Promoción del lavado de cara para prevenir el tracoma activo
Resumen
Antecedentes
El tracoma sigue siendo una de las principales causas de ceguera evitable entre las poblaciones desfavorecidas de muchos países en desarrollo. Se estima que alrededor de 146 millones de personas tienen tracoma activo y casi seis millones de personas están ciegas debido a complicaciones asociadas a infecciones repetidas.
Objetivos
El objetivo de este examen fue evaluar los efectos de la promoción del lavado de cara para la prevención del tracoma activo en las comunidades endémicas.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL (que contiene el registro de ensayos del Grupo Cochrane de Trastornos de los Ojos y la Visión [Cochrane Eyes and Vision Group Trials Register]) (2015, número 1), Ovid MEDLINE, Ovid MEDLINE In‐Process y otras citas no indexadas, Ovid MEDLINE Daily, Ovid OLDMEDLINE (enero de 1946 a enero de 2015), EMBASE (enero de 1980 a enero de 2015), PubMed (enero de 1948 a enero de 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (enero de 1982 a enero de 2015), el metaRegistro de Ensayos Controlados (mRCT) (www.controlled‐trials.com) (consultada el 10 de enero de 2014), ClinicalTrials.gov (www.clinicaltrials.gov) y la Plataforma del Registro Internacional de Ensayos Clínicos (ICTRP) de la Organización Mundial de la Salud (OMS) (www.who.int/ictrp/search/en). No se aplicaron restricciones de fecha o de idioma en las búsquedas electrónicas de ensayos. La última búsqueda en las bases de datos electrónicas se realizó el 26 de enero de 2015.
Para identificar más ensayos relevantes, se revisaron las listas de referencia de los ensayos incluidos. Además, se utilizó el Science Citation Index para buscar referencias de las publicaciones que citaban los ensayos incluidos en la revisión. Se estableció contacto con investigadores y expertos en el área para identificar ensayos adicionales.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) o cuasialeatorizados que compararon el lavado de cara con ningún tratamiento o el lavado de cara combinado con antibióticos con los antibióticos solos. Los participantes en el ensayo eran residentes de comunidades endémicas de tracoma.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, extrajeron los datos y evaluaron la calidad de los ensayos. Cuando fue necesario, se estableció contacto con los autores de los ensayos para obtener información adicional. Dos ensayos cumplieron los criterios de inclusión, pero no se realizó un metanálisis debido a la heterogeneidad metodológica.
Resultados principales
Se incluyeron dos ECA grupales, que proporcionaron datos de 2447 participantes. Ambos ensayos se realizaron en zonas endémicas de tracoma: Norte de Australia y Tanzania. El período de seguimiento fue de tres meses en un ensayo y de 12 meses en el otro; ambos ensayos tuvieron un seguimiento de alrededor del 90% de los participantes en la visita final. En general, la calidad de la evidencia es incierta debido a que los ensayos no informan sobre muchos métodos de diseño y a las diferencias en los resultados informados entre los ensayos.
El lavado de cara combinado con tetraciclina tópica se comparó con la tetraciclina tópica sola en tres pares de pueblos en un ensayo. El ensayo encontró que el lavado de cara combinado con tetraciclina tópica redujo el tracoma activo "grave" en comparación con la tetraciclina tópica sola a los 12 meses (odds‐ratio (OR) ajustado 0,62; intervalo de confianza (IC) del 95%: 0,40 a 0,97); sin embargo, el ensayo no encontró ninguna diferencia importante entre los pueblos de intervención y control en la reducción de otros tipos de tracoma activo (OR ajustado 0,81; IC del 95%: 0,42 a 1,59). Los pueblos de intervención tuvieron una mayor prevalencia de rostros limpios que las aldeas de control entre los niños con tracoma grave (OR ajustado 0,35; IC del 95%: 0,21 a 0,59) y cualquier tracoma (OR ajustado 0,58; IC del 95%: 0,47 a 0,72) a los 12 meses de seguimiento. El segundo ensayo comparó el lavado de ojos con ningún tratamiento o con la tetraciclina tópica sola o con una combinación de lavado de ojos y gotas de tetraciclina en niños con tracoma folicular. A los tres meses, el ensayo no encontró evidencia de beneficio del lavado de ojos solo o en combinación con gotas oftálmicas de tetraciclina en la reducción del tracoma folicular entre los niños con tracoma folicular (riesgos relativos [RR] 1,03; IC del 95%: 0,96 a 1,11; un ensayo, 1143 participantes).
Conclusiones de los autores
Hay evidencia de un ensayo de que el lavado de cara combinado con la tetraciclina tópica puede ser efectivo para reducir el tracoma activo grave y aumentar la prevalencia de las caras limpias en un año de seguimiento. La evidencia actual no es concluyente en cuanto a la efectividad del lavado de cara solo o en combinación con la tetraciclina tópica para reducir el tracoma activo o grave.
PICO
Resumen en términos sencillos
Promoción del lavado de cara para prevenir el tracoma activo
Pregunta de investigación
Se investigó si el lavado de cara previene el tracoma activo en las comunidades endémicas.
Antecedentes
El tracoma es una enfermedad ocular causada por una infección bacteriana. La infección activa suele comenzar en la infancia y se caracteriza por la secreción de los ojos, el eritema y la irritación. La mala higiene facial puede hacer que la enfermedad se propague de una persona a otra a través de moscas que buscan los ojos o dedos contaminados. El lavado de cara se promueve como parte de la estrategia "SAFE" de la Organización Mundial de la Salud para eliminar la ceguera en todo el mundo. El lavado de cara es simple y racional, pero su efectividad para reducir la transmisión del tracoma es incierta.
Características de los estudios
Se incluyeron dos ensayos controlados aleatorizados con un total de 2560 participantes, realizados en Australia y Tanzania. Un ensayo comparó una estrategia combinada de lavado de cara más pomada de tetraciclina (un antibiótico) con la pomada de tetraciclina sola durante un año. El segundo ensayo comparó cuatro grupos de intervención durante tres meses en niños que ya tenían tracoma folicular: una estrategia combinada de lavado de cara más gotas oculares de tetraciclina, lavado de cara solo, gotas oculares de tetraciclina solo y ningún tratamiento. La evidencia está actualizada hasta enero 2015.
Resultados clave
Ambos ensayos informaron del número de niños con tracoma activo como medida de resultado; un ensayo también informó del número de niños con tracoma grave y del porcentaje de rostros limpios después de un año. Un ensayo informó que el lavado de cara era efectivo para aumentar la limpieza facial y reducir el tracoma grave al año; el segundo ensayo no demostró que el lavado de ojos solo o en combinación con gotas oculares de tetraciclina redujera el tracoma folicular entre los niños que tenían tracoma folicular en el momento del reclutamiento.
Calidad de la evidencia
Los dos ensayos incluidos tenían un riesgo incierto de sesgo debido a que no informaban sobre muchos aspectos de los diseños de los ensayos.
Authors' conclusions
Background
Description of the condition
Trachoma is an infective eye disease caused by the bacterial microorganism Chlamydia trachomatis. Trachoma remains a major cause of avoidable blindness among underprivileged populations in many areas of Africa, Asia and the Middle East, where poverty, overcrowding, poor personal and environmental hygiene favor transmission of the disease. It is estimated that about 146 million people have active trachoma and nearly six million people are blind due to complications associated with repeat infections (WHO 1997a). C. trachomatis bacteria is spread from person to person by close contact in overcrowded living conditions, or through contaminated fingers or cloths used by mothers to wipe away discharges on the faces of children (ICEH 1999). Flies, which are attracted to eye and nasal secretions on the faces of infected children, also are believed to be vectors responsible for transmission of the organism (ICEH 1999; West 1991).
In communities where trachoma is endemic, infection usually begins in childhood and repeat episodes of infection cause distortion of the eyelids (entropion), in‐turned eyelashes (trichiasis), corneal abrasion and ultimately blindness due to corneal opacity. Active trachoma is more commonly observed in children (Taylor 1985; West 1991). It is characterized by redness and discharge associated with inflammatory thickening of the upper tarsal conjunctiva (mucous membrane lining the inner surface of the upper eyelids) and follicles (whitish elevations within the conjunctiva). A simplified grading system for the assessment of trachoma and its complications in endemic communities has been published (Thylefors 1987) and discussed in a Cochrane review of antibiotics for trachoma (Evans 2011).
Description of the intervention
Face washing is promoted by the World Health Organization (WHO) program for the global elimination of trachoma as part of the 'SAFE' strategy (WHO 1997b; WHO 2011). The 'SAFE' strategy consists of surgery for trichiasis; antibiotics for infectious trachoma; facial cleanliness to reduce transmission; and environmental improvements (household sanitation and provision of clean water).
How the intervention might work
The face washing component of the SAFE strategy aims to maintain clean faces in the community in order to reduce eye‐seeking flies and person‐to‐person transmission of C. trachomatis. Face washing promotion as a community intervention can be combined with mass treatment of people with antibiotics in areas with high trachoma endemicity. Mass treatment with antibiotics aims to reduce the reservoir of C. trachomatis in the community, while face washing aims to interrupt the cycle of infection and re‐infection in the long term. The antibiotic and environmental arms of the SAFE strategy have been examined in other published Cochrane reviews (Evans 2011; Rabiu 2012).
Why it is important to do this review
The face washing principle appears simple and theoretically sound, but whether this intervention can reduce transmission of trachoma in practice has been the focus of debate (Bailey 2001). Some narrative reviews of the literature have suggested that facial cleanliness may be useful in preventing trachoma (Emerson 2000; Pruss 2000). However, most of the data were obtained from observational studies and the methodological quality of the few controlled trials included was not reported. In this Cochrane review we aim to summarize systematically, research evidence from trials of face washing promotion for preventing active trachoma in endemic communities. In communities where water is scarce, the uptake and practice of face washing may not be as good as in communities where water is freely available. We will consider the potential influence of water availability on outcomes in this review.
Objectives
The objective of this review was to assess the effects of face washing promotion for the prevention of active trachoma in endemic communities.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Trial participants were residents of trachoma endemic communities.
Types of interventions
We considered the following interventions:
-
Face washing promotion versus no intervention;
-
Face washing promotion plus mass antibiotic treatment versus mass antibiotic treatment alone.
Face washing promotion can be delivered by any means appropriate to the local setting, such as: radio or television; health education leaflets; community leaders; religious gatherings; role‐play; drama in village halls; school teachers; women groups; or music. In trials where promotion of face washing was combined with mass antibiotic treatment, antibiotics considered included tetracycline ointment or capsules, azithromycin, or erythromycin, given at any dose or frequency.
Types of outcome measures
We considered the following outcomes for comparison of interventions:
-
Number of participants with active trachoma (TF or TI) at 6, 12 or greater than 12 months post‐treatment allocation (age group as reported in trials). We defined active trachoma using the Thylefors 1987 scale. On this scale, active trachoma is categorized as TF or TI. TF is trachoma follicular inflammation and is defined as the presence of five or more follicles, each of which is at least 0.5 mm in diameter, on the flat surface of the upper tarsal conjunctiva. TI is intense inflammation of the tarsal conjunctiva due to trachoma and is defined as the presence of marked inflammatory thickening of the upper tarsal conjunctiva that obscures more than half of the deep conjunctival vessels. We planned to include trials that used other trachoma grading scales to assess active trachoma, provided the scales used were comparable to the Thylefors 1987 scale;
-
Number of participants with an unclean face at 6, 12 or greater than 12 months post treatment allocation (age group as reported in trials). We defined an unclean face as the presence of eye or nasal discharge (WHO 2001) or any other definition used in trials;
-
Post hoc: Number of participants with severe trachoma. We did not specify severe trachoma among cases of active trachoma as an outcome in the protocol for this review (Ejere 2002). However, one of the two trials that met the inclusion criteria defined and reported this outcome.
Search methods for identification of studies
Electronic searches
We revised the searches of electronic databases from the 2012 update (Ejere 2012). We searched PubMed and the International Clinical Trials Registry Platform, which had not originally been searched. We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily Update, Ovid OLDMEDLINE (January 1946 to January 2015), EMBASE (January 1980 to January 2015), PubMed (January 1948 to January 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to January 2015), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (accessed 10 January 2014), ClinicalTrials.gov (www.clinicaltrial.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 26 January 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), ICTRP (Appendix 8).
Searching other resources
We contacted several trachoma experts, including Hedley Peach and Sheila West, to identify potentially relevant trials missed by the electronic searches. Denise Mabey was a peer reviewer and provided information on potentially relevant trials. We identified existing reviews and checked their citations for relevant trials. We used the Science Citation Index to search for references that cited the trials included in the review.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts found through electronic searches. We retrieved the full‐text of trials that were potentially relevant to the review. Those that met the selection criteria were assessed for methodological quality. We resolved any disagreements by discussion.
Data extraction and management
Two review authors independently extracted data onto a standardized data extraction form. We compared extracted data and reconciled differences. A third review author resolved any disagreements. We entered data into RevMan 2014. Where trials reported the outcomes in different ways, we contacted the primary investigators for further information to allow conversion or transformation of data.
Assessment of risk of bias in included studies
Two review authors independently assessed included trials using the following criteria based on Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Risk of bias domains assessed were selection bias (random sequence generation, allocation concealment before randomization), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting) and other potential sources of bias. While masking of participants and intervention providers was not feasible due to the nature of the intervention of interest, masking of outcome assessors was possible and was assessed post hoc. We judged each included trial for each domain as being at "low", "unclear" "or "high" risk of bias and provided the rationale for each judgement. We provided direct quotes from included trials where appropriate.
Measures of treatment effect
The specified outcomes are dichotomous and therefore we calculated unadjusted risk ratios (RRs) with corresponding 95% confidence intervals (CIs). We also reported adjusted odds ratios (ORs) with corresponding 95% CIs as reported in the included trials.
Unit of analysis issues
Implementation of the intervention meant that clusters rather than individuals were randomized in the included trials. Cluster‐RCTs should be analyzed on the same level as the allocation, using the summary measurement from each cluster, as opposed to the individual level measurement. Analyzing a cluster‐RCT on the individual level could considerably and unnecessarily reduce the power of the trial.
Dealing with missing data
We contacted the trial authors in an effort to obtain missing outcome data or unclearly reported information, e.g. information about random sequence generation, allocation concealment or whether an intention‐to‐treat analyses was conducted.
Assessment of heterogeneity
We compared study designs and found methodological heterogeneity between the included trials that precluded meta‐analysis of outcome data. If more trials are included in future updates of this review and we deem a meta‐analysis appropriate, we will use the Chi2 test to assess statistical heterogeneity but also will consider clinical and methodological heterogeneity of the included trials. Heterogeneity also may be apparent on visual examination of the forest plot.
Assessment of reporting biases
The trial protocol was not available for either included trial. If trial protocols of future included trials are available, we will compare reported outcomes with protocol‐stated outcomes. When 10 or more trials are included in future updates of this review, we will use a funnel plot to display small study effects and potential for publication bias.
Data synthesis
We concluded that meta‐analysis of the two included trials was not appropriate. However, if more trials are included in future updates and meta‐analysis is appropriate, we will combine data using a random‐effects model. When there are fewer than three trials and little evidence of heterogeneity, we will use a fixed‐effect model. In meta‐analyzing cluster‐RCTs, when we encounter trials where the units of allocation and analysis are different (i.e. the unit of allocation was the community and the unit of analysis was individuals in the community) and the cluster design has not been accounted for in the analysis, we will contact primary investigators to obtain data required to develop estimates of intra‐cluster correlation coefficients or design effects to calculate more appropriate CIs on effect estimates. Whenever a meta‐analysis is not possible, we will present a tabulated summary of results.
Subgroup analysis and investigation of heterogeneity
We could not carry out subgroup analysis due to insufficient data. If more trials meet our inclusion criteria in an update of this review and we consider heterogeneity to be present, we will explore outcomes within the following subgroups:
-
Communities with available water supply versus communities with scarce water supply. We have defined water availability in this review as the presence of a functional water source within 30 minutes walk or a distance of less than 4 km from all households within the community (WHO 2001) or any other definition used in the trials;
-
Communities with intense active trachoma versus communities with less intense active trachoma. In this review we have defined intense active trachoma as communities with a baseline prevalence of TF or TI ≥ 20%, while less intense is defined as communities with a prevalence of TF or TI < 20% (WHO 1997b).
Sensitivity analysis
We could not perform sensitivity analysis as only two trials met our inclusion criteria. If a sufficient number of trials are included in future updates and a meta‐analysis is appropriate, we plan to investigate the influence of trials with quasi‐random methods and those without concealment of allocation on the pooled effect size. We also plan to measure the effect of publication bias on the pooled effect size. If feasible, we plan to assess whether the use of individual participant data would affect the direction of effect of interventions.
Results
Description of studies
Results of the search
The original electronic searches in 2004 generated 67 citations and abstracts. We screened these results and retrieved the full text of two potentially relevant articles for further assessment. One of these met the criteria for inclusion (West 1995). The other was not a RCT and therefore excluded (Sutter 1983). A trachoma research expert drew our attention to a RCT that was not published in a journal (Peach 1987). Thus, we included two RCTs in the review.
Updated searches
We performed a search in October 2007 and identified 66 new reports of trials. The Trials Search Co‐ordinator scanned the search results and removed references she judged irrelevant to the scope of the review. We reviewed the full‐text of three articles for potential inclusion; however, we excluded all three. Edwards 2006 and Rubinstein 2006 were reports of health education promotion of face washing and Khandekar 2006 treated face washing and environmental sanitation interventions as one outcome.
In September 2011 electronic searches identified 91 additional references. We assessed the full‐text of one study (King 2011) but excluded it as it evaluated a standardized definition of a clean face for trachoma prevention.
In January 2015 we performed electronic searches and identified 151 additional titles and abstracts and nine trial register records (Figure 1). We classified three titles and abstracts and one trial register record to be potentially relevant and assessed the eligibility further based on the full‐text reports. We excluded one study (Khandekar 2005) as it was not a RCT on face‐washing promotion. Two other reports were from the West 1995 trial which we included in the previous version of the review. Under Characteristics of studies awaiting classification, we have described the trial register record (NCT00348478), which refers to a completed trial for which study results have not been published.
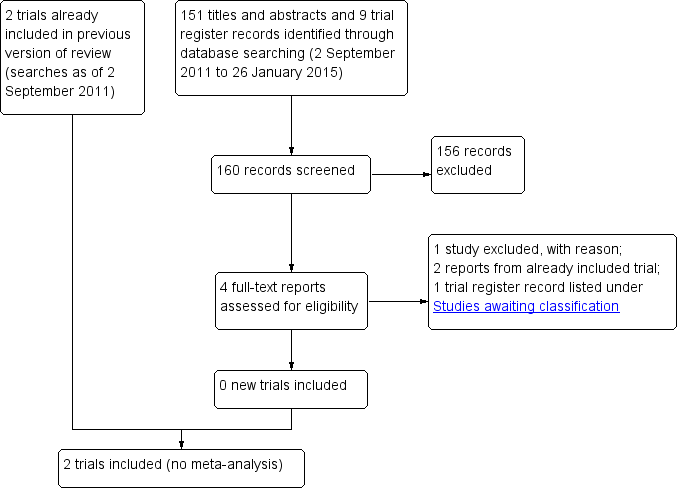
Study flow diagram.
Included studies
See Characteristics of included studies for further details.
Setting and participants
We included two cluster‐RCTs (2560 children in total) conducted in areas endemic to trachoma. West 1995 was undertaken in Kongwa, Tanzania and included a total of 1417 children, aged one to seven years. Six villages were paired based on characteristics; one village of each pair was randomized to intervention and the other assigned to control. Peach 1987 was undertaken in the Northern Territory of Australia. In this trial 36 aboriginal communities were randomized to one of three intervention groups or one control group. A total of 2530 children aged five to 14 years were screened for follicular trachoma. Several children above the age of 14 years and some of preschool age were also screened. Of the total number of children screened in the participating communities, 1143 children with follicular trachoma were recruited into the trial.
Interventions
Both trials incorporated face washing as an intervention in the trial, but used different study designs and implementation methods. In West 1995, three villages (680 children) were randomized to face washing promotion combined with tetracycline and the three remaining villages (737 children) were assigned to tetracycline ointment alone. Face washing promotion was community‐based and consisted of neighborhood meetings to build consensus for increasing face washing and reinforcement activities such as school plays, seminars with the traditional healers and meetings with other village groups. Face washing promotion was carried out for one month during and after mass treatment with tetracycline. Tetracycline ointment was administered topically once daily for 30 days.
In Peach 1987, 36 villages were randomized to either tetracycline eye drops (374 children), eye washing (246 children), eye washing combined with tetracycline eye drops (312 children) or no intervention (211 children). Children in the eye washing group had their eyes washed daily by school teachers for three months. Those in the tetracycline group had tetracycline eye drops applied daily for one week every month for three months. For the purpose of this review, we have reported data for the comparison between eye washing versus no treatment, and eye washing combined with eye drops versus eye drops alone.
Outcome measures
In West 1995, outcomes reported include active trachoma, severe trachoma and clean faces at 12 months. Trachoma was graded using the Thylefors 1987 scale. West 1995 defined severe trachoma as 15 or more follicles, or the presence of inflammation that obscured all vessels of the tarsal plate. We abstracted data from graphs presented in the report of the trial, as rates and raw data were unavailable. These abstracted data should be regarded as best approximations to the true figures. Our protocol specified 'unclean faces' as an outcome of interest, but West 1995 reported 'clean faces'. We elected to present the outcome as reported in the trial because it would be difficult to transform the data without sufficient information from the trialists.
In Peach 1987, the primary outcome was reported as the proportion of children with follicular trachoma at three months after the intervention. The Aboriginal Health Workers used a simplified grading scheme to assess the presence of follicles as indicating active trachoma. Although this scale is crudely comparable to the TF grading on the Thylefors 1987 scale, it may have a lower specificity because participants with fewer than five follicles may have been classified as having active trachoma.
Excluded studies
See Characteristics of excluded studies for further details.
Risk of bias in included studies
Figure 2 gives the results of the assessment of methodological quality of the included trials.
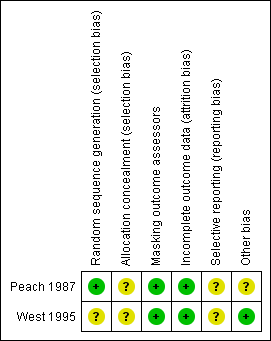
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed West 1995 at unclear risk of selection bias because no details of how randomization was performed or whether allocation assignments to intervention or control groups were concealed before randomization were reported. Authors of Peach 1987 stated that a random number table was used to allocate communities to an intervention or control group. Allocation was done after initial screening by someone who was unaware of the prevalence of trachoma and unfamiliar with the communities, including their school teachers and health workers, but no method of allocation concealment was reported. It is unclear whether baseline prevalence of trachoma was similar among the comparison groups. Information on the number of communities randomized to each experimental group was not available in the report. However, further correspondence with the trial authors suggested that about nine communities were randomized to each of the four trial groups.
Masking (performance bias and detection bias)
Masking of participants in West 1995 and Peach 1987 was not feasible. West 1995 masked outcome assessment by taking photographs of tarsal plates to be read by an examiner who was not aware of the randomization status of the villages. In Peach 1987, trachoma was assessed by trachoma workers who were unaware of treatment allocation to the communities. Steps were taken to ensure that the outcome assessors did not learn to which groups the communities were allocated. Different personnel carried out each intervention, namely eye washing and eye drops. School teachers washed the eyes of the children, while Aboriginal health workers or nurses administered eye drops. Since different personnel carried out each intervention, workers could have become unmasked based on the presence or absence of certain personnel.
Incomplete outcome data
Incomplete outcome data was minimal for both trials. West 1995 reported baseline prevalences in active trachoma between comparison villages were not substantially different. Although 92% of the enrolled participants were followed up for one year, information regarding similarity of follow‐up rates between comparison groups and handling of missing data was not reported.
In Peach 1987 89% of enrolled participants were followed up for three months. All participants lost to follow‐up were assumed to have follicles at the end of the trial when the intention‐to‐treat principle was applied in the primary analysis of the results.
Selective reporting
Neither trial protocol was available for review.
Other potential sources of bias
Investigators in Peach 1987 controlled for observer variation and learning effect by allocating trachoma workers to all four groups in stages. Regardless, sensitivity and specificity among aboriginal trachoma workers in diagnosing follicular trachoma varied.
Effects of interventions
The two trials differed in several respects, particularly with regard to types of intervention, comparison group and definition of outcome measures. Therefore we did not consider a meta‐analysis appropriate. We have presented a narrative summary of the results.
Active trachoma (follicular or TF or TI)
In West 1995, face washing combined with antibiotics was compared to antibiotics alone in three pairs of villages. In pair one, the percentage prevalence of active trachoma was reported to be lower in the village that received a combination of face washing and antibiotics than the village that received antibiotics alone at 12 months follow‐up (approximately 55% compared to 60%; as read from figures; no numerical data provided for this pair or the other two pairs of villages). In a second pair of villages, the percentage of active trachoma also was lower in the combination intervention village than the antibiotics alone village (approximately 40% compared to 50%). However in a third pair of villages, the percentage of active trachoma in the combination intervention village was higher than the antibiotics alone village (approximately 70% compared to 65%). The overall results for all the combination intervention villages compared to the antibiotics alone villages suggest a reduction in the odds of any trachoma but this effect was not statistically significant (adjusted OR 0.81, 95% CI 0.42 to 1.59).
In Peach 1987, 191/246 children (77.6%) in the eye washing group had follicles at three months compared with 160/211 (75.8%) in the no treatment group (Analysis 1.1). The difference was not statistically significant (Analysis 1.1; RR 1.03, 95% CI 0.93 to 1.14). In the eye washing/eye drop combination group, 215/312 (68.9%) had follicles at three months compared to 250/374 (66.8%) in the eye drop only group. The difference was not statistically significant (Analysis 1.1; RR 1.02, 95% CI 0.93 to 1.13). When counting numbers of participants with presence of follicular trachoma, the trial investigators counted all missing cases as having follicular trachoma; the proportions of missing cases ranged from 9.0% to 17.9% among the four intervention groups.
When the Peach 1987 investigators fitted a logit model to the data to account for variations in participant age, geographical location and trachoma outcome assessors, the adjusted odds of having follicular trachoma were slightly higher in the eye drop only group compared to the eye washing/eye drop combination group (adjusted OR 1.17, 95% CI not reported). The adjusted odds of having follicular trachoma in the no treatment group compared to the eye washing group were similar and not statistically significant (adjusted OR 1.02, 95% CI not reported).
Severe trachoma
In West 1995 12‐month prevalence of severe trachoma was compared between the intervention and control villages within each pair of villages. In pair one, the percentage prevalence of severe trachoma was lower in the village that received a combination of face washing and antibiotics than in the village that received antibiotics alone (approximately 8% compared to 14%). In a second pair of villages, the percentage of active trachoma also was lower in the combination intervention village than the antibiotic alone village (approximately 6% compared to 14%). However in the third pair of villages, the percentage of active trachoma in the combination intervention village was slightly higher than the antibiotics alone village (approximately 10% compared to 8%). The CIs and the numbers used to calculate these differences were not reported so that CIs of the differences could not be calculated. The overall results reported after adjustments for age and baseline trachoma status suggest a statistically significant reduction in the odds of severe trachoma by the face washing/antibiotics combination compared to antibiotics alone (adjusted OR 0.62, 95% CI 0.40 to 0.97). At six months follow‐up, the trial authors reported that there was no difference in the prevalence of severe trachoma between the intervention and control groups in the three pairs of villages (data not provided).
Peach 1987 did not report this outcome.
Clean faces
In West 1995 the percentage of children with clean faces was consistently higher in the face washing‐antibiotic combination villages than the antibiotic alone villages. Total results showed an increase in the percentage of children with clean faces in the face washing/antibiotic combination villages from 18% at baseline to 33% at six months and 35% at 12 months follow‐up. There was a smaller increase in the percentage of children with clean faces in the antibiotic alone group (from 19% at baseline to 30% at six months and 26% at 12 months). The difference in the proportion of children with clean faces in the intervention villages compared to the control villages was statistically significant among those with severe trachoma (adjusted OR 0.35; 95% CI 0.21 to 0.59) and any trachoma (adjusted OR 0.58; 95% CI 0.47 to 0.72) at 12 months follow‐up.
Peach 1987 did not report this outcome.
Discussion
Summary of main results
Active trachoma
Two included trials reported active trachoma (defined as TF or TI with five or more follicles or inflammation obscuring at least half of tarsal conjunctiva vessels). The findings were either inconsistent within a trial or showed that the face washing/eye drop combination had no benefit.
It is unclear why face washing promotion combined with tetracycline had an effect on reducing active trachoma in two pairs of villages but no effect in a third pair in West 1995. Differences in baseline characteristics such as prevalence of trachoma, intensity of transmission, availability or access to water supplies between the third pair and the first two pair of villages may be important in explaining the differences in benefit. However, the overall results for the face washing/tetracycline combination villages compared to the tetracycline only villages suggest a modest, but not statistically significant, beneficial effect of face washing in reducing active trachoma at 12 months.
Peach 1987 suggested no benefit for face washing compared with no treatment or for the face washing/eye drops combination in comparison to eye drops alone. The age of participants varied, and there was a higher proportion of severe trachoma among older children. There were variations in the prevalence of trachoma in the different geographical locations from which the participating communities were drawn, as well as small differences in the diagnostic competence of outcome assessors. The trial authors hypothesized that community randomization in the trial may not have adequately controlled for these factors, hence the need to account for them. After fitting the data to a logit model to control for perceived imbalances in the ages of participants, geographical location and outcome assessors, a marginal but not statistically significant benefit was seen for the face washing/eye drops combination compared to the eye drops alone group. However, the report does not state whether the analysis of outcomes via logistic regression models was prespecified or post‐hoc.
Severe trachoma (severe active trachoma)
Only West 1995 reported on severe trachoma (defined as TF or TI with more than 15 follicles or inflammation obscuring all the tarsal conjunctiva vessels). We refer to this outcome as "severe active trachoma". As for all active trachoma, the benefit of face washing in reducing the prevalence of severe trachoma was observed in two pairs of villages at 12 months follow‐up, but not in the third pair. The reasons for this difference in outcome may have been due to differences in baseline characteristics of villages and participants, particularly the severity of trachoma at baseline.
Clean faces
West 1995 reported that the percentage of participants with clean faces increased in both intervention and control groups over 12 months, even though the increase was higher in the intervention group. However, a statistically significant difference in the percentage of clean faces between the intervention and control groups at 12 months suggests a benefit of face washing promotion.
Overall completeness and applicability of evidence
Although two trials are included in this review, we did not perform a meta‐analysis because of clinical heterogeneity between the two trials, particularly with regard to intervention strategies and outcome definition. Although the reports of the design and conduct of the trials suggest that efforts were made by the investigators to assure high quality outcome data, lack of adequately reported information made it difficult to judge trial quality based on key quality parameters specified for this review (see Risk of bias in included studies and Figure 2). Peach 1987 reported outcomes at three months. Although the follow‐up period fell short of what we specified in our protocol, we did not exclude the data from this trial in view of the paucity of RCTs. The two trials were conducted in Australia and Africa. Their generalizability to other countries and settings where trachoma is endemic is unknown.
Quality of the evidence
Active trachoma
If face washing indeed is beneficial with respect to active trachoma, the absence of an effect of face washing in Peach 1987 may be due to a number of factors. Firstly, the trachoma grading system used in the trial may have influenced the results. Participants were recruited into the trial on the basis of whether follicles or papillae were present. Children without trachoma who had follicles/papillae from other causes could have been included. For this group of children, treatment likely had no benefit. Furthermore, participants whose trachoma was less severe may not have experienced a benefit of face washing.
Secondly, in analyzing the results using the intention‐to‐treat principle, the trial authors assumed that all participants lost to follow‐up had follicular trachoma at the end of the trial. If this assumption was incorrect and there were more participants lost to follow‐up in a treatment group compared to control, as was the case with the eye washing group (17% versus 10.4%), treatment might appear to be ineffective compared with control. However, a sensitivity analysis with the missing participants excluded from analysis did not alter the result.
Thirdly, the intervention was administered for only three months. A longer intervention period and follow‐up might have yielded different findings.
Fourthly, Peach 1987 applied face washing to children with established disease rather than the population at risk, and measured the outcome in this group of children. The face washing strategy aims to reduce active trachoma in endemic communities, mainly by reducing the transmission of the disease. The true impact of face washing on active trachoma in the communities might be better evaluated by a study design in which face washing is applied to whole populations at risk rather than only those with the disease and the number of new cases of disease since institution of the intervention determined. It is unclear how much impact on transmission can be achieved by applying face washing only to individuals in endemic communities who already have the disease.
Severe trachoma
The overall results of West 1995 for all the villages after adjusting for age and baseline trachoma status showed a benefit of face washing in reducing severe trachoma in the intervention villages compared to the control villages at 12 months follow‐up. It is probable that participants with severe active trachoma represent a subgroup with more intense transmission and therefore face washing, which aims to break transmission, would be more likely to show a stronger effect within this subgroup. On the other hand, the appropriateness of combining the results from the three pairs of villages is questionable, since presumably the villages were paired because of some differences among them. It is unclear why face washing showed no comparative benefit in the three pairs of villages at six months follow‐up. Apparent benefit at 12 months underscores the importance of a longer follow‐up period to ascertain the impact of the intervention.
Clean faces
The quality of evidence regarding the benefit of cleaning faces daily is largely unknown based on information available for the two included trials. Several methodological details were unreported or unclearly reported (allocation concealment, method of randomizations and attrition). There was inconsistency between the trials regarding the effect of clean faces on active trachoma.
Potential biases in the review process
We used a comprehensive search strategy in multiple databases, thus minimizing missed trials. There was no language restriction in the search for trials in order to reduce bias in locating eligible trials. The two included trials were conducted at least 20 years before this Cochrane review (reported in 1987 and 1995). We identified a completed but not yet reported study in our searches for eligible trials. Inclusion of findings from that study in our next review update may change our conclusions. We contacted trial authors for missing data and two review authors extracted data from the included trials.
Agreements and disagreements with other studies or reviews
A recent systematic review by Stocks 2014 showed a possible beneficial effect of clean faces in reducing the odds for active trachoma (TI/TF). Its objective was to "summarize the evidence in order to devise strategic and cost‐effective approaches to trachoma prevention" (Stocks 2014). It included non‐randomized studies that reported evidence showing that face washing at least once a day has an impact on both TF and TI (OR 0.76, 95% CI 0.57 to 0.96). It also showed that other hygiene‐related exposures affect active trachoma (TI/TF), such as: ocular discharge, nasal discharge, soap use, bathing at least once a day and towel use. The methodological quality of the included studies was not addressed adequately in Stocks 2014.
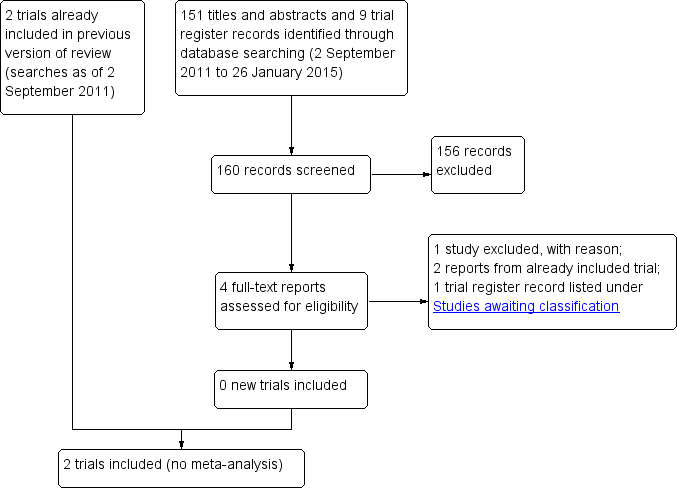
Study flow diagram.
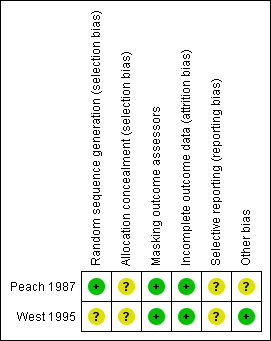
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Eye wash versus control, Outcome 1 Presence of follicular trachoma.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Presence of follicular trachoma Show forest plot | 1 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.11] |
| 1.1 with tetracycline eye drops in both groups | 1 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
| 1.2 without tetracycline eye drops in both groups | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |

