Oxigenoterapia para la fibrosis quística
Resumen
Antecedentes
Las complicaciones más graves de la fibrosis quística (FQ) se relacionan con la insuficiencia respiratoria. Durante décadas el tratamiento de administración de oxígeno suplementario ha sido la atención estándar para los individuos con enfermedades pulmonares crónicas asociadas con hipoxemia. Los médicos les suelen prescribir oxigenoterapia a los pacientes con FQ cuando se produce hipoxemia. Sin embargo, no está claro si hay evidencia empírica disponible para proporcionar indicaciones para este tratamiento debido a sus costes económicos y la frecuente repercusión profunda sobre el estilo de vida.
Objetivos
Evaluar si la oxigenoterapia mejora la longevidad o calidad de vida de los pacientes con FQ.
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos del Grupo Cochrane de Fibrosis Quística (Cochrane Cystic Fibrosis and Genetic Disorders Group) que incluían referencias identificadas de búsquedas exhaustivas en bases de datos electrónicas o búsquedas manuales de revistas relevantes y libros de resúmenes de actas de congresos.
Última búsqueda en el registro de ensayos del Grupo: 15 de mayo 2013.
Criterios de selección
Ensayos controlados aleatorizados o cuasialeatorizados que compararon el oxígeno, administrado en cualquier concentración, por cualquier vía, en pacientes con FQ documentada durante cualquier período de tiempo.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron el riesgo de sesgo de los estudios incluidos y extrajeron los datos.
Resultados principales
Esta revisión incluye 11 estudios publicados (172 participantes); solo uno examinó la oxigenoterapia a largo plazo (28 participantes). No hubo una mejora estadísticamente significativa en la supervivencia, la salud pulmonar o la cardíaca. Hubo una mejora en la asistencia regular a la escuela o al trabajo a los seis y 12 meses en los que recibieron oxigenoterapia. Cuatro estudios examinaron el efecto de la suplementación de oxígeno durante el sueño mediante polisomnografía. Aunque la oxigenación mejoró, se observó una leve hipercapnia. Los participantes se durmieron más rápidamente y pasaron un porcentaje reducido del tiempo total de sueño en sueño REM, pero no hubo mejoras demostrables en los parámetros cualitativos del sueño. Seis estudios evaluaron la suplementación de oxígeno durante el ejercicio. Una vez más, la oxigenación mejoró, pero se produjo una leve hipercapnia. Los participantes que recibieron oxigenoterapia pudieron hacer ejercicio durante un tiempo significativamente mayor. La administración de oxígeno no modificó otros parámetros del ejercicio.
Conclusiones de los autores
No hay datos publicados para guiar la indicación de suplementación de oxígeno crónico a pacientes con enfermedad pulmonar avanzada debido a FQ. La oxigenoterapia a corto plazo durante el sueño y el ejercicio mejora la oxigenación, pero se asocia con hipercapnia moderada, probablemente sin trascendencia clínica. Hay mejoras en la duración del ejercicio, el tiempo para dormirse y la asistencia regular a la escuela o al trabajo. Es necesario realizar ensayos clínicos más grandes y bien diseñados para evaluar los efectos beneficiosos de la oxigenoterapia a largo plazo en pacientes con FQ, administrada de manera continua o durante el ejercicio o el sueño, o ambos. Sin embargo, no se espera que se realicen nuevos estudios de investigación en esta área en el futuro próximo y no está planificado actualizar esta revisión hasta que se disponga de nueva evidencia.
Resumen en términos sencillos
Oxígeno adicional en el tratamiento de la fibrosis quística
Los pacientes con fibrosis quística (FQ) sufren de problemas respiratorios. Brindar oxígeno adicional ha sido durante mucho tiempo la norma de la atención para los pacientes con enfermedades pulmonares crónicas. Es frecuente que los médicos prescriban este tratamiento a los pacientes con FQ cuando no hay suficiente oxígeno en la sangre. Se examinó la evidencia de que este tratamiento mejora la duración y la calidad de vida de los pacientes con FQ. Se encontraron once estudios con 172 participantes para su inclusión en la revisión. Todos los estudios compararon el oxígeno de bajo flujo con el aire de la habitación. Diez de los estudios fueron a corto plazo. Cuatro de los estudios analizaron la administración de oxígeno adicional durante la noche. Durante la noche, los niveles de oxígeno se elevaron durante el sueño con movimientos oculares rápidos (REM, por sus siglas en inglés) y durante el sueño no REM en los pacientes que respiraron oxígeno de bajo flujo. Los participantes que respiraron oxígeno por la noche también pasaron menos tiempo en sueño REM y tardaron menos en dormirse. Seis de los estudios examinaron el efecto del oxígeno extra sobre el ejercicio. Los niveles de oxígeno y dióxido de carbono en la sangre de los participantes aumentaron durante o después del ejercicio cuando respiraron oxígeno de bajo flujo. Los pacientes pudieron hacer ejercicio durante más tiempo cuando respiraron oxígeno de bajo flujo. Hubo una asistencia más regular a la escuela o al trabajo en los que recibieron oxígeno a largo plazo. Hay poca evidencia que apoye o refute la administración a largo plazo de la oxigenoterapia en pacientes con enfermedad pulmonar avanzada por FQ. A corto plazo, el tratamiento ha demostrado cierta mejora en los niveles de oxígeno en la sangre de los pacientes con FQ durante el sueño y el ejercicio. Este aumento de oxígeno también se produjo con un aumento de los niveles de dióxido de carbono, que probablemente no es clínicamente importante. Sin embargo, hay que tener cuidado con los pacientes que tienen una enfermedad pulmonar avanzada, ya que pueden necesitar una mayor monitorización. Se deberían investigar los efectos del tratamiento con oxígeno a largo plazo sobre la calidad del sueño y el ejercicio en pacientes con FQ. Desafortunadamente, no se espera que se realicen estudios de investigación de este tipo en un futuro próximo, por lo que no está previsto volver a actualizar esta revisión hasta que se encuentren nuevos ensayos.
Authors' conclusions
Background
Description of the condition
Cystic fibrosis (CF), the most common life‐threatening genetic illness in Caucasians, is a chronic disease affecting many secretory organs primarily the lungs and organs involved in gastro‐intestinal tract function. With advances in therapy, there has been marked improvement in survival (Fitzsimmons 1996; Frederiksen 1996). However, chronic and recurrent airway‐based infection leads to progressive lung damage, bronchiectasis, and fibrosis (Ramsey 1996). These changes ultimately result in chronic hypoxemia (that is, the condition in which the partial pressure of oxygen in the blood of a person breathing room air is less than 60 millimeters of mercury (mm Hg) (equivalent to 8.0 kilopascal (kPa)) or the oxygen saturation of arterial hemoglobin is less than 90%) and eventually can lead to pulmonary hypertension (an abnormal persistent elevation of the blood pressure within the pulmonary blood vessels) and cor pulmonale (right sided heart failure). Progressive destruction of lung parenchyma, pulmonary vasculature and pulmonary vasoconstriction secondary to chronic hypoxemia may all play a role in the development of pulmonary hypertension in CF (Fraser 1999).
It is important to acknowledge that there is no universally accepted method of detecting or quantitating hypoxemia in CF or other chronic lung diseases. First, transcutaneous pulse oximetry (reported as the percentage of saturation of arterial hemoglobin by oxygen (SaO2)) has become the most common method in clinical practice and research for measuring oxygenation even though partial pressure of oxygen tension measured on an arterial blood gas is a more accurate measure of oxygenation. Within the literature regarding CF, there have been varied measures of oxygenation including the following: minimum SaO2 (Tepper 1983); a lowered mean SaO2 (Coffey 1991; Milross 2001a); percentage of time spent with SaO2 below 90% (Frangiolis 2001); or a fall in baseline SaO2 of ≥ 4% (Narang 2003). This lack of uniformity in definition makes comparison among studies even more difficult. Lastly, gas exchange is a dynamic phenomenon over time when substantial parenchymal lung disease is involved, indicating that the more chronic the study, the more difficult to quantify the adequacy or stability of gas exchange. Nonetheless, oxygen therapy for individuals with hypoxemic lung disease is commonly prescribed and its effects worthy of precise measurement and analysis.
How the intervention might work
The efficacy of chronic oxygen supplementation in people with chronic obstructive pulmonary disease (COPD) and severe hypoxemia has been well demonstrated. The 'British Medical Research Council Trial' randomized people with COPD and severe hypoxemia to receive either 15 hours of oxygen therapy daily or no oxygen (MRC 1981). Similarly, the 'Nocturnal Oxygen Therapy Trial' in the USA randomized participants with severe COPD and chronic hypoxemia to receive either 12 hours or 24 hours continuous oxygen therapy (NOTTG 1980). In both studies, mortality in the treatment group was significantly lower than that seen in the control or lesser‐treated group. Thus, it appears that improvement in survival in individuals with chronic hypoxemia and COPD is related to the use of daily oxygen therapy in people with partial pressure of oxygen in the blood (PaO2) < 55 mm Hg (7.3 kPa). More recent studies have failed to show a survival benefit in people with COPD with milder degrees of hypoxemia (PaO2 56 to 65 mm Hg) (7.4 to 8.7 kPa) (Gorecka 1997).
Oxygen therapy is used in people with CF, particularly in those with chronic hypoxemia, to relieve symptoms of dyspnea and fatigue and to retard the development of cor pulmonale (right heart failure) (Schidlow 1993). However, chronic oxygen therapy has its own costs. The estimated cost of chronic oxygen therapy in the USA in 1995 was $1.8 billion (Tarpy 1995). Oxygen supplementation may be associated with complications or adverse effects, including suppression of respiratory drive in people with hypercarbia (elevated blood carbon dioxide level), psychological sequelae, and decreased mobility due to the tethering of an individual to a device or tank. It remains unclear whether oxygen therapy alleviates symptoms, alters the natural disease progression or reliably accomplishes any benefit in people with CF. In addition, it is unknown whether oxygen supplementation may be beneficial alone or when used in conjunction with non‐invasive ventilation.
An important specific aspect of prescribed oxygen therapy is the context of air travel. Commercial airplanes pressurize cabins to a pressure commensurate with approximately 2500 meters or 8000 feet, which results in modest and clinically insignificant drops in oxygenation in normal individuals. Although there are publications with recommendations regarding the requirement of oxygen therapy in individuals with hypoxemia due to underlying lung disease (Gong 1992), consideration of oxygen therapy during air travel for the individual with CF has not been the subject of study.
Why it is important to do this review
Pulmonary hypertension and cor pulmonale are believed to be pre‐terminal events in the majority of people with CF who die of their respiratory disease. Respiratory failure (the inability of the respiratory system to meet the metabolic demands of the body for oxygen intake and carbon dioxide excretion, leading to hypoxemia and/or elevated partial pressures of carbon dioxide in the blood, usually greater than 50 mm Hg (6.7 kPa)) is the usual mechanism of death. Sleep and exercise are the conditions during which individuals with lung disease are particularly at risk for compromised gas exchange. In chronic severe lung disease of all sorts, including CF, hypoventilation (reduced depth of or frequency of breathing, or both, leading to accumulation of carbon dioxide in the blood) and worsened ventilation perfusion mismatching (an imbalance between air flowing into the air sacs of the lung and the blood flow through the lung which leads to abnormal gas exchange) during sleep often leads to worsened gas exchange and resulting in varying degrees of nocturnal hypoxemia and hypercapnia (Milross 2001b).
It is therefore important to determine whether oxygen therapy alleviates symptoms or reliably accomplishes any benefit in people with CF.
Objectives
To determine whether the use of oxygen therapy in people with CF is associated with changes in mortality, morbidity, quality of life (including sleep quality) or with any adverse effects.
Furthermore, we shall search for evidence as to whether the amount of oxygen supplementation, duration (hours per day), and mode of oxygen delivery determine efficacy of clinical effect.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled studies.
Types of participants
Children and adults with CF diagnosed clinically and by sweat or genetic testing including all ages and all degrees of severity.
Types of interventions
Studies examining therapy with oxygen supplementation in which outcomes are compared with air, for any duration, administered by any means including nasal cannula, face mask, with or without additional ventilatory support such as bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP).
In studies which involve non‐invasive ventilatory support, we will focus predominantly on the impact of oxygen therapy insofar as it can be separated from the actual assistance provided by the mechanical support itself. Studies in which oxygen is administered as therapy for acute respiratory failure or acute hypoxemia will be excluded.
Types of outcome measures
Primary outcomes
-
Survival
-
Changes in lung function (including forced expiratory volume at one second (FEV1) and forced vital capacity (FVC))
-
Changes in gas exchange (including oxygen saturation as measured by pulse oximetry and arterial blood gases)
Secondary outcomes
-
Quality of life (as measured by standardized validated questionnaires)
-
Sleep parameters (as measured by polysomnography)
-
Exercise tolerance (as measured by exercise testing)
-
Nutritional status (as assessed by height, weight and body mass index (BMI) and z scores)
-
Changes in pulmonary artery pressure (as measured by trans‐thoracic or trans‐esophageal echocardiography)
-
Cost of intervention
-
Adverse effects of therapy
Outcome data were grouped into those measured at one, three, six, twelve months and annually thereafter if available. If outcome data were recorded at other time periods then consideration was given to examining these as well.
Search methods for identification of studies
Electronic searches
Relevant studies were identified from the Group's Cystic Fibrosis Trials Register using the term: oxygen and ventilatory support.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (Clinical Trials) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of EMBASE to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's Cystic Fibrosis Trials Register: 15 May 2013.
Searching other resources
In addition the reference lists of all publications found by the above methods were searched for additional references.
Data collection and analysis
Selection of studies
The authors (GM, DV, JF, HE) independently selected the studies to be included in the review (seeContributions of authors). If disagreement arose on the suitability of a study for inclusion in the review, we reached a consensus by discussion.
Data extraction and management
The authors obtained complete texts of all published studies and also texts of ambiguous studies based on title and abstract. Any non‐English language studies obtained were translated into English. The authors independently reviewed each paper in order to ascertain which were relevant to this analysis. Each author, using standard data acquisition forms, independently extracted data. If disagreement arose on the quality of a study, we reached a consensus by discussion.
Assessment of risk of bias in included studies
The authors assessed the risk of bias using the six domains described in the Cochrane Handbook for Systematic Reviews of Interventions (generation of sequence, allocation concealment, blinding, incomplete outcome data, selective reporting and other potential sources of bias) (Higgins 2009). They assessed the risk of bias as high, low or unclear.
Measures of treatment effect
The authors sought data on the number of participants by allocated treated group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up in order to allow an intention‐to‐treat analysis.
For binary outcome measures, we calculated a pooled estimate of the treatment effect for each outcome across studies and reported the odds ratio (OR) (the odds of an outcome among treatment allocated participants to the corresponding odds among controls) and the 95% confidence intervals (CIs) as generated from the meta‐analyses.
For continuous outcomes, we recorded either mean change from baseline for each group or mean post‐treatment or post‐intervention values and standard deviation for each group. Then, where appropriate, we calculated a pooled estimate of treatment effect by calculating the mean difference (MD) and reported this with the 95% CIs as generated by the meta‐analyses. Three studies originally presented data as mean with standard error of the mean (Marcus 1992: McKone 2002; Parsons 1996). We have calculated the standard deviation (SD) from the standard error of the mean (SEM) in order to combine data from these studies in the meta‐analyses.
Where studies measured data longitudinally, we based the analysis on the mean change from baseline at either 6 or 12 months or both if available. As only one long‐term study was identified, the final time point results were used for the majority of studies which were short term. By treating the time points as independent, the correlation between the time points was assumed to be zero.
Unit of analysis issues
Ideally when conducting a meta‐analysis combining results from cross‐over studies, we would have liked to use the methods that are recommended by Elbourne (Elbourne 2002) and Curtin (Curtin 2002). Due to restrictions on the data that were available from the papers, the only method that we were able to use was to treat the cross‐over studies as if they were parallel studies. Elbourne says that this approach will produce conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant will appear in both the treatment and control group, so the two groups will not be independent.
Dealing with missing data
We did not contact any authors for clarification of published results or missing data. In future, if data are missing we would aim to contact the original investigators.
Assessment of heterogeneity
If for future updates of the review we are able to combine data from a sufficient number of trials (at least four), we will test for heterogeneity between trial results using a standard chi‐squared test and the I2 statistic (Higgins 2003). This value describes the percentage of total variation across studies that are due to heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%, and a simplified categorization of heterogeneity that we plan to use is of low (I2 value of 25%), moderate (I2 value of 50%), and high (I2 value of 75%) (Higgins 2003).
Assessment of reporting biases
Publication bias may have been a factor in our review because of the possibility of small unpublished studies which could not be identified in the search. This could be investigated using a funnel plot in the future if more studies are included.
Data synthesis
We have used a fixed‐effect model in our analysis. We planned to use this analysis where there was a low or moderate degree of heterogeneity as defined above. If the I2 statistic showed a high degree of heterogeneity we would have analysed the results using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
If we had been able to combine a sufficient number of studies (at least four) and had identified significant heterogeneity (a value of I2 over 50%), we planned to investigate this by a subgroup analysis according to the degree of disease severity (where FEV1 70% to 80% will be considered mild; 60% to 70% moderate; 50% to 60% moderately severe; 34% to 50% severe; and less than 34% very severe (ATS 1991)). This is a post‐hoc change since development of the original protocol.
Sensitivity analysis
If we had been able to combine a sufficient number of studies we would perform a sensitivity analysis to determine the robustness of our results as follows:
-
with and without quasi‐randomised studies;
-
with and without studies with unclear allocation concealment.
Results
Description of studies
Please see the list of abbreviations in the 'Additional tables' section of the review (Table 1).
| Abbreviations | Definition |
| BiPAP | bileval positive airway pressure |
| BMI | body mass index |
| CO2 | carbon dioxide |
| COPD | chronic obstructive pulmonary disease |
| CPAP | continuous positive airway pressure |
| ETCO2 | end tidal carbon dioxide tension |
| FEV1 | forced expiratory volume in one second |
| FiO2 | fraction of inspired oxygen, or percent of oxygen in the inspired gas |
| FVC | forced vital capacity |
| kPa | kilopascal, a unit of air pressure |
| LVEF | left ventricular ejection fraction |
| mm Hg | millimeter of mercury, a unit of air pressure |
| MVV | maximal voluntary ventilation |
| NIPPV | non‐invasive positive pressure ventilation |
| NREM | non‐rapid eye movement |
| PaCO2 | arterial carbon dioxide tension |
| PaO2 | partial pressure of oxygen in the blood |
| PCO2 | partial pressure of carbon dioxide in the blood |
| PETCO2 | maximum partial pressure of carbon dioxide exhaled during a tidal breath, just prior to inspiration |
| REM | rapid eye movement |
| RVEF | right ventricular ejection fraction |
| SaO2 | saturation of haemoglobin with oxygen |
| SpO2 | pulse oximetry |
| TcCO2 | transcutaneous carbon dioxide |
| tPCO2 | transcutaneous partial pressure of carbon dioxide in the blood |
| VCO2 | carbon dioxide production |
| VE | mean total ventilation |
| VO2 | oxygen consumption |
Results of the search
The original searches identified 15 studies for consideration. These studies examined the use of oxygen therapy in CF either acutely or chronically. Nine studies were included (Gozal 1997; Marcus 1992; McKone 2002; Milross 2001b; Nixon 1990; Parsons 1996; Shah 1997; Spier 1984; Zinman 1989); one study was excluded (Serra 2002); the remaining five studies were listed as 'Awaiting classification'. These five studies were assessed for the review updated in 2008 (Barker 1998;Fauroux 2001;Fauroux 2004;Holland 2003;Stewart 2001); of these, four were excluded and the remaining study was eligible for inclusion (Barker 1998). Another study, in a more recent search, has been assessed as eligible for inclusion in the review (Falk 2006). In total, eleven studies with 172 participants are now included in this review (Barker 1998; Falk 2006; Gozal 1997;Marcus 1992;McKone 2002;Milross 2001b;Nixon 1990;Parsons 1996;Shah 1997;Spier 1984;Zinman 1989). Details of the methods, participants, interventions, and outcomes are shown in the Characteristics of included studies section of the review.
The search run in 2010 identified six new studies, all of which were excluded. No new studies were identified for the 2013 update.
Included studies
Study design
Only one study was a parallel study (Zinman 1989); the remainder being cross‐over in design. With the exception of the Zinman paper, which followed participants for over two years (Zinman 1989), all other studies only examined the efficacy of short‐term oxygen (less than two days), either administered during exercise or overnight during sleep.
Participant characteristics
The diagnosis of CF in all participants in all 11 studies was based on a positive sweat test, typical respiratory or digestive symptoms or a positive family history, or both.
All 11 studies included participants with moderate to severe obstructive lung disease. Of these, four studies included participants with severe obstructive lung disease with baseline measurements of FEV1 reported as follows: two studies reported the mean (SEM) 31 (3) % predicted (Parsons 1996) and 62 (5) % predicted (Shah 1997); one study reported the mean (SD) 29.4 (3.4) % predicted (Gozal 1997); and the remaining study reported just the mean 41% predicted (McKone 2002).
In six studies, specific criteria for lung function or gas exchange were included in the methodology (Falk 2006; Marcus 1992; Milross 2001b; Nixon 1990; Spier 1984; Zinman 1989). Two papers based participant selection on blood gas parameters (Marcus 1992; Spier 1984). In one study participants were included if an arterial blood gas on room air showed a SaO2 less than 92% (Spier 1984). In the Marcus study, participants were included if they had a resting PaO2 less than 70 mm Hg (equivalent to 9.33 kPa), when the arterial carbon dioxide tension (PaCO2) was greater than 45 mm Hg (6 kPa) on room air, or if they had required multiple hospital admissions (Marcus 1992). Three papers used spirometry as an inclusion criterion (Falk 2006; Milross 2001b; Nixon 1990). Participants were included in the Milross study if FEV1 was less than 65% predicted (Milross 2001b) and in the Falk study if FEV1 was between 30 and 70% predicted (Falk 2006). The Nixon paper enrolled equal numbers of participants with FEV1 under 50% and over 50% predicted (Nixon 1990). The Zinman paper enrolled participants based on a combination of spirometry and blood gas measurements, requiring a FEF25‐75 less than 25% predicted or an arterialized capillary blood gas measurement with a PO2 less than 65 mm Hg (8.67 kPa) on two occasions, one week apart (Zinman 1989). The remaining five studies did not state inclusion criteria for lung function (Barker 1998; Gozal 1997; McKone 2002; Parsons 1996; Shah 1997).
Interventions
During sleep
Four studies reported the effects of nocturnal oxygen supplementation on sleep quality and gas exchange during sleep in the short term (Gozal 1997; Milross 2001b; Parsons 1996; Spier 1984). There were differences in the amount of supplemental oxygen given and in the method of delivery of the oxygen. Two studies compared placebo (room air), low‐flow oxygen and oxygen administered via non‐invasive positive pressure ventilation (NIPPV) (Gozal 1997; Milross 2001b). One of these compared the effects of room air, low‐flow nocturnal oxygen and an average of 2.2 L/min oxygen delivered via the NIPPV circuit (Gozal 1997). The second study compared the effects of room air, low‐flow oxygen supplementation titrated to keep SaO2 greater than 90% administered via face mask with low pressure continuous positive airway pressure (CPAP) of 4 to 5 cm H2O and bilevel ventilatory support with or without oxygen to maintain SaO2 greater than 90% (Milross 2001b). From these two studies, we excluded the data which examined the effects of NIPPV from our analysis (Gozal 1997; Milross 2001b). The remaining two studies compared nocturnal low‐flow oxygen and room air (Parsons 1996; Spier 1984). The amount of supplemental oxygen was not stated for one study (Parsons 1996). In the other study, humidified oxygen or compressed air were administered via nasal prongs at 2 L/min (Spier 1984).
One study looked at the effects of long‐term oxygen therapy in hypoxemic CF participants during sleep (Zinman 1989). Twenty‐eight children and adults were enrolled in three Canadian centers. Participants were randomized to receive either oxygen supplementation to achieve a PaO2 of 70 mm Hg or room air administered from a concentrator type device in the home. Treatment was administered during sleep via facemask or nasal cannula. A limitation of the study was the failure to assess the adequacy of oxygenation during sleep with the prescribed therapy. Participants had the option of changing the route of administration, in which case the flow of oxygen necessary to maintain a PaO2³ 70 mm Hg was recalculated. During hospitalizations, the participant's physician determined oxygen requirements. Adherence to therapy was determined by correlating participant logs with timers on the concentrators (Zinman 1989). The aim of the study was to determine the impact of chronic oxygen therapy in advanced CF lung disease on morbidity and mortality. Outcomes measured included death, measures of pulmonary function, anthropometric measurements, exercise tests and radionuclide angiography to assess right heart function. Structured questionnaires were used to assess cognitive function, memory capacity and the participants' sense of self‐esteem. The study design called for outcome variables to be measured at intervals up to 36 months post‐enrolment (Zinman 1989).
During exercise
Six studies looked at the effects of oxygen therapy on exercise; five studies evaluated the effects of short‐term oxygen supplementation on exercise tolerance (Barker 1998; Falk 2006; Marcus 1992; McKone 2002; Nixon 1990) and one study examined the ability of oxygen to enhance recovery from exercise and to improve performance of a subsequent bout of exercise (Shah 1997). None of these studies used control groups but instead looked at participants with CF with and without oxygen supplementation.
The six studies used different oxygen concentrations: five used fraction of inspired oxygen (FiO2) 1.00 (Shah 1997); FiO2 0.30 (Marcus 1992; Nixon 1990); FiO2 0.39 (McKone 2002) and FiO2 0.4 (Barker 1998). The sixth study considered participants at sea level and at Dead Sea level (396 m below sea level) (Falk 2006). The elevated barometric pressure at low altitude was used as a method of increasing the quantity of inspired oxygen and therefore the exact amount of oxygen supplementation can not be quantified (Falk 2006).
Different exercise protocols were used in these six studies. In one study, the participants performed initially to maximal exercise capacity and then underwent two supramaximal (130% of the workload calculated during maximal exercise) exercises 10 minutes apart; after one of which they breathed room air and after the other FiO2 of 1.0 (Shah 1997). Two studies used a protocol of two progressive exercise tests in which the participants exercised to exhaustion (Marcus 1992; Nixon 1990). In a further study, individuals exercised at 80% of maximal workload to exhaustion (McKone 2002). In another study, a graded submaximal test at each of three velocities, followed by a maximal exercise test, followed by a six‐minute walk test were performed (Falk 2006). The final study used submaximal cycle ergometry using pseudo‐random binary sequences of workload (PRBS) (Barker 1998).
The participants in each of the six exercise studies were randomized to receive room air during one exercise and oxygen supplementation during the second session. One of these studies split participants into two groups based on whether or not they desaturated during exercise while breathing normal air (Nixon 1990). If their peak SaO2 remained above 90% during exercise, participants were allocated to the "High Sat" group, but if their peak SaO2 was measured at 90% or below, they were allocated to the "Low Sat" group (Nixon 1990). It is unclear whether this was an a priori division or was made on retrospective analysis (Nixon 1990). We did not anticipate such a division and this is a post hoc change for this review.
Measured parameters in the five studies looking at short‐term oxygen supplementation on exercise tolerance included heart rate, maximal oxygen uptake, maximal carbon dioxide (CO2) production, peak minute ventilation and arterial oxyhemoglobin saturation (Barker 1998; Falk 2006; Marcus 1992; McKone 2002; Nixon 1990). The Barker study measured respiratory exchange ratio and spectral analysis of the PRBS data (Barker 1998). In addition, the Falk study measured peak oxygen consumption (VO2pk), ventilatory equivalent (Ve/VO2), ventilatory reserve utilisation (VE/MVV), blood lactate (La) and a rating of overall perceived exertion (Falk 2006). Maximum partial pressure of carbon dioxide exhaled during a tidal breath (PETCO2) was measured in two studies (Marcus 1992; McKone 2002) and transcutaneous PCO2 (tPCO2) was also measured in two studies (Marcus 1992; Nixon 1990).
Excluded studies
In total 11 studies have been excluded. One study was excluded immediately from the original search because it involved the use of oxygen only in conjunction with assisted ventilation (Serra 2002). Four studies were excluded because they were comparing the use of oxygen in conjunction with two modes of ventilation (Elkins 2004; Fauroux 2001; Fauroux 2004; Young 2008). Two studies were excluded as they compared chest physiotherapy with or without pressure support (Fauroux 1999; Placidi 2006). Another study was excluded because it was comparing two methods of airway clearance (Holland 2003). The ninth study was excluded because it was comparing compressed air with added nitric oxide (Stewart 2001). The remaining two studies were excluded as they looked at aerosolisation with or without pressure support (Fauroux 2000) and the use of dornase alfa with or without pressure support (Riethmueller 2006).
Risk of bias in included studies
Methodological information was abstracted from all studies, and assessed for risk of bias independently by the authors (seeContributions of authors).
Allocation
Randomization
All studies reported that the order of interventions was randomized, but no study reported the method of randomization used. The risk of bias for randomization for all studies is therefore uncertain.
Concealment of allocation
No study reports give details of concealment of allocation and so the risk of bias has been graded as uncertain in the tables for each study (Characteristics of included studies).
Blinding
Due to the nature of the intervention it is difficult to blind participants and physicians to treatment.
Blinding of Participants
Two studies were judged as not having blinded the participants to the gas mixture administered. One study took place at two locations, one at sea level and one at the Dead Sea; therefore participants could not be blinded (Falk 2006). Another study states that participants were aware of the nature of the intervention on the second and third nights of the study (oxygen or NIPPV) due to the interface used (Gozal 1997). These studies were judged to have a high risk of bias from blinding.
Five studies were judged as having blinded participants to the type of therapy administered and to have a low risk of bias (Marcus 1992; McKone 2002; Shah 1997; Nixon 1990; Zinman 1989).
The other four studies did not discuss the degree of blinding used and were judged as unclear (Barker 1998; Milross 2001b; Parsons 1996; Spier 1984).
Blinding of Clinicians
Two studies were judged as not having blinded the clinicians to the gas mixture administered, for the same reasons as the participants stated above, and judged to have a high risk of bias (Falk 2006; Gozal 1997). One study did not blind the primary investigator (McKone 2002).
Two studies were judged as having blinded the supervising clinicians to the type of therapy administered (Nixon 1990; Zinman 1989), although this was only the case for the participants at home in one study (Zinman 1989).
The other six studies did not discuss the degree of blinding used and were judged as having an uncertain risk of bias (Barker 1998; Marcus 1992; Milross 2001b; Parsons 1996; Shah 1997; Spier 1984).
Blinding of Outcome Assessors
All of the studies were judged as having an uncertain risk of bias from the blinding of outcome assessors. In one study it was unclear whether the supervising physician was also the outcome assessor (McKone 2002) and in the remaining 10 studies, blinding of assessors was not discussed (Barker 1998; Falk 2006; Gozal 1997; Marcus 1992; Milross 2001b; Nixon 1990; Parsons 1996; Shah 1997; Spier 1984; Zinman 1989) .
Incomplete outcome data
No study explicitly states that an intention‐to‐treat analysis was conducted.
No study was judged to have a high risk of bias due to incomplete outcome data reporting.
Seven of the studies were judged to have a low risk of bias due to incomplete outcome data reporting. In three studies, data appear to have been analyzed based on all enrolled participants; these studies state that all participants completed all the exercises and therefore were judged to have a low risk of bias (Barker 1998; McKone 2002; Shah 1997). In another study, data were analyzed on all enrolled participants; however, two participants were also analyzed as a separate group due to obstructive sleep apnoea symptoms; this study was also considered to be low risk (Spier 1984). In a fifth study, data from three participants were not analysed as they were unable to complete the exercise tests (Falk 2006). Since a reason was given for non‐inclusion, this also was judged as having a low risk of bias. In one further study, six out of eight participants completed the study; two participants were enrolled, but later excluded from the study as they did not tolerate NIPPV (Gozal 1997). As these participants were withdrawn due to the NIPPV treatment rather than the oxygen treatment, this was judged as having a low risk of bias for the purposes of this review. In the final study, the data were analyzed both on an intention‐to‐treat basis and on the 18 participants who completed the study (10 participants dropped out ‐ four from the oxygen group and six from the room air group) (Zinman 1989). Although a rise in PaCO2 greater than 5 mm Hg was determined a priori to be grounds to exclude a participant from the study, there were no exclusions for this reason because this eventuality did not occur. The follow‐up of all participants with respect to survival data was complete (Zinman 1989).
The remaining studies were judged to have an uncertain risk of bias due to incomplete outcome data reporting. Two studies analyzed data on participants with less than 15% exclusions (Marcus 1992; Nixon 1990). In one of those studies, all but one participant completed the exercise tests; it is not stated whether the results were analyzed to include this participant (Marcus 1992). In the second of those studies, data from two participants were not analyzed, as they were too young to co‐operate sufficiently; it was unclear as to whether these two participants were from the same group and therefore unclear as to whether their withdrawal led to a risk of bias (Nixon 1990). In one study 11 participants underwent full nocturnal polysomnography, but it is not clear if the results reported are from all participants or just the seven who had arterial blood gas measurements taken prior to the first part of the study and after each subsequent part (Parsons 1996). In another study there were incomplete arterial blood gas measurements for four participants and it is unclear if the results reported are for all participants or not (Milross 2001b).
Selective reporting
All studies were judged as uncertain for selective reporting bias as no study mentioned all outcomes. If parameters were not mentioned they were assumed not to have been measured.
Survival data, changes in lung function, changes in gas exchange, quality of life, Wmax, nutritional status and changes in pulmonary artery pressure were reported in full in one study (Zinman 1989). Changes in lung function, changes in gas exchange and exercise tolerance reported in a further study (Falk 2006). Changes in gas exchange and sleep parameters were reported in full in three studies (Gozal 1997; Parsons 1996; Spier 1984). Changes in gas exchange and exercise tolerance were reported in full in four studies (Marcus 1992; McKone 2002; Nixon 1990; Shah 1997).
Barker reported changes in gas exchange and exercise tolerance but no statement of statistical significance was given (Barker 1998). Data were reported for gas exchange and sleep parameters by Milross (Milross 2001b). However, upon inspection the data appear skewed and could not be included in meta‐analysis. Lung function data were measured by McKone but analysis not reported (McKone 2002). This may have been because results were not significant.
Other than in the Zinman paper, survival was not specifically mentioned but data were present for all patients at the end of the study in each paper and it was assumed therefore that survival was not mentioned because all patients survived.
Other potential sources of bias
There were no other potential sources of bias identified in any of the studies. These were all graded as uncertain as it is not possible to ascertain for certain whether other biases were present.
Effects of interventions
Please see the full list of abbreviations in the 'Additional tables' section of the review (Table 1). Only significant results are reported.
Primary outcomes
1. Survival
Long‐term oxygen therapy, as defined in the landmark studies in the 1980s, has never been studied in CF. The long‐term use of nocturnal oxygen supplementation had no discernible effect on mortality (Zinman 1989). Based on intention‐to‐treat, Zinman reported 4 out of 14 (29%) deaths in the oxygen group and 4 out of 14 (29%) deaths in the placebo group (Zinman 1989). Of those who completed the study, there were 2 out of 10 deaths (20%) in the oxygen group as compared to 0 out of 8 (0%) deaths in the air group. Two participants in each group were withdrawn in order to receive oxygen therapy for declining clinical status. When these data are entered into the graphs they give a non‐significant result (Analysis 1.1).
2. Changes in lung function
Only one study reported on changes in lung function; this was the study which examined the effects of long‐term nocturnal oxygen therapy (Zinman 1989). There was no significant change in FEV1 after either six months or after 12 months (Analysis 1.2). Furthermore, there was no significant difference in FVC after six months or after 12 months (Analysis 1.3).
3. Changes in gas exchange
Eight studies reported changes in gas exchange in people with CF with and without supplementary oxygen therapy, of which seven reported short‐term changes after exercise (Falk 2006; Marcus 1992; McKone 2002; Nixon 1990; Shah 1997) or sleep (Gozal 1997; Parsons 1996; Spier 1984). The eighth study reported changes in PO2 and PCO2 on blood gases after 12 months of nocturnal oxygen therapy (Zinman 1989).
Data from the Marcus, McKone and Parsons studies were originally presented as mean (standard error of the mean). We therefore calculated the standard deviation using the formula SD = SE x square root of n, to enable data to be used in the meta‐analysis (Marcus 1992; McKone 2002; Parsons 1996). Data were only entered for the "High Sats" group in the Nixon study unless stated otherwise, as the "High Sats" and "Low Sats" data could not be combined (Nixon 1990).
a. Changes in gas exchange during exercise
i. Gas exchange at the end of submaximal exercise
Three studies reported absolute values for SaO2 post exercise (McKone 2002; Nixon 1990; Falk 2006). There was a significant improvement in SaO2 levels with administration of supplementary oxygen after submaximal exercise, MD 2.11 (95% CI 1.54 to 2.68) (P < 0.00001). There was no significant difference in post‐exercise end‐tidal CO2 tension (PET CO2) (Analysis 1.4).
ii. Gas exchange during maximal exercise
Four studies reported measures of gas exchange during maximal exercise (Falk 2006; Marcus 1992; Nixon 1990; Shah 1997) (Analysis 1.4). The Marcus study reported changes in SaO2, PET CO2 and transcutaneous CO2 tension (tPCO2) during maximal (peak) exercise (Marcus 1992). Nixon reported pooled changes in PET CO2 for the "High Sats" and "Low Sats" groups (Nixon 1990). Supplementary oxygen administration during maximal exercise significantly improved all three parameters: SaO2, MD 7.00 (95% CI 2.61 to 11.39) (P = 0.002) (Marcus 1992); PET CO2, MD 3.71 (95% CI 1.28 to 6.14) (P = 0.003) (Marcus 1992; Nixon 1990); and tPCO2, MD 4.00 (95% CI 1.23 to 6.77) (P = 0.005) (Marcus 1992). Three studies reported absolute SaO2 figures during maximal exercise and the use of supplementary oxygen during maximal exercise also significantly improved SaO2, MD 2.01 (95% CI 1.16 to 2.85) (P < 0.00001) (Falk 2006; Nixon 1990; Shah 1997). Additionally, Nixon reports the absolute values of PETCO2 for the "Low Sat" and "High Sat" groups individually. During peak exercise in room air, PETCO2 was significantly higher in the "Low Sat" group compared to the "High Sat" group, mean (SD) 43.00 (8.00) compared to 34.00 (3.00) mm Hg (P < 0.005) (Nixon 1990).
b. Changes in gas exchange during sleep
Three studies examined changes in SaO2 and CO2 levels during sleep (Gozal 1997; Parsons 1996; Spier 1984). Values were reported for rapid eye movement (REM) sleep, non‐REM sleep and total sleep. Since different units were used for recording transcutaneous CO2 tension (tPCO2 in kPa (Gozal 1997) and TcPCO2 in mm Hg (Spier 1984; Parsons 1996)) results could not be combined for the meta‐analysis (Analysis 1.5).
i. Gas exchange during REM sleep
There was a significant improvement in SaO2 during REM sleep with supplementary oxygen, MD 7.54 (95% CI 4.31 to 10.77) (P < 0.00001) (Gozal 1997; Parsons 1996). The transcutaneous CO2 tension (in kPa) was found to be significantly higher with oxygen therapy in the Gozal study, MD 1.00 (95% CI 0.15 to 1.85) (P = 0.02) (Gozal 1997), but no significant difference was seen in the Spier study which recorded values in mm Hg (Spier 1984).
ii. Gas exchange during non‐REM sleep
A significant improvement in SaO2 readings was also seen in non‐REM sleep with supplementary oxygen, MD 6.00 (95% CI 1.92 to 10.08) (P = 0.004) (Gozal 1997). Transcutaneous CO2 levels were not significantly different in the room air versus oxygen therapy groups (Gozal 1997) and (Spier 1984).
iii. Gas exchange during total sleep
In the original paper, Parsons reported a significant increase in tPCO2 in participants treated with oxygen during sleep (Parsons 1996). Results were presented as mean (SEM); we calculated the SDs in order to enter the data into a meta‐analysis. Entering the maximum value for tPCO2 into the data tables did not give a significant result for this outcome. Parsons also reported the change in PaCO2. The increase in PaCO2 was reported as significant in the oxygen night only and changed from a baseline measurement of mean (SEM) 44.00 (1.00) mm Hg to 49.00 (3.00) mm Hg (P = 0.04) (Parsons 1996). No data were presented for the room air night and so we were unable to enter these results into the meta‐analysis.
c. Changes in gas exchange after 12 months
Zinman measured changes in arterial blood gases (PaO2 and PCO2) after 12 months therapy (Zinman 1989). Oxygen flow was titrated to achieve a defined improvement in oxygenation and PaO2 was measured at 12 months on that flow. There was no significant change in PaO2 (Analysis 1.6) or PaCO2 after 12 months (Analysis 1.7).
Secondary Outcomes
1. Quality of life
Only one study investigated the effects of oxygen therapy on psychosocial, cognitive and behavioral aspects of daily living (Zinman 1989). Nocturnal oxygen therapy did not improve mood, esteem, or cognitive function, as determined by standardized questionnaires. Using a non‐standardized structured questionnaire, significantly more participants receiving oxygen reported regular attendance at school or work after 6 and 12 months therapy, 10 out of 14 compared to 3 out of 14 and 10 out of 11 compared to 2 out of 10 respectively. Data at 6 and 12 months are presented in the graphs and give the following significant results, OR 9.17 (95% CI 1.63 to 51.43) and OR 40.00 (95% CI 3.05 to 524.83) respectively (Analysis 1.8). The paper reports that there was no change in maintenance of social contacts, fulfilment of daily management regimen or frequency of discussion of the impact of their illness (Zinman 1989).
2. Sleep parameters
Four studies measured changes in sleep parameters with oxygen therapy compared to room air (Gozal 1997; Milross 2001b; Parsons 1996; Spier 1984) (Analysis 1.9).
The percentage of total sleep time spent in REM sleep was significantly reduced with oxygen therapy compared to room air, MD ‐4.49 (95% CI ‐6.04 to ‐2.95) (P < 0.00001) (Gozal 1997; Parsons 1996; Spier 1984), but this was not the case for the percentage of total sleep time spent in non‐REM sleep (Gozal 1997; Spier 1984) . Sleep latency, ie the time taken to fall asleep after the start of the study, was also significantly reduced with oxygen therapy, MD ‐6.00 (95% CI ‐11.06 to ‐0.94) (P = 0.02) (Gozal 1997). The other sleep parameters that could be presented in the meta‐analysis included total sleep time, (Gozal 1997; Spier 1984) and arousal index (Gozal 1997). Neither of these parameters was significantly affected by the use of oxygen therapy.
Other outcomes were reported narratively by one study with no actual data that could be included in a meta‐analysis (Parsons 1996). This study reported that there was no difference in respiratory disturbance index, sleep fragmentation, sleep efficiency, total sleep time and time in REM between the room air and low‐flow oxygen groups. There was also no reported improvement in subjective sleep quality (Parsons 1996).
Upon inspection, the data from the Milross study appears to be skewed and thus can not be combined with the data from the other studies in a meta‐analysis. Milross reported no change in total sleep time, sleep latency or arousals between room air and oxygen therapy (Milross 2001b). Neither did the investigators detect any change in sleep architecture (Milross 2001b).
Spier also reported on the number of sleep stage changes. There were an increased mean number of sleep stage changes in the room air group compared to the oxygen group, 10.1 changes compared to 8.1 changes (P < 0.05) (Spier 1984).
3. Exercise tolerance
Five studies measured exercise parameters with and without supplementary oxygen therapy (Falk 2006; Marcus 1992; McKone 2002; Nixon 1990; Shah 1997). One study used a submaximal exercise protocol only and took measurements after exercise (McKone 2002). Two studies measured during maximal exercise only (Marcus 1992; Shah 1997) and two studies recorded measurements both after submaximal exercise and during maximal exercise (Falk 2006; Nixon 1990).
a. Submaximal exercise
Three studies measured exercise parameters after submaximal exercise, but none of the results were significant (Analysis 1.10). The most common parameters to be reported were exercise tolerance, (McKone 2002); oxygen consumption, (McKone 2002; Nixon 1990); CO2 production, (Falk 2006; McKone 2002; Nixon 1990); minute ventilation, (Falk 2006; McKone 2002; Nixon 1990); and heart rate, (Falk 2006; McKone 2002; Nixon 1990). Of these variables, there were no significant differences between room air and supplementary oxygen.
b. Maximal exercise
Four studies reported exercise parameters during maximal exercise (Falk 2006; Marcus 1992; Nixon 1990; Shah 1997) (Analysis 1.11). Two of these studies reported on exercise duration; participants were able to exercise for a significantly longer duration with oxygen therapy, MD 1.03 minutes (95% CI 0.11 to 1.95) (P = 0.03) (Falk 2006; Marcus 1992). The ventilatory equivalent (max VE/VO2) was significantly higher in room air compared to oxygen therapy, MD ‐3.86 (95% CI ‐6.82 to ‐0.91) (P = 0.01) (Falk 2006; Marcus 1992; Nixon 1990). None of the other parameters reported were significantly affected by supplementary oxygen compared with room air.
4. Nutritional status
Only one study examined the effect of oxygen therapy on objective measures of nutrition (Zinman 1989). The percentage change in ideal weight for height was not significant after 6 or 12 months therapy (Analysis 1.12).
5. Changes in right heart function
Only the Zinman study examined whether the use of chronic nocturnal oxygen therapy retarded the development of pulmonary hypertension and right‐sided heart failure (Analysis 1.13). Six out of fourteen participants in the air group and four out of fourteen participants in the oxygen group developed at least one physical sign consistent with cor pulmonale. When entered into the meta‐analysis, the result was not significant. No statistical analysis was mentioned in the original paper and subsequent follow up of participants who died outside the study period was incomplete (Zinman 1989).
Radionuclide angiography was performed on 24 participants (12 in each group). Both the placebo and oxygen group had detectable reductions in right ventricular ejection fraction (RVEF) and left ventricular ejection fraction (LVEF) at baseline, but there were no significant differences in these parameters after one year of oxygen therapy compared to placebo at rest or after exercise (Analysis 1.13).
6. Costs of intervention
None of the studies addressed cost issues, either directly or indirectly.
7. Adverse effects
The major adverse effect of oxygen therapy that investigators measured was an excessive increase in carbon dioxide levels. Comparisons between groups were made more difficult by the use of variations in protocol, duration of therapy and different methods of measurement. The degree of elevation in carbon dioxide during sleep or exercise was generally within 10 mm Hg or 1.0 kPa. One study did report an average of 16 mm Hg rise during exercise in the "Low Sat" group (Marcus 1992). It is unclear whether these rises are of clinical significance over time. Notably, the only paper to study long‐term oxygen therapy did not discover an increase in PaCO2 after one year and reported not adverse clinical effects with the use of oxygen therapy (Zinman 1989).
No study reported local complications arising either from the use of oxygen or other problems relating to the method of administration of the study gas.
Discussion
The frequency of the use of oxygen therapy in CF is unknown. We wished to examine the evidence for its potential benefit in CF.
Eleven studies were included in the review and ten of these were short‐term. Nine of these studies were included in the original review and two were added at a later revision in 2008 (Barker 1998; Falk 2006). Six of the eleven studies reported the effects of oxygen supplementation on exercise performance in CF (Barker 1998; Falk 2006; Marcus 1992; McKone 2002; Nixon 1990; Shah 1997). Four other studies reported the effects of nocturnal oxygen supplementation on gas exchange and sleep quality (Gozal 1997; Milross 2001b; Parsons 1996; Spier 1984). A considerable number of outcomes were tested and to put the results in context, it should be noted that the results for the first two primary outcomes listed in this review were non‐significant. The third primary outcome and some of the secondary outcomes did, however, show some significant differences between treatment and placebo groups which are worthy of discussion.
The results of the review of short‐term studies showed that there was a statistically significant improvement in oxygen saturations during or immediately after exercise in participants with CF inspiring low‐flow oxygen compared with room air. There was, however, a greater increase in both the transcutaneous and end‐tidal CO2 level while breathing low‐flow oxygen during exercise compared to air. The clinical significance of this finding is unclear. During sleep, there was a significant improvement in oxygen saturation levels during both REM and non‐REM sleep in those breathing low‐flow oxygen. Data looking at changes in CO2 levels during sleep could not be combined in a meta‐analysis, but greater increases in CO2 levels while breathing oxygen were reported in some studies. There was a significant reduction in percent of total sleep time spent in REM sleep and a reduced sleep onset latency during sleep with oxygen compared to air, but no significant changes in the mechanics of ventilation during sleep were found. Participants were able to exercise for a significantly longer duration while breathing oxygen compared to air, but no other significant effect on exercise tolerance was found.
One study looked at the long‐term effects of oxygen in CF, and specifically described outcome measures including mortality, changes in lung function, gas exchange after 12 months, nutritional indices and progression of pulmonary hypertension (Zinman 1989). The participants received oxygen only during sleep. In participants followed for a mean duration of 26 months, nocturnal oxygen therapy did not affect any of the outcome variables. There was a trend towards improved right ventricular ejection fraction (RVEF) and cardiac output in the participants receiving oxygen but there was no clear relationship with better clinical outcome. Reasons for a lack of efficacy of oxygen could be explained by a number of the aspects of study design. The study population was relatively small, and may not have been of sufficient size to demonstrate statistical significance. Another reason may relate to the duration of oxygen therapy being too short to deliver a significant clinical benefit ‐ seven hours on average per day compared to the 12 to 24 hours of oxygen demonstrated in the original studies in COPD (MRC 1981; NOTTG 1980). The criterion for study entry included participants who were less severely hypoxemic than those in the COPD studies, which could easily have mixed those who would benefit with those with milder hypoxemia (Gorecka 1997). It is also conceivable that considering the different pulmonary pathophysiology in CF compared to COPD, there might be less therapeutic responsiveness to oxygen therapy.
Despite the presumed widespread use of oxygen therapy in CF, we believe that it is most frequently prescribed for people with documented arterial oxygen desaturation. Based on the successful use of long‐term oxygen therapy in other conditions such as COPD, we hoped to find evidence to support the use of chronic oxygen therapy. We identified only one clinical study of chronic oxygen therapy which had a number of limitations and on this basis, there is an absence of evidence for benefit from long‐term oxygen supplementation in people with advanced lung disease due to CF (Zinman 1989). It is possible that oxygen administered for longer daily durations in more uniformly severely ill individuals would prove to be beneficial.
With respect to oxygen supplementation during sleep in people with CF, it appears that hypoxemia can predictably be eliminated with variable degrees of hypercapnia resulting (Gozal 1997; Parsons 1996; Spier 1984). Based on the levels of hypercapnia noted in studies, there is no strong reason to suspect clinically important untoward side effects in the short term. We cannot be entirely sanguine about the potential effects of oxygen supplementation in all individuals with CF and advanced lung disease. Attention to blood gas analysis would be warranted in such individuals. In people with advanced hypercapnia in CF, nasal ventilation during sleep has become a common therapy (Hodson 1991; Moran 2007). There is no evidence of a consistently demonstrable improvement in sleep quality in people with CF given oxygen supplementation. However, the lack of benefit in the three single night studies in hospital sleep laboratories may derive in part from the artificial nature of the sleep environment. Further study may be warranted to assess sleep quality with oxygen therapy in these participants.
People with CF with advanced lung disease typically have reduced exercise tolerance, a fact corroborated in the four studies reviewed (Marcus 1992; McKone 2002; Nixon 1990; Shah 1997). Reduced exercise tolerance could be related to the underlying condition or ventilatory limits. However, it could also be related to hypoxemia, which can be treated with oxygen therapy. There is evidence of modest enhancement of exercise capacity and duration with oxygen supplementation, especially in participants with more advanced lung disease (Marcus 1992; McKone 2002; Nixon 1990). There is also a tendency for more hypercapnia during exercise when oxygen supplementation is administered, but once again, as with sleep, the degree of hypercapnia is unlikely to be of untoward clinical significance. In the one study that looked at recovery and subsequent exercise, people with CF do tend to recover more quickly when given oxygen supplementation after vigorous exercise and perform at a higher capacity with the next exercise challenge (Shah 1997). If the findings in these studies all of which examine short‐term oxygen use were corroborated in longer‐term use of oxygen in individuals with CF, improved fitness, muscle mass and well‐being might well result. Certainly, this hypothesis would be a worthy one for future study.

Comparison 1 Oxygen therapy versus control, Outcome 1 Survival.

Comparison 1 Oxygen therapy versus control, Outcome 2 Change in FEV1.
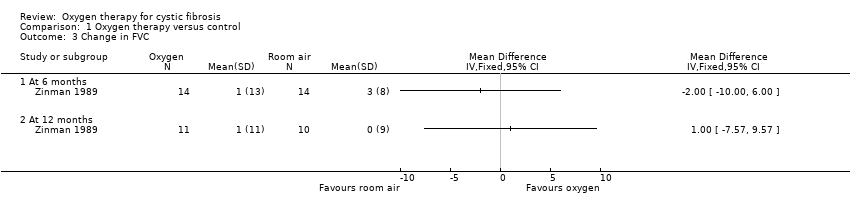
Comparison 1 Oxygen therapy versus control, Outcome 3 Change in FVC.

Comparison 1 Oxygen therapy versus control, Outcome 4 Change in gas exchange during exercise.

Comparison 1 Oxygen therapy versus control, Outcome 5 Change in gas exchange during sleep.
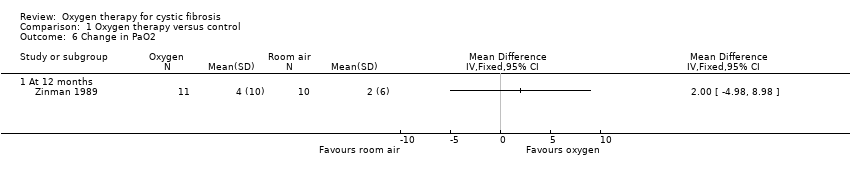
Comparison 1 Oxygen therapy versus control, Outcome 6 Change in PaO2.
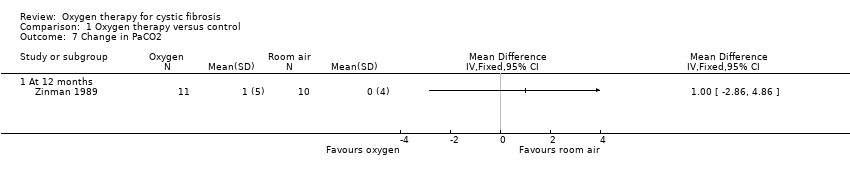
Comparison 1 Oxygen therapy versus control, Outcome 7 Change in PaCO2.
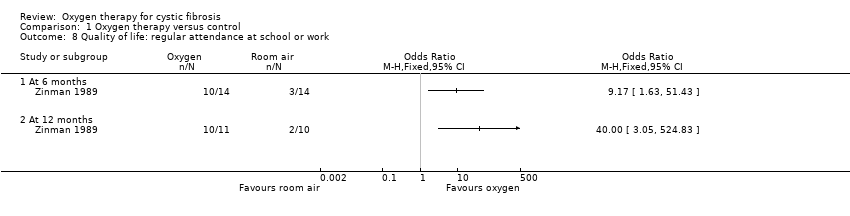
Comparison 1 Oxygen therapy versus control, Outcome 8 Quality of life: regular attendance at school or work.
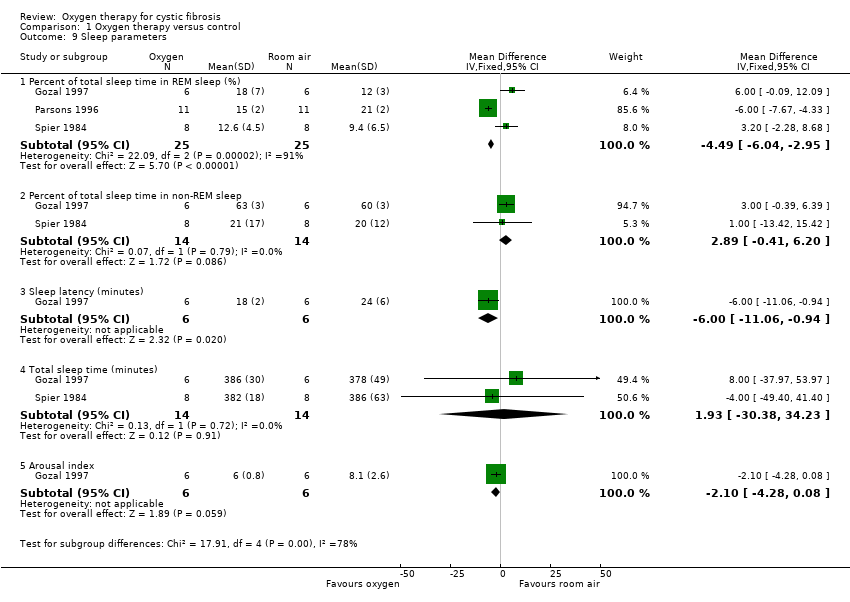
Comparison 1 Oxygen therapy versus control, Outcome 9 Sleep parameters.

Comparison 1 Oxygen therapy versus control, Outcome 10 Exercise parameters after submaximal exercise.
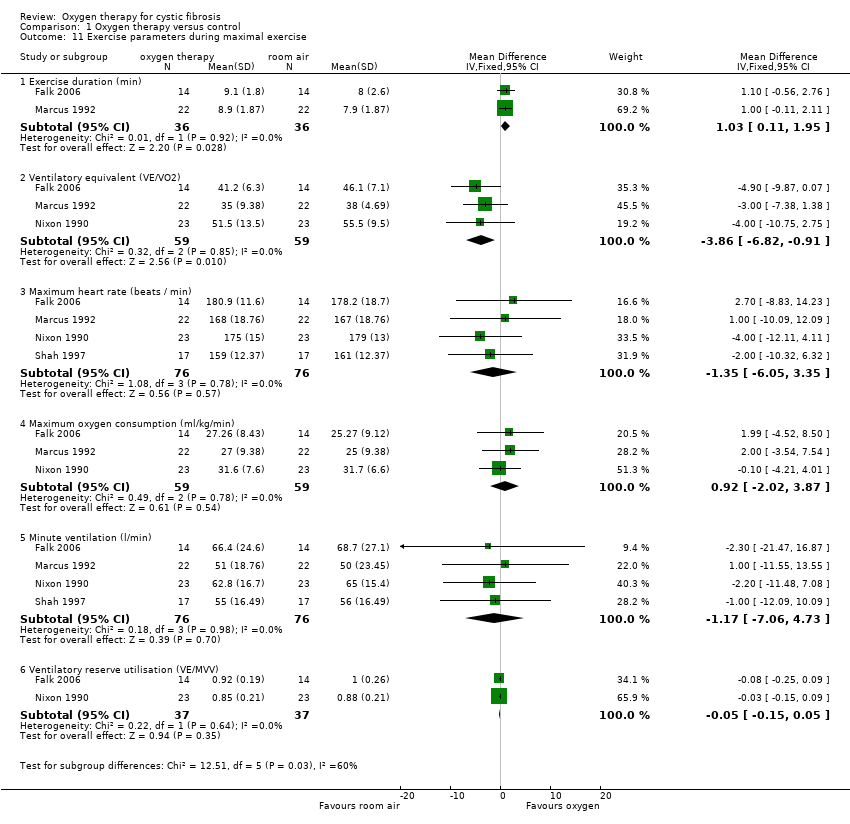
Comparison 1 Oxygen therapy versus control, Outcome 11 Exercise parameters during maximal exercise.

Comparison 1 Oxygen therapy versus control, Outcome 12 Nutritional status: change in % ideal body weight for height.
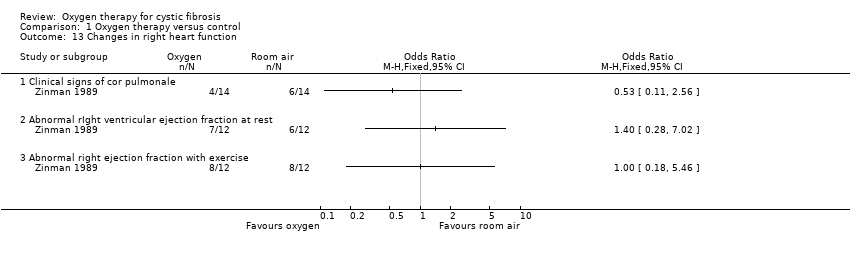
Comparison 1 Oxygen therapy versus control, Outcome 13 Changes in right heart function.
| Abbreviations | Definition |
| BiPAP | bileval positive airway pressure |
| BMI | body mass index |
| CO2 | carbon dioxide |
| COPD | chronic obstructive pulmonary disease |
| CPAP | continuous positive airway pressure |
| ETCO2 | end tidal carbon dioxide tension |
| FEV1 | forced expiratory volume in one second |
| FiO2 | fraction of inspired oxygen, or percent of oxygen in the inspired gas |
| FVC | forced vital capacity |
| kPa | kilopascal, a unit of air pressure |
| LVEF | left ventricular ejection fraction |
| mm Hg | millimeter of mercury, a unit of air pressure |
| MVV | maximal voluntary ventilation |
| NIPPV | non‐invasive positive pressure ventilation |
| NREM | non‐rapid eye movement |
| PaCO2 | arterial carbon dioxide tension |
| PaO2 | partial pressure of oxygen in the blood |
| PCO2 | partial pressure of carbon dioxide in the blood |
| PETCO2 | maximum partial pressure of carbon dioxide exhaled during a tidal breath, just prior to inspiration |
| REM | rapid eye movement |
| RVEF | right ventricular ejection fraction |
| SaO2 | saturation of haemoglobin with oxygen |
| SpO2 | pulse oximetry |
| TcCO2 | transcutaneous carbon dioxide |
| tPCO2 | transcutaneous partial pressure of carbon dioxide in the blood |
| VCO2 | carbon dioxide production |
| VE | mean total ventilation |
| VO2 | oxygen consumption |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Survival Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Deaths at 36 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in FEV1 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FVC Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in gas exchange during exercise Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Post exercise SaO2 (%) | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | 2.11 [1.54, 2.68] |
| 4.2 Post exercise PETCO2 (mm Hg) | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐2.14, 2.36] |
| 4.3 Change in SaO2 during maximal exercise (%) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [2.61, 11.39] |
| 4.4 Change in PETCO2 during maximal exercise (mm Hg) | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [1.28, 6.14] |
| 4.5 Change in tPCO2 during maximal exercise (mm Hg) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [1.23, 6.77] |
| 4.6 SaO2 during maximal exercise (%) | 3 | 108 | Mean Difference (IV, Fixed, 95% CI) | 2.01 [1.16, 2.85] |
| 5 Change in gas exchange during sleep Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 SaO2 in REM sleep (%) | 2 | 34 | Mean Difference (IV, Fixed, 95% CI) | 7.54 [4.31, 10.77] |
| 5.2 tPCO2 in REM sleep (kPa) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.15, 1.85] |
| 5.3 TcPCO2 in REM sleep (mm Hg) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐6.69, 7.29] |
| 5.4 SaO2 in non‐REM sleep (%) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.92, 10.08] |
| 5.5 tPCO2 in non‐REM sleep (kPa) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.09, 1.49] |
| 5.6 TcPCO2 in non‐REM sleep (mm Hg) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.51, 4.51] |
| 5.7 Maximum TcCO2 with total sleep (mm Hg) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐2.07, 12.07] |
| 6 Change in PaO2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in PaCO2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Quality of life: regular attendance at school or work Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Sleep parameters Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Percent of total sleep time in REM sleep (%) | 3 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐4.49 [‐6.04, ‐2.95] |
| 9.2 Percent of total sleep time in non‐REM sleep | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.89 [‐0.41, 6.20] |
| 9.3 Sleep latency (minutes) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐11.06, ‐0.94] |
| 9.4 Total sleep time (minutes) | 2 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.93 [‐30.38, 34.23] |
| 9.5 Arousal index | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐4.28, 0.08] |
| 10 Exercise parameters after submaximal exercise Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Exercise duration (s) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 163.0 [‐66.91, 392.91] |
| 10.2 Oxygen consumption (ml/kg/min) | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐2.06, 1.20] |
| 10.3 CO2 production (l/min) | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 10.4 Minute ventilation (l/min) | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐3.36, 1.53] |
| 10.5 Heart rate (beats / min) | 3 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.96 [‐8.84, 0.92] |
| 11 Exercise parameters during maximal exercise Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Exercise duration (min) | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [0.11, 1.95] |
| 11.2 Ventilatory equivalent (VE/VO2) | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐3.86 [‐6.82, ‐0.91] |
| 11.3 Maximum heart rate (beats / min) | 4 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐6.05, 3.35] |
| 11.4 Maximum oxygen consumption (ml/kg/min) | 3 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [‐2.02, 3.87] |
| 11.5 Minute ventilation (l/min) | 4 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐7.06, 4.73] |
| 11.6 Ventilatory reserve utilisation (VE/MVV) | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.15, 0.05] |
| 12 Nutritional status: change in % ideal body weight for height Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Changes in right heart function Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Clinical signs of cor pulmonale | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Abnormal rIght ventricular ejection fraction at rest | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Abnormal right ejection fraction with exercise | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

