Sulfato de magnesio inhalado en el tratamiento del asma aguda
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, controlled, double blind study, 2 groups. 1 centre in Tenerife. | |
| Participants | 24 patients (Intervention 13, Control 11), adults, acute asthma, moderate obstruction. | |
| Interventions | Intervention: 2 mL of MgSO₄ (isotonic) dose and 400 mcg of salbutamol (delivery probably by MDI). Control: 2 mL of a physiological serum of an inhaled form, 400 mcg of salbutamol (delivery probably by MDI). Nebuliser: no details. | |
| Outcomes | FEV1 and PEF at 0, 15, 30 45 minutes. | |
| Notes | Funding: Gobierno Autonomo Canarias. Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details but stated as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only and not all time points reported. |
| Methods | Double blind, randomised controlled trial, parallel. 1 emergency department in India. | |
| Participants | Inclusion criteria: participants aged 13 to 60, BTS definition acute asthma (PEF and clinical features). Exclusion criteria: first episode of wheeze, chronic bronchitis or emphysema, heart failure, angina, renal failure, temperature > 38 ºC, ET tube required, no consent, pregnancy, failure to do peak flow. Intervention: 50 randomised. Mean age (years): 46.26 (13.96). Men:women: 27:23. Acute severe: 29. Acute life threatening: 21. Smokers: 9. Baseline PEF: 118.6 (41.3). Duration of attack; days (SD) 4.16 (1.69). Control: 50 randomised. Mean age (years): 41.00 (16.66). Men: women: 33:17. Acute severe: 30. Severe life threatening: 20. Smokers: 5. Baseline PEF: 111.6 (43.3). Duration of attack; days (SD) 4.28 (1.99). | |
| Interventions | Intervention: MgSO₄ (1 mL of 500 mg/mL MgSO₄) and salbutamol (1 mL of salbutamol) 8 mL distilled water – 295 mOsmol/kg ×3 in an hour. Control: salbutamol 1 mL, 1.5 mL distilled water, 7.5 mL normal saline – 287 mOsmol/kg ×3 in an hour. Treatment over 1 h; 3 nebulisers 20 minutes apart. Follow‐up for 20 minutes. Ultrasonic nebuliser. | |
| Outcomes | PEF, heart rate, systolic pressure, diastolic pressure, time in ED, blood gases (O₂ and CO₂— 0 and 120 minutes), magnesium levels (0 and 120 minutes). Time points 0, 15, 60, 75, 120 minutes. | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Separate envelopes to ensure concealment until inclusion (where they were kept and whether tamper proof — not mentioned). |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) | Low risk | The 2 researchers were blinded to the treatments so measurements (normal clinical outcomes) remained blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 50 participants both sides at beginning and 50 participants both sides completed the study with full outcome data. |
| Selective reporting (reporting bias) | Low risk | Follow‐up data and longer‐term outcome data not collected. No apparent indication of selective reporting. |
| Methods | Randomised open controlled trial. 1 hospital in Bangladesh. | |
| Participants | Inclusion criteria: severe acute asthma. Exclusion criteria: none stated. 120 randomised. Intervention: 60 randomised. Control: 60 randomised. | |
| Interventions | Intervention: salbutamol with MgSO₄. Control: salbutamol with normal saline. | |
| Outcomes | PEF, respiratory rate, pulse rate, systolic, diastolic blood pressure, adverse effects. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details but states randomized. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear how many participants completed the trial or if any were excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract. no prospective trial registration identified, no outcome measures pre‐specified. |
| Methods | Double‐blind, randomised controlled trial. 1 Paediatric emergency centre, Qatar. | |
| Participants | Inclusion criteria: moderate/severe asthma exacerbation, age 2‐14 years, previous diagnosis of asthma. Exclusion criteria: prematurity, critical illness needing ICU admission for IV bronchodilator, NIV or invasive ventilation, transfer to other institution, history of hypersensitivity to MgSO₄, history of neuromuscular/cardiac/renal disease, underlying structural lung disease, received systemic steroid/theophylline/ipratropium in prior 72 h, consolidation on chest XR, received IV MgSO₄ before randomisation, prior participation in the study, haemodynamic instability. Number randomised: 400. Intervention: 208 randomised. Mean age (years): 5.6 (3.1). Male:female: 133:75. Moderate:severe: 168:40. Mean baseline asthma severity score: 7.6 (1.3). Control: 192 randomised. Mean age (years): 5.8 (3.1). Male:female: 115:77. Moderate:severe: 163:29. Mean baseline asthma severity score: 7.5 (1.3). | |
| Interventions | Intervention: 800 mg MgSO₄ (15 mL). Control: 15 mL 0.9% NaCl. Medication divided into 3 doses over 1 h. Jet nebuliser. | |
| Outcomes | Time to medical readiness for discharge, mean asthma severity score (4, 8, 12, 24, 36, 48 h), mean asthma severity score at discharge, need for revisit or readmission (2 weeks). Adverse events: chest tightness and facial rash (1; intervention group). Excessive cough (1; control group). ICU admission (1; control group). | |
| Notes | Funding: Hamad Medical Corporation; Number: 12095/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation without blocks. |
| Allocation concealment (selection bias) | Low risk | Randomisation list provided to pharmacy resulted in preparation of identical‐appearing sealed numbered vials. |
| Blinding of participants and personnel (performance bias) | Low risk | All study personnel were blinded to treatment. |
| Blinding of outcome assessment (detection bias) | Low risk | All study personnel were blinded to treatment. The paper was not explicit re. outcome assessors ‒ they were assumed to also have been blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Over 90% completed the trial in both arms. Balanced number were excluded from each arm, with reasons given. |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration. All listed outcomes are reported. |
| Methods | Parallel 1 Children’s Assessment Unit, 1 hospital (UHW). | |
| Participants | Inclusion criteria: age range 2 to 16 years, acute severe asthma. Exclusion criteria: chronic lung disease, congenital heart disease, unable to understand English. 17 randomised (8 boys). Intervention: 7 completed. Control: 10 completed. | |
| Interventions | Intervention: 2.5 mL isotonic MgSO₄ (3 occasions at 20‐minute intervals), salbutamol and ipratropium bromide. Control: 2.5 mL isotonic saline (3 occasions at 20‐minute intervals), salbutamol and ipratropium bromide. 3 dosages over 1 h: follow‐up for 240 minutes. | |
| Outcomes | Asthma severity scores (ASS), the sum of wheeze, accessory muscle use and heart rate, were computed on 6 occasions over 4 h. The primary endpoint was the area under the curve of the ASS at the 6 time points for each child. | |
| Notes | Funding: local R and D pilot funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by pharmacy at source ‒ in ED as sequential vials (code in pharmacy). |
| Allocation concealment (selection bias) | Low risk | As above – absolute concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Trial described as double blind: as above. |
| Blinding of outcome assessment (detection bias) | Low risk | Trial described as double blind: as above. |
| Incomplete outcome data (attrition bias) | Low risk | All data collected for the 17 patients. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Outcomes partially reported. |
| Methods | Randomised controlled trial. Outpatient department and Emergency department from 1 hospital, Egypt. | |
| Participants | Inclusion criteria: pregnancy, acute exacerbation of asthma partially or not completely controlled on routine acute asthma therapy. Exclusion criteria: congestive heart failure, history of angina, renal problems, history suggestive of pulmonary oedema, very severe asthma (altered consciousness, respiratory acidosis, needing intubation, arrest), any associated medical illness e.g. diabetes/hypertension, fever > 38°C, inability to perform PEF. Number randomised: 60. All participants female. Intervention: 30 randomised. Mean age (years): 25.7 (3.8). Control: 30 randomised. Mean age (years): 25.9 (4.0). | |
| Interventions | Intervention: 500 mg (1 mL) MgSO₄ with 1 mL salbutamol solution and 8 mL 0.9% NaCl. Control: 1 mL salbutamol solution with 9 mL 0.9% NaCl. Treatments given over 8 minutes; max 3 sets of nebulisation 20 minutes apart. | |
| Outcomes | PEF, FEV1, FVC, FEV1/FVC ratio, FEF 25‐75%, arterial blood pCO2, pO2 and pH, oxygen saturations, serum potassium. Recorded at end of therapy ‒ assumed to be 2 h from baseline. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly classified into groups comparable in socio‐demographic criteria, but no indication is given of random sequence generation. Baseline clinical characteristics are given, and there is no indication that the groups were balanced with regard to clinical criteria. |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomized into 2 groups through sealed opaque envelopes, but no indication is given whether participants or research personnel were aware of group allocation. |
| Blinding of participants and personnel (performance bias) | High risk | Participants were randomized into 2 groups through sealed opaque envelopes, but no mention of procedures to blind personnel. |
| Blinding of outcome assessment (detection bias) | High risk | No mention of procedures to blind personnel. |
| Incomplete outcome data (attrition bias) | Unclear risk | On further correspondence, appropriate exclusion criteria were applied but no indication given whether excluded participants were balanced across groups, and no dropout data were given. |
| Selective reporting (reporting bias) | High risk | No prospective trial registration identified. Primary outcome on which this trial was powered is not stated. On further correspondence, adverse event data were given but no clinical baseline characteristics are given. |
| Methods | Design: parallel randomised controlled trial. | |
| Participants | Location: 1 university hospital in Brooklyn, NY. | |
| Interventions | Treatment: albuterol 2.5 mg/3 mL nebule followed by 384 mg isotonic MgSO₄ every 20 min × 3. | |
| Outcomes | Measured FEV1 every 20 minutes for 2 h. | |
| Notes | Funding: supported by an unrestricted educational grant from Astra Pharmaceutical Company; no Astra Pharmaceutical Company products were used in the study. Mouthpieces for the spirometer were supplied at no charge from Mallinkrodt Nellcor Puritan Bennett. Circulaire nebulizers were supplied by Westmed Inc. at a reduced rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An assigned third party randomised participants by means of a computer‐generated random table (1:1 randomisation) to either the treatment or control group. |
| Allocation concealment (selection bias) | Low risk | An assigned third party randomised participants by means of a computer‐generated random table (1:1 randomisation) to either the treatment or control group. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded, placebo‐controlled. A log of the identification number and specific treatment of each participant was kept and remained closed to the investigators until the completion of the study. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded, placebo‐controlled. A log of the identification number and specific treatment of each participant was kept and remained closed to the investigators until the completion of the study. Outcomes were assessed every 20 minutes for 2 h. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts: 3 in each group. Albuterol plus normal saline solution (3 unable to complete spirometry); and albuterol plus magnesium (2 inappropriate randomisation, 1 unable to perform spirometry). |
| Selective reporting (reporting bias) | High risk | Mean values only given for FEV1, no SDs and the text reports that there were no statistically significant differences in FEV1 between the groups. The text also states "The analysis of continuous safety variables (BP, pulse rate, respiratory rate, oxygen saturation, and serum magnesium concentrations) did not demonstrate any clinically or statistically significant differences between the 2 groups at any point during the study." |
| Methods | Random allocation into 3 groups parallel study. | |
| Participants | Location: 1 emergency department teaching hospital in India. Acute severe asthma , PEF < 50%. Group A = 24 Group B = 26 Group C = 21 | |
| Interventions | Group A: salbutamol; Group B; salbutamol and MgSO₄; Group C MgSO₄ alone; no details on dose or frequency. | |
| Outcomes | FEV1, FVC, FEV1/FVC, PEF, "Vital parameters" | |
| Notes | 2 abstracts only (the same). Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only and no data reported except there was a significant improvement in groups B and C compared to group A. |
| Methods | Parallel. | |
| Participants | A total of 110 participants. | |
| Interventions | Intervention: received the control treatment with the addition of 150 mg of MgSO₄ (0.3 mL of 50% MgSO₄ heptahydrate) to each nebulised dose of medication. Control: received nebulised treatments of albuterol sulfate 0.5% (5 mg/mL) combined with 0.5 mg of ipratropium bromide 0.02% inhalation solution (Atrovent). | |
| Outcomes | Vital signs and peak flow measurements were also assessed at the end of each treatment (a maximum of 3 treatments) and just prior to discharge. A 24‐hour follow‐up call was made to each participant, during which peak flow measurements were again obtained. | |
| Notes | Abstract only. Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no detail. |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised but no detail. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double blind but no detail. |
| Blinding of outcome assessment (detection bias) | Low risk | Described as double blind but no detail. |
| Incomplete outcome data (attrition bias) | Unclear risk | Very limited information ‒ impossible to judge. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Vital signs are mentioned as being recorded but are not reported. |
| Methods | RCT, parallel | |
| Participants | Inclusion criteria: adults, >18 years in the emergency dept with asthmatic crisis, FEV1 < 60% predicted. Exclusion criteria: smokers, those with ambulatory use of systemic steroids, with associated co‐morbidities (neuropathy, nephropathy, heart disease, liver disease), fever at admission, use of dietary supplements with MgSO₄, irreversible airway obstruction (persistent abnormal spirometry), near‐fatal asthma, requirement of endotracheal intubation at admission, anatomic abnormalities of the bronchial tree (bronchiectasis, tuberculosis), history of pulmonary or thoracic surgery, hypersensitivity to MgSO₄, and pregnancy or breastfeeding. Location: National Institute of Respiratory Diseases, a tertiary care teaching hospital and national referral centre in Mexico City. Date of study: June 2008 to March 2009. Intervention: 60 randomised, 30 completed. Mean age (years): 34.3 (12.4). Men:women: 9:21. Control: 52 randomised, 30 completed. Mean age (years): 40.3 (11.6). Men:women: 9:21. | |
| Interventions | Each nebulisation lasted 20 mins. Intervention: standard nebulisation but diluted with 3 mL (333 mg) of 10% isotonic MgSO₄ (Magnefusin PISA, Guadalajara, Mexico; 1 g/10 mL). Also received 125 mg of IV methylprednisolone. Control: 1 IV dose of 125 mg methylprednisolone and nebulisation with 7.5 mg of albuterol and 1.5 mg of ipratropium bromide in 3 divided doses. Standard nebulisation diluted in 3 mL of isotonic saline solution (SS) as placebo. | |
| Outcomes | FEV1 post‐BD (absolute in litres and as percentage of predicted), clinical improvement, oxygen saturation, admission to the ED, admission to the asthma ward, hospital readmissions. At 30‐min post‐nebulisation, patients were clinically and functionally re‐evaluated. Also evaluated at 30 days. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Low risk | After randomisation, diluents were prepared by a physician outside the study who was not responsible for the participants’ care and only had control of the pre‐filled syringes. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind. Both diluents are odourless, tasteless and colourless to the eye and did not differ when transparency was measured. |
| Blinding of outcome assessment (detection bias) | Low risk | The physician responsible for the participants’ care along with the nurse and respiratory therapist were blinded to the type of treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Reasons given for dropouts in both groups in the CONSORT diagram. It seems as though there are a high percentage of dropouts but the majority are post‐randomisation exclusions based on exclusion criteria. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods section are reported. Best judgement with no access to trial protocol. |
| Methods | Parallel RCT. | |
| Participants | Age: 18 to 60 years. Location: emergency department of a tertiary referral centre in India. Acute asthma and FEV1 < 30% predicted. Intervention: 30. Control: 30. | |
| Interventions | Intervention: nebulised similarly using isotonic MgSO₄ (3 mL of 3.2 g%) as a vehicle ‒ unsure if this is “Nebulized salbutamol and ipratropium”. Control: nebulised salbutamol and ipratropium using isotonic saline as a vehicle thrice at 20‐min intervals. | |
| Outcomes | FEV% predicted at 120 minutes, pooled discharge rate proportion of groups attaining PEF > 60% predicted and relief in dyspnoea at 30, 60, 90, 120 min). | |
| Notes | Abstract only. Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Single blind – no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single blind – no further details. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | High risk | 1 outcome partially reported and not significant. Abstract only. |
| Methods | Double blind, randomised controlled trial. 34 emergency departments, UK. | |
| Participants | Inclusion criteria: severe (BTS/SIGN quantified) asthma attack, age ≥16 years. Exclusion criteria: life‐threatening features, contraindication to MgSO₄, participant unable to give verbal/written consent, previous participation in the study; criteria amended to exclude those who had received MgSO₄ in the past 24 h. 1109 randomised. Intervention 1 (nebulised MgSO₄): 339 randomised. Mean age (years): 36.5 (14.8). Men:women: 107:232. Smokers: 98. Mean predicted PEF (L/min): 430 (118.8). Intervention 2 (intravenous MgSO₄): 406 randomised. Mean age (years): 35.6 (13.1). Men:women: 130:279. Smokers: 138. Mean predicted PEF (L/min): 431.8 (116.9). Control: 364 randomised. Mean age (years): 36.4 (14.1). Men:women: 112:252. Smokers: 127. Mean predicted PEF (L/min): 435.0 (110.8). | |
| Interventions | Intervention 1: 100 mL 0.9% NaCl IV and 2 mmol MgSO₄ in 7.5 mL 0.9% NaCl nebulised. Intervention 2: 8 mmol MgSO₄ in 100 mL 0.9% NaCl IV and 7.5 mL 0.9% NaCl nebulised. Control: 100 mL 0.9% NaCl IV and 7.5 mL 0.9% NaCl nebulised. IV infusion given once over 20 mins, nebulisers given 3 times, each over 20 minutes. | |
| Outcomes | Admission (4 h, 7 days); change in participant's assessment of breathlessness via visual analogue scale, change in PEF, heart rate, respiratory rate, BP, oxygen saturations (1, 2 h); adverse events (2 h); mortality, length of hospital stay, admission to HDU or ICU. Adverse events: treatment group 41 adverse events; control group 36 adverse events. | |
| Notes | Funding: UK National Institute for Health Research Health Technology Assessment Programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple and blocked randomisation sequences used to allocate participants to numbered treatment packs. |
| Allocation concealment (selection bias) | Low risk | Allocated treatment pack numbers were only revealed after participant details recorded and the participant irreversibly entered into the trial. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, hospital staff, and research staff were masked to allocated treatment. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, hospital staff, and research staff were masked to allocated treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Over 95% of randomised participants in each arm were included in primary analysis. All participants clearly accounted for in flow diagram. There was an inevitable 'drop off' in participants available at each time point for many of the secondary outcomes; it is unclear what impact this may have had on the results. |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration identified. All primary and secondary outcomes listed in the trial register were reported. |
| Methods | Double blind, randomised controlled trial. 2 emergency departments, Iran. | |
| Participants | Inclusion criteria: moderate/severe asthma exacerbation defined by PEFR < 40% to 69% predicted or limiting speech/normal activity, age > 16 years. Exclusion criteria: need for immediate intubation, significant impairment of heart function, kidney or liver disease, fever > 38.3 °C, chronic lung disease, pregnancy, lactation, pneumonia. 50 randomised. Intervention: 25 randomised. Mean age (years): 52.4 (16.9). Men:women: 11:14. Acute moderate: 3. Acute severe: 22. Mean predicted PEF (%): 15.1 (4.7). Control: 25 randomised. Mean age (years): 53.9 (16.2). Men:women: 14:11. Acute moderate: 3. Acute severe: 21 Mean predicted PEF (%): 14.7 (6.4). | |
| Interventions | Intervention: 3 mL MgSO₄ solution (260 mmol/L) nebulised. Control: 3 mL 0.9% NaCl nebulised. Nebulised medication given every 20 to 60 minutes. | |
| Outcomes | Predicted PEFR (%), oxygen saturations, respiratory rate, dyspnoea severity index (20, 60 minutes); need for admission, serious side‐effect rate (60 minutes). Adverse effects: no "serious side‐effects" reported. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation software used. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) | Low risk | Both patients and investigators were blinded to the content of identical treatment vials. |
| Blinding of outcome assessment (detection bias) | Low risk | Both patients and investigators were blinded to the content of identical treatment vials. |
| Incomplete outcome data (attrition bias) | Unclear risk | The report does not state that all 25 randomised participants in each arm completed the trial, but as the trial finished at 60 mins is it likely that they did. |
| Selective reporting (reporting bias) | Unclear risk | Trial registered while recruiting. Pre‐specified primary and secondary outcomes are reported; note omission of PEFR/dyspnoea scale reporting at 40 mins and no data given to support report of "no treatment‐related complications". Clear mistakes in reporting of vital signs. |
| Methods | Design: parallel randomised controlled trial. | |
| Participants | Location: 2 university hospitals in New Zealand. | |
| Interventions | Standard of care: salbutamol 2.5 mg nebulised ×1 or more, hydrocortisone 100 mg IV at presentation. | |
| Outcomes | Measured at baseline and after each treatment (every 30 min ×3): FEV1, % predicted FEV1, BP, heart rate, O₂ saturation. | |
| Notes | Funding: the study was funded by a research grant from the University of Otago. The study sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to their treatment groups in accordance with the allocation sequence determined by the hospital pharmacy. |
| Allocation concealment (selection bias) | Unclear risk | No information. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded, placebo‐controlled. Participants and investigators were unaware of treatment allocation through provision by the hospital pharmacy of pre‐prepared identical unmarked syringes containing the study drug. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded, placebo‐controlled. Participants and investigators were unaware of treatment allocation through provision by the hospital pharmacy of pre‐prepared identical unmarked syringes containing the study drug. Outcomes assessed every 30 minutes. |
| Incomplete outcome data (attrition bias) | Low risk | 6 in total. MgSO₄ (1 COPD, 1 pneumonia). Saline (3 COPD, 1 pneumonia). |
| Selective reporting (reporting bias) | High risk | The primary outcome, FEV1, was fully reported but other outcomes were not. "The change in blood pressure and heart rate did not differ between the two groups. No clinically significant adverse events were reported." |
| Methods | Parallel RCT. | |
| Participants | Location: authors based in Iran. Participants: 40 asthmatic children in total between 2 groups. Mean age: 3.55 years. | |
| Interventions | Intervention: nebulised salbutamol, as a vehicle isotonic MgSO₄ mixed with salbutamol. Control: nebulised salbutamol, as a vehicle 2.5 mL of normal saline. | |
| Outcomes | Days of hospital stay, hours of need for oxygen, respiratory distress. Measured 1 h before and 1 h after the second course of treatment. | |
| Notes | Abstract only. Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly enrolled. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Double blind. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Low risk | Outcomes stated as measured, reported. Abstract only. |
| Methods | Parallel RCT | |
| Participants | Inclusion criteria: moderate to severe asthma attacks, 18 to 60 years. Exclusion criteria: patients with febrile disease, diabetes, congestive heart failure, atherosclerotic heart disease, intractable hypertension, chronic obstructive lung disease, renal and hepatic failure and arrhythmia were excluded from the study. Pregnant and breast‐feeding women, patients who had already taken theophylline, antihistaminics, and systemic steroids in the previous 24 h, who had acute or chronic respiratory failure, who had been on long‐term oxygen therapy, and a history of allergy to salbutamol and MgSO₄ have been excluded as well. Location: emergency department, Turkey. Intervention: 14. Mean age: 46.43 (years) (3.31) range 18 to 3. Men:women: 4:10. Control: 12. Mean age: 37.83 (years) (9.26) range 20 to 52. Men:women: 3:9. | |
| Interventions | Every 20 mins for first hour and every hour for the rest of 4 h. Intervention: isotonic MgSO₄ (2.5mL) + salbutamol (2.5 mL). Control: salbutamol (2.5 mL) + saline (2.5 mL). | |
| Outcomes | PEF, clinical scores, discharge rates, admission rates. 20th, 60th, 120th, 180th, 240th minute (180 and 240 not compared as most patients completed study in 2 h). | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised ‒ details of sequence generation not included in trial report. |
| Allocation concealment (selection bias) | Unclear risk | Information not available in trial report. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Single blind – no further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Single blind – no further details. |
| Incomplete outcome data (attrition bias) | Unclear risk | Information provided in trial report on discharges from both groups up to 240 minutes. |
| Selective reporting (reporting bias) | Low risk | No apparent indication of selective reporting. |
| Methods | Design: parallel randomised controlled trial. | |
| Participants | Location: 1 paediatric emergency department in Detroit, Michigan. | |
| Interventions | Treatment: albuterol 2.5 mg nebule with 2.5 mL isotonic MgSO₄ (6.3% solution); 1 dose. | |
| Outcomes | Lung function (FEV1 and % predicted FEV1) at baseline, then at 10 and 20 minutes after treatment. | |
| Notes | Funding: this work was funded by an unrestricted grant from the Division of Pediatric Emergency Medicine, Children’s Hospital of Michigan, Detroit, Michigan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used to provide randomisation and this was performed by a senior research pharmacist at the institution. |
| Allocation concealment (selection bias) | Low risk | A table of random numbers was used to provide randomisation and this was performed by a senior research pharmacist at the institution. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded, placebo controlled. The study medications were provided in identical syringes and both the pharmacy and the investigator were blinded to their contents. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded, placebo controlled. The study medications were provided in identical syringes and both the pharmacy and the investigator were blinded to their contents. Outcomes assessed at 10 and 20 minutes after treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | None described. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section are reported. |
| Methods | Design: parallel randomised controlled trial. | |
| Participants | Location: emergency department, St John's Medical College Hospital, India. | |
| Interventions | Standard of care: hydrocortisone 100 mg IV. | |
| Outcomes | Clinical score: Fischl Index, clinical examination. Pulmonary function: PEF. Vitals: respiratory rate, heart rate, BP, pulsus paradoxus. Admission rates, vital signs. Adverse events/side effects:
| |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind, placebo‐controlled. Outcomes assessed at 20 minute intervals. |
| Incomplete outcome data (attrition bias) | Unclear risk | None described. |
| Selective reporting (reporting bias) | Unclear risk | Pulsus paradoxus and BP are mentioned but not reported, but pulsus paradoxus is included as part of the Fischl index. |
| Methods | Design: randomised controlled trial. | |
| Participants | Location: Department of Paediatric Asthma of Ege University Hospital, Turkey. | |
| Interventions | Treatment: MgSO₄ 2 mL (280 mmol/L, 258 mOsm, pH 6.7). | |
| Outcomes | Evaluations at: 5, 15, 30, 60, 180,240 and 360 minutes. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly selected for the study and divided into 2 groups. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | None described. |
| Selective reporting (reporting bias) | High risk | No statistical differences were found between the groups for respiratory rate, heart rate and BP. It is also unclear as to the time point reported as although 5 minutes was prespecified, there were also several other time points specified and only the maximum values were presented. |
| Methods | Randomised controlled trial. 1 hospital, Iran. | |
| Participants | Inclusion criteria: moderate to severe asthma (GINA‐defined) with acute attack. Exclusion criteria: corticosteroid therapy, steroid/theophylline/ipratropium in past 72 h, chronic lung disease e.g. bronchopulmonary dysplasia/CF, allergy to MgSO₄ or salbutamol, not co‐operative. 80 randomised. Intervention 1 (nebulised MgSO₄): 40 randomised. Mean age (years): 9 (2.2). Male:female: 10:30. Control: 40 randomised. Mean age (years): 8.5 (2.4) Male:female: 17:23. | |
| Interventions | Intervention: 3 mL 7.5% MgSO₄, 0.15 mg/kg salbutamol. Control: 3 mL normal saline, 0.15 mg/kg salbutamol. 3 doses at 20 minute intervals. | |
| Outcomes | Pulmonary index, PEFR, adjusted PEFR at 30, 60 and 90 minutes | |
| Notes | Funding: Babol University of Medical Sciences ‒ Research and Technology Institute. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "divided into two groups randomly" but no details given. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double‐blind" in prospective trial registration but no details given. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double‐blind" in prospective trial registration but no details given. |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered; planned outcomes were fully reported. |
| Methods | Design: randomised controlled trial. | |
| Participants | Location: emergency departments in 4 Argentinian hospitals. | |
| Interventions | Standard of care: all patients received supplemental oxygen. If patient condition worsened patient may receive salbutamol 2.5 mg nebulised at discretion of physician. | |
| Outcomes | Measurements made at baseline, 10 minutes after treatment and 20 minutes after treatment. | |
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind, placebo‐controlled. |
| Incomplete outcome data (attrition bias) | High risk | 3 patients were enrolled more than once, only the initial visit was used in the analysis but treatment group not stated. |
| Selective reporting (reporting bias) | High risk | There were no significant differences between the groups in changes in BP, heart rate, or respiratory rate at either 10 minutes or 20 minutes. |
| Methods | Parallel. | |
| Participants | Inclusion criteria: patients in age group of 15 to 60 years with severe bronchial asthma, as judged by Fischl index having PEF < 300 L/min or FEV in 1st second less than 40% of the predicted value were included in the study. Exclusion criteria: all patients who had received oral inhaler or parenteral bronchodilators in the past 6 h or steroid in the previous 12 h were excluded from the study. Adults and children with severe asthma (15 to 60 years) ‒ 40 participants. 30 female and 10 male but unclear how divided between groups. Intervention: 20 completed. Control: 20 completed. | |
| Interventions | Intervention: given 4 doses of nebulised solution of "3.2G%" MgSO₄, 20 minutes apart. Control: received 4 doses of nebulised salbutamol (each dose of 3 mL containing 25 mg), 20 minutes apart. | |
| Outcomes | PEF (L/min), respiratory rate, Fischl index and SaO₂. | |
| Notes | Abstract only. Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of random sequence generation not included in trial report. There is no reference to randomisation in trial report and trial not reported as randomised – seeking clarification from author. |
| Allocation concealment (selection bias) | Unclear risk | Details of allocation concealment not included in trial report. There is no reference to randomisation in trial report and trial not reported as randomised – seeking clarification from author. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No details of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | If the trial was not blinded, there is a strong likelihood that outcome assessment was not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. No apparent indication of selective reporting. |
| Methods | Double blind, randomised controlled trial. 30 emergency departments or children's assessment units, UK. | |
| Participants | Inclusion criteria: severe (BTS/SIGN quantified) asthma exacerbation after conventional treatment, age 2 to 16 years. Exclusion criteria: coexisting respiratory disease, severe renal disease, severe liver disease, known pregnancy, known previous reaction to magnesium, inability to give informed consent, previous randomisation into the trial, life‐threatening symptoms, current or previous (in the 3 months preceding screening) involvement with a trial of a medicinal product. 508 randomised. Intervention: 252 randomised. Median age (years): 4 (3 to 7). Male:female: 143:109. Control: 256 randomised. Median age (years): 4 (3 to 7). Male:female: 150:106. | |
| Interventions | Intervention: 2.5 mL MgSO₄ (250 mmol/L) nebulised. Control: 2.5 mL isotonic saline nebulised. 3 doses given at roughly 20 minute intervals. | |
| Outcomes | Mean Yung asthma severity score, treatment step‐down (60 minutes); length of stay, need for additional intravenous bronchodilator, admission to PICU/HDU or intubation, adverse events (until discharge). Adverse events: treatment group 47, control group 59 | |
| Notes | Funding: National Institute of Health Research Health Technology Assessment Programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated blocked randomisation sequence stratified by centre was generated by an independent statistician who had no further involvement in the study. |
| Allocation concealment (selection bias) | Low risk | Treatment packs were identical in appearance and numbered sequentially for each centre. |
| Blinding of participants and personnel (performance bias) | Low risk | All participants (patients, clinicians, research team, and statisticians) were masked to the treatment allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | The statistical analyses were completed with masked data, with treatment groups revealed only after final analyses had been completed. |
| Incomplete outcome data (attrition bias) | Low risk | Over 90% randomised participants in each arm were included in the adjusted primary analysis. All participants who withdrew or were excluded are clearly accounted for in the flow diagram. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial. All listed outcomes are reported. |
| Methods | Double blind, randomised controlled trial. Chest and emergency departments at 1 hospital, Egypt. | |
| Participants | Inclusion criteria: diagnosis of asthma. Exclusion criteria: fever, lower respiratory tract infection, cardiac/renal/hepatic dysfunction, needed NIV/intubation, near‐fatal asthma, pregnancy, lactation, failed to use PEF meter, inhaled/oral/intravenous bronchodilator use within past 6 h or steroid use within past 12 h. 30 randomised. Intervention 1 (magnesium): 10 randomised. Mean age (years): 33.5 (17.8). Men:women: 4:6. Mean % of predicted PEF at presentation: 33.9 (9.8). Intervention 2 (salbutamol and placebo) : 10 randomised. Mean age (years): 48.6 (9.9). Men:women: 3:7. Mean % of predicted PEF at presentation: 36.4 (10.5). Intervention 3 (salbutamol and magnesium): 10 randomised. Mean age (years): 51.3 (15.8). Men:women: 7:3. Mean % of predicted PEF at presentation: 34.1 (9.4). | |
| Interventions | Intervention 1: 3 mL MgSO₄ (3.3% solution) nebulised. Intervention 2: 0.5 mL salbutamol (0.5% solution) in 2.5 mL isotonic saline nebulised. Intervention 3: 0.5 mL salbutamol (0.5% solution) in 2.5 mL MgSO₄ (4% solution) nebulised. 4 doses given at 20 minute intervals. Ultrasonic nebuliser. | |
| Outcomes | PEF improvement, respiratory rate, heart rate, blood pressure, oxygen saturations, improvement in Fischl index of clinical severity, adverse event rate (all at "final" time point, assumed to be 2 h). Adverse events: no events "severe enough to warrant withdrawal" reported. | |
| Notes | Funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports patients were randomised into 3 groups but no details given. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double blind" but no details given about who was blinded or how. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double blind" but no details given about who was blinded or how. |
| Incomplete outcome data (attrition bias) | Unclear risk | The report does not specify how many randomised participants completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | No prospective trial registration identified. Primary and secondary outcomes not defined. No power calculation reported. |
| Methods | Double blind, randomised controlled trial. 1 emergency department, Turkey | |
| Participants | Inclusion criteria: children aged 3 to 15 years with asthma admitted to the emergency department due to a moderate asthma exacerbation. Exclusion criteria: any associated chronic diseases such as cystic fibrosi and bronchiectasis. 100 randomised. Intervention: 50 randomised. Mean age, months (SD): 76.06 (27.33). Male:female: 25:25. Median (IQR) modified pulmonary index score at presentation 8 (7‐8). Control: 50 randomised. Mean age, months (SD): 74.96 (33.65). Male:female: 29/21. Median (IQR) modified pulmonary index score at presentation 7 (7 to 9). | |
| Interventions | Intervention: nebulised salbutamol (0.15 mg/kg) + 1 mL magnesium sulfate (15%) + 1.5 mL isotonic saline. Control: nebulised salbutamol (0.15 mg/kg) + 1.5 mL isotonic saline. 3 doses given at 20 min intervals. | |
| Outcomes | Primary outcome: Modified Pulmonary Index Score (MPIS); secondary outcomes: hospitalisation rates, symptoms of magnesium imbalance such as nausea, vomiting, abdominal pain, chest pain, headache, fatigue, hypotension and fever. | |
| Notes | Funding: "this research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were assigned consecutively to the control or intervention group based on a stratified randomisation procedure" but no further detail about how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Data not given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as "double‐blind" but no details of who was blinded and the blinding procedure. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as "double‐blind" but no details of who was blinded and the blinding procedure. |
| Incomplete outcome data (attrition bias) | Low risk | "All patients enrolled in the study completed it". |
| Selective reporting (reporting bias) | High risk | No trial registration or prospective protocol identified. Adverse events reported as: "no side effect caused by magnesium was observed in any of the patients in the study". Modified pulmonary index score reported numerically at 120 minutes only; other time points presented graphically with no measure of variance |
ASS: Asthma Severity Score (ASS)
ATS: American Thoracic Society
BP: blood pressure
BTS: British Thoracic Society
COPD: Chronic obstructive pulmonary disease
ED: emergency department
FEV1: Forced expiratory volume in 1 second
FVC: Forced vital capacity
h: hour(s)
IV: intravenous
MDI: metered dose inhaler
MgSO₄: magnesium sulfate
PEF: Peak Expiratory Flow Rate
R&D: research and development
sBP: systolic blood pressure
SD: standard deviation
SIGN: Scottish Intercollegiate Guidelines Network
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Review | |
| Oral supplementation in chronic asthma | |
| Oral supplementation in chronic asthma | |
| Oral supplementation in chronic asthma | |
| Study does not assess people with acute asthma | |
| Study does not assess people with acute asthma | |
| Letter to the Editor | |
| Study of stable asthma and methacholine challenge tests | |
| Review | |
| Not a randomised controlled study | |
| Study not a randomised trial and in mild‐to‐moderate persistent asthma rather than acute asthma | |
| Not a randomised controlled trial | |
| Exercise induced bronchospasm and challenge test. Not a randomised controlled trial. | |
| Oral supplementation in chronic asthma | |
| Study does not assess people with acute asthma and is not a randomised controlled trial | |
| Study of stable asthma and methacholine challenge tests | |
| Randomised controlled trial of intravenous MgSO4 | |
| Review | |
| Review | |
| Stable asthma histamine‐induced bronchospasm adults | |
| Study does not assess people with acute asthma. Dose response study in 20 normal individuals and 19 with chronic asthma. | |
| Study does not assess people with acute asthma. Stable asthma histamine challenge tests. | |
| Stable adult asthma with histamine challenges | |
| Randomised controlled trial of intravenous MgSO₄ | |
| Randomised controlled trial of intravenous MgSO₄ | |
| Review | |
| Review | |
| Paediatric exercise‐induced bronchospasm. Not a randomised controlled trial. | |
| Review | |
| Study does not assess people with acute asthma | |
| Not a randomised controlled trial | |
| Study does not assess people with acute asthma | |
| Review | |
| Oral supplementation in uncontrolled and partly controlled atopic asthma | |
| Review | |
| Review | |
| Oral supplementation on people with unstable asthma | |
| Systematic review, includes intravenous MgSO₄ | |
| Study does not assess people with acute asthma | |
| Study does not assess people with acute asthma | |
| Study does not assess people with acute asthma | |
| Letter to the editor | |
| Randomised controlled trial of intravenous MgSO₄ | |
| Intravenous MgSO₄ | |
| Oral supplementation in children with uncontrolled asthma | |
| Intravenous MgSO₄ | |
| Comparison between inhaled versus intravenous MgSO₄ | |
| Randomised controlled trial of intravenous MgSO₄ | |
| Not a randomised controlled trial | |
| Editorial | |
| Study of stable asthma and methacholine challenge tests | |
| Not a randomised controlled trial | |
| Review | |
| Study does not assess people with acute asthma | |
| Looking at ipratropium bromide mixed with either MgSO₄ or saline for bronchiolitis (up to age 11.5 months) | |
| Study does not assess people with acute asthma | |
| Study does not assess people with acute asthma | |
| Randomised controlled trial of intravenous MgSO₄ | |
| Randomised controlled trial of intravenous versus nebulised MgSO₄ | |
| No response to attempts made to contact first author from 2002 to 2012. First author sadly died in 2014. | |
| Randomised controlled trial of nebulized magnesium sulfate versus ipratropium bromide/fenoterol in children with severe asthma exacerbation | |
| Not a randomised controlled trial | |
| Study does not assess people with acute asthma | |
| Study does not assess people with acute asthma (stable chronic asthma) and is not a randomised controlled trial | |
| Intravenous MgSO₄ and not a randomised controlled trial |
MgSO₄: magnesium sulfate
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | "Ventilatory, cardiovascular and metabolic responses to salbutamol, ipratropium bromide and magnesium sulfate in bronchial asthma: comparative study" |
| Participants | No details |
| Interventions | No details |
| Outcomes | No details |
| Notes | Full‐text unobtainable |
| Methods | "Inhaled magnesium sulfate as adjunct therapy for moderate to severe asthma exacerbations, a randomized control clinical trial" |
| Participants | No details |
| Interventions | No details |
| Outcomes | No details |
| Notes | Full‐text unobtainable |
| Methods | Prospective double‐blind placebo controlled trial |
| Participants | Children diagnosed as asthmatic according to The Global Initiative for Asthma (GINA) guidelines, aged 5 to 14 years old, capable of measuring PEFR, presenting with moderate to severe acute exacerbation according to paediatric asthma severity score and PEFR |
| Interventions | Group A: participants receive inhaled salbutamol solution (0.15 mL/kg) plus isotonic magnesium sulfate (2 mL) in a nebulizer chamber; Group B: participants receive inhaled salbutamol solution (0.15 mL/kg), diluted with placebo (normal saline 2 mL) in a nebulizer chamber. |
| Outcomes | 1. Asthma severity measured using the Pediatric Asthma Severity Score (PASS) at baseline, 20, 40 and 60 minutes post‐nebulisation 2. Oxygen saturation measured using pulse oximetry at baseline, 20, 40 and 60 minutes post‐nebulisation 3. Lung function assessed through measuring peak expiratory flow rate (PEFR) at baseline, 20, 40 and 60 minutes post‐nebulisation |
| Notes | Trial stated as complete February 2016 but no associated publication identified. Contact person emailed on 7 September 2017 to enquire about status of results/publication. No response received at time of review publication. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Comparison of clinical and spirometric response between nebulized salbutamol, MgSO₄ and nebulized salbutamol alone in acute asthma attack |
| Methods | Randomized, double‐blind controlled trial 1 hospital, Iran |
| Participants | Inclusion criteria: having a history of asthma, a minimum 18 years and maximum 65 years Exclusion criteria: COPD; kidney disease; CHF; pneumonitis; underlying respiratory disease 146 randomised |
| Interventions | Intervention: nebulized MgSO₄ 1/5 mL (20 g / 100 mL) with salbutamol 2/5 mL Control: normal saline with nebulized salbutamol 2/5 mL |
| Outcomes | Clinical state, FEV1, PEFR |
| Starting date | 22 March 2014 |
| Contact information | |
| Notes |
| Trial name or title | Nebulized Magnesium Sulfate as an Adjunct to Standard Therapy in Asthma Exacerbation |
| Methods | Randomized, double‐blind controlled trial 1 paediatric emergency department, Mexico |
| Participants | Inclusion criteria: clinical history of asthma, clinical diagnosis of moderate or severe asthma exacerbations, age 2 to 15 years Exclusion criteria: coexistence of lung disease, severe kidney or liver disease, pregnancy, previous reaction to magnesium, no parental consent, prior inclusion in this study, presence of life‐threatening co‐morbidities, need for advanced airway management, life‐threatening symptoms. Estimated enrolment: 152 |
| Interventions | Intervention: nebulized salbutamol 2.5 mg (2 to 5 years) or 5 mg (≥ 6 years) and ipratropium bromide 250 mcg mixed with 2.5 mL of isotonic MgSO₄ (150 mg) per dose every 20 minutes during the first hour, continued with nebulized standard treatment every hour for 4 h, plus IV methylprednisolone or PO prednisolone 2 mg/kg/day for each treatment. Control: nebulized salbutamol 2.5 mg (2‐5 years) or 5 mg (≥ 6 years) and ipratropium bromide 250 mcg mixed with 2.5 mL of isotonic saline per dose every 20 minutes during the first hour, continued with nebulized standard treatment every hour for 4 h, plus IV methylprednisolone or PO prednisolone 2 mg/kg/day for each treatment. |
| Outcomes | Primary outcome measure: change from Baseline Preschool Respiratory Assessment Measure (PRAM) at 20, 40, 60, 120, 180 and 240 minutes after beginning treatment. Secondary outcome measures: rate of hospitalisation at 4 h, change from baseline heart rate, respiratory rate and blood pressure at 20, 40, 60, 120, 180 and 240 minutes after beginning treatment. |
| Starting date | September 2015 |
| Contact information | |
| Notes | Estimated study completion date: January 2018 |
| Trial name or title | Magnesium nebulization utilization in management of paediatric asthma (MagNUM PA) trial: study protocol for a randomized controlled trial |
| Methods | Randomized double‐blind controlled trial in 7 Canadian paediatric emergency departments |
| Participants | The trial will include 816 otherwise healthy children who are 2 to 17 years old, having had at least 1 previous wheezing episode, have received systemic corticosteroids, and have a Pediatric Respiratory Assessment Measure (PRAM) ≥ 5 points after 3 salbutamol and ipratropium treatments for a current acute asthma exacerbation. |
| Interventions | 3 doses nebulized salbutamol with either 600 mg MgSO₄ or placebo 20 min apart. |
| Outcomes | Primary outcome: hospitalisation within 24 h of the start of the experimental therapy for persistent respiratory distress or supplemental oxygen. Secondary outcomes include all‐cause hospitalisation within 24 h, PRAM, vital signs, number of bronchodilator treatments by 240 min, association between the difference in the primary outcome between the groups, age, gender, baseline PRAM, atopy, and “viral induced wheeze” phenotype. |
| Starting date | November 2014 |
| Contact information | Suzanne Schuh: [email protected] |
| Notes | Estimated completed: December 2017 |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary function (% FEV1) Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 3.28 [1.06, 5.49] |
| Analysis 1.1  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 1 Pulmonary function (% FEV1). | ||||
| 1.1 90 minutes | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 8.57 [1.99, 15.15] |
| 1.2 120 minutes | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [0.25, 4.95] |
| 2 Pulmonary function % predicted PEF Show forest plot | 2 | 636 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐2.33, 2.42] |
| Analysis 1.2  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 2 Pulmonary function % predicted PEF. | ||||
| 3 Clinical severity scores (closest to 60 mins) Show forest plot | 2 | 1130 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.12] |
| Analysis 1.3  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 3 Clinical severity scores (closest to 60 mins). | ||||
| 3.1 Yung ASS at 60 minutes | 1 | 472 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.35, 0.02] |
| 3.2 Change in dyspnoea VAS at 60 minutes | 1 | 658 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.02, 0.28] |
| 4 Admission at first presentation Show forest plot | 4 | 1308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.91, 1.00] |
| Analysis 1.4  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 4 Admission at first presentation. | ||||
| 4.1 Adults | 3 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.03] |
| 4.2 Children | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.92, 1.01] |
| 5 HDU/ITU admission Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 5 HDU/ITU admission. | ||||
| 5.1 Admission to HDU (adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Admission to ICU (adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Admission to PICU/HDU or intubation (children) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Readmission Show forest plot | 2 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.84, 3.87] |
| Analysis 1.6  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 6 Readmission. | ||||
| 7 Respiratory rate at 60 mins Show forest plot | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.14, 1.53] |
| Analysis 1.7  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 7 Respiratory rate at 60 mins. | ||||
| 8 Heart rate at 60 mins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 8 Heart rate at 60 mins. | ||||
| 9 Systolic blood pressure at 60 mins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 9 Systolic blood pressure at 60 mins. | ||||
| 10 Diastolic blood pressure at 60 mins Show forest plot | 1 | 674 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.29, 4.51] |
| Analysis 1.10  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 10 Diastolic blood pressure at 60 mins. | ||||
| 11 Serious adverse events (during admission) Show forest plot | 2 | 557 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| Analysis 1.11  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 11 Serious adverse events (during admission). | ||||
| 11.1 Adults | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 11.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.06, ‐0.01] |
| 12 Any adverse event (during admission) Show forest plot | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| Analysis 1.12  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 12 Any adverse event (during admission). | ||||
| 12.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.02, 0.07] |
| 12.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 13 Serious adverse events (within 30 days) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 13 Serious adverse events (within 30 days). | ||||
| 14 Any adverse event (within 30 days) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 14 Any adverse event (within 30 days). | ||||
| 15 Adverse event: hypotension Show forest plot | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.01, 0.04] |
| Analysis 1.15  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 15 Adverse event: hypotension. | ||||
| 15.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 15.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 16 Adverse event: flushing Show forest plot | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| Analysis 1.16  Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 16 Adverse event: flushing. | ||||
| 16.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 16.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary function % predicted FEV1 Show forest plot | 4 | 208 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [‐1.58, 8.26] |
| Analysis 2.1  Comparison 2 MgSO4 + SABA versus SABA, Outcome 1 Pulmonary function % predicted FEV1. | ||||
| 1.1 Adults | 3 | 146 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐3.30, 7.67] |
| 1.2 Children | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 8.10 [‐3.03, 19.23] |
| 2 % predicted FEV1: subgroup: severity Show forest plot | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | 4.12 [‐1.81, 10.06] |
| Analysis 2.2  Comparison 2 MgSO4 + SABA versus SABA, Outcome 2 % predicted FEV1: subgroup: severity. | ||||
| 2.1 Severe (FEV1 <50% predicted) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [0.05, 19.75] |
| 2.2 Moderate | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐6.59, 8.27] |
| 3 Pulmonary function PEF L/min Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 MgSO4 + SABA versus SABA, Outcome 3 Pulmonary function PEF L/min. | ||||
| 3.1 Adults | 3 | 155 | Mean Difference (IV, Fixed, 95% CI) | 11.91 [‐4.12, 27.95] |
| 3.2 Children | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 11.90 [‐6.86, 30.66] |
| 4 Admission to hospital Show forest plot | 6 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.15] |
| Analysis 2.4  Comparison 2 MgSO4 + SABA versus SABA, Outcome 4 Admission to hospital. | ||||
| 4.1 Adults | 4 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.07] |
| 4.2 Children | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.44, 2.98] |
| 5 Heart rate at 120 mins Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 MgSO4 + SABA versus SABA, Outcome 5 Heart rate at 120 mins. | ||||
| 6 Respiratory rate at 120 mins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 MgSO4 + SABA versus SABA, Outcome 6 Respiratory rate at 120 mins. | ||||
| 7 Diastolic blood pressure at 120 mins Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [‐1.35, 2.80] |
| Analysis 2.7  Comparison 2 MgSO4 + SABA versus SABA, Outcome 7 Diastolic blood pressure at 120 mins. | ||||
| 8 Systolic blood pressure at 120 mins Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [‐2.69, 4.48] |
| Analysis 2.8  Comparison 2 MgSO4 + SABA versus SABA, Outcome 8 Systolic blood pressure at 120 mins. | ||||
| 9 Serious adverse events Show forest plot | 5 | 243 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| Analysis 2.9  Comparison 2 MgSO4 + SABA versus SABA, Outcome 9 Serious adverse events. | ||||
| 9.1 Adults | 4 | 181 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.2 Children | 1 | 62 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 10 Any adverse events Show forest plot | 5 | 694 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| Analysis 2.10  Comparison 2 MgSO4 + SABA versus SABA, Outcome 10 Any adverse events. | ||||
| 10.1 Adults | 4 | 329 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 10.2 Children | 1 | 365 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical severity score Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.62, 0.36] |
| Analysis 3.1  Comparison 3 MgSO4 versus SABA, Outcome 1 Clinical severity score. | ||||
| 1.1 Fischl index final score (120 mins) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.07, 0.41] |
| 1.2 Fischl index score (time point unclear) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.11, 0.71] |
| 1.3 Change in Fischl index at 120 mins | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.67, 1.27] |
| 2 Admission to hospital Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 MgSO4 versus SABA, Outcome 2 Admission to hospital. | ||||
| 3 Heart rate (120 mins) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 21.20 [0.17, 42.23] |
| Analysis 3.3  Comparison 3 MgSO4 versus SABA, Outcome 3 Heart rate (120 mins). | ||||
| 4 Respiratory rate Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.91, ‐0.89] |
| Analysis 3.4  Comparison 3 MgSO4 versus SABA, Outcome 4 Respiratory rate. | ||||
| 5 Systolic pressure (120 mins) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 MgSO4 versus SABA, Outcome 5 Systolic pressure (120 mins). | ||||
| 6 Diastolic pressure (120 mins) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 MgSO4 versus SABA, Outcome 6 Diastolic pressure (120 mins). | ||||
| 7 Serious adverse events Show forest plot | 2 | 53 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| Analysis 3.7  Comparison 3 MgSO4 versus SABA, Outcome 7 Serious adverse events. | ||||
| 8 Mild‐Moderate Side Effects Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3 MgSO4 versus SABA, Outcome 8 Mild‐Moderate Side Effects. | ||||
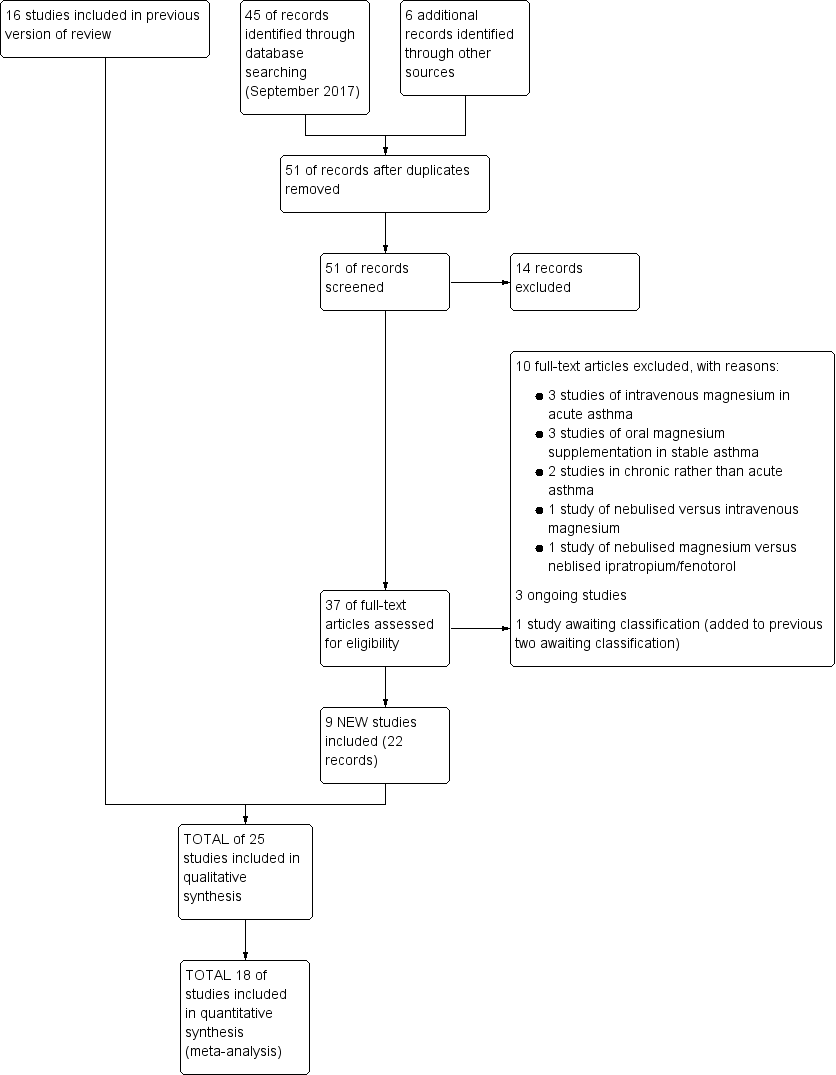
Study flow diagram: review update

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

In the control group 82 people out of 100 had hospital admission , compared to 78 (95% CI 75 to 82) out of 100 for the active treatment group.
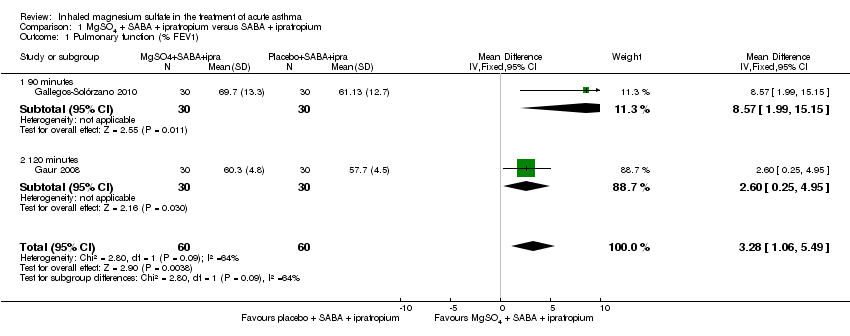
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 1 Pulmonary function (% FEV1).

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 2 Pulmonary function % predicted PEF.

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 3 Clinical severity scores (closest to 60 mins).
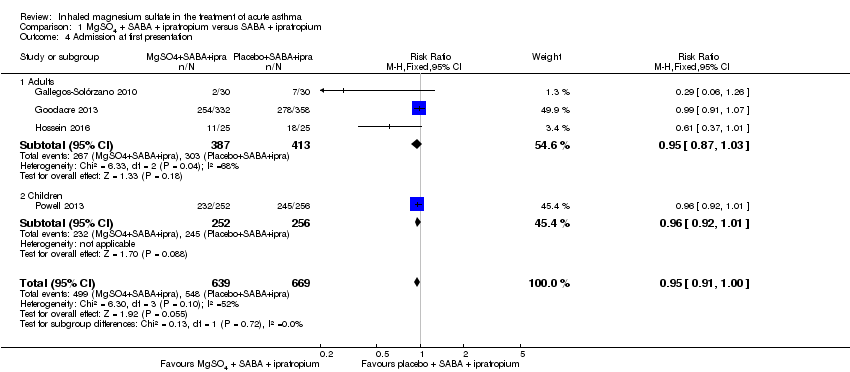
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 4 Admission at first presentation.

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 5 HDU/ITU admission.

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 6 Readmission.
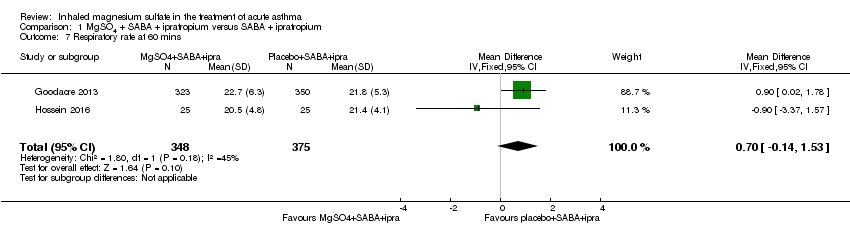
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 7 Respiratory rate at 60 mins.
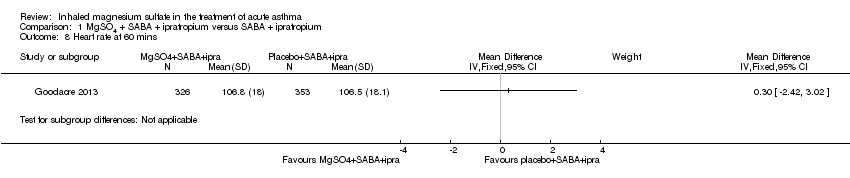
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 8 Heart rate at 60 mins.

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 9 Systolic blood pressure at 60 mins.

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 10 Diastolic blood pressure at 60 mins.
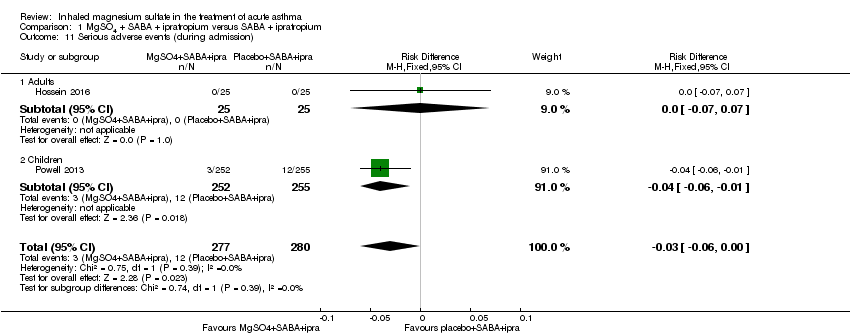
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 11 Serious adverse events (during admission).

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 12 Any adverse event (during admission).
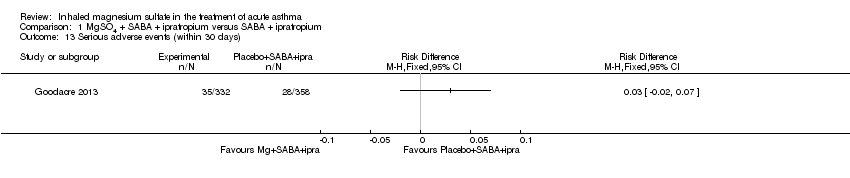
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 13 Serious adverse events (within 30 days).

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 14 Any adverse event (within 30 days).
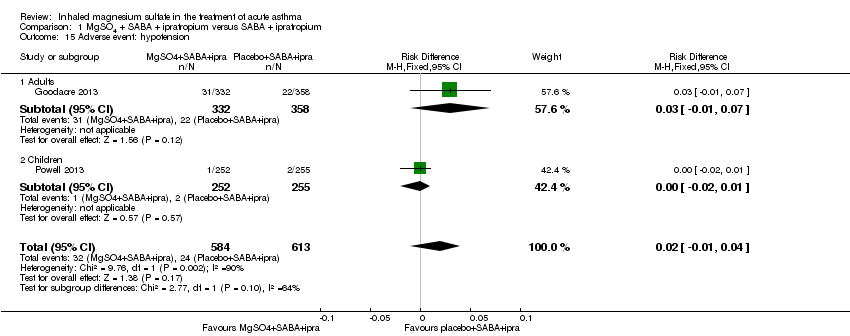
Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 15 Adverse event: hypotension.

Comparison 1 MgSO4 + SABA + ipratropium versus SABA + ipratropium, Outcome 16 Adverse event: flushing.

Comparison 2 MgSO4 + SABA versus SABA, Outcome 1 Pulmonary function % predicted FEV1.
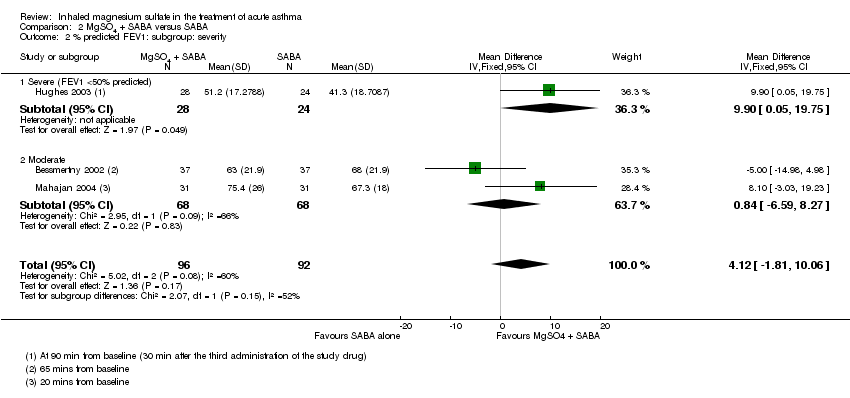
Comparison 2 MgSO4 + SABA versus SABA, Outcome 2 % predicted FEV1: subgroup: severity.

Comparison 2 MgSO4 + SABA versus SABA, Outcome 3 Pulmonary function PEF L/min.

Comparison 2 MgSO4 + SABA versus SABA, Outcome 4 Admission to hospital.
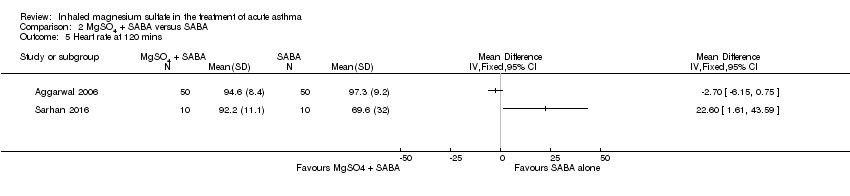
Comparison 2 MgSO4 + SABA versus SABA, Outcome 5 Heart rate at 120 mins.
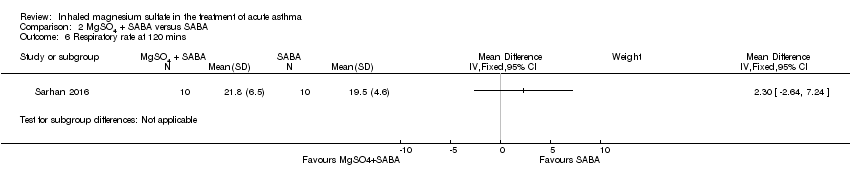
Comparison 2 MgSO4 + SABA versus SABA, Outcome 6 Respiratory rate at 120 mins.

Comparison 2 MgSO4 + SABA versus SABA, Outcome 7 Diastolic blood pressure at 120 mins.

Comparison 2 MgSO4 + SABA versus SABA, Outcome 8 Systolic blood pressure at 120 mins.

Comparison 2 MgSO4 + SABA versus SABA, Outcome 9 Serious adverse events.
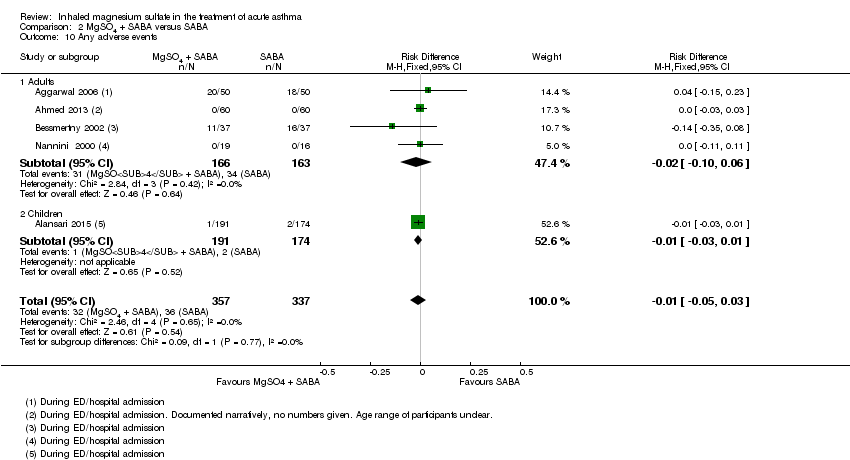
Comparison 2 MgSO4 + SABA versus SABA, Outcome 10 Any adverse events.

Comparison 3 MgSO4 versus SABA, Outcome 1 Clinical severity score.

Comparison 3 MgSO4 versus SABA, Outcome 2 Admission to hospital.

Comparison 3 MgSO4 versus SABA, Outcome 3 Heart rate (120 mins).
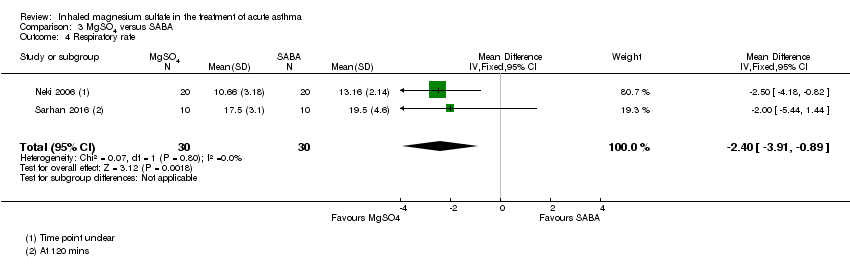
Comparison 3 MgSO4 versus SABA, Outcome 4 Respiratory rate.
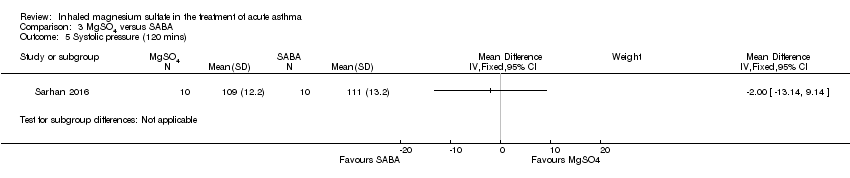
Comparison 3 MgSO4 versus SABA, Outcome 5 Systolic pressure (120 mins).
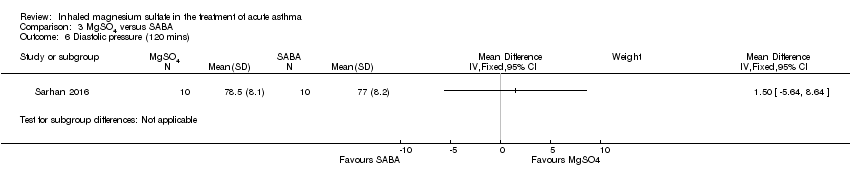
Comparison 3 MgSO4 versus SABA, Outcome 6 Diastolic pressure (120 mins).
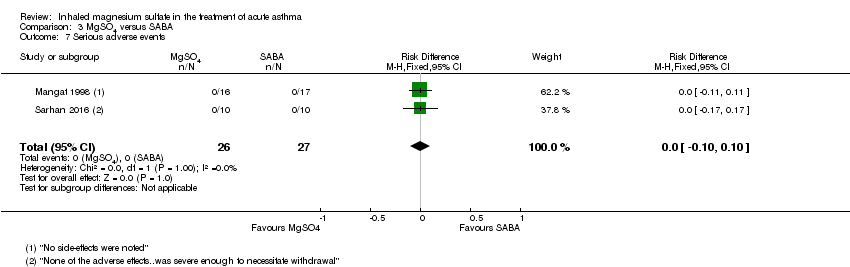
Comparison 3 MgSO4 versus SABA, Outcome 7 Serious adverse events.

Comparison 3 MgSO4 versus SABA, Outcome 8 Mild‐Moderate Side Effects.
| MgSO₄+ SABA + ipratropium compared to SABA + ipratropium in the treatment of acute asthma | ||||||
| Patient or population: adults and children with acute exacerbation of asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with SABA + ipratropium | Risk with MgSO4 + SABA + ipratropium | |||||
| Pulmonary function (% predicted FEV1) (90 to 120 minutes) | The mean pulmonary function (% predicted FEV1) was 65% | % predicted FEV1 was 3.28% higher | ‐ | 120 | ⊕⊝⊝⊝ | Outcome measured at 90 mins in 1 study and 120 mins in the other. 1 study (Gaur 2008) has reported much smaller standard deviations and contributes almost 90% of analysis weight |
| Pulmonary function % predicted PEF (60 minutes) | The mean pulmonary function % predicted PEF was 50.45% | % predicted PEF was 0.05 higher | ‐ | 636 | ⊕⊕⊕⊝ | Both studies in adults Mean control group % predicted PEF was 36% in 1 study and 64.9% in the other |
| Clinical severity scores (60 minutes) | The mean dyspnoea VAS was 31.8; the mean Yung ASS was 4.95 | SMD 0.01 higher | ‐ | 1130 | ⊕⊕⊝⊝ | 1 study reported Yung ASS and the other change in dyspnoea VAS |
| Admission at first presentation | 819 per 1000 | 778 per 1000 | RR 0.95 | 1308 | ⊕⊕⊕⊝ | Adults vs children test for subgroup difference: P = 0.72, I² = 0% |
| Readmission (7 to 30 days) | 26 per 1000 | 46 per 1000 | RR 1.80 | 750 | ⊕⊕⊝⊝ | Outcome measured at 7 days in 1 study and 30 days in the other. |
| Serious adverse events (during admission) | 43 per 1000 | Not estimable. See comment. | ‐ | 557 | ⊕⊕⊕⊝ | Risk difference: −0.03 (95% CI −0.06 to 0.00) Adults vs children test for subgroup difference: P = 0.39, I² = 0% Goodacre 2013 also reported participants with 1 or more SAE within 30 days: 35/332 in the MgSO₄ group and 28/358 in the placebo group (RD: 0.03; 95% CI −0.02 to 0.07) |
| Any adverse event (during admission) | 144 per 1000 | Not estimable. See comment. | ‐ | 1197 | ⊕⊕⊕⊕ | Risk Difference: 0.01 (95% CI −0.03 to 0.05) Adults vs children test for subgroup difference: P = 0.34, I² = 0% Goodacre 2013 also reported participants with 1 or more adverse event within 30 days: 52/332 in the MgSO₄ group and 36/358 in the placebo group (OR 1.66, 95% CI 1.05 to 2.62) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One study contributing most of weight at unclear risk of bias in multiple domains (−1 study limitations) 2 I² > 50% (−1 inconsistency) 3 Studies equal size but one study contributes almost 90% of weight to analysis due to much smaller standard deviations. Result no longer significant if random‐effects model applied (−1 imprecision) 4 Although one study at unclear risk of bias in several domains, the larger study, which contributes vast majority of weight to analysis, if of high methodological quality (no downgrade) 5 Although confidence interval includes no difference, they are sufficiently tight to effectively rule out an important between‐group difference (no downgrade) 6 Confidence intervals include both harm and benefit of intervention (−1 imprecision) 7 Although two of the studies at unclear risk of bias in several domains the two large studies contributing nearly 95% of weight in analysis are both of high methodological quality (no downgrade) 8 Although the I² = 52%, the two large studies contributing to this analysis show consistent results (no downgrade) 9 Confidence intervals include no difference (−1 imprecision) 10 Confidence intervals include no difference and appreciable harm or benefit of the intervention (−2 imprecision) 11 Events rare and confidence intervals include no difference (−1 imprecision) | ||||||
| MgSO₄+ SABA compared to SABA in the treatment of acute asthma | ||||||
| Patient or population: adults and children with acute exacerbation of asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with SABA | Risk with MgSO4 + SABA | |||||
| Pulmonary function % predicted FEV1 (20 minutes to 2 to 3 h) | The mean pulmonary function % predicted FEV1 was 56.55% | % predicted FEV1 was 3.34% higher | ‐ | 208 | ⊕⊕⊝⊝ | Adults vs children test for subgroup difference: P = 0.35, I² = 0% Severe vs moderate asthma exacerbation test for subgroup difference: P = 0.15, I² = 51.8% (favouring a greater effect in the more severe subgroup) |
| Pulmonary function PEF L/min ‐ Adults (20 minutes to 2 to 3 h) | The mean pulmonary function PEF was 233 L/min | PEF was 11.91 L/min higher | ‐ | 155 | ⊕⊕⊝⊝ | |
| Pulmonary function PEF L/min ‐ Children (60 minutes) | The mean pulmonary function PEF was 143.5 | PEF was 11.9 L/min higher | ‐ | 80 | ⊕⊕⊝⊝ | |
| Admission to hospital at initial presentation | 202 per 1000 | 158 per 1000 (105 to 233) | RR 0.78, (0.52 to 1.15) | 375 | ⊕⊕⊝⊝ | Adults vs children test for subgroup difference: P = 0.35, I² = 0% |
| Serious adverse events (During ED/hospital admission) | Not estimable | Not estimable. See comment | ‐ | 243 | ⊕⊕⊝⊝ | Risk difference: 0.00 (95% CI −0.04 to 0.04) No events reported |
| Any adverse events (During ED/hospital admission) | 107 per 1000 | Not estimable. See comment | ‐ | 694 | ⊕⊕⊝⊝ | Risk difference: −0.01 (95% CI −0.05 to 0.03) Adults vs children test for subgroup difference: P = 0.77, I² = 0% |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Several studies were at unclear or high risk of bias in one or more domain (−1 study limitations) 2 Confidence intervals include both possible harm and benefit of the intervention (−1 imprecision) 3 Study at unclear risk of bias in several domains (−1 study limitations) 4 No events reported but less than 250 participants in total. Risk difference confidence intervals include a possible important harm or benefit of the intervention (−1 imprecision) | ||||||
| MgSO₄compared to SABA in the treatment of acute asthma | ||||||
| Patient or population: adults and children with acute exacerbation of asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with SABA | Risk with MgSO4 | |||||
| Lung function | Reported narratively in text | |||||
| Clinical severity score ‐ Fischl index (120 minutes) | The Fischl index score was 2.1 | Fischl index score 0.13 lower | ‐ | 93 | ⊕⊝⊝⊝ | Time point 120 minutes in 2 studies and unclear in the third study Wide range of control group scores (0.3, 0.76 and 4.81). Scale out of 7 with higher score indicating more severe symptoms. 4.81 reported in study with unclear time point. |
| Admission to hospital at initial presentation | 118 per 1000 | 62 per 1000 | RR 0.53 | 33 | ⊕⊝⊝⊝ | |
| Serious adverse events (During ED/hospital admission) | Not estimable | Not estimable. See comment | 53 | ⊕⊕⊝⊝ | Risk difference: 0.00 (95% CI −0.10 to 0.10) No events reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Several studies at unclear or high risk of bias in one or more domains (−1 study limitations) 2 Confidence intervals include both possible harm and benefit of the intervention (−1 imprecision) 3 Time‐point for measurement unclear in one study (−1 indirectness) 4 Study at unclear risk of bias in several domains (−1 study limitations) 5 One small study. Confidence intervals include appreciable harm or benefit of the intervention (−2 imprecision) 6 Two small studies. No events reported. Risk difference confidence intervals include appreciable harm or benefit of the intervention (−1 for imprecision) | ||||||
| Study | Severity of asthma exacerbation | Diagnosis based on | Population (adult/mixed/paediatric) |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | |||
| Severe | BTS definition clinical features | Paediatric (2 to 16) | |
| Unclear | PEF and clinical signs | Adults | |
| Moderate to severe | FEV1 < 60% | Adults >18 | |
| Severe | FEV1 < 30% | Adults (18 to 60) | |
| Severe | BTS definition | Adult (≥ 16) | |
| Moderate to severe | PEF < 70% and clinical signs | Adult (> 16) | |
| Severe after conventional treatment | BTS definition | Paediatric (2 to 16) | |
| MgSO4 and SABA versus SABA | |||
| Moderate | FEV1 and PEF at baseline | Adults | |
| Severe and life threatening | BTS definition clinical features and PEF | Mixed (13 to 60) | |
| Severe | PEF | Not documented | |
| Moderate to severe | Clinical score | Paediatric (2 to 14) | |
| Unclear | N/A | Adult | |
| Moderate to severe | PEF between 40% to 80% | Adults (18 to 65) | |
| Severe | PEF < 50% | Adults | |
| Severe | FEV1 < 50% | Adults (16 to 65) | |
| Unclear | Clinically defined as respiratory distress | Paediatric (mean age 3.55 years) | |
| Moderate to severe | Clinical scores and PEF | Adults (18 to 60) | |
| Moderate to severe | FEV1 between 45% and 75% | Paediatric (5 to 17) | |
| Moderate to severe | GINA definition | Paediatric (5 to 14) | |
| Severe | PEF < 50% | Adult (> 18) | |
| Unclear | PEF < 300L/min | Mixed (11 to 70) | |
| Moderate | Not described | Children (3 to 15) | |
| MgSO₄ versus SABA | |||
| Severe | PEF < 50% | Adults | |
| Moderate to severe | PEF < 300 L/Min | Mixed (12 to 60) | |
| Moderate to severe | PEF < 75% | Paediatric | |
| Severe | FEV1 < 40% or PEF < 300 L/Min | Adult (15 to 60) | |
| Unclear | PEF < 300L/min | Mixed (11 to 70) | |
| BTS: British Thoracic Society GINA: Global Initiative for Asthma | |||
| Study | Presentation to which department? | Origin | Primary outcome(s) | Total n randomised | Side effects (patients in study) | Pharmaceutical exclusions | Other Interventions |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | |||||||
| Children’s Assessment Unit after GP referral | Cardiff, Wales | ASS (Yung) | 17 | 1 tingling in fingers and 1 transient hypotension | None stated | All management followed the BTS/SIGN guidelines; all children received 2 mg/kg prednisolone | |
| ED | USA | PEF, admissions | 110 | No comment on side effects in paper | Not stated | All subjects received 50 mg of oral prednisone at the onset of the treatment | |
| ED | Mexico City, Mexico | % change FEV1, O₂ post treatment, admission rates | 112 | Dry and bitter mouth (MgSO₄ group 1), dizziness (MgSO₄ 1; placebo 1) | Use of steroids prior to presentation | All participants received one IV dose of 125 mg methylprednisolone at admission and 1 mg/kg/day for 10 days prednisolone,on discharge. Other treatments were administered according to the treating physician | |
| ED | Delhi, India | FEV1 | 60 | None reported | None stated | All participants received IV hydrocortisone on arrival | |
| ED | UK | Admission within 7d, visual analogue scale for breathlessness at 2 h | 703 | AEs (41 MgSO₄/salbutamol; 36 placebo/salbutamol) | MgSO₄ in the past 24 h | All participants were managed according to BTS/SIGN guidelines (consisting of | |
| ED | Tehran, Iran | PEFR improvement, admission rate | 50 | No serious side effects reported | None stated | All participants received 50 mg oral prednisolone | |
| ED and children's assessment units | UK | Yung asthma severity score | 508 | 47 in MgSO₄ group and 59 in control group | None | Hospital‐defined conventional treatment | |
| MgSO4 and SABA versus SABA | |||||||
| ‐ | Tenerife Spain | FEV1, PEF | 24 | None reported | None stated | Not stated | |
| ED | New Delhi India | PEF | 100 | Palpitations (MgSO₄/salbutamol 13; salbutamol/placebo 11) and tremors (7; 7). | None stated | Clinicians free to administer steroids, salbutamol, IV hydrocortisone if judged to be required | |
| ‐ | Mymensingh, Bangladesh | PEF | 120 | None reported | None stated | Not stated | |
| Paediatric emergency centre | Doha, Qatar | Time to readiness for discharge | 400 | Chest tightness and facial rash (MgSO₄/salbutamol 191), excessive cough (placebo/salbutamol 174) | None stated | All participants received methylprednisolone 1 mg/kg IV every 12h and additional nebulised albuterol at clinicians' discretion | |
| Outpatient department and ED | Sohag, Egypt | Exacerbations post intervention, delivery outcome, post‐partum health status | 60 | None reported | None stated | All participants received 100 mg hydrocortisone IV, 500 mg aminophylline IV | |
| ED | Brooklyn, USA | FEV1 (% pred) | 74 | No SAEs reported | No theophylline or anticholinergics 2 h prior to presentation | Intravenous hydrocortisone, 2 mg/kg | |
| ED | Ajmer India | PEF | 71 | "Side effects were self limiting" | Not stated | Not stated | |
| ED | Wellington New Zealand | FEV1 | 52 | None reported | None | All participants received 100 mg hydrocortisone IV | |
| ‐ | Urmia, Iran | Reduced mean duration of O₂ therapy in MgSO₄ group, no change in Respiratory Distress Score) | 40 | No side effects | Not stated | Not stated | |
| ED | Gazi, Turkey | PEF difference | 26 | Transient hypotension (1 MgSO₄), palpitation (1 salbutamol) | None | All participants received 1 mg/kg prednisolone. Theophylline, anticholinergics and salbutamol given at clinicians discretion | |
| ED | Detroit, USA | % change in FEV1 | 62 | No side effects | Steroids, ipratropium or theophylline in the last 3 days. | All participants received 2 mg/kg of prednisone | |
| ‐ | Babol, Iran | Pulmonary index, PEFR, adjusted PEFR | 80 | ‐ | Corticosteroids; steroids, theophylline or ipratropium use within last 72 h | Not stated | |
| ED | 4 hospitals in Argentina | PEF, admissions | 35 | None reported | Oral or parenteral steroids in the last 7 days | No other medications were permitted during the study except | |
| Chest and ED | Minia, Egypt | Clinical improvement, PEFR | 30 | None severe enough to warrant withdrawal | Bronchodilators in last 6 h, steroids in last 12 h | Nebulised salbutamol, IV hydrocortisone, IV aminophylline at clinicians' discretion | |
| ED | Turkey | Modified pulmonary index score | 100 | "No side effect caused by magnesium was observed in any of the patients in the study" | Not stated | Nebulised salbutamol (0.15 mg/kg), methylprednisolone 1 mg/kg IV; Oxygen was given to patients with SaO2≤ 95% | |
| MgSO₄ versus SABA | |||||||
| ED | Ajmer India | PEF | 71 | "Side effects were self limiting" | Not stated | Not stated | |
| ED | St John’s College, India | PEF, Fischl index score, admissions | 33 | Transient self limiting hypotension (1) palpitation (1) tremors (2) all in control group and only 1 transient hypotension in MgSO₄ group (33) | Oral parenteral bronchodilators (6 h) steroids (last 12 h) | All participants received 100 mg hydrocortisone IV | |
| ‐ | Izmir, Turkey | % change in PEF ASS (Davies Leffert, Dabbous score) | 40 | No side effects | Beta2‐agonists or theophylline in the last 12 h | No other medication given | |
| ‐ | Amritsar Punjab | PEF, RR, Fischl index | 40 | ‐ | Oral, inhaled or parenteral steroids in last 12 h | All participants received 100 mg hydrocortisone IV | |
| Chest and ED | Minia, Egypt | Clinical improvement, PEFR | 30 | None severe enough to warrant withdrawal | Bronchodilators in last 6 h, steroids in last 12 h | Nebulised salbutamol, IV hydrocortisone, IV aminophylline at clinicians' discretion | |
| ASS: Asthma Severity Score; BP: blood pressure; ED: emergency department; FEV1: Forced expiratory volume in 1 second; h: hour(s) | |||||||
| Study (N) | Magnesium sulfate | Control | ||||
| Dose | N | Co‐interventions | Dose | N | Co‐interventions | |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | ||||||
| 2.5 mL isotonic MgSO₄ (151 mg /dose) | 7 | 500 mcg Ipratropium bromide 2.5 mg salbutamol or 5 mg salbutamol (depending on age) 3 times per h | 2.5 mL of isotonic saline) | 10 | Same as for MgSO₄ group | |
| 150 mg MgSO₄ (0.3 mL of 50% MgSO₄ heptahydrate) | 60 | Albuterol sulfate (0.5%) 5 mg/mL) and 0.5 mg ipratropium bromide (0.02% inhalation solution) (frequency*) | No placebo so volume will be less: i.e. blinding may be an issue) | 50 | Same as for MgSO₄ group | |
| 3 mL (333 mg) of 10% isotonic MgSO₄ (1 g/10 mL) | 60 (30 withdrawals) | 2.5 mg albuterol and 500 mcg ipratropium 3 doses per hour | 3 mL isotonic saline | 52 (22 withdrawals) | Same as for MgSO₄ group | |
| 3 mL (3.2 g%) isotonic MgSO₄ | 30 | Salbutamol and ipratropium (dose*, frequency*) | Saline | 30 | Same as for MgSO₄ group | |
| 2 mmol MgSO₄ | 339 (7 withdrawal) | 7.5 mL 0.9% NaCl nebulised, 3 doses; 100 mL 0.9% NaCl IV once, BTS/SIGN standard treatments plus others at clinicians' discretion | 7.5 mL 0.9% saline nebulised, 3 doses, 100 mL 0.9% NaCl IV once | 364 (7 withdrawal) | BTS/SIGN standard treatments plus others at clinicians' discretion | |
| 3 mL (260 mmol/L) MgSO4 | 25 | 2.5 mg salbutamol, 0.5 mg ipratropium nebulised every 20 to 60 minutes, 50 mg oral prednisolone (once*) | 3 mL 0.9% NaCl | 25 | Same as for MgSO₄ group | |
| 2.5 mL 250 mmol/L MgSO₄ | 252 (13 withdrawals) | 3 doses every 20 min. Hospital‐defined conventional treatment | 2.5 mL isotonic saline | 256 (10 withdrawals) | Same as for MgSO₄ group | |
| MgSO4 and SABA versus SABA | ||||||
| 2 mL MgSO₄ (isotonic) | 13 | 400 mcg salbutamol (once*) | 2 mL of a physiological serum of an inhaled form 11 patients | 11 | 400 mcg salbutamol | |
| 1 mL of 500 mg/mL MgSO₄ | 50 | 1 mL salbutamol (dose*, 8 mL distilled water, (295 mOsml/kg) 3 times per h ultrasonic nebuliser | 7.5 mL normal saline | 50 | 1 mL salbutamol (dose*), 1.5 mL distilled water (287 mOsml/kg) 3 times per h | |
| MgSO₄ (dose* frequency*) | 60 | Not recorded | Normal saline (dose* frequency*) | 60 | Not recorded | |
| 800 mg (15 mL) MgSO₄ | 208 (17 withdrawals) | 5 mg albuterol, divided into 3 doses over 1 h. Methylprednisolone 1 mg/kg IV every 12 h. 3 doses nebulized 1 mL albuterol (5 mg/mL), 250 mcg ipratropium, 2 mL normal saline before trial doses started | 15 mL 0.9% NaCl | 192 (18 withdrawals) | Same as for MgSO₄ group | |
| 500 mg (1mL) MgSO₄ | 30 | 1 mL salbutamol solution (dose*), 8 mL 0.9% NaCl, max 3 doses with 20 mins apart. 100 mg hydrocortisone IV, 500 mg aminophylline IV (once*) | 1 mL 0.9% NaCl | 30 | Same as for MgSO₄ group | |
| MgSO₄ (384 mg) | 37 (3 withdrawals) | Followed by ( i.e. not mixed) albuterol 2.5 mg/mL 3 times per h | Normal saline (no volume documented) | 37 (3 withdrawals) | Same as for MgSO₄ group | |
| MgSO₄ | 26 | No doses in any group or co‐interventions described | Not stated | 24 | No doses in any group or co‐interventions described | |
| 2.5 mL isotonic MgSO₄ (250 mmol/L 151 mg) 28 patients | 28 | 2.5 mg salbutamol 3 times per 30 minutes | 2.5 mL normal saline | 24 | Same as for MgSO₄ group | |
| Isotonic MgSO₄ (dose*, frequency*) | * | Salbutamol (dose*) | 2.5 mL normal saline (frequency*) | * | Same as for MgSO₄ group | |
| Isotonic MgSO₄ (2.5 mL) | 14 | Salbutamol (dose*) 3 times per h then 1 per h for 3 h | 2.5 mL normal saline | 12 | Same as for MgSO₄ group | |
| 3 mL 7.5% MgSO₄ | 40 | 0.15 mg/kg salbutamol 3 doses, every 20 min | 3 mL normal saline | 40 | Same as for MgSO₄ group | |
| 2.5 mL Isotonic (6.3%) MgSO₄ solution | 31 | Albuterol 2.5 mg 1 dose | 2.5 mL normal saline | 31 | Same as for MgSO₄ group | |
| 3 mL isotonic MgSO₄ (286 mOsml, 7.5%, 225 mg) | 19 | 0.5 mL 2.5 mg salbutamol 1 dose* | 3 mL normal saline | 16 | Same as for MgSO₄ group | |
| 2.5 mL MgSO4 (100 mg), 0.5 mL salbutamol (2.5 mg) | 10 | 4 doses at 20 min intervals. If needed: additional nebulised salbutamol, IV hydrocortisone, IV aminophylline | 2.5 mL isotonic saline | 10 | Same as for MgSO4 group | |
| 1 mL magnesium sulfate (15%) + 1.5 mL isotonic saline | 50 | 3 doses at 20 min intervals. Also nebulised salbutamol (0.15 mg/kg), methylprednisolone 1 mg/kg IV; Oxygen was given to patients with SaO2 ≤ 95% | 1.5 mL isotonic saline | 50 | Same as for MgSO₄ group | |
| MgSO₄ versus SABA | ||||||
| MgSO₄ | 21 | No doses in any group or co‐interventions described | Not stated | 24 | No doses in any group or co‐interventions described | |
| 3.2% solution MgSO₄ = 95 mg) | 16 | 4 doses every 20 minutes | 3 mL (2.5 mg) salbutamol | 17 | Four doses every 20 minutes | |
| 2 mL MgSO₄ (280 mmol/L) | 20 | 1* dose given over 10 to 15 minutes | Salbutamol 2.5 mg in 2.5 mL | 20 | 1 dose* given over 10 to 15 minutes | |
| 20 patients 3.2 G % MgSO₄ | 20 | 4 doses every 20 min | 3 mL of 25 mg* salbutamol (likely decimal point missing) | 20 | Same as for MgSO4 group | |
| 3 mL (100 mg) MgSO4 | 10 | 4 doses at 20 min intervals. If needed: additional nebulised salbutamol, IV hydrocortisone, IV aminophylline | 0.5 mL salbutamol (2.5 mg) | 10 | Same as for MgSO₄ group | |
| TOTAL: 2907 randomised to comparisons of interest. 130 withdrawn, 2777 completed | TOTAL: 1476 randomised, 70 withdrawn = 1406 completed intervention | TOTAL: 1431 randomised, 60 withdrawn = 1371 completed control | ||||
| * denotes uncertainty | ||||||
| Study ID (author, date of publication) | Review primary outcomes | Review secondary outcomes | |||||
| FEV1 | PEF | Clinical severity scores | Hospital admissions | Duration of symptoms | Vital signs | Adverse effects | |
| MgSO₄ and SABA and Ipratropium bromide versus SABA and Ipratropium | |||||||
| N | N | Y | N | N | N | Y | |
| N | P | N | N | N | N | P | |
| Y | N | N | N | N | N | Y | |
| Y | N | N | N | N | N | N | |
| N | Y | N | Y | N | Y | Y | |
| N | Y | Y | Y | N | Y | N | |
| N | N | Y | P | N | N | Y | |
| MgSO4 and SABA versus SABA | |||||||
| Y | Y | N | N | N | N | N | |
| N | Y | N | Y | N | Y | Y | |
| N | P | N | N | N | N | N | |
| N | N | Y | P | N | N | Y | |
| Y | Y | N | N | N | Y | N | |
| P | N | N | N | N | N | Y | |
| P | P | N | N | N | N | Y | |
| Y | N | N | Y | N | N | Y | |
| N | N | N | N | N | N | N | |
| N | Y | P | Y | N | N | Y | |
| Y | N | N | Y | N | N | Y | |
| N | Y | Y | N | N | N | N | |
| N | Y | N | Y | N | N | Y | |
| N | Y | Y | N | N | Y | N | |
| N | N | Y | Y | N | N | Y | |
| MgSO₄ versus SABA | |||||||
| P | P | N | N | N | N | Y | |
| N | Y | N | Y | N | N | Y | |
| N | Y | N | N | N | N | Y | |
| N | Y | N | N | N | Y | N | |
| N | Y | Y | N | N | Y | N | |
| N ‒ the study did not report the outcome but it is not clear whether the outcome was measured or not Y ‒ full reporting P ‒ partial reporting | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary function (% FEV1) Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 3.28 [1.06, 5.49] |
| 1.1 90 minutes | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 8.57 [1.99, 15.15] |
| 1.2 120 minutes | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [0.25, 4.95] |
| 2 Pulmonary function % predicted PEF Show forest plot | 2 | 636 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐2.33, 2.42] |
| 3 Clinical severity scores (closest to 60 mins) Show forest plot | 2 | 1130 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.12] |
| 3.1 Yung ASS at 60 minutes | 1 | 472 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.35, 0.02] |
| 3.2 Change in dyspnoea VAS at 60 minutes | 1 | 658 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.02, 0.28] |
| 4 Admission at first presentation Show forest plot | 4 | 1308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.91, 1.00] |
| 4.1 Adults | 3 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.87, 1.03] |
| 4.2 Children | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.92, 1.01] |
| 5 HDU/ITU admission Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Admission to HDU (adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Admission to ICU (adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Admission to PICU/HDU or intubation (children) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Readmission Show forest plot | 2 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.84, 3.87] |
| 7 Respiratory rate at 60 mins Show forest plot | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.14, 1.53] |
| 8 Heart rate at 60 mins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Systolic blood pressure at 60 mins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 Diastolic blood pressure at 60 mins Show forest plot | 1 | 674 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [0.29, 4.51] |
| 11 Serious adverse events (during admission) Show forest plot | 2 | 557 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 11.1 Adults | 1 | 50 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.07, 0.07] |
| 11.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.06, ‐0.01] |
| 12 Any adverse event (during admission) Show forest plot | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 12.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.02, 0.07] |
| 12.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 13 Serious adverse events (within 30 days) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Any adverse event (within 30 days) Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Adverse event: hypotension Show forest plot | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.01, 0.04] |
| 15.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 15.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 16 Adverse event: flushing Show forest plot | 2 | 1197 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 16.1 Adults | 1 | 690 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 16.2 Children | 1 | 507 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary function % predicted FEV1 Show forest plot | 4 | 208 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [‐1.58, 8.26] |
| 1.1 Adults | 3 | 146 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐3.30, 7.67] |
| 1.2 Children | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 8.10 [‐3.03, 19.23] |
| 2 % predicted FEV1: subgroup: severity Show forest plot | 3 | 188 | Mean Difference (IV, Fixed, 95% CI) | 4.12 [‐1.81, 10.06] |
| 2.1 Severe (FEV1 <50% predicted) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [0.05, 19.75] |
| 2.2 Moderate | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐6.59, 8.27] |
| 3 Pulmonary function PEF L/min Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Adults | 3 | 155 | Mean Difference (IV, Fixed, 95% CI) | 11.91 [‐4.12, 27.95] |
| 3.2 Children | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 11.90 [‐6.86, 30.66] |
| 4 Admission to hospital Show forest plot | 6 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.15] |
| 4.1 Adults | 4 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.07] |
| 4.2 Children | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.44, 2.98] |
| 5 Heart rate at 120 mins Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Respiratory rate at 120 mins Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Diastolic blood pressure at 120 mins Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [‐1.35, 2.80] |
| 8 Systolic blood pressure at 120 mins Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [‐2.69, 4.48] |
| 9 Serious adverse events Show forest plot | 5 | 243 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.1 Adults | 4 | 181 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.2 Children | 1 | 62 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 10 Any adverse events Show forest plot | 5 | 694 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 10.1 Adults | 4 | 329 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 10.2 Children | 1 | 365 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical severity score Show forest plot | 3 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.62, 0.36] |
| 1.1 Fischl index final score (120 mins) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.07, 0.41] |
| 1.2 Fischl index score (time point unclear) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.11, 0.71] |
| 1.3 Change in Fischl index at 120 mins | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.67, 1.27] |
| 2 Admission to hospital Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Heart rate (120 mins) Show forest plot | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 21.20 [0.17, 42.23] |
| 4 Respiratory rate Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.91, ‐0.89] |
| 5 Systolic pressure (120 mins) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Diastolic pressure (120 mins) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Serious adverse events Show forest plot | 2 | 53 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 8 Mild‐Moderate Side Effects Show forest plot | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |

