Profilaxis con antibióticos para prevenir la endocarditis bacteriana tras intervenciones dentales
Resumen
Antecedentes
La endocarditis infecciosa es una infección grave que surge en el recubrimiento de las cavidades del corazón. Pueden causarla hongos, pero la mayoría de las veces la causa es bacteriana. Muchas intervenciones dentales causan bacteriemia, que podría dar lugar a endocarditis bacteriana en un pequeño porcentaje de personas. La incidencia de la endocarditis bacteriana es baja, pero tiene una alta tasa de mortalidad.
Las guías de muchos países han recomendado la administración de antibióticos a personas con alto riesgo de endocarditis antes de realizar intervenciones dentales invasivas. Sin embargo, las guías del National Institute for Health and Care Excellence (NICE) en Inglaterra y Gales no recomiendan la profilaxis antibiótica sistemática contra la endocarditis infecciosa en personas a las que se les realizan intervenciones dentales. Esta es una actualización de una revisión elaborada por primera vez en 2004 y actualizada por última vez en 2013.
Objetivos
Objetivo principal
Determinar si la administración de antibióticos profilácticos en comparación con ninguna administración de antibióticos o placebo antes de intervenciones dentales invasivas en personas con riesgo o con alto riesgo de endocarditis bacteriana influye en la mortalidad, las enfermedades graves o la incidencia de endocarditis.
Objetivos secundarios
Determinar si el efecto de la profilaxis dental con antibióticos difiere en personas con diferentes afecciones cardíacas que les predisponen a un mayor riesgo de endocarditis, y en personas sometidas a diferentes intervenciones dentales de alto riesgo.
Efectos perjudiciales
Si no se hubiera encontrado evidencia de ensayos controlados aleatorizados o estudios de cohortes sobre si los antibióticos profilácticos afectaron a la mortalidad o a la enfermedad grave, y se hubiera encontrado evidencia de estos ensayos o de estudios de casos y controles que indicara que la profilaxis con antibióticos redujo la incidencia de endocarditis, en ese caso también se habría evaluado si los efectos perjudiciales de la profilaxis con dosis única de antibióticos, como con penicilina (amoxicilina 2 ó 3 g), antes de intervenciones dentales invasivas, en comparación con ningún antibiótico o placebo, fueron equivalentes a los efectos beneficiosos en la prevención de la endocarditis en personas con alto riesgo de esta enfermedad.
Métodos de búsqueda
Un documentalista realizó búsquedas en cuatro bases de datos bibliográficas hasta el 10 de mayo de 2021 y utilizó métodos de búsqueda adicionales para identificar estudios publicados, no publicados y en curso.
Criterios de selección
Debido a la baja incidencia de endocarditis bacteriana, se previó que se identificarían pocos ensayos, de haber alguno. Por este motivo, se incluyeron estudios de cohortes y de casos y controles con grupos de comparación o control emparejados de forma apropiada. La intervención fue la profilaxis antibiótica comparada con ninguna profilaxis antibiótica o placebo antes de una intervención dental en personas con un riesgo elevado de endocarditis bacteriana. Los estudios de cohortes debían hacer un seguimiento de las personas en riesgo y evaluar los desenlaces tras una intervención dental invasiva, con el agrupamiento de los participantes en función de si habían recibido o no la profilaxis. Los estudios de casos y controles incluidos debían emparejar a las personas que habían presentado endocarditis tras pasar por una intervención dental invasiva (y de quienes se sabía que tenían un riesgo alto antes de realizársele la intervención) con aquellas con riesgo similar que no habían presentado endocarditis.
Los desenlaces de interés fueron la mortalidad o los eventos adversos graves que requirieron ingreso hospitalario; la aparición de endocarditis después de cualquier intervención dental en un período de tiempo definido; la aparición de endocarditis debido a otras causas no dentales; cualquier efecto adverso registrado de los antibióticos; y el coste de la provisión de antibióticos en comparación con el de la atención a los pacientes que presentan endocarditis.
Obtención y análisis de los datos
Dos autores de la revisión analizaron de forma independiente las entradas, seleccionaron los estudios para inclusión, evaluaron el riesgo de sesgo de los estudios incluidos y extrajeron los datos de los estudios incluidos. El equipo de autores evaluó la certeza de la evidencia identificada para la comparación principal y los desenlaces clave mediante el método GRADE. Los resultados principales se presentaron en una tabla "Resumen de los hallazgos".
Resultados principales
La nueva búsqueda no encontró estudios nuevos para inclusión desde la última versión de esta revisión en 2013.
No se incluyeron ensayos controlados aleatorizados (ECA), ensayos clínicos controlados (ECC) ni estudios de cohortes en las anteriores versiones de la revisión, pero un estudio de casos y controles cumplió con los criterios de inclusión. Los autores del ensayo recopilaron información sobre 48 personas que habían contraído endocarditis bacteriana en un periodo específico de dos años y se habían sometido a una intervención médica o dental con indicación de profilaxis en los últimos 180 días. Estas personas fueron emparejadas con un grupo similar de personas que no había contraído endocarditis bacteriana. A todos los participantes del estudio se les había realizado una intervención médica o dental invasiva. Los dos grupos se compararon para establecer si quienes habían recibido antibióticos preventivos (penicilina) tenían menos probabilidades de presentar endocarditis. Los autores no observaron un efecto significativo de la profilaxis con penicilina sobre la incidencia de endocarditis. No se proporcionaron datos sobre otros desenlaces.
El nivel de certeza que se tiene con respecto a esta evidencia es muy bajo.
Conclusiones de los autores
Aún no hay evidencia clara de si la profilaxis con antibióticos es efectiva o no contra la endocarditis bacteriana en personas con riesgo que se someten a una intervención dental invasiva. No es posible determinar si los efectos perjudiciales potenciales y los costes de la administración de antibióticos superan cualquier efecto beneficioso. Desde el punto de vista ético los médicos deberían comentar los posibles efectos beneficiosos y perjudiciales de la profilaxis con antibióticos con sus pacientes antes de decidir acerca de su administración.
PICO
Resumen en términos sencillos
Antibióticos para la prevención de la endocarditis bacteriana (infección o inflamación grave del recubrimiento de las cavidades del corazón) en odontología
Pregunta de la revisión
Esta revisión Cochrane quería averiguar si las personas con riesgo alto de endocarditis bacteriana (una infección o inflamación grave del recubrimiento de las cavidades del corazón que puede ser mortal), deben recibir antibióticos de forma habitual antes de intervenciones dentales invasivas para reducir la incidencia de endocarditis, el número de muertes y la cantidad de enfermedades graves que experimenta este grupo de personas.
Antecedentes
La endocarditis bacteriana es una infección que tiende a producirse en áreas del corazón con malformaciones o daños previos. Se suele tratar con antibióticos. Aunque muy poco frecuente, la endocarditis bacteriana es potencialmente mortal. Hasta el 30% de las personas que la contraen podrían morir, incluso con tratamiento antibiótico.
Las intervenciones dentales invasivas pueden causar endocarditis bacteriana en personas con riesgo de presentarla. Se desconoce el número de casos de endocarditis bacteriana (si los hay) causados directamente de esta manera. Muchas intervenciones dentales causan bacteriemia, que consiste en la presencia de bacterias en la sangre. Aunque la bacteriemia suele resolverse rápidamente gracias al sistema inmunitario del organismo, algunos expertos creen que podría provocar una endocarditis bacteriana en algunas personas de riesgo.
Las guías de muchos países recomiendan que las personas con alto riesgo de endocarditis bacteriana reciban antibióticos antes de someterse a intervenciones dentales invasivas. Pero otras autoridades han cuestionado el uso habitual de los antibióticos y argumentan que su prescripción excesiva ha provocado que muchos microorganismos desarrollen resistencia a los antibióticos comunes y también que los efectos adversos ocasionales de los antibióticos (reacciones alérgicas graves) podrían superar los posibles beneficios.
En 2007, las guías de la American Heart Association cambiaron para recomendar que solo se administren antibióticos antes de las intervenciones dentales a las personas con riesgo alto de presentar endocarditis bacteriana. Las guías del National Institute for Health and Care Excellence (NICE) en Inglaterra y Gales fueron un paso más allá y aconsejaron no prescribir de manera habitual antibióticos preventivos para las intervenciones dentales o quirúrgicas invasivas.
Características de los estudios
No existen nuevos estudios para incluir en esta revisión actualizada. La revisión original incluyó un estudio en Países Bajos que comparó el tratamiento de personas con alto riesgo de endocarditis que presentaron o no endocarditis bacteriana. Los autores recopilaron información sobre 48 personas que habían contraído endocarditis bacteriana en un periodo específico de dos años y se habían sometido a una intervención médica o dental con indicación de profilaxis en los últimos 180 días. Estas personas fueron emparejadas con un grupo similar de personas que no había contraído endocarditis bacteriana. A todos los participantes del estudio se les había realizado una intervención médica o dental invasiva. Los dos grupos se compararon para establecer si quienes habían recibido antibióticos preventivos tenían menos probabilidades de presentar endocarditis.
Resultados clave
No está claro si la administración de antibióticos como medida preventiva antes de las intervenciones dentales invasivas es o no efectiva contra la endocarditis bacteriana en las personas con alto riesgo.
No se encontraron estudios que evaluaran el número de muertes, episodios adversos graves que requirieran el ingreso hospitalario, otros efectos adversos ni las implicaciones económicas del tratamiento.
No está claro si los posibles efectos perjudiciales y los costes de la administración de antibióticos superan cualquier efecto beneficioso. Desde el punto de vista ético los médicos deberían analizar los posibles efectos beneficiosos y perjudiciales del tratamiento preventivo con antibióticos con sus pacientes antes de decidir acerca de su prescripción.
Limitaciones de la evidencia
La evidencia se basa en un estudio que tiene algunas limitaciones en su diseño. Por ejemplo, los participantes que recibieron antibióticos podrían haber tenido un peor estado de salud general que los que no los recibieron. No se confía en la evidencia que se encontró. Tan solo se puede concluir que no se conocen los efectos de la profilaxis con antibióticos para la prevención de la endocarditis bacteriana.
Fecha de la evidencia
Esta revisión actualiza a la que se elaboró originalmente en 2004 y se actualizó por última vez en 2013. Ahora la evidencia está actualizada hasta el 10 de mayo de 2021.
Authors' conclusions
Summary of findings
| Antibiotic prophylaxis compared with no antibiotic prophylaxis for the prevention of bacterial endocarditis in dentistry | ||||
| Population: adults or children at risk of endocarditis Setting: dental setting Intervention: antibiotic prophylaxis Comparison: no antibiotic prophylaxis | ||||
| Outcome | Results | No of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Mortality or serious adverse events requiring hospitalisation | No data reported | 248 (1 study) | ‐ | ‐ |
| Development of endocarditis (in those with definite indication for prophylaxis) | There was no difference in the number of people (with a definitive indication for prophylaxis) who developed endocarditis between those receiving prophylaxis and those not receiving prophylaxis (OR 1.62; 95% CI 0.57 to 4.57). | 248 (1 study) | ⊕⊝⊝⊝ | ‐ |
| Adverse effects of antibiotics | No data reported | 248 (1 study) | ‐ | ‐ |
| CI: confidence interval; OR: odds ratio | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded 3 levels for high risk of bias and serious imprecision. | ||||
Background
Description of the condition
Infective endocarditis is a rare disease caused by infected vegetations (growths) that often occur on previously damaged or congenitally malformed cardiac valves or endocardium (heart chamber lining). The infecting organisms are usually bacteria and less commonly fungi, particularly of the Candida species. Bacterial endocarditis is infective endocarditis caused by bacteria that enter the blood (bacteraemia). Bacteria may enter the blood through a variety of points of entry but especially mucosal surfaces. The gingiva (gums) and periodontal ligaments, which surround all teeth, experience an almost constant degree of inflammation and as such are a potential point of entry for bacteria to the blood. Indeed, everyday activities such as toothbrushing can cause bacteraemia (Lucas 2000; Roberts 1999). Bacterial endocarditis is a rare but potentially life‐threatening condition. A 2019 systematic review reported bacterial endocarditis incidence of 15 cases per 100,000 in the USA in 2011, with six‐month mortality of up to 30%, even with antibiotic treatment (Jamil 2019).
In the past, the majority of people who developed endocarditis had a known pre‐existing cardiac defect. More recently, however, this trend has shifted, with nearly half of endocarditis cases having no known previous cardiac disease (Duval 2012). The growth of the aging population with comorbidities and their subsequent increased interactions with healthcare may have contributed to the increased incidence of the disease (Jamil 2019).
Common cardiac conditions that put people at risk include previous endocarditis, prosthetic heart valves, valvular stenosis, ventricular septal defect and valvular damage following rheumatic fever (Danchin 2005; Farook 2012). In particular, people with previous endocarditis and prosthetic heart valves are considered to have a high risk of developing endocarditis (Durack 1994). These predisposing conditions either cause changes in the surface of the heart lining (endocardium) or changes in blood flow that damage the endocardium and enable organisms in the blood to adhere and multiply, forming bacterial vegetations. This leads to severe systemic illness and directly affects the functioning of the heart. Fragments of the vegetations may break away and become lodged elsewhere in the circulatory system, potentially resulting in an embolism.
Description of the intervention
Most dental procedures cause bacteraemia, which, it has been hypothesised, may lead to bacterial endocarditis. The proportion of bacterial endocarditis cases arising as a result of dental treatment is uncertain, though a recent study estimated it at 12% (Delahaye 2016). For many years, there was a well‐established practice of administering antibiotics, typically penicillins, to individuals at risk of developing bacterial endocarditis before any dental procedures that carried a risk of a bacteraemia developing. Early clinical guidelines supported this practice (Dajani 1997; EWP 1993), based on the rationale that a high circulating dose of antibiotic would prevent the development of an infected vegetation on damaged endocardium and thus prevent endocarditis.
Some population‐based case studies questioned the routine use of antibiotics for endocarditis prophylaxis (e.g. Strom 1998a), arguing that the adverse effects of antibiotics may outweigh their potential benefits. This point of view was given some support after the original 2004 publication of this review, which did not find sufficient evidence to draw any conclusions about the effectiveness or ineffectiveness of the intervention. Across Europe, the USA and Australia, clinical guidelines moved away from recommending antibiotic prophylaxis for all at‐risk patients, instead advising that they be given only to those at 'high risk' (Farook 2012), though 'high risk' was defined differently by different regulatory bodies (e.g. American Heart Association; European Society of Cardiology). In 2008, the National Institute for Health and Care Excellence (NICE) went even further when it published guidance for England and Wales stating that no antibiotic prophylaxis was required for any interventional procedure (NICE 2008). Initially, despite marked reductions in the use of prophylactic antibiotics, the incidence of bacterial endocarditis after dental treatment did not appear to increase (Duval 2012; Thornhill 2011). Recent evidence from a systematic review investigating the impact of guideline changes on the global incidence of infective endocarditis suggests that restricting prophylactic antibiotics to only high‐risk patients has not resulted in an increase in the incidence of streptococcal cases in North American populations; however, the authors indicate that further research is needed to clarify the impact of guideline changes in the UK and some European countries (Williams 2021). The NICE guidance was updated in 2016 (NICE 2008).
How the intervention might work
Antibiotic prophylaxis before invasive procedures has been a key strategy for preventing bacterial endocarditis for several decades, and remains so in many parts of the world (Thornhill 2011). Antibiotic therapy for the treatment of bacterial endocarditis is not in question: without antibiotic therapy, infective endocarditis is fatal (Durack 1994). However, the use of antibiotic prophylaxis for the prevention of bacterial endocarditis remains controversial.
Why it is important to do this review
In 2004, Cochrane Oral Health first undertook this systematic review, initially looking at penicillin for the prevention of bacterial endocarditis in people having dental treatment. The review was updated and expanded to include all antibiotics in 2008, and updated again in 2013. The review included only one case‐control study and was inconclusive about the place of antibiotic prophylaxis in the prevention of bacterial endocarditis. The initial review created much debate around the prescribing of antibiotic prophylaxis for the prevention of bacterial endocarditis. Changes in clinical guidelines in various countries led to restrictions in the use of prophylactic antibiotics, but many dentists were concerned that this could put patients at increased risk of bacterial endocarditis due to a dental intervention.
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of priority titles (Worthington 2015). This review was one of those identified at that time and its importance was confirmed in our second comprehensive prioritisation process, which was undertaken in 2020 (see Cochrane Oral Health priority review portfolio).
Objectives
Primary objective
To determine whether prophylactic antibiotic administration, compared to no antibiotic administration or placebo, before invasive dental procedures in people at risk or at high risk of bacterial endocarditis, influences mortality, serious illness or the incidence of endocarditis.
Secondary objectives
To determine whether the effect of dental antibiotic prophylaxis differs in people with different cardiac conditions predisposing them to increased risk of endocarditis, and in people undergoing different high risk dental procedures.
Harms
Had we found no evidence from randomised controlled trials or cohort studies on whether prophylactic antibiotics affected mortality or serious illness, and we had found evidence from these or case‐control studies suggesting that prophylaxis with antibiotics reduced the incidence of endocarditis, then we would also have assessed whether the harms of prophylaxis with single antibiotic doses, such as with penicillin (amoxicillin 2 g or 3 g) before invasive dental procedures, compared with no antibiotic or placebo, equalled the benefits in prevention of endocarditis in people at high risk of this disease.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) and controlled clinical trials (CCTs) where these were available, though we anticipated these may not have been possible due to the low incidence of bacterial endocarditis. We planned to include cohort and case‐control studies where suitably matched control or comparison groups had been studied.
Types of participants
RCTs and CCTs
Studies must have involved adults or children, or both, who had any of the following pre‐existing cardiac defects (i.e. patients known to be at risk): congenital heart defects, a history of rheumatic fever, prosthetic heart valves (tissue and mechanical) or previous endocarditis. We excluded studies of people with pacemakers (and no other risk factors).
The dental procedures that the participants may have undergone in the studies included: supragingival and subgingival scaling of teeth, extensive restorations of teeth, endodontics and oral surgery including dental extractions. We considered procedures performed under local and general anaesthetic.
Types of interventions
RCTs and CCTs
The intervention assessed was the administration of an antibiotic, compared with no such administration or placebo, before a dental procedure. We included studies in which an antibiotic was administered postoperatively if this was part of a protocol including preoperative administration. The antibiotics could be administered by oral, intravenous, or intramuscular routes, but not topically.
Co‐interventions could include preoperative use of mouthwash or mechanical cleaning of teeth.
Types of outcome measures
RCTs and CCTs
-
Mortality or serious adverse events (from any cause) requiring hospital admission
-
Development of endocarditis following any dental procedure in a defined time period
-
Development of endocarditis due to other non‐dental cause
Secondary outcomes
RCTs and CCTs
-
Any recorded adverse effects of the antibiotics
-
Cost implications of antibiotic provision for prophylaxis compared with the cost of care of patients who develop bacterial endocarditis
Assessment of harms would have included all studies where potentially serious adverse effects (such as would be expected to result in hospitalisation) or fatal adverse effects of a single antibiotic dose had been reported or assessed.
Cohort studies and case‐control studies
To be included, cohort studies would have had to fulfil the following criteria.
-
Participants would be people at increased risk of endocarditis (as above).
-
Their progress would be followed (no minimum time period) and invasive dental procedures carried out.
-
Use (or not) of prophylactic antibiotics at these visits and occurrence or not of bacterial endocarditis, death or serious illness would be recorded (as a minimum).
-
It would be possible to compare incidence of bacterial endocarditis, and death or serious illness in those who received invasive dental procedures with and without antibiotics.
The case‐control study included fulfilled the following criteria.
-
The groups that were compared included a group of people at increased risk of endocarditis who did develop bacterial endocarditis, and a group of people at increased risk of endocarditis who did not develop bacterial endocarditis.
-
Information was provided on the numbers of people in each group who had undergone an invasive dental procedure within a (stated) set period, and the numbers who had received antibiotic prophylaxis before the procedure.
We decided post hoc that studies that excluded cases when they died due to endocarditis would be excluded from our review as up to 30% of people who contract endocarditis will die of it and these participants may be different from those who survive.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
-
Cochrane Oral Health’s Trials Register (searched 10 May 2021) (Appendix 1);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 4) in the Cochrane Library (searched 10 May 2021) (Appendix 2);
-
MEDLINE Ovid (1946 to 10 May 2021) (Appendix 3);
-
Embase Ovid (1980 to 10 May 2021) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. We opted not to use a filter to limit the search to randomised controlled trials as the yield from the subject search was low.
Searching other resources
The following trial registries were searched for ongoing studies:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 10 May 2021) (Appendix 5);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 10 May 2021) (Appendix 6).
For the 2013 update of this review, we had searched the metaRegister of Controlled Trials to 21 January 2013 (see Appendix 7), but this resource is no longer available.
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We checked that none of the included studies in this review had been retracted due to error or fraud.
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts obtained from the searches. The review authors were not blinded to the authors, institution or journal. Full‐text papers that were retrieved were similarly screened for inclusion independently by two review authors. Any disagreements over inclusion would have been resolved by discussion between the review authors, with a third review author being consulted if necessary.
Data extraction and management
Two review authors independently extracted data and quality information onto a custom‐designed data collection form. In addition to bibliographic details of the paper, the key items of data we recorded were the study design, country of origin, details of the antibiotic intervention, type of dental procedure and study population details including risk factors. The outcome data collected from RCTs and CCTs would have included number of deaths; number of hospital admissions; number of serious illnesses that would be expected to result in hospital admission; number of cases of endocarditis; any other adverse events noted and number of people originally randomised to each group. The outcome data collected from cohort studies would have included the same information as for RCTs plus adjusted odds ratios or risk ratios and information about the factors for which adjustments were made. The outcome data collected from the case‐control study included the adjusted odds ratio of a person at increased risk of endocarditis having had antibiotic prophylaxis prior to invasive dentistry before either developing endocarditis (cases) or not (controls).
Where necessary, we contacted study authors for further details of their studies to assess inclusion.
Assessment of risk of bias in included studies
We planned to rank included studies according to study design: RCT, CCT, cohort study, case‐control study.
Two review authors independently assessed the risk of bias in the included study using the Cochrane risk of bias assessment tool for non‐randomised studies. The domains we assessed were: sequence generation, allocation concealment, confounding, blinding of outcome assessment, completeness of outcome data, risk of selective outcome reporting, and risk of other potential sources of bias.
We described the risk of bias in each domain for the included study, along with a judgement of low, high or unclear risk of bias. We considered the risk of bias overall according to whether there was a low risk of bias for all key domains (overall low), unclear risk of bias for one or more key domains (overall unclear) or high risk of bias for one or more key domains (overall high) (Higgins 2011).
Had data appeared ambiguous or incomplete, we would have contacted the study authors for clarification.
Measures of treatment effect
We planned to use the risk ratio as the effect estimate measure for dichotomous data, and the mean difference (or standardised mean difference) for continuous data.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
We planned to contact study authors for missing data if required.
Assessment of heterogeneity
We planned to test for heterogeneity between trial results using a standard Chi2 test, considered significant where the P value was less than 0.1.
For case‐control studies, we planned that the odds of antibiotic prophylaxis before dental treatment in the previous three months for cases and controls would not be pooled with data from other types of studies. We planned to tabulate any harms data according to study design, but not to pool them.
Assessment of reporting biases
We did not assess publication bias.
Data synthesis
We planned to seek data on the number of participants with each outcome event, by allocated treatment group (RCTs) or quantile (cohort studies). We aimed to calculate a pooled estimate of the treatment effect for each outcome (separately) across RCTs, CCTs, cohort studies and case‐control studies in a random‐effects meta‐analysis as an odds ratio (the ratio of odds of developing bacterial endocarditis in the prophylaxis group to the odds in the no prophylaxis group), since the odds ratio is the only good measure of association that works across prospective studies and case‐control studies (Fleiss 1981).
Subgroup analysis and investigation of heterogeneity
If sufficient data had been identified, we would have conducted subgroup analysis according to:
-
different dosages, e.g. 2 g and 3 g amoxicillin;
-
different underlying causes of at‐risk and high‐risk status for endocarditis; and
-
different invasive dental techniques.
Sensitivity analysis
If sufficient data had been identified, we would have conducted a sensitivity analysis by removing any studies where risk factors for endocarditis were significantly different between the groups being compared and the trial authors had not adjusted adequately for this difference.
Summary of findings and assessment of the certainty of the evidence
We judged the certainty of the evidence we found according to GRADE criteria and created a summary of findings table to summarise our findings for the main comparison and our key outcomes of mortality, endocarditis and serious adverse events.
Results
Description of studies
Results of the search
For this update, we identified a total of 530 unique references between 21 January 2013 (which was the search date used in the 2013 version of this review) and 10 May 2021. None of the references were suitable for inclusion in the review. A flow diagram of this study selection combined with the study selection for the previous version is presented in Figure 1.
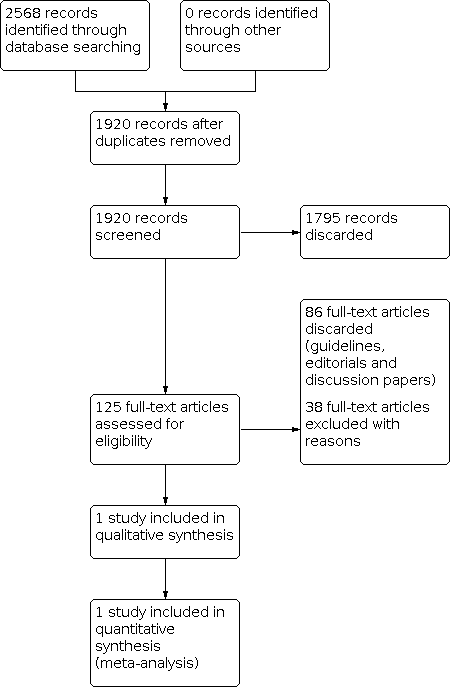
Study flow diagram
Included studies
We included one case‐control study in the original review (Van der Meer 1992a). Since the time of the last review, no further evidence has been produced to determine the effects of antibiotic prophylaxis for dental procedures.
Van der Meer 1992a involved 349 people with definite native‐valve endocarditis, 197 of whom had previous heart disease (proxy responders, i.e. spouses or general practitioners, were interviewed for 10 of these). Of these 197 at‐risk individuals, 54 had undergone a medical or dental procedure with an indication for prophylaxis within the past 180 days. Within this group, a causal relationship was ruled out in six people as the agent isolated from the blood was unlikely to have originated in the area of the procedure. Of the remaining 48 people with endocarditis, who formed the case group, 44 had undergone a dental procedure which the paper identified as having a definite (24) or possible (20) indication for prophylaxis (none of these cases had used a proxy responder). Indications for definite prophylaxis were dental extractions and dental root work, while indications for possible prophylaxis were defined as dental scaling.
Of 889 potential controls who were sent an introductory letter, 689 were ineligible (53 had died, 29 had a prosthetic heart valve, 62 could not be located, 102 could not be contacted by phone, and 418 had not undergone an invasive dental or medical procedure within the past 180 days). The remaining 200 were interviewed by phone two to five days later; 181 of these controls had undergone a dental procedure with definite (79) or possible (102) indications for prophylaxis.
Seven of 24 cases and 16 of 79 controls had had appropriate prophylaxis for a dental procedure requiring definite prophylaxis within the previous 180 days.
The characteristics of the cases and controls were not well described, as those who had received a dental procedure (rather than a medical one) were not separated out in the publication (the separated data were provided by Professor Van der Meer). The median time between a dental procedure requiring definite prophylaxis and onset of endocarditis was 10 days in the cases, and the median time between a dental procedure requiring definite prophylaxis and interview was 71 days in the controls (data missing for 12 controls). The following procedures were performed.
-
Apical surgery in one case (4%) and one control (1%)
-
Dental avulsion in one case (4%) and 12 controls (15%)
-
Dental extraction in nine cases (38%) and 15 controls (19%)
-
Dental abscess in one case (4%) and one control (1%)
-
Removal of subgingival calculus in three cases (13%) and eight controls (33%)
-
Removal of calculus plus polishing of teeth in six cases (25%) and 34 controls (43%)
-
Root canal therapy in three cases (13%) and eight controls (10%).
Including the cases and controls undergoing either definite or possible indication for prophylaxis, and including the four cases and 19 controls who underwent a non‐dental procedure, 69% of cases and 55% of controls were male. The median age of this larger group was 41 years for cases and 40 for controls (the controls were age‐matched).
Seven of 21 cases and 9 of 46 controls had had appropriate prophylaxis for a dental procedure requiring definite prophylaxis within the previous 90 days. Seven of 44 cases and 17 of 181 controls had had appropriate prophylaxis for a dental procedure requiring definite or possible prophylaxis within the previous 180 days. Seven of 32 cases and 9 of 100 controls had had appropriate prophylaxis for a dental procedure requiring definite or possible prophylaxis within the previous 90 days. No information was presented on the adjunctive use of mouthwash
Excluded studies
We did not identify any new 'excluded studies'. Details of previously excluded studies are presented in the Characteristics of excluded studies tables.
Risk of bias in included studies
Van der Meer 1992a, our one included study, used a case‐control design. Overall, the observational and retrospective nature of the design conferred a high risk of bias (Characteristics of included studies; Figure 2).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Van der Meer 1992a used a case‐control design and therefore we judged it to be at high risk of selection bias.
Blinding
Outcome assessors in Van der Meer 1992a were not blinded and therefore we judged it to be at high risk of detection bias.
Incomplete outcome data
We judged Van der Meer 1992a to be at high risk of attrition bias. Potential cases who were very ill or who died were included in the selection process via the use of proxy responders (i.e. spouses or general practitioners); however, this did not occur for the 53/889 potential controls who died. In addition, it was unclear how similar the groups were with regard to proportions of males and females, different types of cardiac risk factor, and different dental interventions.
Selective reporting
Van der Meer 1992a reported expected outcomes in full and so we judged it to be at low risk of reporting bias.
Other potential sources of bias
We judged Van der Meer 1992a to be unclear in terms of other potential sources of bias. Participant sex and cardiac risk factor type were not described for the subgroup who had had a dental procedure, and the types of dental intervention appear to have been different for the cases and controls, although the two groups were matched for age. Both groups were required to have undergone invasive dental techniques within 180 days prior to onset of symptoms or interview and data were split by time period for both groups.
Effects of interventions
We included one case‐control study (Van der Meer 1992a).
In each of the ways of assessing the data (as presented above under Included studies), the proportion of people receiving prophylaxis was greater in the cases than in the controls. When we calculated the odds of developing endocarditis in those receiving prophylaxis compared with those not receiving prophylaxis, we found an odds ratio (OR) that was not significantly different from the OR for any of the groupings (OR 1.62, 95% confidence interval (CI) 0.57 to 4.57 for those with a definite indication for prophylaxis within the previous 180 days).
Only four cases developed endocarditis following non‐dental medical interventions (within the past 180 days and with pre‐existing cardiac indications for the use of prophylaxis), so assessment of the effects of prophylaxis in these cases was not possible.
It was unclear whether antibiotic prophylaxis was effective or ineffective against bacterial endocarditis in people at risk who were about to undergo an invasive dental procedure.
The study did not provide any data on mortality, adverse events requiring hospitalisation, adverse effects of antibiotics or cost implications of treatment.
Because we observed no significant protective effect of antibiotic prophylaxis against endocarditis, we did not perform a wide‐ranging search to pool information on the potential harmful effects of antibiotic prophylaxis as prespecified in the protocol.
Discussion
Summary of main results
This review update has identified no additional studies that meet the review's inclusion criteria.
The one included case‐control study included all people in the Netherlands who developed endocarditis following an invasive dental procedure while at known cardiac risk over a two‐year period (24 individuals who underwent a procedure that definitely required prophylaxis, and a further 20 who may have required prophylaxis). The study provided no conclusive evidence about whether antibiotic prophylaxis is effective or ineffective against bacterial endocarditis in high risk individuals about to undergo an invasive dental procedure.
The evidence regarding the development of endocarditis over 180 days in those who received prophylaxis compared with those who did not receive prophylaxis is uncertain, with an odds ratio in favour of prophylaxis but with a confidence interval that includes both benefit and harm (OR 1.62, 95% CI 0.57 to 4.57).
There are currently insufficient primary data to determine whether antibiotic prophylaxis before invasive dental procedures in people at increased risk of endocarditis prevents endocarditis, deaths or other serious illness.
Overall completeness and applicability of evidence
As the usefulness of prophylaxis could not be established, we have not examined the harms of antibiotic administration in detail; this would be a systematic review in itself. Such a review would, however, be extremely valuable and could potentially be used by a wide spectrum of research workers and other systematic reviewers. In the absence of a systematic review on the harms of penicillins, the most authoritative source is Meyler's Side Effects of Drugs (Aronson 2006). The range of potential side effects from the administration of antibiotics is vast, and while the aetiology is largely hypersensitive, some direct toxic effects may also occur.
The effects of the NICE guidance on the incidence of endocarditis in the UK are going to be monitored using Hospital Episode Statistics (HES). Tracking endocarditis incidence rates over a number of years and comparing them with rates recorded in the pre‐NICE guideline era might be one method to answer this conundrum, albeit in a rather crude fashion.
Another underexplored area is the cost of prophylaxis, both in terms of finance and health. The financial cost to health services of providing large quantities of prophylactic antibiotics must be weighed against the cost of treating patients who develop endocarditis. Although endocarditis is a serious disease, it occurs in appreciably fewer patients than those potentially at risk. The health costs should also be considered, particularly the potential harms of administering antibiotics compared to endocarditis. The involvement of health economists would be beneficial. This was explored in depth in the NICE guidance (NICE 2008, updated 2016).
Despite the varying guidelines produced over the years and the change recommended by NICE, it is important for medical and dental practitioners to remember that patients remain at risk of developing endocarditis. Many patients will develop endocarditis via organisms that enter the blood through the oral cavity. Whilst there is no evidence that dental treatment is or is not directly related to the development of the disease, nor that prophylactic antibiotics can or cannot prevent the development of the disease, achieving and maintaining the highest level of oral health in at‐risk patients is a logical objective.
Following the update of the NICE guidance in 2016, the Scottish Dental Clinical Effectiveness Programme produced guidance for clinicians in Scotland to help overcome any concerns regarding implementation (SDCEP 2018).
Quality of the evidence
The overall certainty of the evidence is very low, as the evidence comes from a single study at high risk of bias. While it would be useful to have higher levels of certainty about the effectiveness of antibiotic prophylaxis of endocarditis in dentistry, it is not feasible because the incidence of endocarditis is so low. A randomised controlled trial run over two years would require approximately 60,000 participants with a cardiac risk factor for endocarditis (a cohort study over 10 years would require approximately 18,000 participants). Such a trial would require an intense international effort.
A larger, well conducted case‐control study might be more feasible, but would still require a large effort and multicentre participation. If including every endocarditis case in the Netherlands for two years produces only 24 appropriate cases, then the area or time span covered in a suitably sized study would be very large indeed. Selection of appropriate controls is probably the most challenging aspect; ideally, as in Van der Meer 1992a, they should have had dental treatment within a predefined time period and be matched very closely for sex, age and type of cardiac risk factor. Additionally, neither cases nor controls should be excluded for death or serious illness (use of proxy respondents would be ideal and this would require retrospective identification of controls as well as ongoing prospective identification of cases) and dental records should be available and be explicit about the use (or not) of prophylaxis. Full details should be collected on other factors that may compound the risk such as general well‐being, coexisting medical problems, socioeconomic status and oral health status.
The fact that neither of these study types has been attempted since this review began suggests it is highly unlikely that they ever will.
Potential biases in the review process
We identified no potential biases in the review process.
Agreements and disagreements with other studies or reviews
Most experts agree that there is little scientific evidence to support the effectiveness of antibiotic prophylaxis for the prevention of bacterial endocarditis (Cahill 2017; Duval 2012; Farook 2012; Thornhill 2011). This lack of evidence has led to variations in guideline recommendations with regard to who should or should not be prescribed antibiotic prophylaxis and who is or is not considered high‐risk for bacterial endocarditis. However, one area where most guidelines agree is with regard to the need for regular dental surveillance to promote good oral hygiene, thus reducing the need for invasive dental procedures and subsequently reducing the risk of bacterial endocarditis (SDCEP 2018).
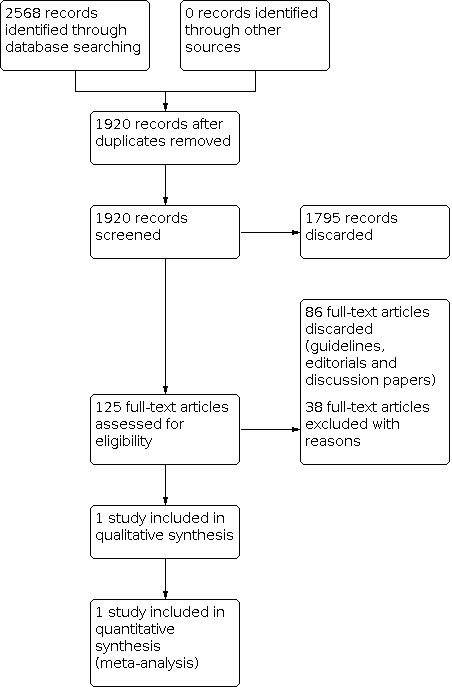
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Antibiotic prophylaxis compared with no antibiotic prophylaxis for the prevention of bacterial endocarditis in dentistry | ||||
| Population: adults or children at risk of endocarditis Setting: dental setting Intervention: antibiotic prophylaxis Comparison: no antibiotic prophylaxis | ||||
| Outcome | Results | No of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Mortality or serious adverse events requiring hospitalisation | No data reported | 248 (1 study) | ‐ | ‐ |
| Development of endocarditis (in those with definite indication for prophylaxis) | There was no difference in the number of people (with a definitive indication for prophylaxis) who developed endocarditis between those receiving prophylaxis and those not receiving prophylaxis (OR 1.62; 95% CI 0.57 to 4.57). | 248 (1 study) | ⊕⊝⊝⊝ | ‐ |
| Adverse effects of antibiotics | No data reported | 248 (1 study) | ‐ | ‐ |
| CI: confidence interval; OR: odds ratio | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded 3 levels for high risk of bias and serious imprecision. | ||||

