled local infection. In one trial (Sebban 2009*) a MASCC score of 21 or lower was an inclusion criterion.
The oral antibiotics differed between trials: antipneumococcal quinolones in two trials (Paganini 2003; Sebban 2009*), other quinolones in 10 trials (Brack 2012*; Cagol 2009*; Flaherty 1989; Giamarellou 2000; Gupta 2009*; Hidalgo 1999; Malik 1992; Mullen 1999; Petrilli 2000). Quinolones were given in combination with ampicillin‐clavulanate, ampicillin‐sulbactam, or penicillin V in nine trials (Brack 2012*; Cagol 2009*; Freifeld 1999; Gupta 2009*; Innes 2003; Kern 1999; Niho 2004; Rolston 1995; Samonis 1997; Velasco 1995) and in combination with clindamycin in one trial (Rubenstein 1993). The antibiotics given orally were different in most studies from the drugs given intravenously.
The setting of therapy also varied. All patients were treated as outpatients in six trials (Cornely 2003; Gupta 2009*; Mullen 1999; Paganini 2003; Petrilli 2000; Rolston 1995; Rubenstein 1993). Patients randomised to oral therapy were treated as outpatients while the control group was treated in hospital in three trials (Hidalgo 1999; Innes 2003; Samonis 1997). Therapy was initiated in hospital and continued at home in two trials (Brack 2012*; Sebban 2009*). In the rest of the trials all patients were treated in hospital.
With both regimens few studies had high mortality rates (5% to 8.8%) (Flaherty 1989; Giamarellou 2000; Kern 1999; Malik 1992). This can be explained by the design of the trials: randomisation of patients not episodes (Giamarellou 2000; Kern 1999; Malik 1992), a longer follow‐up period (Kern 1999), and not applying most low risk criteria for inclusion (Malik 1992).
Excluded studies
Fifty‐eight trials were excluded from this review (Characteristics of excluded studies). Reasons for their exclusion were the following.
-
Not randomised trials (Ammann 2004; Aquino 1997; Aquino 2000; Bash 1994; Berrahal 1996; Chamilos 2005; Cornelissen 1995; Gardembas‐Pain 1991; Escalante 2004; Freifeld 2011; Horowitz 1996; Lau 1994; Malik 1994; Malik 1997; Montalar Salcedo1999; Marra 2000; Mustafa 1996; Nepokul'chitskaia; Paganini 2001b; Papadimitris 1999; Vallejo 1997; Wacker 1997).
-
Sequential oral ‐ intravenous antibiotics were used for all the patients in both trial arms (Paganini 2001a).
-
No intravenous treatment arm (Malik 1995). All patients received oral antibiotics, and were randomised to outpatient versus inpatient therapy.
-
Sequential oral antibiotic therapy was compared to no treatment (or placebo) (Klaassen 2000; Santolaya 1997).
-
No oral treatment arm (IATCG‐EORTC 1994; Kibbler 1987; Meunier 1991; Rapoport 1999; Santolaya 2004; Sato 2008).
-
Review (Cometta 2004; Copper 2011; Freifeld 1997; Leverger 2004; Mullen 2001; Tamura 2005).
-
One study (Minotti 1999) included all patients with fever post‐chemotherapy, neutropenic and non‐neutropenic, and did not report the outcomes of neutropenic patients separately.
-
Prophylaxis (Timmers 2007).
Risk of bias in included studies
Adequate allocation concealment was reported in six trials (Brack 2012*; Cagol 2009*; Cornely 2003; Giamarellou 2000; Kern 1999; Sebban 2009*); information regarding adequate allocation concealment was provided by the contact authors in five other trials (Hidalgo 1999; Innes 2003; Paganini 2000; Shenep 2001; Velasco 1995). Two trials (Malik 1992; Niho 2004) reported how the allocation concealment was undertaken but its adequacy was unclear. These trials used sealed envelopes, however, no mention was made about whether the envelopes were opaque and the trials were assessed as at unclear risk of bias. There was no information regarding concealment of allocation in the other nine trials. Two trials were double blinded (Cagol 2009*; Freifeld 1999) and in one the outcomes assessors were blinded to the treatment arm (Kern 1999). Blinding was not specified in the other trials.
Duration of follow‐up was predefined only in three trials (Cornely 2003; Kern 1999 (reported); Paganini 2000 (data from author)). In the other trials follow‐up varied according to the length of the neutropenic febrile episode. In two trials (Giamarellou 2000; Innes 2003) the patients were followed for a predefined time after resolution of fever. In the other trials patients were followed until the end of the febrile neutropenic episode or until the end of antibiotic treatment.
Five per cent (median, range 0 to 0.18) of the patients were excluded from the final analysis. One trial provided no information about exclusions from the final analysis (Rolston 1995).
The unit of randomisation was the patient in four trials (Giamarellou 2000; Kern 1999; Malik 1992; Samonis 1997) and the episode of febrile neutropenia in the other trials. The later trials included 2050 episodes in 1407 patients; the number of patients included was not provided in three trial (Cornely 2003; Hidalgo 1999; Rolston 1995).
Graphical representation of the risk of bias is provided in Figure 1 and Figure 2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Mortality (9 trials, 1392 patients or episodes)
No difference in mortality (from any cause or caused by the infectious episode) between oral and intravenous treatment was demonstrated (RR 0.95, 95% CI 0.54 to 1.68, Analysis 1.1).
Treatment failure (all trials, 3135 patients or episodes)
There was no significant difference in failure rate between the two interventions (comparison 01, outcome 02, Analysis 1.2). The RR of treatment failure for 'initial oral' studies was 0.89 (95% CI 0.79 to 1.03, N = 2189), and 1.07 (95% CI 0.90 to 1.27, N = 946) for 'sequential' studies. A comparable RR was calculated for failure not due to modification of the initial regimen.
Per protocol analysis gave similar results to those of the ITT analysis (per protocol analysis: RR 0.98, 95% CI 0.86 to 1.11, all trials, 2912 patients, Analysis 1.3).
Adverse events
No deaths or permanent damage were attributed to the oral therapy in any of the trials. Adverse effects that required discontinuation of the assigned antibiotic therapy were reported in 15 (Flaherty 1989; Freifeld 1999; Giamarellou 2000; Hidalgo 1999; Innes 2003; Malik 1992; Mullen 1999; Niho 2004; Paganini 2000; Rubenstein 1993; Shenep 2001; Velasco 1995) (also Cornely 2003;Gupta 2009*; Kern 1999; Paganini 2003; Petrilli 2000), which reported side effects, of the 17 trials. Separate analysis of the 'initial oral' studies revealed significantly more adverse events requiring discontinuation among orally treated patients (RR 2.78, 95% CI 1.14 to 6.75, Analysis 1.4). This finding is consistent with the high rate of gastrointestinal adverse events with oral antibiotics as shown in Analysis 1.5, and with the fact that these events hamper any oral but not intravenous treatment (Cornely 2003; Freifeld 1999; Giamarellou 2000; Innes 2003; Kern 1999; Malik 1992; Niho 2004; Paganini 2000; Paganini 2003; Petrilli 2000; Rubenstein 1993; Shenep 2001; Velasco 1995) ('post‐protocol' analysis).
Dropouts (lost to follow‐up) before end of study
Nineteen trials reported the number of patients who were lost to follow‐up before the end of the study in each group (Cornely 2003; Freifeld 1999; Giamarellou 2000; Hidalgo 1999; Innes 2003; Kern 1999; Malik 1992; Niho 2004; Paganini 2000; Paganini 2003; Petrilli 2000; Rubenstein 1993; Samonis 1997; Shenep 2001; Velasco 1995). No significant difference in the number of dropouts was found between the oral and intravenous (IV) treatment (RR 0.82, 95% CI 0.61 to 1.10, N = 2802).
Treatment failure ‐ age
The outcomes of patients younger than 60 years of age versus older patients could not be extracted from the original publications.
There was no difference in the failure rates between the assigned treatments in the trials that included only children (RR 1.02, 95% CI 0.82 to 1.28, N = 1013, 8 trials) as well as in the trials in adults (RR 0.98, 95% CI 0.85 to 1.12, N = 1652, 12 trials, Analysis 2.1). One study (Kern 1999) included a low number of children and was considered for the purpose of the analysis as addressing an adult population. One death was documented among children (Brack 2012*) treated with intravenous antibiotics.
Treatment failure ‐ source of infection
Treatment failure in relation to evidence of documented infection was addressed in trials (Freifeld 1999; Giamarellou 2000; Hidalgo 1999; Kern 1999; Malik 1992; Rolston 1995; Rubenstein 1993; Samonis 1997). There were no significant differences in treatment failure rate among the patients with unexplained fever (RR 1.03, 95% CI 0.79 to 1.33, N = 924) and those with documented infections (RR 1.00, 95% CI 0.84 to 1.19, N = 641, Analysis 2.2). In one trial (Freifeld 1999) the assessment was made at presentation while in the other trials assessment was done after 48 hours and therefore could not serve as a tool to assess the risk of patients ahead of treatment (unless switching to IV antibiotic treatment after 48 hours).
Treatment failure ‐ severity of neutropenia
Failures according to the absolute neutrophil count (ANC) were analysed in three studies (Kern 1999; Rubenstein 1993; Shenep 2001).
No significant difference in treatment failure rate was found among patients with severe neutropenia (RR 1.07, 95% CI 0.76 to 1.49, N = 370). Among patients with ANC greater than 0.1 (109 cells/L) the risk of infection was not increaed with oral antibiotics (RR 0.66, 95% CI 0.45 to 0.98, N = 328, Analysis 2.3).
No deaths had occurred in patients with ANC greater than 0.1 (109 cells/L) at presentation.
Treatment failure ‐ type of malignancy
Two trials (Hidalgo 1999; Samonis 1997) included only patients with solid tumours and patients with lymphoma. One trial (Petrilli 2000) included 96% patients and another trial (Cagol 2009*) included 90% of patients with solid tumours and were analysed as such; two trials (Rolston 1995; Rubenstein 1993) provided the failure rates of both oral and intravenous treatments among patients in accordance with the background malignancy; one trial had included only haematological cancer patients (Giamarellou 2000). No difference in treatment failure was demonstrated in patients with solid tumours (RR 0.89, 95% CI 0.70 to 1.12, N = 990) and in patients with haematological malignancy (RR 1.04, 95% CI 0.84 to 1.28, N = 312, Analysis 2.4).
Comparison of mortality in subgroups could not be performed due to the low rate of deaths.
Continuous data
Due to insufficiently reported continuous outcome data, such as duration of fever, duration of antibiotic therapy, and length of hospital stay, these data could not be analysed.
Sensitivity and subgroup analyses
Sensitivity analyses of studies by the risk of bias (according to the adequacy of allocation concealment: low risk of bias, unclear; and according to blinding: blinding versus no blinding) showed no significant impact on the risk of treatment failure. Sensitivity analyses on different case definitions (trials including only patients with solid tumours versus trials including patients with solid and haematological malignancies; trials including patients with acute leukaemia versus trials excluding them; trials excluding patients with any identified source of infection at presentation versus trials including patients regardless of the source of infection; the above mentioned subgroups) showed similar RRs.
Treatment setting
Treatment setting (in or out of hospital) had no effect on the results (Analysis 4.1).
Antibiotics used in the trials
Quinolones alone were tested in nine (Cornely 2003; Flaherty 1989; Giamarellou 2000; Hidalgo 1999; Malik 1992; Mullen 1999; Paganini 2003; Petrilli 2000; Sebban 2009*) of the 22 trials in the pooled analysis. The dosage used varied from 400 to 800 mg of ofloxacin, and 600 to 2250 mg of ciprofloxacin daily, and in one trial 400 mg moxifloxacin daily. The quinolones were most commonly used with ampicillin‐clavulanate at a maximal daily dosage of 1500 to 1875 mg. When analyses were performed according to oral antibiotic regimen, we observed no significant impact of quinolones treatment alone versus quinolones in combination with other antibiotics ('post‐protocol' analysis, Analysis 4.2).
Funnel plot asymmetry
No significant heterogeneity was found in any of the outcomes evaluated (Figure 3, Figure 4).
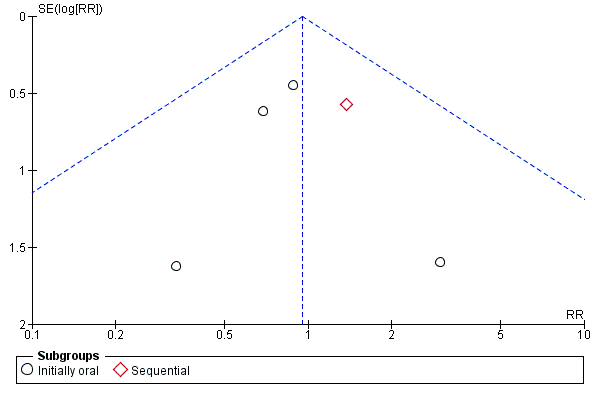
Funnel plot of comparison: 1 Oral versus intravenous antibiotic therapy, outcome: 1.1 Mortality.
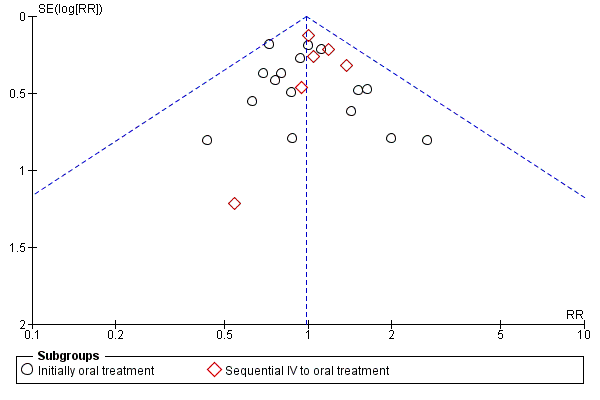
Funnel plot of comparison: 1 Oral versus intravenous antibiotic therapy, outcome: 1.3 Treatment failure ‐ per protocol analysis.
Discussion
The rates of treatment failure and mortality were not statistically significantly different in neutropenic patients given oral and intravenous antibiotic treatments. The RR for treatment failure in patients treated with oral antibiotics was 0.95. The RR for a fatal outcome was 0.95. No significant heterogeneity was shown for both comparisons. ITT analysis might favour equivalence, however the results of the per protocol analysis were similar to those of the primary analysis.
These effects were stable in a range of patient subgroups. The RR was similar across different case definitions of the underlying disease and the cause of fever. The RR did not depend on the age of the patients or on the antibiotic regimen. It was similar in studies that started patients on immediate oral treatment and in those that switched to oral treatment after a short time of intravenous treatment. Introduction of bias by inadequate randomisation or allocation concealment was shown to be unlikely by the sensitivity analysis. There was a trend toward more adverse effects in patients given oral treatment. However the majority were gastrointestinal complaints that did not necessitate discontinuation of therapy.
One limitation of the analysis is its inability to define the patients who may be offered oral antibiotics. This is due to the variations in the inclusion and exclusion criteria of the included trials. The difference in low risk criteria is not surprising, the concept of low risk neutropenic fever and its definition developed during the years in which the studies were performed. Different prognostic criteria evolved based on observational studies. An international collaboration had led to the development of a validated weighted scoring system identifying low risk patients, adopted by the Infectious Diseases Society of America (IDSA) (Chamilos 2005; Hughes 2002; Klastersky 2000; Klastersky 2006). Only one of the trials added in the current version (Sebban 2009*) incorporated that scoring system as an inclusion criterion.
Exclusion criteria that were common to all studies were criteria defining severe sepsis (mainly haemodynamic instability and organ failure: altered mental status, respiratory failure, renal and liver abnormalities), inability to swallow or take oral medication, allergy to study drugs, pregnancy and lactation. Most studies did not include patients with acute leukaemia and about half excluded patients with pneumonia, severe cellulitis or intravascular infection.
The low mortality rate led to wide CIs in the absolute risk reduction. To confirm equivalence of two treatments, we should ideally be able to show that any estimate of risk that is included within the CI lies within a predefined range of equivalence (as the CI of the effect of the recommended treatment) and has no clinical significance (Jones 1996). In a population with an expected mortality of less than 1% this uncertainty may have no real consequences.
For treatment failure the CI is narrower and probably has no clinical importance since failure means mainly a change in the antibiotic regimen.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Oral versus intravenous antibiotic therapy, outcome: 1.1 Mortality.

Funnel plot of comparison: 1 Oral versus intravenous antibiotic therapy, outcome: 1.3 Treatment failure ‐ per protocol analysis.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 1 Mortality.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 2 Treatment failure.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 3 Treatment failure ‐ per protocol analysis.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 4 Adverse events requiring discontinuation of antibiotics.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 5 Gastrointestinal adverse events ('post‐protocol' analysis).

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 6 Lost to follow‐up.

Comparison 1 Oral versus intravenous antibiotic therapy, Outcome 7 Treatment failure not dt modification in update.

Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 1 Treatment failure ‐ age.

Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 2 Treatment failure ‐ source of infection.

Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 3 Treatment failure ‐ severity of neutropenia.

Comparison 2 Oral versus intravenous antibiotic therapy ‐ subgroup analysis, Outcome 4 Treatment failure ‐ type of malignancy.

Comparison 3 Methodological quality of studies, Outcome 1 Allocation concealment.

Comparison 4 Post hoc subgroup analyses, Outcome 1 Setting.

Comparison 4 Post hoc subgroup analyses, Outcome 2 Type of oral antibiotics.
| Oral compared to intravenous antibiotic therapy for febrile neutropenia in cancer patients | ||||||
| Patient or population: patients with febrile neutropenia in cancer patients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| intravenous antibiotic therapy | Oral | |||||
| Mortality | Study population | RR 0.95 | 1392 | ⊕⊕⊕⊝ | ||
| 32 per 1000 | 30 per 1000 | |||||
| Low risk | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment failure | Study population | RR 0.96 | 3142 | ⊕⊕⊕⊝ moderate1 | ||
| 284 per 1000 | 272 per 1000 | |||||
| Moderate | ||||||
| 211 per 1000 | 203 per 1000 | |||||
| Treatment failure ‐ per protocol analysis | Study population | RR 0.98 | 2912 | ⊕⊕⊕⊝ | ||
| 225 per 1000 | 221 per 1000 | |||||
| Moderate | ||||||
| 184 per 1000 | 180 per 1000 | |||||
| Adverse events requiring discontinuation of antibiotics | Study population | RR 1.45 | 1823 | ⊕⊕⊝⊝ low1, 2 | ||
| 21 per 1000 | 31 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Treatment failure not dt modification in update | Study population | RR 0.95 | 3041 | ⊕⊕⊕⊝ | ||
| 267 per 1000 | 254 per 1000 | |||||
| Moderate | ||||||
| 180 per 1000 | 171 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 High risk of detection bias in most of the trials 2 A wide CI | ||||||
| Common criteria |
| Haemodynamic stablity |
| No organ failure |
| Ability to take oral medications |
| No pneumonia |
| No infection of a central line |
| No severe soft‐tissue infection |
| No acute leukaemia as the background malignancy |
| No known drug allergy |
| Not pregnant or lactating women |
| Study ID | Evident infection | Previous AB | Prolonged neutropeni | Performance status | Active malignancy | BMT/PSCT | Other |
| Kern 1999 | Infected catheter or CNS infection, known bacterial /viral/fungal infection | yes | yes | no | no | yes | Need of IV supportive therapy, expected to die within 48 hours, HIV, fever unrelated to infection and protocol violation |
| Mullen 1999 | A source of infection that required hospitalisation as: tunnelitis, pneumonia, perirectal cellulitis, typhlitis, resistant microorganism to one of the study's drugs | no | no | no | yes | yes | >10% dehydration, bleeding requiring platelet transfusion, |
| Paganini 2000 | Infected catheter, perineal/ facial cellulitis, uncontrolled local infection, positive blood cultures at 72 hours | no | no | no | yes | yes | Persistance of fever >48 hours, incorrectable bleeding; refractory hypoglycemia or hypocalcemia |
| Rubenstein 1993 | Known resistant microorganism | no | no | no | no | no | Na<128, uncontrolled hypercalcemia, more than 30 miles away |
| Samonis 1997 | Pneumonia, deep organ infection | yes | yes | no | yes | no* | Prior hospitalisation |
| Shenep 2001 | Pneumonia, clinical or radiographic evidence of focal bacterial infection, severe mucositis, positive blood cultures at 48 hours | no | no | no | no | no | MRSA or P.Aeroginosa in any culture obtained in preceding 12weeks |
| Velasco1995 | Meningitis, pyelonephritis | yes | no | yes | no | no* | Long term central vein catheter |
| Petrilli 1999 | no | no | no | no | no | no* | |
| Flaherty 1989 | no | yes | no | no | no | no | |
| Freifeld 1999 | Intravascular infection, tunnelitis, pneumonia, neurologic symtoms, | no | yes | no | no | yes | Treatment with Ca‐Mg or probenecid or alluporinol or theophylline, HIV |
| Giamarelou 2000 | Suspected anaerobes | no | no | yes | no | no | Moribund and high probability of dying within 48 hours |
| Hidalgo 1999 | Pneumonia, extensive cellulitis, meningitis, pyelonephritis | no | no | yes | yes | no | Clotting abnormalities, acidosis, hypercalcaemia, uncontrolled bleeding, live >2h apart from hospital; Hx of tumour fever, other severe extra hematologic chemotherapy induced toxicity, no 24 hours home companion |
| Innes 2003 | Tunnelitis, cellulitis, abcess, clinically documented infection likely to require prolonged antibiotic therapy | yes | yes | no | no | yes | Need for the use of G/GM‐CSF and cytokines; no responsible adult living with them (carer); |
| Malik 1992 | no | yes | no | no | no | no | Recurrent FUO |
| Cornely 2003 | not excluded | excluded (except cotrimoxazole prophylaxis) | yes | yes | excluded | excluded | potential compromised absorption; inability to take oral medication; tenopathy, epilepsy; aplastic anaemia, acute leukaemia; septic shock or signs of sever infection; HIV carrier; serious concomitant disease, liver transaminase> x5 of norm. |
| Niho 2004 | not excluded | excluded | no | not excluded | no | yes | Recurrent FUO; renal insufficiency; hepatic insufficiency; hypotension or peripheral circulatory failure; uncontrolled hypercalcaemia; altered sensorium; respiratory rate >30 breaths/min; serum sodium <128 mg/dl; inability to take oral medications; intestinal malabsorption |
| Paganini 2003 | Fascial, perineal, or catheter‐associated cellulites; uncontrolled local infection; positive blood cultures within the first 48 hours; infection with microorganisms known as resistant to ceftriaxone or ciprofloxacin | included | yes | not excluded | not excluded | excluded | severe comorbidity factors; respiratory failure |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 9 | 1392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.68] |
| 1.1 Initially oral | 6 | 961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.62] |
| 1.2 Sequential | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.45, 4.22] |
| 2 Treatment failure Show forest plot | 22 | 3142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
| 2.1 Initially oral treatment | 16 | 2196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.03] |
| 2.2 Sequential IV to oral treatment | 6 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.27] |
| 3 Treatment failure ‐ per protocol analysis Show forest plot | 22 | 2912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 3.1 Initially oral treatment | 16 | 1991 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
| 3.2 Sequential IV to oral treatment | 6 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.29] |
| 4 Adverse events requiring discontinuation of antibiotics Show forest plot | 15 | 1823 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.61, 3.46] |
| 4.1 Initially oral treatment | 10 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.14, 6.75] |
| 4.2 Sequential IV to oral treatment | 5 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.25] |
| 5 Gastrointestinal adverse events ('post‐protocol' analysis) Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Initially oral treatment | 11 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [2.87, 7.04] |
| 5.2 Sequential IV to oral treatment | 4 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [1.03, 7.66] |
| 6 Lost to follow‐up Show forest plot | 19 | 2810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.10] |
| 7 Treatment failure not dt modification in update Show forest plot | 21 | 3041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.06] |
| 7.1 Initially oral treatment | 15 | 2095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 7.2 Sequential IV to oral treatment | 6 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Treatment failure ‐ age Show forest plot | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Children | 8 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 1.2 Adults | 12 | 1652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.12] |
| 2 Treatment failure ‐ source of infection Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Unexplained fever | 10 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.33] |
| 2.2 Documented infection | 10 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 3 Treatment failure ‐ severity of neutropenia Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Absolute neutrophil count >=10^9/L | 3 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| 3.2 Absolute neutrophil count <10^9/L | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.49] |
| 4 Treatment failure ‐ type of malignancy Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Solid tumour | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.12] |
| 4.2 Haemetologic malignancy | 4 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Allocation concealment Show forest plot | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adequate (A) | 12 | 1651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 1.2 Unclear (B) | 10 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Setting Show forest plot | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oral‐outpatient, IV‐inpatients | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.43] |
| 1.2 Inpatients | 6 | 1128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
| 1.3 Outpatients | 7 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.85, 1.50] |
| 1.4 Only first dose in | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.34] |
| 2 Type of oral antibiotics Show forest plot | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quinolones only | 7 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 2.2 Quinolones in combination with augmentin, ampicillin‐sulbactam, penicillin V or clindamycin | 11 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 2.3 Cefixime | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.64, 1.56] |
| 2.4 New quinolones | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.50, 1.86] |

