Suplementos de vitamina A para la reducción del riesgo de transmisión maternoinfantil de la infección por VIH
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Described as randomised double‐blind. | |
| Participants | 728 HIV‐infected women enrolled at 17‐39 weeks' gestation in KwaZulu‐Natal Province of South Africa; 30.6% of whom had serum retinol levels < 20 µg/dl. | |
| Interventions | Daily oral vitamin A (5000 IU retinyl palmitate and 30 mg beta‐carotene) or placebo. At delivery, women in the vitamin A group received a dose of 200,000 IU of retinyl palmitate while the placebo arm received an identical placebo. | |
| Outcomes | Stillbirths, HIV infection in the child, neonatal deaths, preterm birth, birthweight, low birthweight. | |
| Notes | No woman received any antiretroviral therapy. It is not stated in the trial reports whether the women also received iron, folic acid, and/or chloroquine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised, placebo‐controlled, double‐blind. | |
| Participants | 1075 pregnant HIV‐infected women enrolled at 12‐27 weeks' gestation in Dar es Salam, Tanzania. Of 1085 women intially randomised, 10 were eventually excluded for either being HIV‐negative (n=7) or nonpregnant (n=3). The prevalence of vitamin A deficiency (< 0.70 µmol/L) was about 34% during the second trimester. | |
| Interventions | Daily oral dose of one of: vitamin A (30mg beta carotene + 5000 IU retinyl palmitate) alone, multivitamins ( 20mg B1, 20mg B2, 25mg B6, 100mg niacin, 50microg B12, 500mg C, 30 mg E, and 0.8 mg folic) plus vitamin A, multivitamins without vitamin A, or placebo. At delivery, women receiving any vitamin A were given an additional 200,000 IU oral dose of vitamin A while the others received an extra dose of placebo. | |
| Outcomes | Stillbirths, HIV infection in child, preterm delivery, low birthweight, postpartum CD4 levels. | |
| Notes | It is not mentioned in this trial whether any woman received antiretroviral therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised, placebo‐controlled, double‐blind. One hundred and seventy‐three (32.5%) HIV‐infected women were lost to follow‐up and excluded from the analysis. | |
| Participants | 533 HIV‐infected pregnant women enrolled at 22‐35 weeks' gestation in Harare, Zimbabwe. | |
| Interventions | Daily oral tablet containing either vitamin A (3000 micrograms retinol equivalents and 3.5 mg beta‐carotene) and the recommended daily allowance of 11 micronutrients (1.5mg thiamine, 1.6mg riboflavin, 2.2mg B‐6, 4.0micrograms B‐12, 17mg niacin, 80mg C, 10micrograms D, 10mg E, 15mg zinc, 1.2micrograms copper, 65micrograms selenium), or placebo. | |
| Outcomes | Gestational length, birthweight, preterm delivery | |
| Notes | All women received iron and folic acid as part of routine antenatal care. No woman received antiretroviral therapy. Information is not available on the HIV status of the children due to technical difficulties and problems with follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial. Patients were assigned to treatment using computer‐generated random numbers, and treatment was concealed by prepacking study supplements in sequentially numbered series assigned to study indentification numbers. Sixty three women (9%) were lost to follow‐up before delivery and excluded from the analyses. The 14 pairs of twins in the study were excluded from the birth weight and mortality analyses because twins are known to have lower birth weights and higher mortality rates. | |
| Participants | 697 pregnant HIV‐infected women enrolled at 18‐28 week's gestation in Blantyre, Malawi. The prevalence of vitamin A deficiency (< 0.70 µmol/L) was 51% during the second trimester. | |
| Interventions | All women received orally administered daily doses of iron (30mg of elemental iron) and folate (400 µg) from enrollment until delivery. One‐half of the women were randomised to receive daily doses of orally administered vitamin A (10,000 IU). | |
| Outcomes | Stillbirths, HIV infection in child, preterm delivery, low birthweight, postpartum CD4 levels. | |
| Notes | All women received oral vitamin A (100,000 IU) at 6 weeks postpartum, as per policy of the Malawi Ministry of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The participants in this randomised controlled trial were not pregnant women, but those who had just delivered and their newborn babies. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Bloemfontein Vitamin A Trial |
| Methods | |
| Participants | 303 HIV‐positive pregnant women from metropolitan Bloemfontein, South Africa. The majority (56%) lived in informal settlements, and all attended public health facilities. For the trial, women were asked to volunteer for HIV testing during their first antenatal visit. Pretest counseling was done in groups, and posttest counseling was done individually. Seropositive women were asked to participate in the trial. All trial participants gave separate informed consent for the trial. All patients were recruited by 1 study physician and received verbal or written information (Sesotho, English, or Afrikaans information sheets). |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HIV infection in child Show forest plot | 3 | 2022 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.41] |
| Analysis 1.1  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection in child. | ||||
| 2 HIV infection or death Show forest plot | 1 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.27] |
| Analysis 1.2  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 HIV infection or death. | ||||
| 3 Stillbirth Show forest plot | 4 | 2855 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.68, 1.43] |
| Analysis 1.3  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Stillbirth. | ||||
| 4 Preterm less than 34 weeks Show forest plot | 2 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.24, 2.00] |
| Analysis 1.4  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Preterm less than 34 weeks. | ||||
| 5 Preterm less than 37 weeks Show forest plot | 3 | 2110 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| Analysis 1.5  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm less than 37 weeks. | ||||
| 6 Low birth weight less than 2500g Show forest plot | 4 | 2606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.68, 1.01] |
| Analysis 1.6  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Low birth weight less than 2500g. | ||||
| 7 Low birthweight less than 2000g Show forest plot | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.27] |
| Analysis 1.7  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Low birthweight less than 2000g. | ||||
| 8 Birthweight Show forest plot | 3 | 1809 | Mean Difference (IV, Fixed, 95% CI) | 89.78 [84.73, 94.83] |
| Analysis 1.8  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Birthweight. | ||||
| 9 Maternal death Show forest plot | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.37] |
| Analysis 1.9  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 9 Maternal death. | ||||
| 10 Postpartum CD4 count Show forest plot | 1 | 727 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐51.06, 43.06] |
| Analysis 1.10  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 10 Postpartum CD4 count. | ||||
| 11 Infant death Show forest plot | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.50] |
| Analysis 1.11  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 11 Infant death. | ||||
| 12 Neonatal admission to neonatal unit | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Death of child by 24 months Show forest plot | 2 | 1635 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| Analysis 1.13  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 13 Death of child by 24 months. | ||||
| 14 Side effects in child | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Later death of child Show forest plot | 1 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.53, 2.81] |
| Analysis 1.15  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 15 Later death of child. | ||||
| 16 Maternal post‐partum infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Acceptance of supplementation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
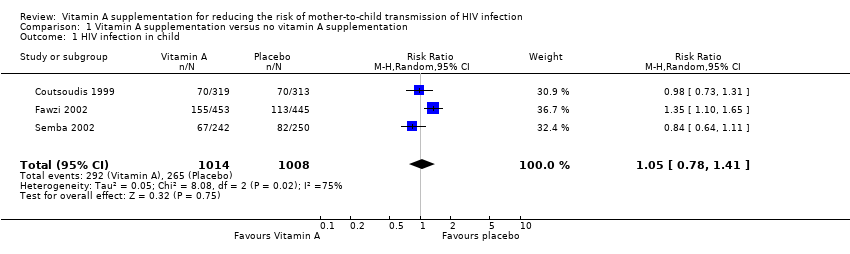
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection in child.
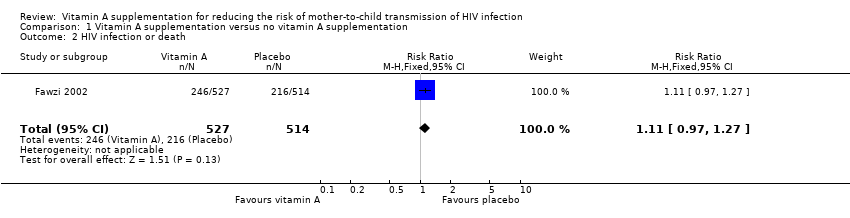
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 HIV infection or death.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Stillbirth.
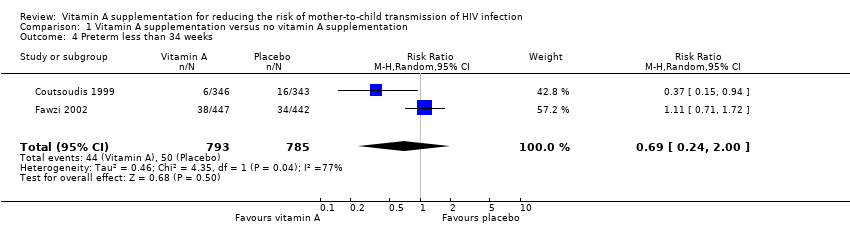
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Preterm less than 34 weeks.
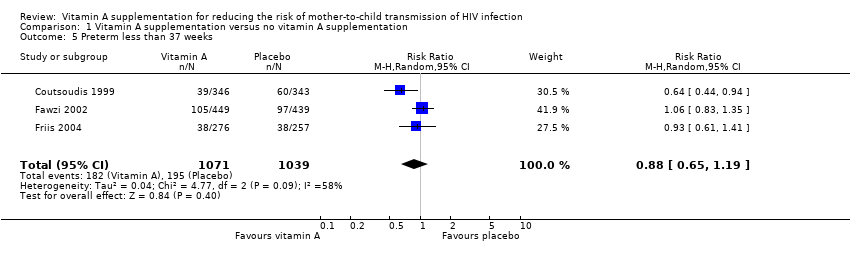
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm less than 37 weeks.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Low birth weight less than 2500g.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Low birthweight less than 2000g.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Birthweight.
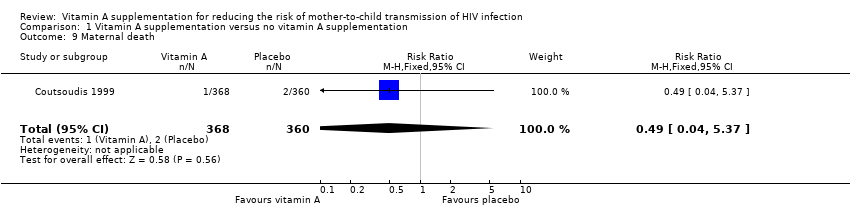
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 9 Maternal death.
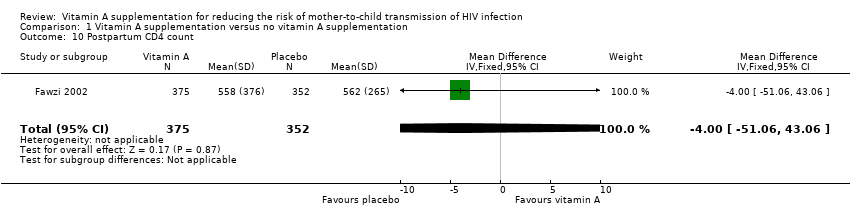
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 10 Postpartum CD4 count.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 11 Infant death.
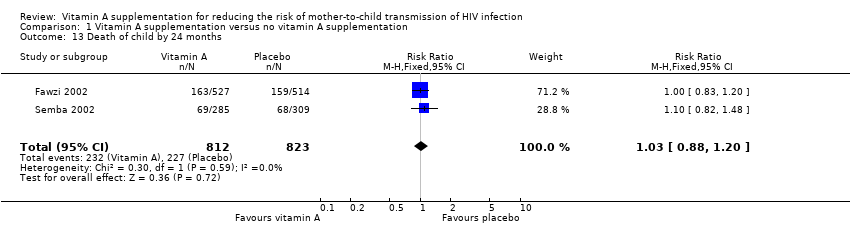
Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 13 Death of child by 24 months.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 15 Later death of child.
| Search | Details |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] |
| #2 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #3 | Search (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | Search #1 AND #2 AND #3 AND #4 AND #5 Limits: Publication Date from 2003 to 2008 |
| Search | Details |
| #1 | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection')) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'))) OR ((('b cell lymphoma'/exp OR 'b cell lymphoma') OR ('b cell lymphoma'/exp OR 'b cell lymphoma'))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human |
| #2 | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')))) OR (((('randomized controlled trial'/exp OR 'randomized controlled trial') OR ('randomized controlled trial'/exp OR 'randomized controlled trial')) OR (('randomized controlled trial'/exp OR 'randomized controlled trial') OR ('randomized controlled trial'/exp OR 'randomized controlled trial')))) OR (allocat*:ti OR allocat*:ab) AND [2003‐2008]/py |
| #3 | 'mother‐to‐child transmission' OR mtct OR 'vertical disease transmission' AND [2003‐2008]/py |
| #4 | caroten* OR retinoic OR ('retinol'/exp OR 'retinol') OR vitamin* OR micronutrient* AND [2003‐2008]/py |
| #5 | pregnant OR ('pregnancy'/exp OR 'pregnancy') OR antepartum OR ('ante partum') OR antenatal OR ('ante natal') OR prenatal OR ('pre natal') AND [2003‐2008]/py |
| #6 | #1 AND #2 AND #3 AND #4 AND #5 |
| Search | Details |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) |
| #3 | (MOTHER‐TO‐CHILD TRANSMISSION) OR MTCT OR (VERTICAL DISEASE TRANSMISSION) |
| #4 | CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | PREGNANT OR PREGNANCY OR ANTEPARTUM OR (ANTE‐PARTUM) OR ANTENATAL OR (ANTE‐NATAL) OR PRENATAL OR (PRE‐NATAL) |
| #6 | #1 AND #2 AND #3 AND #4 AND #5 |
| Search | Details |
| #1 | Search: (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw]) AND (acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH]) |
| #2 | Search: (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh])) |
| #3 | Search: (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search: CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search: PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | #1 and #2 and #3 and #4 and #5 Limit: 2003:2008 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HIV infection in child Show forest plot | 3 | 2022 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.41] |
| 2 HIV infection or death Show forest plot | 1 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.27] |
| 3 Stillbirth Show forest plot | 4 | 2855 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.68, 1.43] |
| 4 Preterm less than 34 weeks Show forest plot | 2 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.24, 2.00] |
| 5 Preterm less than 37 weeks Show forest plot | 3 | 2110 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| 6 Low birth weight less than 2500g Show forest plot | 4 | 2606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.68, 1.01] |
| 7 Low birthweight less than 2000g Show forest plot | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.27] |
| 8 Birthweight Show forest plot | 3 | 1809 | Mean Difference (IV, Fixed, 95% CI) | 89.78 [84.73, 94.83] |
| 9 Maternal death Show forest plot | 1 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.37] |
| 10 Postpartum CD4 count Show forest plot | 1 | 727 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐51.06, 43.06] |
| 11 Infant death Show forest plot | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.50] |
| 12 Neonatal admission to neonatal unit | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Death of child by 24 months Show forest plot | 2 | 1635 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 14 Side effects in child | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Later death of child Show forest plot | 1 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.53, 2.81] |
| 16 Maternal post‐partum infection | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Acceptance of supplementation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

