Ácido 5 aminosalicílico oral para el mantenimiento de la remisión inducida quirúrgicamente en la enfermedad de Crohn
Resumen
Antecedentes
La prevención de la recurrencia es un aspecto fundamental en el tratamiento de la enfermedad de Crohn. Los corticosteroides, que son el tratamiento principal de las exacerbaciones agudas, no son efectivos para el mantenimiento de la remisión y su uso crónico está limitado por numerosos eventos adversos. Los ensayos controlados aleatorizados que evalúan la eficacia de los agentes orales ácido 5 aminosalicílico (5‐ASA) para el mantenimiento de la remisión médicamente inducida en la enfermedad de Crohn han producido resultados contradictorios.
Objetivos
Realizar una revisión sistemática para evaluar la eficacia y la seguridad de los agentes orales 5‐AAS para el mantenimiento de la remisión médicamente inducida en la enfermedad de Crohn.
Métodos de búsqueda
Se realizaron búsquedas en MEDLINE, EMBASE, CENTRAL y en el registro especializado del Grupo Cochrane de EII desde su creación hasta el 8 de junio de 2016. También se realizaron búsquedas en listas de referencias y en actas de los congresos.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados que compararon los agentes orales 5‐ASA con placebo o sulfasalazina en pacientes con enfermedad de Crohn inactiva. Los ensayos debían tener una duración de tratamiento de al menos seis meses.
Obtención y análisis de los datos
Dos autores, de forma independiente, extrajeron los datos y realizaron la evaluación del riesgo de sesgo. Cualquier desacuerdo se resolvió mediante discusión y consenso. La principal medida de resultado fue la ocurrencia de una recurrencia, según la definición de los estudios primarios. Los resultados secundarios incluyeron el tiempo hasta la recurrencia, los eventos adversos, el retiro debido a eventos adversos y los eventos adversos graves. Se calculó el riesgo relativo (RR) combinado y el intervalo de confianza del 95% (IC del 95%) correspondiente mediante un modelo de efectos fijos. Todos los datos se analizaron sobre la base de la intención de tratar y los abandonos se consideraron como recurrencia. Los análisis de sensibilidad incluyeron un análisis de los casos disponibles en el que se ignoraron los abandonos y se utilizó un modelo de efectos aleatorios. La calidad general de la evidencia que respalda los resultados se evaluó mediante los criterios GRADE.
Resultados principales
Se incluyeron 12 estudios (2146 participantes) que compararon 5‐ASA con placebo. No se identificaron estudios que compararan la sulfasalazina con placebo. Siete estudios se consideraron con bajo riesgo de sesgo. Se consideró que los demás estudios tuvieron riesgo incierto de sesgo en diversos ítems debido a la falta de detalles suficientes para poder evaluarlos. No se encontraron diferencias estadísticamente significativas en las tasas de recurrencia a los 12 meses. El 53% (526/998) de los pacientes con 5‐ASA (dosis de 1,6 g a 4 g/día) presentaron una recurrencia a los 12 meses, en comparación con el 54% (544/1016) de los pacientes con placebo (RR 0,98; IC del 95%: 0,91 a 1,07; 11 estudios; 2014 pacientes; evidencia de calidad moderada). Los análisis de sensibilidad basados en un análisis de los casos disponibles y un modelo de efectos aleatorios no tuvieron repercusión en los resultados. Un estudio no encontró diferencias en las tasas de recurrencia a los 24 meses. El 54% (31/57) de los pacientes con 5‐ASA (dosis de 2 g/día) presentaron una recurrencia a los 24 meses, en comparación con el 58% (36/62) de los pacientes con placebo (RR 0,94; IC del 95%: 0,68 a 1,29; 119 pacientes; evidencia de calidad baja). Un estudio pediátrico no encontró diferencias estadísticamente significativas en las tasas de recurrencia a los 12 meses. El 62% (29/47) de los pacientes pediátricos con 5‐ASA (dosis de 50 mg/kg/día) presentaron una recurrencia a los 12 meses, en comparación con el 64% (35/55) de los pacientes pediátricos con placebo (RR 0,97; IC del 95%: 0,72 a 1,31; 102 pacientes; evidencia de calidad moderada). No hubo diferencias estadísticamente significativas en la proporción de pacientes que presentaron un evento adverso, retiro debido a eventos adversos o eventos adversos graves. El 34% (307/900) de los pacientes con 5‐ASA tuvieron al menos un evento adverso en comparación con el 33% (301/914) de los pacientes con placebo (RR 1,05; IC del 95%: 0,95 a 1,17; diez estudios; 1814 pacientes). El 14% (127/917) de los pacientes con 5‐ASA se retiraron debido a eventos adversos, en comparación con el 13% (119/916) de los pacientes con placebo (RR 1,11; IC del 95%: 0,88 a 1,38; nueve estudios; 1833 pacientes). El 1% (3/293) de los pacientes con 5‐ASA tuvieron un evento adverso grave en comparación con el 0,7% (2/283) de los pacientes con placebo (RR 1,43; IC del 95%: 0,24 a 2,83; tres estudios; 576 pacientes). Entre los efectos adversos más frecuentes que se informaron en los estudios se encontraban diarrea, náuseas y vómitos, dolor abdominal, dolor de cabeza y erupción cutánea.
Conclusiones de los autores
En esta revisión no se encontró evidencia que indicara que las preparaciones orales de 5‐AAS son superiores a placebo en el mantenimiento de la remisión médicamente inducida en pacientes con enfermedad de Crohn. Es posible que no se justifique la realización de ensayos aleatorizados adicionales.
PICO
Resumen en términos sencillos
Ácido 5 aminosalicílico oral para el mantenimiento de la remisión inducida quirúrgicamente en la enfermedad de Crohn
¿Qué es la enfermedad de Crohn?
La enfermedad de Crohn es un trastorno inflamatorio crónico que puede comprometer cualquier segmento del tracto gastrointestinal. Puede afectar a personas de cualquier edad. Cuando los pacientes tienen la enfermedad de Crohn activa presentan síntomas como dolor abdominal, diarrea y pérdida de peso. Cuando los síntomas cesan, se considera que los pacientes están en remisión. La enfermedad de Crohn activa se puede tratar mediante tratamiento médico (p.ej., con fármacos como esteroides, inmunosupresores o biológicos) o mediante cirugía para extirpar las porciones enfermas del intestino. El objetivo del tratamiento médico de la enfermedad de Crohn es inducir la remisión y mantenerla durante el mayor tiempo posible.
¿Qué son los fármacos ácido 5 aminosalicílico (5‐ASA)?
Los fármacos 5‐ASA son un grupo de compuestos que se piensa que tratan la enfermedad de Crohn reduciendo la inflamación en el tracto gastrointestinal. Estos fármacos a menudo se administran por vía oral (es decir, por la boca).
¿Qué examinaron los investigadores?
Se estudió si el 5‐ASA oral mantiene la remisión en los pacientes con enfermedad de Crohn y si causa algún efecto perjudicial (efectos secundarios). Se buscó extensamente en la literatura médica hasta el 8 de junio de 2016.
¿Qué encontraron los investigadores?
Se encontraron 12 estudios con un total de 2146 participantes. Once estudios que incluyeron 2014 participantes adultos compararon 5‐ASA oral con placebo (es decir, píldoras o comprimidos inactivos). Un estudio con 132 niños comparó 5‐ASA oral con placebo. Se realizaron 11 estudios durante 12 meses y un estudio durante 24 meses. Siete estudios se consideraron de calidad alta y los demás estudios se consideraron de calidad incierta porque no se informaron detalles suficientes para poder emitir un juicio sobre la calidad. Los estudios con detalles insuficientes en general fueron estudios más antiguos que se publicaron hace 20 años o más. Un análisis combinado de 11 estudios con 2014 participantes adultos no encontró diferencias entre 5‐ASA oral (a dosis diarias entre 1,6 g y 4 g) y placebo en la proporción de participantes que permanecían en remisión a los 12 meses. Igualmente, en un estudio en el que participaron 161 adultos no se encontraron diferencias entre 5‐ASA oral (a una dosis de 2 g por día) y placebo en la proporción de participantes que permanecían en remisión a los 24 meses. El estudio con niños no encontró diferencias entre 5‐ASA oral (a una dosis diaria de 50 mg/kg) y placebo en la proporción de participantes que permanecían en remisión a los 12 meses. No parece haber un mayor riesgo de efectos secundarios en los pacientes que toman 5‐ASA oral en comparación con placebo. Entre los efectos adversos más frecuentes que se informaron en los estudios se encontraban diarrea, náuseas y vómitos, dolor abdominal, dolor de cabeza y erupción cutánea.
En conclusión, no hay evidencia de que 5‐ASA oral sea superior a placebo para ayudar a los pacientes con enfermedad de Crohn a permanecer en la remisión lograda con el tratamiento médico.
Authors' conclusions
Summary of findings
| 5‐ASA compared to placebo for maintenance of medically‐induced remission in Crohn's disease | ||||||
| Patient or population: patients with maintenance of medically‐induced remission in Crohn's disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 5‐ASA compared to placebo | |||||
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ 12 months | 535 per 10001 | 525 per 1000 | RR 0.98 | 2014 | ⊕⊕⊕⊝ | |
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ 24 months | 679 per 10003 | 672 per 1000 | RR 0.99 | 161 | ⊕⊕⊝⊝ | |
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ Pediatric | 688 per 10003 | 736 per 1000 | RR 1.07 | 132 | ⊕⊕⊕⊝ | |
| Adverse events | 329 per 10001 | 346 per 1000 | RR 1.05 | 1814 | ⊕⊕⊝⊝ | |
| Withdrawals due to adverse events | 130 per 10001 | 144 per 1000 | RR 1.11 | 1833 | ⊕⊕⊝⊝ | |
| Serious adverse events | 7 per 10001 | 10 per 1000 | RR 1.43 | 576 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
Background
Crohn's disease is a chronic inflammatory disorder that can involve any part of the gastrointestinal tract. Common symptoms include abdominal pain, diarrhoea and weight loss. Crohn's disease is characterized by chronicity and recurrences. There is no cure for Crohn's disease.Thus, treatment is directed towards inducing and maintaining remission, and addressing complications.
The prevention of relapse is a major issue in the management of Crohn's disease. Corticosteroids, the main treatment for acute exacerbations, are not effective for maintenance of remission in Crohn's disease (Steinhart 2003), and long‐term use of corticosteroids is limited by numerous adverse events.
5‐aminosalicylates are a group of compounds that have long been used in inflammatory bowel disease. The first 5‐aminosalicylate agent used in clinical practice was sulphasalazine, which is composed of sulphapyridine linked by an azo bond to 5‐aminosalicylic acid (5‐ASA). Sulphasalazine was first used in the 1940s as a treatment for arthritis (Svartz 1942). Improvement in gastrointestinal symptoms was noted in patients who had concurrent ulcerative colitis leading to further use of this agent in inflammatory bowel disease.
The majority of an oral dose of sulphasalazine reaches the colon where the azo‐bond is split by an azo reductase released by colonic bacteria, yielding 5‐ASA and sulphapyridine. Virtually all the sulphapyridine is absorbed from the colon whereas most of 5‐ASA remains within the colon and is excreted in the stool. Several studies have shown that 5‐ASA is the active therapeutic moiety of sulphasalazine and that sulphapyridine acts only as a carrier molecule to deliver 5‐ASA to the colon (Azad Khan 1977; Klotz 1980; van Hees 1980). Yet it is the sulphapyridine which is responsible for most of the severe adverse effects of sulphasalazine (Schroder 1972). Sulphapyridine undergoes acetylation in the liver with subsequent excretion in the urine. Slow acetylators show more of the adverse effects of this component secondary to accumulation of sulphapyridine in the blood prior to excretion in the kidneys (Das 1973).
Recognition that 5‐ASA is the active ingredient of sulphasalazine and that sulphapyridine is responsible for most of the side effects led to several investigations of the use of 5‐ASA as a single agent for the treatment of inflammatory bowel disease. 5‐ASA in an unprotected form is, however, readily absorbed in the proximal small intestine (Myers 1987), and does not reach the distal bowel in therapeutic concentrations. There have been several formulations of 5‐ASA designed to inhibit proximal absorption and to ensure delivery to distal sites of inflammation. There are two basic ways in which 5‐ASA can be protected: by linking it to itself or to another carrier and by the use of slow release preparations of 5‐ASA. Different 5‐ASA preparations may allow delivery of 5‐ASA to different locations in the gastrointestinal tract.
5‐ASA formulations include azo compounds, mesalazine delayed‐release agents and mesalazine slow‐release formulations. For azo compounds a carrier molecule is linked to 5‐ASA by an azo bond using the same principle as sulphasalazine. Olsalazine consists of two molecules of 5‐ASA joined together whilst balsalazide is a pro‐drug in which a 5‐ASA molecule is linked to 4‐aminobenzoyl‐B‐alanine, an inert and biologically inactive carrier molecule. Like sulphasalazine, the azo bond of these drugs is split in the colon by bacterial azo‐reductases, releasing 5‐ASA to exert local therapeutic activity. Mesalazine delayed‐release agents (Eudragit‐coated) are coated with a resin designed to dissolve at a certain pH. Asacol is coated with Eudragit S which dissolves above pH 7.0 to release 5‐ASA in the terminal ileum and colon. Eudragit‐L coated mesalazine (Salofalk) dissolves above pH 6.0 to release 5‐ASA in the terminal ileum and colon. Mesalazine slow‐release formulations include drugs such as Pentasa. Pentasa contains microgranules of 5‐ASA that are individually coated with ethylcellulose. The microgranules are dispersed in the gut providing a slow, steady release of 5‐ASA along the length of the intestine from the upper small bowel to the colon.
These 5‐ASA preparations were intended to avoid the adverse side effects of sulphasalazine whilst maintaining its therapeutic benefits. Several randomised controlled trials have been published, comparing various 5‐ASA agents to placebo, with conflicting results (De Franchis 1997; Gendre 1993; Mahmud 2001; Sutherland 1997). Three previous meta‐analyses have suggested that 5‐ASA may be beneficial for the maintenance of remission in Crohn's disease (Camma 1997; Messori 1994; Steinhart 1994), but in one report the only bibliographic database searched was MEDLINE (Camma 1997), and another did not report how the quality of included studies was assessed (Messori 1994). Furthermore, a number of recent randomised controlled trials have been published since these meta‐analyses were reported. An up to date systematic review using the Cochrane Collaboration format is indicated to summarise the current evidence on the use of 5‐ASA agents for the maintenance of medically‐induced remission in Crohn's disease. When possible, data on outcomes were pooled together for meta‐analyses. This systematic review is an update of a previously published Cochrane review (Akobeng 2005). The use of 5‐ASA agents for the prevention of recurrences following surgery for Crohn's disease was not the subject of this review and is covered by a separate systematic review (Gordon 2011).
Objectives
1. To evaluate the efficacy of 5‐ASA agents for the maintenance of medically‐induced remission in Crohn's disease.
2. To determine adverse events associated with 5‐ASA treatment in Crohn's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were considered for inclusion.
Types of participants
Patients of any age with Crohn's disease in remission as defined by a recognized Crohn's disease activity index or endoscopy were considered for inclusion.
Types of interventions
Trials that compared oral 5‐ASA agents to placebo or sulphasalazine with a treatment duration of at least six months were considered for inclusion.
Types of outcome measures
The primary outcome measure was the occurrence of clinical or endoscopic relapse as defined by the primary studies. Secondary endpoints included time to relapse, adverse events, withdrawals due to adverse events and serious adverse events.
Search methods for identification of studies
A. Electronic searching
We searched the following electronic databases from inception to 8 June 2016 for relevant studies:
1. MEDLINE;
2. EMBASE;
3. CENTRAL; and
4. Cochrane IBD Group Specialized Register.
The search strategy was not limited by language (see Appendix 1).
B. Reference searching
The references of all identified studies were inspected for more trials.
C. Abstracts of major gastroenterology meetings
A manual search of abstracts submitted to major gastroenterology meetings (1995 to 2016) was performed in the following journals to identify more trials:
1. Gastroenterology (American Gastroenterological Association);
2. Gut (British Society of Gastroenterology);
3. American Journal of Gastroenterology (American College of Gastroenterology);
4. Canadian Journal of Gastroenterology (Canadian Association of Gastroenterology);
5. Journal of Pediatric Gastroenterology and Nutrition (European Society of Paediatric Gastroenterology, Hepatology and Nutrition); and
6. Journal of Pediatric Gastroenterology and Nutrition (North American Society of Paediatric Gastroenterology, Hepatology and Nutrition).
D. Personal contacts
Leaders in the field were contacted to try to identify other studies.
E. Drug companies
The manufacturers of 5‐ASA agents were contacted for additional data.
Data collection and analysis
Papers (or abstracts) that appeared to be potentially relevant were identified by two authors (DZ and JKM). The authors (DZ and JKM), after reading the full texts, independently assessed the eligibility of all trials identified using the inclusion criteria above. Disagreement among authors was discussed and agreement reached by consensus.
Quality assessment
The methodological quality of the included studies was independently evaluated by two authors (DZ and JKM) using the Cochrane risk of bias tool (Higgins 2011). Each trial was rated as high, low, or unclear risk of bias for each of the following criteria:
-
Randomisation sequence generation;
-
Allocation concealment;
-
Blinding;
-
Incomplete outcome data;
-
Selective reporting; and
-
Other sources of bias.
The overall quality of the evidence supporting the primary outcomes was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). Randomised trials are considered to provide high quality evidence, but may be downgraded due to: (1) risk of bias, (2) indirectness of evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision (sparse data), and (5) reporting bias (publication bias). The different quality ratings are interpreted as the likelihood that future research would change the effect estimate. Further research is unlikely to change the effect estimate if the evidence is high quality. If the overall evidence is of moderate quality further research may have an impact on our confidence in the effect estimate and may change the estimate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate when the evidence is rated as low quality. Very low quality research means that we are very uncertain about the finding (Guyatt 2008; Schünemann 2011).
DATA COLLECTION
A data extraction form was developed and used to extract information on relevant features and results of included studies. Two authors (DZ and JKM) independently extracted and recorded data on the predefined checklist. Extracted data included the following items:
a. characteristics of patients: age, sex, disease distribution, disease duration, disease activity index;
b. total number of patients originally assigned to each treatment group;
c. intervention: type and dose of 5‐aminosalicylate;
d. control: placebo, other drugs;
e. concurrent medications; and
f. outcomes: time of assessment, length of follow up, type of Crohn's disease activity index used, definitions of remission and relapse, relapse rates, adverse events.
STATISTICAL ANALYSIS
The Cochrane Collaboration review manager (RevMan) software (version 5.3.5) was used for data analysis. Patients with final missing outcomes were assumed to have relapsed. Analyses were grouped by length of follow‐up.
Dichotomous variables
We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. We pooled studies for meta‐analysis when patients, outcomes, and interventions were deemed to be sufficiently similar (determined by consensus). A fixed‐effect model was used to pool data.
Heterogeneity
Heterogeneity among trial results was assessed by visual inspection of forest plots and by calculating the Chi2 and I2 statistics. We aimed to further investigate potential sources of heterogeneity.
Publication Bias
The possibility of a publication bias was investigated through the construction of funnel plots (trial effects versus trial size).
Sensitivity analyses
Sensitivity analyses were conducted based on the following:
a. only including patients whose outcome is known (i.e. an available case analysis where the number of patients who completed the study are used as the denominator); and
b. random‐effects versus fixed‐effect models.
We also planned to consider the effect of:
c. allocation concealment;
d. type of 5 ASA;
e. dose of 5 ASA; and
f. concurrent medications (especially immunosuppressants such as azathioprine, 6‐mercaptopurine, methotrexate, cyclosporine and mycophenolate mofetil).
Results
Description of studies
A literature search conducted on 8 June 2016 identified 10,137 studies. Five additional studies were identified through searching of references. After duplicates were removed a total of 7023 reports remained for review of titles and abstracts. Two authors independently reviewed titles and abstracts and 37 potentially relevant reports on the use of 5‐ASA agents for the maintenance of medically‐induced remission in Crohn's disease were selected for full text review (See Figure 1). Twenty reports of 15 studies were excluded (See Characteristics of excluded studies). Five studies were excluded because allocation of patients to treatment was not random (Bresci 1991; Bresci 1994; Hanauer 1993; Lichtenstein 2009; Nakshabendi 1992). One study was excluded because the duration of treatment was four months (Brignola 1992). Camma 1997 was excluded because it was a systematic review and Luthra 2002 was excluded because it was a commentary on a published trial. Five studies were excluded because they compared sulphasalazine to placebo (Ewe 1976; Lennard‐Jones 1977; Malchow 1981; Malchow 1984; Summers 1979). Schreiber 1994 was excluded because it compared 5‐ASA to 4‐ASA. Anthonisen 1974 was excluded the participants had active Crohn's disease and were not in remission.
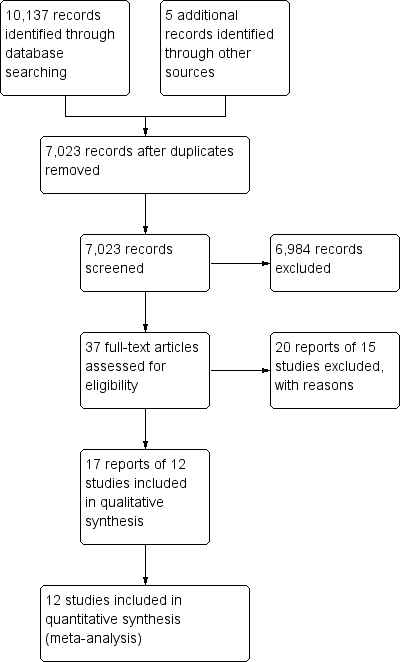
Study flow diagram.
Eighteen reports of 12 studies involving a total of 2146 patients, were selected for inclusion (Anonymous 1990; Arber 1995; Bondesen 1991; Cezard 2009; De Franchis 1997; Gendre 1993; Mahmud 2001; Modigliani 1996; Prantera 1992; Sutherland 1997; Thomson 1995; Wellman 1988). All twelve included studies were randomised controlled trials that compared oral 5‐ASA agents to placebo for the maintenance of medically‐induced remission in Crohn's disease (see Characteristics of included studies). None of the included studies compared 5‐ASA to sulphasalazine. The participants of eleven of the included studies were adults (aged >18). Cezard 2009 enrolled paediatric participants (aged < 18 years). In 10 of the studies, the duration of follow‐up was 12 months (Anonymous 1990; Arber 1995; Cezard 2009; De Franchis 1997; Mahmud 2001; Modigliani 1996; Prantera 1992; Sutherland 1997; Thomson 1995; Wellman 1988). Gendre 1993 followed up patients at 12 and 24 months. Bondesen 1991 followed up patients at 6 month intervals for 12 to 18 months. In 11 of the included studies, the diagnosis of Crohn's disease was established by conventional clinical, radiologic, endoscopic or histologic criteria. Bondesen 1991 did not describe how the diagnosis Crohn's disease was established. In two studies (Arber 1995; Cezard 2009), the Harvey Bradshaw activity index (Softley‐Clamp modification) was used to measure relapse whilst the other studies used the Crohn's Disease Activity Index (CDAI).
Studies with 12 months follow‐up
Anonymous 1990
Sample
Two hundred and forty‐eight patients were randomised from eight centres in Europe, Canada and South Africa. Patients had to be in remission at time of entry (CDAI <150) and their disease had to be been controlled (i.e. no steroids or stable low dose prednisone 2.5 mg/day or less) for the preceding month prior to entry. Exclusion criteria included ileostomy or colostomy, obstruction, perforation or haemorrhage, total parental nutrition, treatment with metronidazole, azathioprine or disodium cromoglycate, known sensitivity to salicylates, other significant disease or unsuitability to participate in the trial. Patients subsequently found not to meet the inclusion criteria after randomisation were excluded from the analysis. The age of the 206 patients (115 males, 91 females) who were included in the per protocol analysis ranged from 16 to 75 years.
Treatment
Patients were randomised to receive either 5‐ASA (Mesasal/Claversal) 1.5 g/day or placebo. Only anti‐diarrhoeal agents were allowed as additional medications.
Endpoints
Patients were followed up for 12 months and were considered to have relapsed if the CDAI became >150 and had increased 60 points from baseline. Secondary outcomes included adverse events and withdrawal due to adverse events.
Arber 1995
Sample
Fifty‐nine patients (37 men, 22 women) were recruited from 9 gastroenterology centres in Israel. All patients had proven Crohn's disease of at least one years duration and were in continuous remission for at least six months (Harvey‐Bradshaw index < 4), while being treated with only 5‐ASA, sulphasalazine or no therapy. Other exclusion criteria were not reported.
Treatment
Participants were randomised to receive either mesalazine (Rafasal) 250 mg tablets (2 x 2 per day) (n = 28) or placebo (n = 31). Restrictions on concomitant medications were not reported.
Endpoints
Patients were followed up for 12 months and primary trial endpoints were 1‐year follow‐up, or clinical relapse (rise of more than four points on the Harvey Bradshaw index). Secondary outcomes included adverse events and withdrawal due to adverse events.
Bondesen 1991
Sample
Two hundred and two patients were recruited from eight gastroenterology centres in Denmark. Patients had to have clinically inactive Crohn's disease. There were no differences between the treatment groups with respect to sex, age, disease duration or location, prior medical or surgical treatment, length of remission, CDAI at entry or adherence with medication regimen.
Treatment
Patients were randomised to receive either 5‐ASA (Pentasa) 1.5 g twice a day (n = 101) or placebo (n = 101).
Endpoints
Patients were follow up at 6 month intervals for 12 to 18 months and trial endpoints were relapse and adverse events.
Sample
One hundred and thirty‐two paediatric patients (age < 18 years) were recruited from 17 centres (16 from France and 1 from Switzerland). Patients had to be diagnosed with Crohn's disease before the age of 16 and were in clinical remission within six months of flare‐up treatment. Exclusion criteria included previous treatment with mesalazine or immunosuppressants or known hypersensitivity to salicylates.
Treatment
Patients were randomised to receive either 5‐ASA 50 mg/kg/day (n = 68) or placebo (n = 64).
Endpoints
Patients were followed up for 12 months and trial endpoints included relapse, adverse events, withdrawal due to adverse events and serious adverse events.
Mahmud 2001
Sample
Three hundred and twenty‐seven patients (150 males, 177 females; age >18 years) were recruited from 3 European countries (Ireland, United Kingdom and France). Patients were diagnosed with Crohn's disease within five years of entry, and had to be in remission for at least one month prior to randomisation (CDAI < 150). Steroids, azathioprine or other immunosuppressive therapy was not allowed within one month of the pre‐study visit nor was concomitant therapy with antibiotics for more than one month. Other exclusion criteria included pregnancy, intending to be pregnant or breast feeding, clinically significant hepatic or renal insufficiency, strictures causing mechanical obstruction, fistulae, oral or symptomatic anal Crohn's disease, stoma or significant small bowel disease apart from terminal ileal disease and patients with known hypersensitivity to salicylates.
Treatment
Participants were randomised to receive either 2 g/day of 5‐ASA (Olsalazine) or placebo. No other active medication for Crohn's disease was allowed, but antidiarrhoeal agents were permitted.
Endpoints
Patients were followed up for a total of 12 months. The primary endpoint of efficacy was relapse defined as a CDAI > 150 or an increase in the CDAI score by 60 points or more from the baseline at visit 2 (week 0), or the need for additional therapy or surgery.
Prantera 1992
Sample
One hundred and twenty‐five patients (78 males, 47 females; age range 23 to 48 years) were recruited from 8 Italian centres. All patients were in remission (defined as a CDAI < 150) for at least 3 months, but not more than 2 years and had not taken corticosteroids, sulphasalazine or metronidazole for at least 3 months or azathioprine for at least 6 months prior to entry. Other exclusion criteria included intestinal strictures 12 months prior to entry; Crohn's disease close to the ileum; active perianal or extraintestinal Crohn's disease; internal and external fistulas; sensitivity to aminosalicylates; and "other usual criteria for excluding participation in a clinical trial".
Treatment
Participants were randomised to receive either 5‐ASA (Asacol) 2.4 g/day (n = 64) or placebo (n = 61). Restrictions on concomitant medications were not reported.
Endpoints
Patients were followed up for 12 months. The primary study endpoint was clinical relapse defined as CDAI > 150 with an increase of 100 points over the baseline. Secondary outcomes included adverse events and withdrawal due to adverse events.
Sutherland 1997
Sample
Two hundred and ninety‐three patients ( aged ≥18 years old) were recruited from 31 Canadian centres. Patients had to be in remission (CDAI < 150 and no symptoms for 30 days prior to entry) and have reported at least two flares within the last four years, with one flare or a recent resection within 18 months. Participants should not have taken immunosuppressives within 90 days, corticosteroids within 30 days or mesalamine or metronidazole within 7 days of entry. Other exclusion criteria included total proctocolectomy, short‐bowel syndrome, three or more resections within the last 10 years, chronic perianal disease, ulcerative colitis, positive stool tests for pathogens, parasites or Clostridium difficile toxin, drug or alcohol abuse, hepatic, neurological, endocrine, renal or other major systemic disease, and cancer (excluding basal or squamous cell skin), inability to provide informed consent, and sensitivity to salicylates.
Treatment
Patients were randomised to receive either 5‐ASA (capsules of microsphere coated with ethylcellulose) 3 g/day or placebo. Other active medications for Crohn's disease were not allowed but codeine and loperamide were permitted for the control of diarrhoea.
Endpoints
Patients were followed up for 12 months. The primary outcome measure was relapse defined as CDAI >150 or an increase of at least 60 points from baseline. Secondary outcomes included adverse events, withdrawal due to adverse events and serious adverse events.
Thomson 1995
Sample
Two hundred and seven participants (101 females, 106 males; age range 18 to 71 years) were recruited from 7 European countries, Canada, South Africa and Israel. Patients had to be in remission (CDAI < 150), and have had one flare within 18 months of study entry. Azathioprine, other immunosuppressives or corticosteroids were not permitted within one month of entry. Other exclusion criteria included pregnancy and previous gastrointestinal surgery with more than 100 cm of bowel excised.
Treatment
Patients were randomised to receive either 3 g/day of 5‐ASA (Claversal/Mesasal) or placebo. Other active medications for Crohn's disease were not allowed with the exception of antidiarrhoea drugs. Short‐term antibiotics were allowed for non‐intestinal infections.
Endpoints
Patients were followed up for 12 months. The primary outcome measure was relapse defined as a CDAI score greater than 150 with at least a 60‐point increase from the baseline index score. Secondary outcomes included adverse events and withdrawal due to adverse events.
Study with 24 months follow‐up
Gendre 1993
Sample
One hundred and sixty‐one patients (77 men, 84 women; age range 17 to 50 years) were recruited from 16 centres in France. All patients had been in remission (CDAI < 150) for less than 24 months prior to entry. Steroids or immunosuppressive therapy were not permitted for at least one month before entry. Other exclusion criteria included curative surgery, perianal disease or planned pregnancy.
Treatment
Patients were randomised to receive either mesalazine (Pentasa), 2 g/day (n = 80) or placebo (n = 81). Antispasmodics, antidiarrhoeal drugs, cholestyramine and sedatives were allowed.
Endpoints
Patients were followed up for 24 months and trial endpoints included surgery for acute complications, clinical relapse (defined as CDAI of > 250 or a CDAI between 150 and 250 with an increase of > 50 points from baseline), adverse events, and withdrawal due to adverse events.
Risk of bias in included studies
The results of the risk of bias analysis are summarized in Figure 2. Random sequence generation was rated as low risk of bias in seven studies (Anonymous 1990; Arber 1995; Cezard 2009; Mahmud 2001; Prantera 1992; Sutherland 1997; Thomson 1995) and as unclear risk of bias in five studies (Bondesen 1991; De Franchis 1997; Gendre 1993; Modigliani 1996; Wellman 1988). Allocation concealment was rated as low risk of bias in seven studies (Anonymous 1990; Arber 1995; Cezard 2009; De Franchis 1997; Prantera 1992; Sutherland 1997; Thomson 1995) and as unclear risk of bias in five studies (Bondesen 1991; Gendre 1993; Mahmud 2001; Modigliani 1996; Wellman 1988). Blinding of participants and personnel was judged to be adequate in 10 studies and unclear risk of bias in two studies (Bondesen 1991; Wellman 1988). The blinding of outcome assessors was judged to be adequate in nine studies and unclear risk of bias in three studies (Bondesen 1991; De Franchis 1997; Wellman 1988). Bondesen 1991 did not describe drop‐outs and was rated as unclear risk of bias for incomplete outcome data. Mahmud 2001 was rated as unclear risk of bias for incomplete outcome data because more patients in the olsalazine group failed to complete the study compared to placebo patients. Reasons for patients withdrawal were given in all studies, though no study reported post‐withdrawal data for any patient. The percentage of randomised patients with unknown outcome at the end of study ranged from 3% (Wellman 1988) to 34% (Modigliani 1996; Thomson 1995). For most studies more patients in the 5‐ASA arm had unknown outcomes compared to the placebo arm, this difference ranged from 3% (Sutherland 1997) to 16% (Mahmud 2001). See additional Table 1 for more information. Five studies were judged to be at low risk of bias for selective reporting (Cezard 2009; Mahmud 2001; Modigliani 1996; Thomson 1995; Wellman 1988). Six studies were judged to be at unclear risk of bias for selective reporting for reporting on some post hoc subgroup analyses (Anonymous 1990; Arber 1995 ; De Franchis 1997; Gendre 1993; Prantera 1992; Sutherland 1997). However, these subgroup analyses would generally be expected for this type of study. Bondesen 1991 was judged to be at unclear risk of bias for selective reporting and other bias because it was an abstract publication that provided insufficient details to allow a judgement. The other studies were rated as low risk of bias for other potential sources of bias.
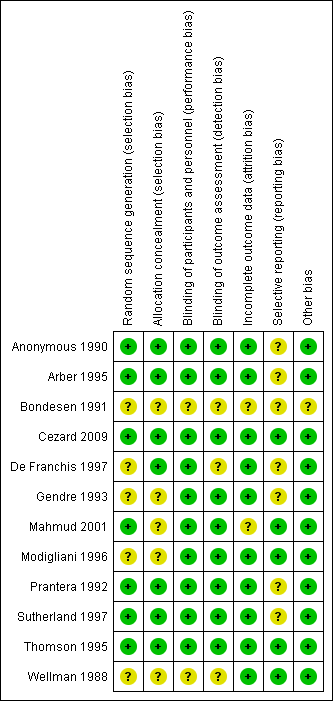
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Study | Total Unknown ‐ n(%) | 5‐ASA unknown ‐ n(%) | Placebo unknown ‐ n(%) |
| Anonymous 1990 | 55 (22%) | 23 (19%) | 32 (26%) |
| Arber 1995 | 10 (17%) | 6 (21%) | 4 (13%) |
| Cezard 2009 | 30 (23%) | 21 (31%) | 9 (14%) |
| De Franchis 1997 | 17 (14%) | 8 (14%) | 9 (15%) |
| Gendre 1993 (Eng) | 42 (26%) | 23 (29%) | 19 (23%) |
| Mahmud 2001 | 82 (25%) | 55 (33%) | 27 (17%) |
| Modigliani 1996 | 44 (34%) | 17 (26%) | 27 (42%) |
| Prantera 1992 | 15 (12%) | 10 (16%) | 5 (8%) |
| Sutherland 1997 | 92 (31%) | 47 (33%) | 45 (30%) |
| Thompson 1995 | 98 (34%) | 52 (38%) | 46 (31%) |
| Wellman 1988 | 2 (3%) | 2 (6%) | 0 (0%) |
Effects of interventions
Efficacy
Occurrence of relapse
For the main analysis, we used as the denominator the total number of patients randomised. We assumed that participants who dropped out of the study, and on whom there were no post withdrawal information, had relapsed during the study period. There was no statistically significant difference in relapse rates in patients who were followed for 12 months. Fifty‐three per cent (526/998) of 5‐ASA patients relapsed at 12 months compared to 54% (544/1016) of placebo patients (RR 0.98, 95% CI 0.91 to 1.07; 11 studies; 2014 patients; Figure 3). No significant heterogeneity was detected for this comparison (I2 = 33%, P = 0.14). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to an unknown risk of bias in some studies in the pooled analysis (See summary of findings Table for the main comparison). A sensitivity analysis using a random‐effects model had little effect on the results (RR 0.98, 95% CI 0.88 to 1.08). There was no statistically significant difference in relapse rates at 24 months. Sixty‐eight per cent (54/80) of 5‐ASA patients relapsed at 24 months compared to 68% (55/81) of placebo patients (RR 0.99, 95% CI 0.80 to 1.23, 1 study; 161 patients). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to an unknown risk of bias (i.e. random sequence generation, allocation concealment and selective reporting) and sparse data (109 events; See summary of findings Table for the main comparison). There was no statistically significant difference in relapse rates at 12 months in the paediatric study (Cezard 2009). Seventy‐four per cent (50/68) of paediatric 5‐ASA patients relapsed at 12 months compared to 69% (44/64) of paediatric placebo patients (RR 1.07, 95% CI 0.86 to 1.33; 1 study; 132 patients).
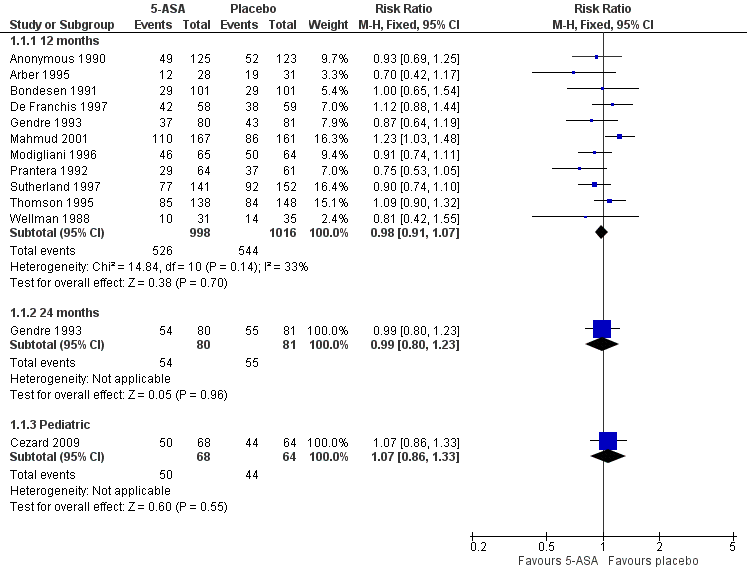
Forest plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
In further sensitivity analyses, we analysed only participants who completed the study and ignored the dropouts (i.e. an available case analysis). There was no statistically significant difference in relapse rates in patients who were followed for 12 months. Thirty‐eight per cent (263/689) of 5‐ASA patients relapsed at 12 months compared to 42% (316/749) of placebo patients (RR 0.90, 95% CI 0.79 to 1.01; 11 studies; 1438 patients). Significant heterogeneity was detected for this comparison (I2 = 40%, P = 0.09). A sensitivity analysis using a random‐effects model had little effect on the results (RR 0.89, 95% CI 0.76 to 1.05). There was no statistically significant difference in relapse rates at 24 months. Fifty‐four per cent (31/57) of 5‐ASA patients relapsed at 24 months compared to 58% (36/62) of placebo patients (RR 0.94, 95% CI 0.68 to 1.29, 1 study; 119 patients). There was no statistically significant difference in relapse rates at 12 months in the paediatric study (Cezard 2009). Sixty‐two per cent (29/47) of paediatric 5‐ASA patients relapsed at 12 months compared to 64% (35/55) of paediatric placebo patients (RR 0.97, 95% CI 0.72 to 1.31; 1 study; 102 patients).
Time to relapse
Although the initial intention was to summarise time to relapse using the log hazard ratio, since a relationship between treatment and withdrawal of treatment was evident we did not consider this further.
Safety
Adverse events
There was no statistically significant difference in the proportion of patients who experienced at least one adverse event. Thirty‐four per cent (307/900) of 5‐ASA patients had at least one adverse event compared to 33% (301/914) of placebo patients (RR 1.05, 95% CI 0.95 to 1.17; 10 studies; 1814 patients; Figure 4). Statistically significant heterogeneity was detected for this comparison (I2 = 55%, P = 0.02). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to an unknown risk of bias in the studies in the pooled analysis and unexplained heterogeneity (See summary of findings Table for the main comparison). Common adverse events reported in the studies included diarrhoea (Anonymous 1990; Cezard 2009; Gendre 1993; Mahmud 2001; Prantera 1992; Sutherland 1997; Thomson 1995), nausea or vomiting (Anonymous 1990; Gendre 1993; Sutherland 1997; Thomson 1995), abdominal pain (Anonymous 1990; Prantera 1992; Thomson 1995; Wellman 1988), headache (Arber 1995; Prantera 1992; Sutherland 1997; Wellman 1988); skin rash (Prantera 1992; Wellman 1988), constipation (Anonymous 1990), bloating (Anonymous 1990), loss of appetite (Anonymous 1990), and arthralgia (Prantera 1992).
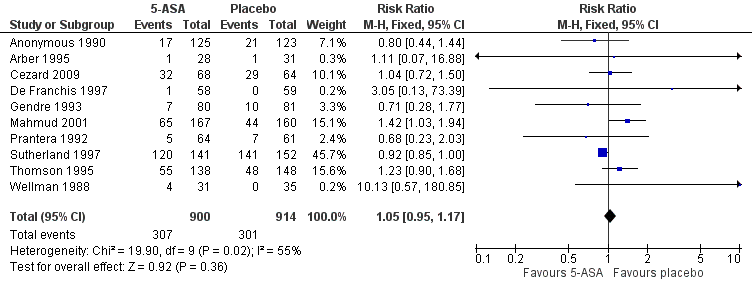
Forest plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.5 Adverse events.
Withdrawal due to adverse events
There was no statistically significant difference in the proportion of patients who withdrew due to an adverse event. Fourteen per cent (127/917) of 5‐ASA patients withdrew due to adverse events compared to 13% (119/916) of placebo patients (RR 1.11, 95% CI 0.88 to 1.38; 9 studies; 1833 patients). Statistically significant heterogeneity was detected for this comparison (I2 = 55%, P = 0.02). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to an unknown risk of bias in some studies in the pooled analysis and unexplained heterogeneity (See summary of findings Table for the main comparison). Common adverse events leading to withdrawal included diarrhoea (Anonymous 1990; Gendre 1993; Mahmud 2001; Sutherland 1997), headache (Anonymous 1990; Arber 1995; Sutherland 1997), nausea and vomiting (Anonymous 1990; Gendre 1993; Sutherland 1997) and abdominal pain (Anonymous 1990).
Serious adverse events
There was no statistically significant difference in the proportion of patients who had a serious adverse event. One per cent (3/293) of 5‐ASA patients had a serious adverse event compared to 0.7% (2/283) of placebo patients (RR 1.43, 95% CI 0.24 to 2.83; 3 studies; 576 patients). No heterogeneity was detected for this comparison (I2 = 0%, P = 0.70). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (5 events; See summary of findings Table for the main comparison). Of the three studies that reported on serious adverse events, De Franchis 1997 reported that there were no serious adverse events in either group. There were two serious adverse events in the Cezard 2009 study including one interstitial nephritis in the 5‐ASA group and one interstitial pneumopathy in the placebo group. Mahmud 2001 reported three serious adverse events, two in the 5‐ASA group and one in the placebo group. None of these serious adverse events were thought to be related to the study medication.
Subgroup analysis
Due to the potential impact of the unknown outcome of patients who withdrew early on the treatment effect, we did not feel it was valid to consider effects within subgroups.
Heterogeneity
When all dropouts are assumed to have relapsed, there was no significant heterogeneity (I2 = 33%; P =0.14) across the 11 trials that followed up participants for 12 months . However, there was significant heterogeneity in the sensitivity analyses where dropouts were ignored (I2 = 40%; P = 0.09). Further investigations of heterogeneity were not felt applicable given the implication of the unknown outcomes on the treatment effect.
Funnel Plots
A funnel plot analysis for the primary outcome (relapse) provides no convincing evidence of publication bias (see Figure 5).
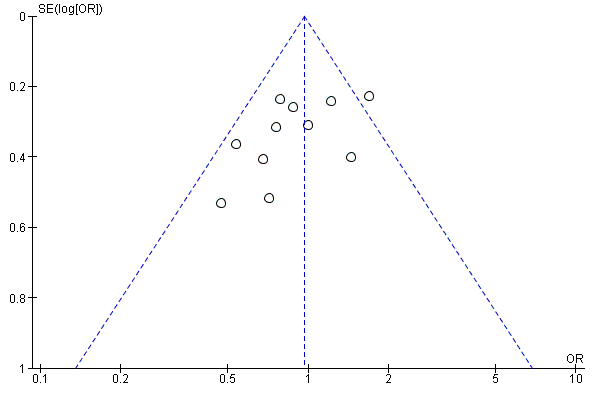
Funnel plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
Discussion
There is no known cure for Crohn's disease, and the disorder is characterised by recurrent flare‐ups of symptoms. Lichtenstein 2004 demonstrated in a recent study that being in remission is associated with improved quality of life in patients with Crohn's disease. Preventing relapses should, therefore, be an important goal in the management of this disease.
Whilst a number of treatment regimens are efficacious in inducing clinical remission in active Crohn's disease, no treatment is available that can completely prevent relapse of the disorder (Biancone 2003). Corticosteroids, the mainstay of treatment for active Crohn's disease are not effective as maintenance therapy (Steinhart 2003). Although the antimetabolites, 6‐mercaptopurine and its prodrug, azathioprine are effective in maintaining remission in Crohn's disease, these drugs might cause significant adverse events (Feagan 2003). Infliximab, a monoclonal antibody against tumour necrosis factor, is an effective maintenance therapy for patients with Crohn's disease with or without fistulas (Hanauer 2002; Rutgeerts 1999; Sands 2004). Methotrexate is also effective for maintenance of remission in Crohn's disease (Feagan 2000). 5‐ASA preparations have been found to be superior to placebo for the maintenance of remission in ulcerative colitis (Wang 2016), but its efficacy in Crohn's disease is controversial (Sandborn 2003). Randomised controlled trials which compared 5‐ASA agents with placebo for the maintenance of medically‐induced remission in Crohn's disease have yielded conflicting results (de Franchis 1997; Gendre 1993; Mahmud 2001; Sutherland 1997). In this review, we have summarised the findings of available randomised controlled trials on the subject.
For the main analyses, we used as the denominator the total number of patients randomised, and assumed that participants who dropped out of the study, and on whom there were no post withdrawal information, had relapsed during the study period. This main analysis demonstrated that, in 11 randomised controlled trials where 2014 participants were followed up for 12 months, there was no statistically significant difference between 5‐ASA agents and placebo with regard to the prevention of relapse (RR 0.98, 95% CI 0.91 to 1.07). A GRADE analysis indicates that the overall quality of the evidence supporting this outcome was moderate. Sensitivity analyses using a random‐effects model and an available case analysis had no impact on the results. A single paediatric study (132 participants) did not find any difference in relapse rates between 5‐ASA and placebo treated participants (Cezard 2009). A single study in which patients were followed up for 24 months also did not demonstrate any statistically significant difference in relapse rates between the two groups in either the main analysis or sensitivity analyses. We therefore found no evidence from this study to suggest that 5‐ASA agents are superior to placebo for maintenance of medically‐induced remission in Crohn's disease.
The primary studies had a number of limitations. The drop‐out rates in most studies were quite high and as no follow‐up information was available on these patients, we assumed in the main analysis that drop out patients had relapsed. Exactly how this assumption might have affected the results of the meta‐analysis is unclear. This highlights the importance of following‐up patients after they withdraw from a study. The assessment of disease activity was not uniform amongst the primary studies. Whilst 10 of the included studies used the CDAI to measure disease activity, two studies used the Harvey Bradshaw Index (Arber 1995; Cezard 2009). The studies which used CDAI defined relapse as a CDAI > 150 or a variable minimum rise in the CDAI score between 50 and 100 points from baseline along with a CDAI > 150.
We found no evidence to suggest that more adverse events occurred in patients on 5‐ASA agents compared to placebo. Although the reporting of adverse events was somewhat inconsistent across the studies we were able to pool data for the proportion of patients who experienced at least one adverse event, withdrawal due to adverse events and serious adverse events. There was no statistically significant differences between 5‐ASA and placebo for any of these outcomes. GRADE analyses indicate that the overall quality of the evidence supporting these outcomes was low.
Different preparations and different doses of 5‐ASA were used in the included studies. The doses ranged from 1 g to 3 g per day. A linear dose‐response relationship has been suggested for 5‐ASA agents in the maintenance of remission in ulcerative colitis (Hanauer 2004). None of the included studies compared different doses of 5‐ASA, and there was no evidence to suggest that studies which used higher doses of 5‐ASA preparations demonstrated greater benefits or more adverse events. There was no strong indication to suggest that more adverse events occurred in patients on 5‐ASA agents compared to placebo. Future studies should report adverse events in a more consistent manner.
We found no evidence in this review to suggest that 5‐ASA preparations are superior to placebo for the maintenance of medically‐induced remission in Crohn's disease. The use of 5‐ASA agents for the prevention of recurrences following surgery for Crohn's disease was not the subject of this review and has been assessed in a separate systematic review (Gordon 2011).
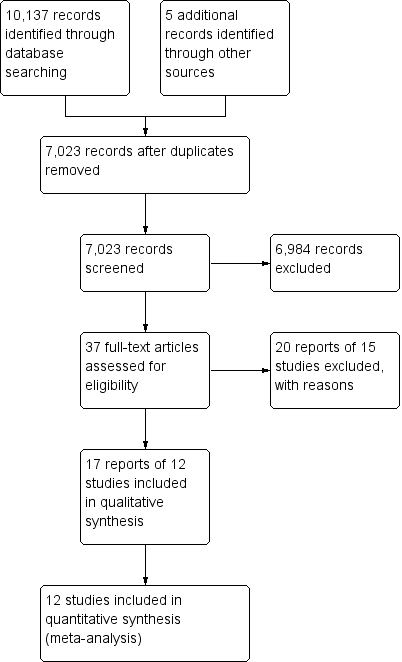
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
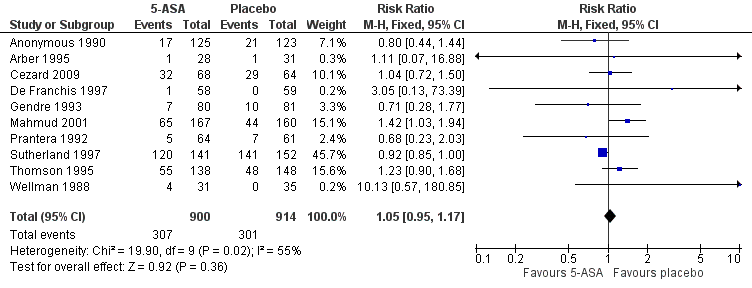
Forest plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.5 Adverse events.

Funnel plot of comparison: 1 5‐ASA compared to placebo, outcome: 1.8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.

Comparison 1 5‐ASA compared to placebo, Outcome 1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
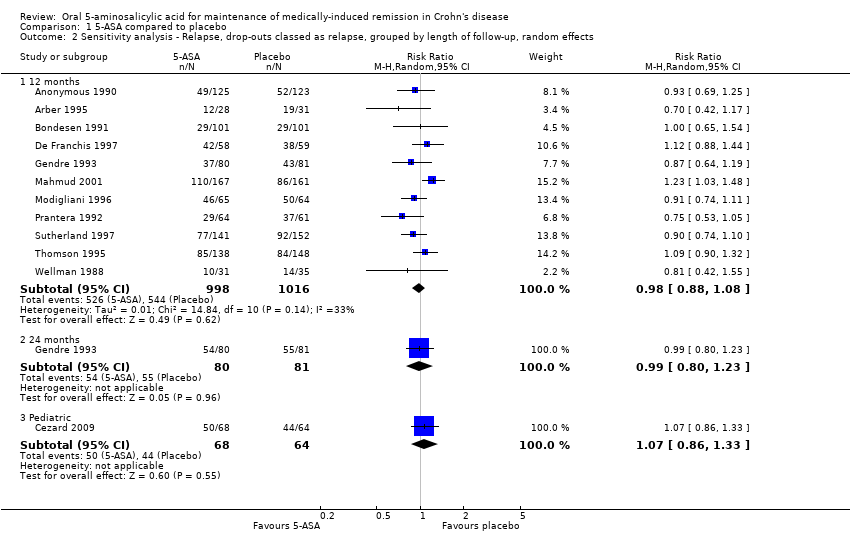
Comparison 1 5‐ASA compared to placebo, Outcome 2 Sensitivity analysis ‐ Relapse, drop‐outs classed as relapse, grouped by length of follow‐up, random effects.

Comparison 1 5‐ASA compared to placebo, Outcome 3 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up.

Comparison 1 5‐ASA compared to placebo, Outcome 4 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up, random effects.

Comparison 1 5‐ASA compared to placebo, Outcome 5 Adverse events.

Comparison 1 5‐ASA compared to placebo, Outcome 6 Withdrawals due to adverse events.

Comparison 1 5‐ASA compared to placebo, Outcome 7 Serious adverse events.

Comparison 1 5‐ASA compared to placebo, Outcome 8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up.
| 5‐ASA compared to placebo for maintenance of medically‐induced remission in Crohn's disease | ||||||
| Patient or population: patients with maintenance of medically‐induced remission in Crohn's disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 5‐ASA compared to placebo | |||||
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ 12 months | 535 per 10001 | 525 per 1000 | RR 0.98 | 2014 | ⊕⊕⊕⊝ | |
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ 24 months | 679 per 10003 | 672 per 1000 | RR 0.99 | 161 | ⊕⊕⊝⊝ | |
| Relapse, drop‐outs classed as relapse, grouped by length of follow‐up ‐ Pediatric | 688 per 10003 | 736 per 1000 | RR 1.07 | 132 | ⊕⊕⊕⊝ | |
| Adverse events | 329 per 10001 | 346 per 1000 | RR 1.05 | 1814 | ⊕⊕⊝⊝ | |
| Withdrawals due to adverse events | 130 per 10001 | 144 per 1000 | RR 1.11 | 1833 | ⊕⊕⊝⊝ | |
| Serious adverse events | 7 per 10001 | 10 per 1000 | RR 1.43 | 576 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Control group risk comes from control arm of meta‐analysis, based on included trials. | ||||||
| Study | Total Unknown ‐ n(%) | 5‐ASA unknown ‐ n(%) | Placebo unknown ‐ n(%) |
| Anonymous 1990 | 55 (22%) | 23 (19%) | 32 (26%) |
| Arber 1995 | 10 (17%) | 6 (21%) | 4 (13%) |
| Cezard 2009 | 30 (23%) | 21 (31%) | 9 (14%) |
| De Franchis 1997 | 17 (14%) | 8 (14%) | 9 (15%) |
| Gendre 1993 (Eng) | 42 (26%) | 23 (29%) | 19 (23%) |
| Mahmud 2001 | 82 (25%) | 55 (33%) | 27 (17%) |
| Modigliani 1996 | 44 (34%) | 17 (26%) | 27 (42%) |
| Prantera 1992 | 15 (12%) | 10 (16%) | 5 (8%) |
| Sutherland 1997 | 92 (31%) | 47 (33%) | 45 (30%) |
| Thompson 1995 | 98 (34%) | 52 (38%) | 46 (31%) |
| Wellman 1988 | 2 (3%) | 2 (6%) | 0 (0%) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 12 months | 11 | 2014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.07] |
| 1.2 24 months | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 1.3 Pediatric | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 2 Sensitivity analysis ‐ Relapse, drop‐outs classed as relapse, grouped by length of follow‐up, random effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 12 months | 11 | 2014 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 24 months | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.23] |
| 2.3 Pediatric | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.86, 1.33] |
| 3 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 12 Months | 10 | 1438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.01] |
| 3.2 24 Months | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
| 3.3 Pediatric | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.31] |
| 4 Sensitivity analysis ‐ Relapse, drop‐outs ignored, grouped by length of follow‐up, random effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 12 Months | 10 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.05] |
| 4.2 24 Months | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.29] |
| 4.3 Pediatric | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.31] |
| 5 Adverse events Show forest plot | 10 | 1814 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.95, 1.17] |
| 6 Withdrawals due to adverse events Show forest plot | 10 | 1833 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.88, 1.38] |
| 7 Serious adverse events Show forest plot | 3 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.24, 8.44] |
| 8 Relapse, drop‐outs classed as relapse, grouped by length of follow‐up Show forest plot | 11 | 2014 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |

