Fluorures dans la prévention des caries précoce (lésions blanches déminéralisées) pendant un traitement avec un appareil dentaire fixe
Résumé scientifique
Contexte
Les lésions blanches déminéralisées peuvent apparaître sur les dents pendant un traitement avec un appareil fixe en raison d'une carie précoce autour des supports qui relient les bagues aux dents. Le fluorure est efficace dans la réduction des caries chez les personnes sensibles dans l’ensemble de la population. Les personnes recevant un traitement orthodontique peuvent être prescrites diverses formes de traitement au fluorure. Cette revue compare les effets de diverses formes de fluorure utilisées pendant le traitement orthodontique pour prévenir le développement des lésions blanches déminéralisées. Ceci est une mise à jour d'une revue Cochrane publiée pour la première fois en 2004.
Objectifs
L'objectif principal de cette revue était d'évaluer les effets du fluorure pour réduire l'incidence de lésions blanches déminéralisées sur les dents pendant le traitement orthodontique.
Les objectifs secondaires étaient d'examiner l'efficacité de différents modes de fluorure pour réduire l'incidence de lésions blanches déminéralisées, ainsi que la taille des lésions. Les critères de jugement évalués par les participants, tels que la perception de lésions blanches déminéralisées et la qualité de vie liée à la santé buccale, ainsi que les rapports sur les effets indésirables devaient être inclus.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre des essais du groupe Cochrane sur la santé bucco‐dentaire (jusqu' au 31 janvier 2013); le registre Cochrane des essais contrôlés (CENTRAL) (La Bibliothèque Cochrane 2012, numéro 12); MEDLINE via OVID (de 1946 au 31 janvier 2013); et EMBASE via OVID (de 1980 au 31 janvier 2013).
Critères de sélection
Nous avons inclus les essais s'ils répondaient aux critères suivants: (1) groupes parallèles des essais cliniques randomisés comparant l'utilisation d'un produit contenant du fluorure par rapport à un placebo, à l'absence de traitement ou à un autre type de traitement au fluorure, dans lesquels (2) le critère de jugement de la déminéralisation de l’émail a été évalué au début et à la fin du traitement orthodontique.
Recueil et analyse des données
Au moins deux auteurs de la revue ont évalué les risques de biais et extrait les données de manière indépendante et en double. Les auteurs des essais ont été contactés pour obtenir des données manquantes ou clarifier certains aspects de la méthodologie des essais. Les directives statistiques de la Collaboration Cochrane ont été suivies.
Résultats principaux
Pour la mise à jour de 2013, trois changements ont été effectués au protocole concernant les critères d'inclusion. Quatorze études incluses dans la version précédente de la revue ont été exclues de cette mise à jour pour les raisons suivantes : cinq études précédemment incluses étaient quasi‐randomisées, cinq autres étaient sur bouche divisée, trois mesuraient les critères de jugement uniquement sur les dents extraites et pour une étude, la même intervention au fluorure était utilisée dans chaque groupe d'intervention de l'étude.
Trois études et 458 participants ont été inclus dans cette revue mise à jour. Une étude a été évaluée à faible risque de biais pour tous les domaines. Dans une étude, le risque de biais n'était pas clair et dans l'autre étude, le risque de biais était élevé.
Une étude contrôlée par placebo de vernis au fluorure appliqué toutes les six semaines (253 participants, faible risque de biais), a fourni des preuves de qualité modérée d'une réduction de lésions blanches déminéralisées de presque 70% (risque relatif (RR) 0,31, intervalle de confiance à 95% (IC) de 0,21 à 0,44, valeur P < 0,001). Ce résultat fournit des preuves de qualité modérée pour cette intervention, car il n'a pas encore été répliqué par d'autres études de participants orthodontiques.
Une étude a comparé deux différentes formulations de dentifrice au fluorure et de bain de bouche prescrits aux participants subissant un traitement orthodontique (97 participants, risque de biais incertain) et n'a trouvé aucune différence entre la combinaison d’un dentifrice / bain de bouche au fluorure d’amines avec le fluorure stanneux et un dentifrice / bain de bouche au fluorure de sodium pour les critères de jugement des taches blanches, de la plaque dentaire et des saignements gingivaux.
Une étude de petite taille (37 participants) comparait l'utilisation d'un dispositif de bille de verre intra‐buccal fixé à l'appareil dentaire et libérant du fluorure par rapport à un bain de bouche quotidien au fluorure. L'étude a été évaluée avec un risque de biais élevé parce qu’un nombre important de participants avaient été perdu de vue durant le suivi et l'observance de l’utilisation du bain de bouche n'a pas été mesurée.
Ni les critères de jugement secondaires de cette revue, ni les effets indésirables des interventions n’ont été rapportés dans aucune des études incluses.
Conclusions des auteurs
Cette revue a trouvé certaines preuves de qualité modérée indiquant que le vernis au fluorure, appliqué toutes les six semaines au moment de la revue orthodontique pendant le traitement, est efficace, mais ce résultat est basé sur une seule étude. Des essais contrôlés randomisés supplémentaires, suffisamment puissants et en double aveugle sont nécessaires pour déterminer le meilleur moyen de prévenir les lésions blanches déminéralisées chez les patients subissant un traitement orthodontique et le moyen le plus précis pour évaluer l'observance du traitement et les effets indésirables potentiels. Les futures études devraient effectuer un suivi des participants au‐delà de la fin du traitement orthodontique pour déterminer l'effet des lésions blanches déminéralisées sur la satisfaction des participants avec le traitement.
PICO
Résumé simplifié
Fluorures dans la prévention des caries précoce (lésions blanches déminéralisées) pendant un traitement avec un appareil dentaire fixe
Question de la revue
Des marques blanches déplaisantes apparaissent parfois sur les dents au cours d’un traitement orthodontique (appareil dentaire). Elles sont causées par une carie dentaire précoce et surviennent généralement avec des appareils dentaires fixes (ou collés) lorsque les dents ne sont pas correctement nettoyées.
Nous savons que le fluorure dans le dentifrice aide à prévenir la formation de caries dentaires; par conséquent, un supplément en fluorure chez les patients portant un appareil dentaire fixe devrait les protéger de ces taches. Cette revue, produite par le groupe Cochrane sur la santé bucco‐dentaire, examine les preuves dans les recherches existantes. L'objectif de cette revue est d'évaluer l'efficacité des fluorures dans la prévention des caries précoces pendant le traitement orthodontique (appareil dentaire) et de déterminer la meilleure façon de l’effectuer.
Contexte
La carie dentaire précoce autour des supports qui relient les bagues aux dents peut provoquer des taches blanches ou brunes (lésions blanches déminéralisées) sur les dents pendant un traitement avec un appareil fixe. L’accumulation de plaque dentaire autour de ces supports est associée à un risque accru de déminéralisation rapide de l'émail des dents. La déminéralisation est un stade précoce, mais réversible, dans le développement de caries. Le port d'un appareil orthodontique fixe peut être associé à de la douleur, ce qui ne facilite pas le brossage des dents et il est donc plus difficile de prévenir l'accumulation de plaque dentaire. Les personnes portent souvent un appareil dentaire pendant une durée de 18 mois ou plus et les caries dentaires peuvent abimer les dents, des obturations et des plombages seront donc nécessaires.
Le fluorure est efficace pour réduire la carie dentaire chez les personnes qui risquent de la développer. Les personnes recevant un traitement orthodontique peuvent être prescrites diverses formes de traitement au fluorure. Il est important de tenir compte de la façon dont le fluorure doit être appliqué et si les enfants et les adolescents (recevant un traitement avec un appareil fixe) sont enclins et capables d’appliquer régulièrement les quantités nécessaires par eux‐mêmes pour prévenir la formation précoce de caries dentaires.
Les caractéristiques de l'étude
Les preuves sur lesquelles cette revue est basée ont été actualisées le 31 janvier 2013. Trois études totalisant 458 participants ont été inclues dans cette revue mise à jour. Les participants subissaient un traitement orthodontique avec un appareil fixe et les lésions blanches déminéralisées ont été évaluées sur les dents restantes à la fin du traitement orthodontique.
Différentes méthodes d'applications de fluorure qui ont été évaluées incluaient:
1. La fluoration, par exemple, du vernis, un bain de bouche, du gel ou du dentifrice contenant du fluor
2. Du fluor fixé sur les attelles;
3. Différentes approches des groupe de contrôle ‐ les personnes n'avaient pas reçu de fluorure comme décrit, ou avaient reçu un placebo ou une autre forme de fluorure.
Résultats principaux
Une étude a montré que lorsque le dentiste vernit le contour de la dent et l’appareil dentaire au fluorure et à chaque ajustage, le risque de développer des taches blanches est réduit de presque 70%; toutefois, d'autres essais bien conçus sont nécessaires pour confirmer ce résultat.
Le reste des preuves est faible et des études supplémentaires sont nécessaires pour établir la meilleure façon d'administrer un supplément de fluorure à des personnes portant un appareil dentaire fixe. Les effets indésirables ou les inconvénients des interventions n'étaient rapportés dans aucune des études incluses.
Qualité des preuves
Les preuves identifiées sont de qualité modérée dans le cas d'une étude bien planifiée et faibles dans les études restantes. Les recommandations indiquent que d'autres recherches bien réalisées devraient être menées dans ce domaine.
Authors' conclusions
Summary of findings
| Fluoride varnish versus placebo varnish for the prevention of demineralised white lesions on teeth during fixed brace treatment | ||||||
| Patient or population: Participants undergoing orthodontic treatment with fixed appliances Comparison: Placebo varnish | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo varnish | Fluoride varnish | |||||
| Number of patients with new demineralised white lesions | Study population | RR 0.31 | 253 | ⊕⊕⊕⊝ | ||
| 640 per 1000 | 198 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 A single study with 253 participants evaluated this outcome. Risk of bias was assessed as low. However, this finding should be interpreted with caution until the study has been replicated. | ||||||
Background
Description of the condition
During orthodontic treatment with fixed appliances, brackets are attached to the teeth to hold the wires that provide the forces required to straighten the teeth. One of the adverse effects of fixed braces is that dental plaque collects around the attachments, leading to accumulation of the types of bacteria that cause dental disease (Naranjo 2006). Build‐up of dental plaque around orthodontic brackets is associated with increased risk of demineralised white lesions (DWLs), which can be visible within six months (Tufekci 2011). Demineralisation is an early, but reversible, stage in the development of dental decay (caries). Cariogenic bacteria present in the dental plaque transform sugar in the diet into organic acids, which start to damage the tooth enamel. Effective removal of plaque will prevent DWLs from occurring; however, the presence of orthodontic appliances in the mouth and associated dental pain may make it more difficult for individuals to adequately clean their teeth and braces. DWLs developing on the buccal surfaces of teeth during orthodontic treatment can become a significant problem over the course of treatment, which may last for 18 months or longer, resulting in a poor appearance of the teeth following straightening (Maxfield 2012). In severe cases, caries can develop, requiring restoration (filling); this may be both painful and costly.
A recent study by Enaia and colleagues (Enaia 2011) using clinical photographs of the teeth taken before and after fixed brace treatment found that the prevalence of DWLs was 32% in participants before treatment; however, this proportion rose to 74% after treatment. Most of the DWLs were minor, but a significant minority of participants (10%) had cavities in their teeth, which may have required a filling. Although DWLs tend to fade with time as they heal, Ogaard 1989 found that, even five years after treatment, orthodontic participants had a significantly higher incidence of DWLs than a control group of participants who did not undergo orthodontic treatment.
Description of the intervention
Orthodontists are keen to prevent the development of DWLs so their patients may have the best possible outcome following orthodontic treatment ‐ well‐aligned caries‐free teeth. Fluoride is important in the prevention of dental decay (ten Cate 2013). Marinho and colleagues (Marinho 2003) found a definite reduction in caries in children and adolescents who performed regular supervised rinsing with a fluoride mouthwash. It has also been shown that fluoride may reduce the number of DWLs that develop during brace treatment. When orthodontic participants used a mouthrinse, Geiger et al (Geiger 1992) found a 30% reduction in the number of participants and a 25% reduction in the incidence of teeth affected by DWLs. Many orthodontists recommend the use of a daily fluoride mouthrinse throughout brace treatment to prevent DWLs (Kerbusch 2012).
Several methods (in addition to fluoridated toothpaste) are used to deliver fluoride to teeth in patients during orthodontic treatment. These include the following.
-
Topical fluorides (e.g. mouthrinse, gel, varnish).
-
Fluoride‐releasing materials (e.g. glues used to bond the brackets onto the teeth and orthodontic elastics that are impregnated with fluoride).
-
Dietary fluoride supplementation (e.g. fluoridated milk).
How the intervention might work
Fluoride present in the mouth reduces caries development via three mechanisms: inhibition of the demineralisation of dental enamel, enhancement of the remineralisation of dental enamel producing a remineralised layer that is resistant to acid attack and inhibition of the bacterial enzymes that produce the acid (Lynch 2006; ten Cate 2013).
Most children undergoing orthodontic treatment will be exposed to some fluoride ‐ low concentrations in the water supply, higher concentrations from fluoridated dentifrices (toothpaste), or both. Use of additional topical fluorides and/or fluoride sources designed to deliver additional fluoride to the at‐risk area near orthodontic brackets are likely to reduce the risk of DWL development. Topical fluorides include fluoride mouthrinses, varnishes, gels, dentifrices and dietary sources (e.g. fluoridated milk). Specific orthodontic sources of fluoride include bracket adhesives and orthodontic elastic bands (elastomeric ligatures), which slowly release fluoride into the mouth. All of these fluoride sources release fluoride into saliva that is distributed throughout the mouth.
Why it is important to do this review
Several systematic reviews have investigated the effects of delivering fluoride in various modes on dental caries in children and adolescents (Marinho 2003; Marinho 2003a; Marinho 2003b; Marinho 2004); however, these systematic reviews did not examine the effects of fluoride on participants wearing fixed orthodontic braces.
Some orthodontists routinely recommend to their patients the use of fluoride mouthrinses. However, clear evidence is lacking regarding the optimum concentration of fluoride in mouthrinses, the optimum frequency of mouthrinse use and the effects of mouthrinses and other topical fluorides over the duration of orthodontic treatment.
This is an update of a Cochrane review first published in 2004 to summarise evidence of the effects of the use of any topical fluoride on the prevention of demineralised white lesions in patients undergoing orthodontic treatment.
Objectives
The primary objective of this review was to evaluate the effects of fluoride in reducing the incidence of demineralised white lesions (DWLs) on the teeth during orthodontic treatment.
The secondary objectives were to examine the effectiveness of different modes of fluoride delivery in reducing the incidence of DWLs, as well as the size of lesions. Participant‐assessed outcomes, such as perception of DWLs, and oral health–related quality of life data were to be included, as would reports of adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in which topical fluoride was delivered by any method to prevent enamel demineralised white lesion (DWL) formation during orthodontic treatment with fixed braces. As topical fluorides are distributed throughout the mouth by saliva, the use of a split‐mouth study design to evaluate these interventions is inappropriate. (Split‐mouth studies included in the previous version of this review found no difference between teeth with fluoridated bracket adhesives and those without, supporting the view that this design is inappropriate for evaluating topical fluorides.) We have excluded split‐mouth studies from the 2013 update of this review.
Types of participants
Included were participants of any age who were undergoing orthodontic treatment with fixed braces in situations where DWLs were assessed on teeth remaining in the mouth at the end of orthodontic treatment (at debonding, immediately after the active fixed brace is removed). We excluded studies that evaluated demineralisation of extracted teeth (ex vivo).
Types of interventions
-
Topical fluoride in the form of toothpaste, mouthrinse, gel, varnish or dietary sources at any dose, frequency, duration or method of administration, and with any of the following active agents/ingredients: NaF (sodium fluoride), SMFP (sodium monofluorophosphate), SnF (stannous fluoride), APF (acidulated phosphate fluoride) and amine F (amine fluoride).
-
Materials containing fluoride that is released during treatment, including fluoride‐releasing composite resin‐bonding materials, glass ionomer cements, compomers and resin‐modified glass ionomers for bonding or banding, slow‐release fluoride devices and fluoride‐releasing elastomeric ligatures.
-
The control group comprises individuals not subjected to the fluoride intervention but instead treated with a placebo, such as a non‐fluoride toothpaste and mouthrinse, or given no intervention. Studies involving a control subjected to an alternative fluoride intervention were also included.
Types of outcome measures
Primary outcomes
-
The outcome measure was the presence/absence of new DWLs by participant. This can be assessed directly from the participant or preferably from start and finish photographs or fluorescent images of the teeth (immediately after the active fixed brace is removed). If the number of DWLs was not recorded at the start of treatment, the outcome was the presence or absence of DWLs at the end of the orthodontic treatment period, again assessed directly from the participant or indirectly from photographs or fluorescent images of the teeth.
Secondary outcomes
-
Differences in size and severity of DWLs between experimental and control groups.
-
Any quantitative assessment of enamel mineral loss, such as fluorescent light techniques or microradiography, used with in situ caries models (Benson 2010).
-
Any participant‐assessed outcomes, such as perception of DWLs and oral health–related quality of life data.
-
Adverse effects.
Search methods for identification of studies
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. No restrictions were placed on the language of publication when searching the electronic databases. Searches were originally done in July 2003 and were undertaken again in May 2012 and January 2013 for this update of the review.
Electronic searches
We searched the following electronic databases.
-
The Cochrane Oral Health Group's Trials Register (to 31 January 2013) (Appendix 1).
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 12) (Appendix 2).
-
MEDLINE via OVID (1946 to 31 January 2013) (Appendix 3).
-
EMBASE via OVID (1980 to 31 January 2013) (Appendix 4).
Searching other resources
The bibliographies of identified RCTs and review articles were checked for additional studies.
Unpublished studies
The US National Institutes of Health Trials Register was searched in June 2013 for ongoing studies. Personal contacts were used to identify unpublished RCTs.
Handsearching
The following journals were identified as important to be handsearched for this review.
-
American Journal of Orthodontics and Dentofacial Orthopedics
-
The Angle Orthodontist
-
European Journal of Orthodontics
-
[British] Journal of Orthodontics
-
Clinical Orthodontics and Research
-
Journal of Dental Research
-
Journal of Dentistry
-
Caries Research
-
Journal of Clinical Orthodontics.
These journals are included in the Cochrane Worldwide Handsearching Programme. See the Cochrane Masterlist for details of issues searched to date. Only handsearching done as part of this programme and uploaded to CENTRAL was included.
Data collection and analysis
Selection of studies
Two review authors independently examined the title, keywords and abstract of reports identified through electronic searching for evidence of three criteria.
-
A randomised clinical trial of participants undergoing orthodontic treatment.
-
A trial comparing the use of a fluoride‐containing product versus no use or use of a non‐fluoride control.
-
A trial that assessed enamel demineralisation at the start and at the end of orthodontic treatment.
For studies that appeared to meet the inclusion criteria, or for which data in the title and abstract were insufficient to allow a clear decision, the full report was obtained. Disagreements were resolved by discussion.
No language restrictions were applied. Translations of foreign language articles were produced by contacts within the Cochrane Oral Health Group.
Data extraction and management
Data were extracted by two review authors independently, in duplicate, using specially designed data extraction forms. The data extraction forms were piloted on several papers and were modified as required before use. Any disagreement was discussed, and a third review author was consulted when necessary. All authors were contacted for clarification of missing information. Data from studies in which the reporting was incomplete were not included in the analysis until the corresponding author of the study had supplied adequate clarification. If agreement could not be reached, data were excluded from the review. All studies that met the inclusion criteria underwent validity assessment and data extraction. Studies rejected at this or subsequent stages were recorded, along with reasons for exclusion, in the 'Characteristics of excluded studies' table.
For each trial, the following data were recorded.
-
Year of publication and country of origin.
-
Study design.
-
Unit of randomisation.
-
Details of participants, including demographic characteristics and criteria for inclusion.
-
Details of types of interventions (method of delivery of fluoride, dose, duration of use).
-
Details of outcomes reported (number, size and severity of white spot lesions), including method of assessment and mean duration of the study.
Assessment of risk of bias in included studies
This assessment was conducted by using the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011). We used the two‐part tool to address the six specific domains (namely, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other bias). Each domain includes one or more specific entries in a 'Risk of bias' table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry: either low risk, unclear risk or high risk.
The domains of sequence generation, allocation concealment, incomplete outcome data and selective outcome reporting are addressed in the tool by a single entry for each study. For blinding, two entries were used because assessments need to be made separately for (1) participants and operators/orthodontists and (2) outcome assessors. When the operator/orthodontist assessed the outcome of the trial, this was noted. The final domain ('other sources of bias') was assessed as a single entry for studies as a whole.
The risk of bias assessment was undertaken independently and in duplicate by two review authors as part of the data extraction process. Disagreements were resolved by discussion.
After taking into account additional information provided by the authors of the trials, review authors grouped studies into the following categories.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information comes from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information comes from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
A risk of bias table was completed for each included study. Results were also presented graphically (Figure 1).
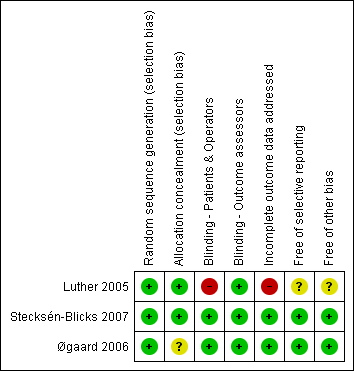
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For dichotomous outcomes, the estimate of effect of an intervention was expressed as risk ratios (RRs) together with 95% confidence intervals (CIs). For continuous outcomes, we estimated mean differences (MDs) and 95% CIs.
Unit of analysis issues
In parallel‐group trials in which participants are randomly assigned to intervention or to control and a single outcome measure per participant is reported, the analysis is straightforward. When individuals are randomly assigned to treatment, each individual has a number of teeth exposed to the intervention or to the control. When the outcome is reported per number of teeth, the data should be adjusted for clustering within the mouth of each individual to avoid unit of analysis errors. If it is unclear from the reports of included trials whether clustering has been considered, authors were contacted to clarify how this dependence has been accounted for in the analysis.
Dealing with missing data
When data were not available in the printed report, or when data were unclear, we contacted the corresponding author of the study to obtain the missing data. The analysis generally includes only available data (ignoring missing data); however, we would have used methods of estimating missing standard deviations as provided in Section 7.7.3 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), if appropriate. Otherwise, we did not undertake any imputations or use statistical methods to allow for missing data.
Assessment of heterogeneity
Pooling of data and meta‐analysis were carried out only if sufficient similarities were noted between studies in types of participants, interventions and outcomes, including the time of the outcome measurement. If any trials were pooled, the significance of discrepancies in the estimates of treatment effects from the different trials was to be assessed by using Cochran's test for heterogeneity, by which heterogeneity was considered significant if P value < 0.1 (Higgins 2011).
The I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than to chance, was used to quantify heterogeneity, with I2 greater than 50% considered to show substantial heterogeneity (Higgins 2011: Section 9.5.2).
Assessment of reporting biases
Only a proportion of research projects conducted are ultimately published in an indexed journal and become easily identifiable for inclusion in systematic reviews. Reporting biases arise when reporting of research findings is influenced by the nature and direction of the findings of the research. We investigated and attempted to minimise in this review potential reporting biases, including publication bias, time lag bias, multiple (duplicate) publication bias and language bias.
If more than ten studies were included for one outcome, we would have constructed a funnel plot. Any asymmetry in the funnel plot indicating possible publication bias would have been investigated by statistical analysis using the methods introduced by Egger 1997 (continuous outcome) and Rücker 2008 (dichotomous outcome) (such analysis would have been done in STATA 11.0).
Data synthesis
A meta‐analysis was to be conducted only if studies of similar comparisons reported the same outcome measures. Risk ratios would have been combined for dichotomous data and mean differences for continuous data, using random‐effects models, provided more than three studies were included in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to investigate clinical heterogeneity by examining the different sources of fluoride. Provided sufficient studies were identified for each intervention and outcome, we planned a priori to conduct subgroup analyses for different sources of fluoride (mouthrinse, gel, varnish dentifrice, bracket adhesive, elastomeric ligature).
Sensitivity analysis
It was planned to undertake sensitivity analyses to examine the effects of quality assessment items on the assessment of overall estimates of effect.
In addition, the effect on findings of the review of including unpublished literature was to be examined.
Summary of findings table
A 'Summary of findings' table was developed for the primary outcomes of this review using GRADEProfiler software. The quality of the body of evidence was assessed with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias, the magnitude of the effect and whether evidence of a dose response was found. The quality of the body of evidence for each of the primary outcomes was categorised as high, moderate, low or very low.
Results
Description of studies
Results of the search
The original search identified 191 publications, of which 101 were excluded after removal of duplicates and review of the title or abstract. Full‐text articles were obtained for the remaining 90. From the 90 full articles, 58 references were assessed as ineligible for inclusion in this review. We contacted 18 study authors concerning 29 references. On the basis of information provided, 15 references were excluded and three were pending further information, leaving 14 trials, involving 613 participants, included in the original version of this review.
For the 2013 update of this review, three changes to the protocol regarding inclusion criteria resulted in the exclusion of all previously included studies. Five previously included quasi‐randomised trials were excluded (Banks 2000; Dyer 1982; Hirschfield 1978; Millett 2000; Sonis 1989), five previously included split‐mouth studies were excluded (Chung 1998; Czochrowska 1998; Gillgrass 2001; Marcusson 1997; Twetman 1997) and three previously included studies were excluded because outcomes were measured ex vivo on extracted teeth (Gorton 2003; Øgaard 1986; Pascotto 2004). Øgaard 2001 was excluded because investigators compared fluoride versus fluoride plus antiseptic.
Three additional studies were identified by the updated search (Luther 2005; Øgaard 2006; Stecksén‐Blicks 2007), for a total of 458 participants included in the 2013 update of this review. Three ongoing studies were identified (NCT01925924; NCT00268138; NCT01768390).
For details of the studies examined and reasons for inclusion or exclusion, please see Characteristics of included studies and Characteristics of excluded studies tables.
Included studies
Three parallel‐group randomised controlled trials (Luther 2005; Øgaard 2006; Stecksén‐Blicks 2007) were included in the update of this review.
Characteristics of the trial participants and setting
Two of the included studies were conducted in Sweden (Øgaard 2006; Stecksén‐Blicks 2007) and one in the United Kingdom (Luther 2005). Two of the studies included adolescent participants with an age range at baseline of 12 to 15 years, and the study by Luther 2005 also included adults up to the age of 45 years. All participants in the included trials were recruited at the start of their orthodontic treatment with fixed appliances, and in all trials, participants were followed until the end of treatment.
Characteristics of the interventions
One of the included studies was placebo‐controlled (Stecksén‐Blicks 2007), and two conducted head‐to‐head comparisons.
We have grouped these trials into three comparisons.
-
Fluoride‐containing varnish versus non–fluoride‐containing placebo varnish (Stecksén‐Blicks 2007).
-
Amine fluoride and stannous fluoride toothpaste/mouthrinse combination versus sodium fluoride toothpaste/mouthrinse combination (Øgaard 2006).
-
Intraoral fluoride‐releasing glass bead device versus fluoride mouthrinse (Luther 2005).
Characteristics of the outcomes
One of the studies reported the outcome of new demineralised white lesions (DWLs) in each participant (Stecksén‐Blicks 2007), and one study (Luther 2005) planned to report this outcome but actually reported only that no statistically significant difference was observed between the groups. The third study (Øgaard 2006) reported a white spot index.
None of the three included studies reported data for the secondary outcomes of this review: differences in size and severity of DWLs, quantitative assessment of enamel mineral loss, participant perception of DWLs and any measure of oral health–related quality of life, and adverse effects.
Excluded studies
A total of 57 studies were excluded from this updated review for the following reasons (for details, see the Characteristics of excluded studies table).
-
Split‐mouth studies (Banks 1997; Buyukyilmaz 1994; Chung 1998; Czochrowska 1998; Demito 2011; Gillgrass 2001; Marcusson 1997; Mattick 2001; Millett 1999; Millett 2000; Mitchell 1992; Shan 2008; Trimpeneers 1996; Turner 1993; Twetman 1997; van der Linden 1998; Vivaldi‐Rodrigues 2006).
-
Not truly randomised (controlled clinical trials (CCTs)) (Banks 2000; Blanco 1988; Leizer 2010).
-
Not randomised controlled trials (RCTs) (Boyles 2007; Dyer 1982; Farhadian 2008; Fricker 1985; Fricker 1987; Gaworski 1999; Geiger 1988; Geiger 1992; Hirschfield 1978; Maijer 1988; Øgaard 1992; Øgaard 1996; Shannon 1978; Shannon 1979; Sonis 1989; Underwood 1989; Wenderoth 1999).
-
Outcomes reported on extracted teeth (ex vivo) (Gorton 2003; O'Reilly 1987; Øgaard 1986; Pascotto 2004).
-
Outcomes assessed at the end of fluoride treatment, rather than at debonding (Marini 1999; Robertson 2011; Sköld‐Larsson 2013).
-
Outcomes assessed some weeks after debonding (Alexander 2000; Boyd 1992; Boyd 1993).
-
Abstracts with insufficient information for inclusion and no response from authors (Alwi 1994; Neumann 1976; Salzmann 1976).
-
DMFS/DMFT (decayed, missing and filled surfaces/teeth) outcome measures, not DWLs (D'Agostino 1988; Dénes 1988; Dénes 1989; Dénes 1991).
-
Fluoride intervention confounded (Øgaard 1997; Øgaard 2001; Ullsfoss 1994).
Risk of bias in included studies
Overall risk of bias assessments for all included studies are shown in Figure 1. One study (Stecksén‐Blicks 2007) was assessed at low risk of bias for all domains, for another study (Øgaard 2006) the overall risk of bias was unclear and the remaining included study (Luther 2005) was assessed at high risk of bias.
Allocation
Two of the included studies clearly reported the method of sequence generation and clear allocation concealment and were assessed at low risk of selection bias (Luther 2005; Stecksen‐Blicks 2007).
One study (Øgaard 2006) reported the method of sequence generation (randomisation table) but did not mention allocation concealment. This study was assessed at unclear risk of selection bias.
Blinding
Two studies were considered truly triple‐blind (participant, clinician and assessor) (Øgaard 2006; Stecksén‐Blicks 2007) because placebos and comparison interventions were identical in appearance to the experimental intervention.
In Luther 2005, participants and clinicians were not blinded, and the resulting risk of performance bias was high; outcome assessors, however, were blinded, so the risk of detection bias was assessed as low.
Incomplete outcome data
Few postrandomisation exclusions were reported in Stecksén‐Blicks 2007 and Øgaard 2006, and numbers and reasons for exclusion were similar for each group; therefore, the risk of attrition bias was assessed as low. In Luther 2005, the overall rate of postrandomisation exclusions was very high, so this study was assessed at high risk of attrition bias.
Selective reporting
Both Stecksén‐Blicks 2007 and Øgaard 2006 reported all planned outcomes in full and were assessed at low risk of reporting bias. In the report of the study by Luther 2005, some information was missing and the denominators were not stated, so this study was assessed at unclear risk of reporting bias.
Other potential sources of bias
In two studies (Øgaard 2006; Stecksén‐Blicks 2007), no other sources of bias were identified. Luther 2005 was assessed at unclear risk of other bias because of possible differences between the groups in terms of compliance, duration of orthodontic treatment and exposure to topical fluorides.
Effects of interventions
See: Summary of findings for the main comparison Fluoride varnish versus placebo varnish
Topical fluorides
The three studies included in this review evaluated different modes of application of topical fluorides.
-
Fluoride‐containing varnish versus non–fluoride‐containing placebo varnish (Stecksén‐Blicks 2007).
-
Amine fluoride and stannous fluoride toothpaste/mouthrinse combination versus sodium fluoride toothpaste/mouthrinse combination (Øgaard 2006).
-
Intraoral fluoride‐releasing glass bead device versus fluoride mouthrinse (Luther 2005).
Fluoride varnish/paste versus placebo
The trial by Stecksén‐Blicks 2007 compared application of fluoride varnish (Fluor Protector containing 0.1% fluoride (F) as difluorosilane in a polyurethane varnish base, Ivoclar Vivadent) every six weeks versus a non–fluoride‐containing placebo varnish, in a double‐blind study assessed as being at low risk of bias. The outcome was new demineralised white lesions (DWLs) identified from clinical photographs taken before and after orthodontic treatment. A reduction of DWLs (almost 70%) was associated with regular application of fluoride varnish during orthodontic treatment in this study (risk ratio (RR) 0.31, 95% confidence interval (CI) 0.21 to 0.44; Analysis 1.1). No adverse effects were reported. The study result should be interpreted cautiously until further clinical trials confirm this finding.
Different types of fluoride administered in toothpaste and mouthrinse
Øgaard 2006 compared two different types of fluoride compounds provided as toothpaste (twice daily) and mouthrinse (once daily) and supplied to participants for use during orthodontic treatment. One group received amine fluoride/stannous fluoride toothpaste (Meridol 140 parts per million (ppm) F, pH 4.5) and amine fluoride/stannous fluoride mouthrinse to be used after toothbrushing at bedtime (250 ppm F, pH 4.0). The other group received neutral sodium fluoride toothpaste (1400 ppm, pH 6.7) and a sodium fluoride mouthrinse (250 ppm F, pH 6.3) to be used after brushing at bedtime.
The outcome of new DWLs was reported at the tooth level with no indication of correction for clustering of teeth within the mouth. The mean change in the white spot lesion index from baseline was greater in the sodium fluoride group, suggesting that this compound was less effective than amine fluoride/stannous fluoride (Analysis 2.1). A slightly larger increase in both the visible plaque index and the gingival bleeding index was reported over the duration of treatment in the group exposed to sodium fluoride (Analysis 2.2; Analysis 2.3). However, these differences should be interpreted cautiously until the results can be independently replicated.
Fluoride‐releasing intraoral device
In the study by Luther and colleagues (Luther 2005), the experimental group received a carbonate‐based glass bead containing 13.3% fluoride glass (Telsol (UK) Ltd), which was attached to the orthodontic brace. The control group was requested to use a daily fluoride mouthrinse (Endekay rinse 0.05% w/v NaF, Stafford‐Miller Ltd). The number and size of new DWLs were determined using computerised image analysis of before and after cross‐polarised (to reduce flash reflections) photographic images of six anterior teeth in each participant. Results were available for only 37 participants of the original 70 recruited (53%) (Analysis 3.1). The glass beads proved to be fragile, and 18 breakages were reported.
Secondary outcomes
None of the three included studies reported data for the secondary outcomes of this review: any differences in size and severity of DWLs, quantitative assessment of enamel mineral loss, participant perception of DWLs and any measure of oral health–related quality of life, and adverse effects.
Sensitivity analyses
Insufficient trials were included in the review for a sensitivity analysis to be undertaken.
Publication bias
Insufficient trials were included in this review to enable the review authors to investigate publication bias.
Discussion
Summary of main results
Moderate‐quality evidence from one trial at low risk of bias indicates that fluoride varnish applied every six weeks is associated with a reduction in new demineralised white lesions (DWLs) (summary of findings Table for the main comparison).
Evidence is insufficient to show whether amine fluoride and stannous fluoride toothpaste/mouthrinse combination is more or less effective than sodium fluoride toothpaste/mouthrinse combination in preventing DWLs, visible plaque or gingivitis.
Evidence is also insufficient to show whether a reduction in the development of DWLs occurs with the use of an intraoral fluoride‐releasing glass bead device.
Overall completeness and applicability of evidence
The latest update of the review has included only parallel‐group trials, in which the individual participant is the unit of randomisation. This was decided upon because of the possibility of cross‐contamination between experimental and control teeth in the same mouth, either between upper and lower arches or between sides of the mouth, which might lead to under‐estimation of the effectiveness of any fluoride products.
Interventions that rely on the patient for delivery, including fluoride mouthrinse and toothpaste, will work only if they are used regularly. They rely greatly on patient compliance to succeed; however, evidence suggests that compliance with mouthrinsing is poor among orthodontic patients. One study (Geiger 1992) found that only 42% of participants rinsed with a sodium fluoride mouthrinse at least every other day. Results also showed that those who complied least with fluoride rinsing regimens tended to have more DWLs. It is important to consider the acceptability of interventions to both adolescents and adults with a view toward increasing compliance with recommended dental hygiene practices.
Interventions that are professionally applied and deliver fluoride 'passively', such as fluoride varnish, fluoride‐releasing bracket cements and fluoride‐releasing elastics, avoid the need for patient compliance. In addition, these materials deliver fluoride close to the bracket, where it is most needed; however, many fluoridated materials release large amounts of fluoride initially, but the level drops rapidly and might not be sufficient to prevent decay over the whole course of orthodontic treatment. Reapplication of fluoride varnish and frequent replacement of fluoride‐releasing elastics are likely to be required. In the parallel‐group trial of a fluoride varnish intervention included in this review, varnish was reapplied every six weeks at each orthodontic check‐up appointment. We found no parallel‐group trials of fluoride‐releasing cements or elastics that met the inclusion criteria for this review.
An interesting addition, since the initial review was carried out, is the further development of materials that produce a slow and sustained release of fluoride (Luther 2005). This trial was small and at high risk of bias, and evidence was insufficient to reveal whether these devices are more or less effective than a mouthrinse in reducing the development of DWLs. It is possible, that with further refinement, this technique could potentially be effective. Intraoral fluoride‐releasing devices should be evaluated by double‐blind parallel‐group randomised controlled trials.
When examining the effectiveness of a fluoride product in preventing dental decay, one should consider two aspects: first, whether the fluoride product reduces the number of DWLs appearing during treatment, and second, whether it reduces the severity of DWLs in terms of the size or area of the tooth surface affected, the amount of mineral lost or the depth of decay. Banks et al (Banks 2000) developed the Enamel Decalcification Index, which is an ordinal index that includes an assessment of the area covered. Assessment of the size of the lesion is a useful outcome measure, but none of the studies included in this review reported this outcome.
Ideally the appearance of the tooth should be recorded before and after orthodontic treatment, so that the change in appearance of the tooth is measured (incidence), not just its appearance at the end (prevalence). There are many different causes of white lesions on the teeth, many of which occur during the development of the teeth. It is important that these development lesions, as well as decay that has occurred before the brace is fitted, are excluded from the analysis, hence the need for the images (photographs or fluorescent) taken before treatment. Measurement of both incidence and severity will depend on the method used to record DWLs. Two main methods may be used: visual inspection and clinical images. Both methods are associated with problems. One problem with visual inspection is that the examiner or examiners will require calibration at the start and regular recalibration throughout the experimental period to ensure consistency of measurement. The duration of the experiment might be quite long because, as discussed later, the product should ideally be tested over the entire length of orthodontic treatment. This can take between 18 and 30 months ‐ sometimes longer. Another problem with visual recording involves masking of the assessor to the allocated intervention. To reduce bias, the examiner should not know whether the participant has received a fluoride product and this will complicate the way the experiment is run.
Images have the advantage of providing a permanent record of the appearance of the tooth. Assessments can be carried out by several people independently or in groups, whereby a consensus is achieved. The images can be placed in a random order and the judges masked to group allocation. In addition, because the assessment can be performed over a short period of time the problem of examiner drift, whereby an assessor might subtly change his or her assessment over time, is reduced. The challenge of using clinical photographs consists of achieving consistency in lighting and reducing reflections that can mask or mimic DWLs; however, when a careful photographic technique is applied, the advantages of photographs outweigh their potential disadvantages. Several optical and fluorescent methods are available for measuring lesions on the teeth (Angmar‐Mansson 1996). These methods require specialised equipment, which would add considerably to the cost of a clinical study, but they provide an objective measurement of the amount of decay in terms of mineral loss or lesion depth or both.
Quality of the evidence
It is important to note that only one study included in this review was judged to be at low risk of bias, and this assessment was made only after contact with the author resulted in clarification of two issues. Both the design and the reporting of trials of fluorides for preventing DWLs were generally poor, even in the most recent trials published in journals for which the use of CONSORT guidelines has been adopted. In particular, the methods of random allocation, sequence generation and concealment were rarely explained. Few studies provided a flow diagram to show withdrawals and drop‐outs.
Potential biases in the review process
We undertook a sensitive search of several electronic sources, supplemented by searches of references lists. We placed no restriction on language or publication status. The review authors have tried, as far as possible, to identify all possible studies that might meet the inclusion criteria for this review. Study authors have been contacted, and many have replied; however, some were not able to supply the requested information, as their records have been destroyed or lost.
When a product, such as a bonding material, can be applied to single teeth, it is tempting to use an experimental design whereby the material being tested is used in two quadrants of the mouth and the control material is used in the other two quadrants. This is called a split‐mouth design. The main advantage of the split‐mouth design over a conventional parallel‐group study design, in which the two materials are tested in two separate groups of individuals, is that the experimental material is tested in the same mouth, under the same conditions as the control material. In theory, any differences in outcome between the two materials are due only to their properties ‐ not to other factors, such as differences in oral hygiene and diet between participants (with a parallel design) or even differences in oral hygiene and diet over time within the same participants (with a cross‐over design).
Unfortunately, when one is examining the ability of fluoride products to reduce decay, it is highly unlikely that the fluoride released will be confined to only the quadrants/teeth in which the experimental material has been placed, and some contamination of the 'untreated' teeth is inevitable. This contamination will reduce the difference in outcomes between treated and untreated teeth. The previous version of this review included split‐mouth studies, which failed to show any difference between treated and untreated teeth; this may be due to cross‐over contamination between control and experimental sides and may reflect our contention of contamination. For this reason, we have decided to exclude split‐mouth studies from this update of our review, and we recognise that changes to the protocol introduce a risk of bias to the review process.
Agreements and disagreements with other studies or reviews
Three other systematic reviews gathering evidence for the most effective means of preventing caries/demineralisation during fixed orthodontic appliance treatment have been reported in the literature. Derks et al (Derks 2004) examined all preventive measures for preventing demineralisation ‐ not just fluoride products. These review authors had to exclude many published studies as well because of inappropriate research design or poor reporting and were unable to provide firm, evidence‐based recommendations as to the prevention of DWLs during fixed orthodontic treatment.
A second systematic review (Chadwick 2005) investigated the effectiveness of topical fluorides used alone in preventing demineralisation during orthodontic treatment. These review authors included seven studies in their review; however, these studies were excluded from our review because the outcomes were not appropriate (DMFT/DMFS), or the participants were not examined immediately after removal of the fixed appliance(s). Although they suggest that according to their outcome measure (preventive fraction), some evidence shows that the addition of a topical fluoride preparation helps in the prevention of demineralisation during fixed orthodontic treatment, this conclusion must be viewed with caution, because these review authors were not able to calculate confidence intervals. We support their request that researchers design and report their studies using standard outcomes, so that in the future, data may be pooled and overall recommendations on preventive measures may be provided.
Rogers et al (Rogers 2010) included 10 studies in their systematic review investigating the effectiveness of fluoride‐containing bonding adhesives used in orthodontics to prevent demineralisation. Five of these studies were excluded from our review because they were not randomised, and a further three studies were excluded because data in the report were insufficient, and the study authors, when contacted, were unable to provide requested data. Rogers' conclusions are consistent with ours with regard to the design of trials and the quality of reporting and statistical analyses.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fluoride varnish versus placebo varnish, Outcome 1 Number of participants with new DWLs.

Comparison 2 Amine fluoride/stannous fluoride toothpaste/mouthrinse combination versus sodium fluoride toothpaste/mouthrinse combination, Outcome 1 White spot index.

Comparison 2 Amine fluoride/stannous fluoride toothpaste/mouthrinse combination versus sodium fluoride toothpaste/mouthrinse combination, Outcome 2 Visible plaque index.

Comparison 2 Amine fluoride/stannous fluoride toothpaste/mouthrinse combination versus sodium fluoride toothpaste/mouthrinse combination, Outcome 3 Gingival bleeding index.
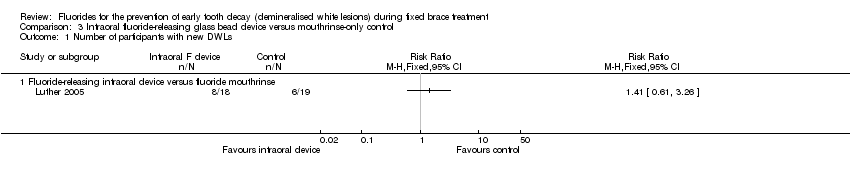
Comparison 3 Intraoral fluoride‐releasing glass bead device versus mouthrinse‐only control, Outcome 1 Number of participants with new DWLs.
| Fluoride varnish versus placebo varnish for the prevention of demineralised white lesions on teeth during fixed brace treatment | ||||||
| Patient or population: Participants undergoing orthodontic treatment with fixed appliances Comparison: Placebo varnish | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo varnish | Fluoride varnish | |||||
| Number of patients with new demineralised white lesions | Study population | RR 0.31 | 253 | ⊕⊕⊕⊝ | ||
| 640 per 1000 | 198 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1 A single study with 253 participants evaluated this outcome. Risk of bias was assessed as low. However, this finding should be interpreted with caution until the study has been replicated. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with new DWLs Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 White spot index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Visible plaque index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Gingival bleeding index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants with new DWLs Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fluoride‐releasing intraoral device versus fluoride mouthrinse | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

