Tratamiento con ondas de choque para el dolor lateral del codo
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | 60 (1 withdrew prior to treatment) participants | |
| Interventions | Group 1 (active ESWT) (31 participants): 3 treatment sessions for each affected arm (one each week for 3 weeks). Treatments were applied using a low energy shock wave machine (Sonocur Basic (Siemens AG, Erlangen, Germany)). Conducting gel was applied to the site of pain and the treatment head of the machine placed on the point of maximum pain as identified by the subject. Subjects received 2000 pulses of 0.03 to 0.17 mJ/mm2 per affected arm in each session. The energy flux density used to treat each subject was determined by the subjects own pain tolerance, as per the manufacturer's protocol. | |
| Outcomes | Outcome assessed at baseline, 4 and 8 weeks after initiation of therapy i.e. baseline and 1 and 5 weeks after the completion of treatment). | |
| Notes | Median values and interquartile ranges were presented in tabular format (and are shown in Additional tables 04). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial | |
| Participants | 93 participants | |
| Interventions | Group 1 (steroid injection group): injection of 20mg of triamcinolone (made up to 1.5ml with 1% lignocaine) into the point of maximal tenderness at the extensor origin of the lateral epicondyle of the humerus. | |
| Outcomes | Outcome assessed at baseline, 6 weeks and 3 months after the end of treatment (i.e 6 weeks and 3 months from baseline in steroid group but 8 weeks and 3.5 months from baseline in ESWT group. | |
| Notes | Mean pain values were presented without any measured of variance. Therefore the study was included in the review but only the proportion of participants with a reduction in pain of 50% at 3 months could be included in the meta‐analysis (noting that the timing of the 3 month assessment was 3.5 months in the ESWT group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Multicentre randomised controlled trial | |
| Participants | 271 participants (1 participant withdrew prior to the intervention) | |
| Interventions | Group 1 (ESWT group) (135 participants): "Low energy" ESWT with 3 treatments of 2000 pulses at ED+ = 0.07‐0.09 mJ/mm2. 6‐8 days between each treatment. Performed under local anaesthesia (3ml, 1% mepivacaine). Positioning was performed with the use of continuous ultrasound imaging to focus the shock waves at the insertion of the muscles at the lateral epicondyle of the humerus. | |
| Outcomes | Outcome assessed at baseline, 6 and 12 weeks and 12 months after last intervention. | |
| Notes | Roles and Maudsley subjective pain scale: 1 = excellent (no pain, full movement and activity), 2 = good (occasional discomfort, full movement, full activity), 3 = fair (some discomfort after prolonged activity), 4 = poor (pain limiting activities). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised multi‐centre placebo‐controlled trial (number of sites not specified) | |
| Participants | 183 participants | |
| Interventions | Each subject received a local anaesthetic or a bier block prior to the study procedure. The affected arm was draped from the view of the study subject. | |
| Outcomes | Outcome was assessed at 4 and 8 weeks after the treatment. | |
| Notes | Unpublished data extracted from the FDA report. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised controlled trial | |
| Participants | 24 participants | |
| Interventions | Group 1 (ESWT group) (13 participants): 2000 shock waves at 2.5 bars of air pressure and frequency of 8‐10 Hz. Three treatments given at intervals of 2 weeks, each lasting for 3‐4 minutes. Electro Medical Systems (EMS) Swiss DolorClast System used. | |
| Outcomes | Outcome assessed at baseline, 3 and 6 months after the final treatment but only the 6 month data was reported. | |
| Notes | Trial also recruited 23 participants with plantar fasciitis. Data presented separately for the two conditions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Randomised controlled trial | |
| Participants | 86 participants randomised but only 74 participants completed treatment and were included in analysis. | |
| Interventions | Group 1 (ESWT group) (37 participants): shock waves were focussed on the common extensor origin under ultrasound guidance. Ultrasound gel used as conductive medium between the skin and treatment head. All treatment sessions were started at a low energy level (1‐3) and the intensity was gradually increased according to each participant's tolerance, not exceeding level 6. A fixed amount of energy (333 mJ/mm2) was delivered at each session, totalling 1000 mJ/mm2 at the end of 3 sessions. | |
| Outcomes | Outcome assessed at baseline, 1, 3 and 12 months after end of treatment. | |
| Notes | Mean values were presented in graphical format but without any measure of variance. Therefore the study was included in the review but only the proportion of participants eventually requiring surgery could be included in the meta‐analysis. The authors reported no significant difference between the two treatment groups for any of the measured outcomes at any timepoint and hence the results are consistent with the conclusion of the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised multi‐centre placebo‐controlled trial (3 sites) but open cross‐over to active treatment if not improved by at least 50% with respect to pain elicited by Thomsen test at 12 weeks following completion of treatment. | |
| Participants | 114 participants | |
| Interventions | Group 1 (ESWT group) (56 participants): 2000 impulses at 0.06mJ/mm2 using Sonocur ESWT system (Siemens, USA) weekly for 3 weeks. The treatment head of the device was directed to the point of maximal tenderness on the lateral epicondyle as identified by physician palpation and patient report. An ultrasound coupling gel was used. During treatment, the technique of clinical focussing was employed by adjusting the shock wave focus every 200‐400 impulses, redirecting the shock waves to the most symptomatic site. | |
| Outcomes | Outcome was assessed at baseline, 1, 4, 8, 12 weeks and 6 and 12 months after completion of treatment (i.e. 3, 6, 10, 14 weeks and 6.5 and 12.5 months from baseline). | |
| Notes | The Thomsen test was performed with the shoulder flexed to 60 degrees, the elbow extended, the forearm pronated and the wrist extended to 30 degrees. Pressure was applied on the dorsum of the hand to stress the extensor carpi radialis and brevis. The test was performed 3 times, with participants recording their pain on a 10cm VAS after the third test. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial | |
| Participants | 115 participants | |
| Interventions | Group 1 (Experimental group): ESWT 1000 impulses of 0.08mJ/mm2 at weekly intervals for 3 weeks | |
| Outcomes | Assessments were made at baseline (6 weeks before treatment), at end of 3‐week treatment (0 weeks) and at 3, 6 & 24 weeks after the completion of treatment. | |
| Notes | 3 publications of identical trial design probably reporting subsets of same trial therefore reference with largest sample size and longest follow‐up reported in review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomised controlled trial | |
| Participants | 78 participants | |
| Interventions | Group 1 (38 participants) (ESWT group): 3 treatments at weekly intervals of low energy ESWT with 3 x 2000 pulses applied using a device‐dependent energy flux density of 0.09 mJ/mm2. Repetition frequency of shock wave pulses was 4 Hz. The total dose applied was 0.54 mJ/mm2. | |
| Outcomes | Outcome assessed at baseline, 3 and 12 months after last treatment. | |
| Notes | The Thomsen Test data was converted to 100mm VAS scale for the purpose of pooling of data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomised controlled trial | |
| Participants | 75 participants | |
| Interventions | Group 1 (ESWT group) (40 participants): 1500 pulses at 0.18 mJ/mm2 applied. Target area was located via ultrasonographic localisation and finding the area of maximum reproduction of local pain at initiation of the treatment. | |
| Outcomes | Outcome assessed at baseline and 3 months (one month after completion of therapy). Also assessed prior to each treatment, ie: at one month and 2 months. | |
| Notes | All treatments using a Sonocur Plus Unit (Siemens) which generates mechanical shock waves using an electromagnetic generator. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised controlled trial. | |
| Not a randomised controlled trial. | |
| Not a randomised controlled trial. Case series of 512 patients with soft tissue pain in proximity to bones. Included 66 patients with epicondylitis | |
| Not a randomised controlled trial. | |
| Not looking at treatment efficacy of ESWT. | |
| Not a randomised controlled trial. Case series of 812 patients with enthesopathy of whom 525 suffered from radial epicondylitis. Abstract only. | |
| Not a randomised controlled trial. Case series of 19 patients with tennis elbow and 44 patients with painful heel. | |
| Not a randomised controlled trial. | |
| Not a randomised controlled trial. Case series of 124 patients with lateral epicondylitis, 26 patients with medial epicondylitis and 60 patients with plantar fasciitis. | |
| Not a randomised controlled trial. Case series of 53 patients with lateral epicondylitis (56 elbows). | |
| Not a randomised controlled trial. Case series of 30 patients with chronic medial epicondylitis and first 30 of 101 patients with lateral epicondylitis. | |
| No information regarding efficacy of ESWT ‐ a comparison of two ultrasound localisation techniques. | |
| Not a randomised controlled trial. Case series of 30 patients treated operatively and 30 patient treated with extracorporeal shock waves for chronic lateral epicondylitis. | |
| Not a randomised controlled trial. Case series of 16 patients. | |
| Not a randomised controlled trial. Case series of 150 patients. | |
| Not a randomised controlled trial | |
| Not specific to tennis elbow | |
| Not a randomised controlled trial. A review article. | |
| Not a randomised controlled trial. | |
| Not a randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The efficacy of ultrasound guided extra‐corporeal shock wave therapy (ESWT) in the treatment of lateral epicondylitis (tennis elbow): a randomised double‐blind placebo‐controlled trial |
| Methods | |
| Participants | lateral epicondylitis |
| Interventions | ultrasound guided ESWT versus |
| Outcomes | overall pain on 100 mm VAS; overall functional level of upper limb on 100 mm VAS; 8‐item pain‐free function index; DASH; SF‐36; maximum pain‐free grip strength; additional treatments including surgery; adverse effects |
| Starting date | 1999 |
| Contact information | Rachelle Buchbinder |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean pain at rest (100 point scale) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 ESWT VERSUS PLACEBO, Outcome 1 Mean pain at rest (100 point scale). | ||||
| 1.1 4‐6 weeks | 3 | 446 | Mean Difference (IV, Random, 95% CI) | ‐9.42 [‐20.70, 1.86] |
| 1.2 12 weeks | 1 | 271 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐5.43, 7.43] |
| 1.3 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐25.2 [‐30.59, ‐19.81] |
| 1.4 12 months | 1 | 271 | Mean Difference (IV, Random, 95% CI) | 7.0 [2.83, 11.17] |
| 2 Mean pain with resisted wrist extension (Thomsen test)(100 point scale) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 ESWT VERSUS PLACEBO, Outcome 2 Mean pain with resisted wrist extension (Thomsen test)(100 point scale). | ||||
| 2.1 1 week | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐8.42 [‐17.31, 0.47] |
| 2.2 4 weeks | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐11.48 [‐21.08, ‐1.88] |
| 2.3 6 weeks | 2 | 371 | Mean Difference (IV, Random, 95% CI) | ‐16.20 [‐47.75, 15.36] |
| 2.4 8 weeks | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐14.04 [‐23.95, ‐4.13] |
| 2.5 12 weeks | 3 | 455 | Mean Difference (IV, Random, 95% CI) | ‐9.04 [‐19.37, 1.28] |
| 2.6 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐29.3 [‐35.83, ‐22.77] |
| 2.7 12 months | 1 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐7.08, 5.08] |
| 3 Mean pain with typical daily activities Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 ESWT VERSUS PLACEBO, Outcome 3 Mean pain with typical daily activities. | ||||
| 3.1 4‐6 weeks | 2 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐1.78 [‐6.70, 3.14] |
| 3.2 8 weeks | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐8.40 [‐16.20, ‐0.60] |
| 3.3 12 weeks | 1 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐7.14, 7.14] |
| 3.4 12 months | 1 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐8.48, 2.48] |
| 4 Mean pain with resisted middle finger extension (100 point scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 ESWT VERSUS PLACEBO, Outcome 4 Mean pain with resisted middle finger extension (100 point scale). | ||||
| 4.1 6 weeks | 2 | 371 | Mean Difference (IV, Random, 95% CI) | ‐20.51 [‐56.57, 15.56] |
| 4.2 12 weeks | 1 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐9.62, 5.62] |
| 4.3 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐40.0 [‐45.52, ‐34.48] |
| 4.4 12 months | 1 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐6.48, 4.48] |
| 5 Mean pain with resisted supination of the wrist (Mills test)(100 point scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 ESWT VERSUS PLACEBO, Outcome 5 Mean pain with resisted supination of the wrist (Mills test)(100 point scale). | ||||
| 5.1 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of patients with significant improvement Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 ESWT VERSUS PLACEBO, Outcome 6 Number of patients with significant improvement. | ||||
| 6.1 50% improvement in overall pain at 1 month (4 weeks) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.55, 1.90] |
| 6.2 50% improvement in night pain at 1 month (4 weeks) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.40] |
| 6.3 Success (at least 50% improved overall pain AND pain>4cm AND no pain meds for 2/52) (5 weeks) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.62, 2.51] |
| 6.4 Success (defined as no pain/occasional discomfort and no additional Rx at 3 months) (12 weeks) | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.56] |
| 6.5 Success (defined as at least 50% improvement in pain elicited by Thomsen test ) (12 weeks) | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.55, 3.12] |
| 6.6 significant improvement in pain of 3 or more points (on 10‐point VAS) (6 months) | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.49, 67.29] |
| 6.7 Number with at least 50% improvement in investigator‐assessed pain and 4 or less on 10cm VAS (8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.08, 2.37] |
| 6.8 Number with at least 50% improvement in pain with activities and 4 or less on 10 cm VAS (8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.94, 1.78] |
| 6.9 Number with no or rare use of pain medications (8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.94, 1.35] |
| 6.10 Success (at least 50% improvement in investigator and participant ‐assessed pain and rare pain meds)(8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.99, 2.56] |
| 7 Failure of treatment defined by Roles and Maudsley score of 4 Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 ESWT VERSUS PLACEBO, Outcome 7 Failure of treatment defined by Roles and Maudsley score of 4. | ||||
| 7.1 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.50] |
| 7.2 6 weeks | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 1.91] |
| 7.3 12 weeks | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.32, 1.16] |
| 7.4 24 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.06, 0.33] |
| 7.5 12 months | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.17] |
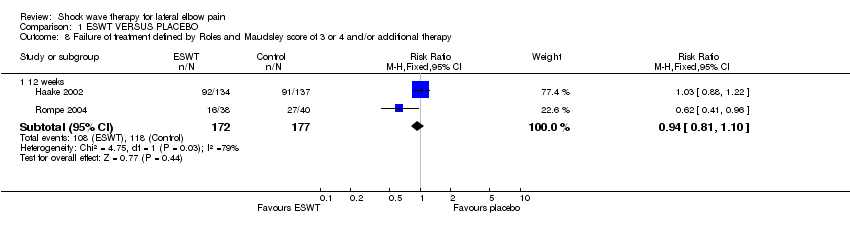
| 8 Failure of treatment defined by Roles and Maudsley score of 3 or 4 and/or additional therapy Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 ESWT VERSUS PLACEBO, Outcome 8 Failure of treatment defined by Roles and Maudsley score of 3 or 4 and/or additional therapy. | ||||
| 8.1 12 weeks | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.10] |
| 9 Number of patients who eventually underwent surgical release of common extensor origin Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 ESWT VERSUS PLACEBO, Outcome 9 Number of patients who eventually underwent surgical release of common extensor origin. | ||||
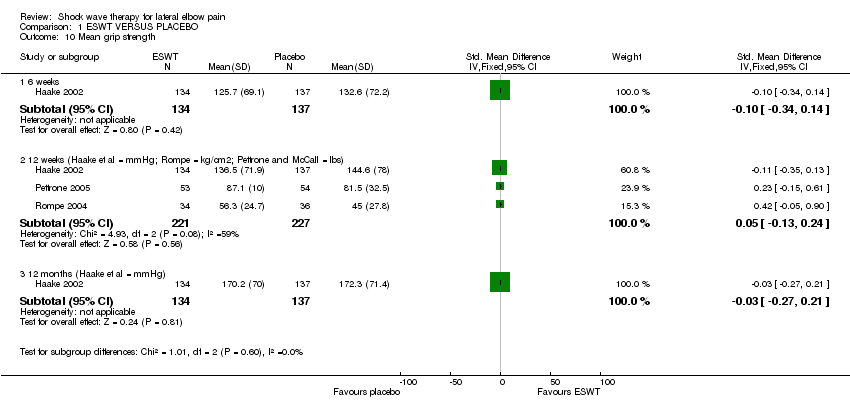
| 10 Mean grip strength Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 ESWT VERSUS PLACEBO, Outcome 10 Mean grip strength. | ||||
| 10.1 6 weeks | 1 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 10.2 12 weeks (Haake et al = mmHg; Rompe = kg/cm2; Pettrone and McCall = lbs) | 3 | 448 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.13, 0.24] |
| 10.3 12 months (Haake et al = mmHg) | 1 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.27, 0.21] |
| 11 Mean Upper Extremity Function Scale (range 8‐80) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 ESWT VERSUS PLACEBO, Outcome 11 Mean Upper Extremity Function Scale (range 8‐80). | ||||
| 11.1 1 week | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐9.32, 1.64] |
| 11.2 4 weeks | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐13.47, ‐2.37] |
| 11.3 8 weeks | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐13.57, ‐2.43] |
| 11.4 12 weeks | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐9.20 [‐13.56, ‐4.84] |
| 12 Mean patient‐specific activity score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 ESWT VERSUS PLACEBO, Outcome 12 Mean patient‐specific activity score. | ||||
| 12.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Mean patient evaluation of their disease status (100mm VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 ESWT VERSUS PLACEBO, Outcome 13 Mean patient evaluation of their disease status (100mm VAS). | ||||
| 13.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mean pain with palpation over the lateral epicondyle (100 point scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 ESWT VERSUS PLACEBO, Outcome 14 Mean pain with palpation over the lateral epicondyle (100 point scale). | ||||
| 14.1 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Mean pain with Chair test (100 point scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.15  Comparison 1 ESWT VERSUS PLACEBO, Outcome 15 Mean pain with Chair test (100 point scale). | ||||
| 15.1 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Mean pain at night (100 point scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 ESWT VERSUS PLACEBO, Outcome 16 Mean pain at night (100 point scale). | ||||
| 16.1 3‐4 weeks | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐10.01 [‐34.23, 14.21] |
| 16.2 6 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐27.40 [‐32.98, ‐21.82] |
| 16.3 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐25.2 [‐30.59, ‐19.81] |
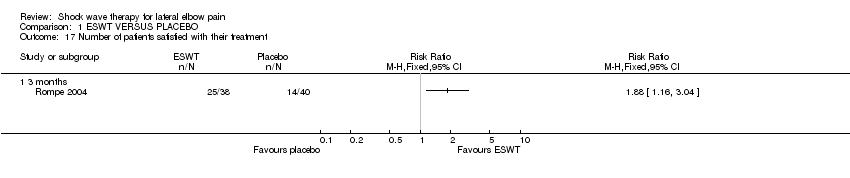
| 17 Number of patients satisfied with their treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.17  Comparison 1 ESWT VERSUS PLACEBO, Outcome 17 Number of patients satisfied with their treatment. | ||||
| 17.1 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Mean investigator assessment of pain to pressure over lateral epicondyle (10 cm VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.18  Comparison 1 ESWT VERSUS PLACEBO, Outcome 18 Mean investigator assessment of pain to pressure over lateral epicondyle (10 cm VAS). | ||||
| 18.1 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Number of patients reported pain during treatment Show forest plot | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.48, 2.62] |
| Analysis 1.19  Comparison 1 ESWT VERSUS PLACEBO, Outcome 19 Number of patients reported pain during treatment. | ||||
| 20 Number of patients reported nausea during treatment Show forest plot | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.89 [2.50, 66.47] |
| Analysis 1.20  Comparison 1 ESWT VERSUS PLACEBO, Outcome 20 Number of patients reported nausea during treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with successful treatment (defined as reduction in pain of 50% or greater at 3 months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 ESWT VERSUS STEROID INJECTION, Outcome 1 Number of patients with successful treatment (defined as reduction in pain of 50% or greater at 3 months). | ||||

Comparison 1 ESWT VERSUS PLACEBO, Outcome 1 Mean pain at rest (100 point scale).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 2 Mean pain with resisted wrist extension (Thomsen test)(100 point scale).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 3 Mean pain with typical daily activities.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 4 Mean pain with resisted middle finger extension (100 point scale).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 5 Mean pain with resisted supination of the wrist (Mills test)(100 point scale).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 6 Number of patients with significant improvement.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 7 Failure of treatment defined by Roles and Maudsley score of 4.
Comparison 1 ESWT VERSUS PLACEBO, Outcome 8 Failure of treatment defined by Roles and Maudsley score of 3 or 4 and/or additional therapy.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 9 Number of patients who eventually underwent surgical release of common extensor origin.
Comparison 1 ESWT VERSUS PLACEBO, Outcome 10 Mean grip strength.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 11 Mean Upper Extremity Function Scale (range 8‐80).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 12 Mean patient‐specific activity score.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 13 Mean patient evaluation of their disease status (100mm VAS).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 14 Mean pain with palpation over the lateral epicondyle (100 point scale).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 15 Mean pain with Chair test (100 point scale).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 16 Mean pain at night (100 point scale).
Comparison 1 ESWT VERSUS PLACEBO, Outcome 17 Number of patients satisfied with their treatment.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 18 Mean investigator assessment of pain to pressure over lateral epicondyle (10 cm VAS).

Comparison 1 ESWT VERSUS PLACEBO, Outcome 19 Number of patients reported pain during treatment.

Comparison 1 ESWT VERSUS PLACEBO, Outcome 20 Number of patients reported nausea during treatment.

Comparison 2 ESWT VERSUS STEROID INJECTION, Outcome 1 Number of patients with successful treatment (defined as reduction in pain of 50% or greater at 3 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean pain at rest (100 point scale) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 4‐6 weeks | 3 | 446 | Mean Difference (IV, Random, 95% CI) | ‐9.42 [‐20.70, 1.86] |
| 1.2 12 weeks | 1 | 271 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐5.43, 7.43] |
| 1.3 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐25.2 [‐30.59, ‐19.81] |
| 1.4 12 months | 1 | 271 | Mean Difference (IV, Random, 95% CI) | 7.0 [2.83, 11.17] |
| 2 Mean pain with resisted wrist extension (Thomsen test)(100 point scale) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 week | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐8.42 [‐17.31, 0.47] |
| 2.2 4 weeks | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐11.48 [‐21.08, ‐1.88] |
| 2.3 6 weeks | 2 | 371 | Mean Difference (IV, Random, 95% CI) | ‐16.20 [‐47.75, 15.36] |
| 2.4 8 weeks | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐14.04 [‐23.95, ‐4.13] |
| 2.5 12 weeks | 3 | 455 | Mean Difference (IV, Random, 95% CI) | ‐9.04 [‐19.37, 1.28] |
| 2.6 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐29.3 [‐35.83, ‐22.77] |
| 2.7 12 months | 1 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐7.08, 5.08] |
| 3 Mean pain with typical daily activities Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 4‐6 weeks | 2 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐1.78 [‐6.70, 3.14] |
| 3.2 8 weeks | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐8.40 [‐16.20, ‐0.60] |
| 3.3 12 weeks | 1 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐7.14, 7.14] |
| 3.4 12 months | 1 | 271 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐8.48, 2.48] |
| 4 Mean pain with resisted middle finger extension (100 point scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 6 weeks | 2 | 371 | Mean Difference (IV, Random, 95% CI) | ‐20.51 [‐56.57, 15.56] |
| 4.2 12 weeks | 1 | 271 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐9.62, 5.62] |
| 4.3 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐40.0 [‐45.52, ‐34.48] |
| 4.4 12 months | 1 | 271 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐6.48, 4.48] |
| 5 Mean pain with resisted supination of the wrist (Mills test)(100 point scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of patients with significant improvement Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 50% improvement in overall pain at 1 month (4 weeks) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.55, 1.90] |
| 6.2 50% improvement in night pain at 1 month (4 weeks) | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.40] |
| 6.3 Success (at least 50% improved overall pain AND pain>4cm AND no pain meds for 2/52) (5 weeks) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.62, 2.51] |
| 6.4 Success (defined as no pain/occasional discomfort and no additional Rx at 3 months) (12 weeks) | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.56] |
| 6.5 Success (defined as at least 50% improvement in pain elicited by Thomsen test ) (12 weeks) | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.55, 3.12] |
| 6.6 significant improvement in pain of 3 or more points (on 10‐point VAS) (6 months) | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.49, 67.29] |
| 6.7 Number with at least 50% improvement in investigator‐assessed pain and 4 or less on 10cm VAS (8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.08, 2.37] |
| 6.8 Number with at least 50% improvement in pain with activities and 4 or less on 10 cm VAS (8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.94, 1.78] |
| 6.9 Number with no or rare use of pain medications (8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.94, 1.35] |
| 6.10 Success (at least 50% improvement in investigator and participant ‐assessed pain and rare pain meds)(8 weeks) | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.99, 2.56] |
| 7 Failure of treatment defined by Roles and Maudsley score of 4 Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.50] |
| 7.2 6 weeks | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 1.91] |
| 7.3 12 weeks | 2 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.32, 1.16] |
| 7.4 24 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.06, 0.33] |
| 7.5 12 months | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.17] |
| 8 Failure of treatment defined by Roles and Maudsley score of 3 or 4 and/or additional therapy Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 12 weeks | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.10] |
| 9 Number of patients who eventually underwent surgical release of common extensor origin Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Mean grip strength Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 6 weeks | 1 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 10.2 12 weeks (Haake et al = mmHg; Rompe = kg/cm2; Pettrone and McCall = lbs) | 3 | 448 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.13, 0.24] |
| 10.3 12 months (Haake et al = mmHg) | 1 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.27, 0.21] |
| 11 Mean Upper Extremity Function Scale (range 8‐80) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 1 week | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐3.84 [‐9.32, 1.64] |
| 11.2 4 weeks | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐13.47, ‐2.37] |
| 11.3 8 weeks | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐13.57, ‐2.43] |
| 11.4 12 weeks | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐9.20 [‐13.56, ‐4.84] |
| 12 Mean patient‐specific activity score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Mean patient evaluation of their disease status (100mm VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mean pain with palpation over the lateral epicondyle (100 point scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Mean pain with Chair test (100 point scale) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Mean pain at night (100 point scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 3‐4 weeks | 2 | 175 | Mean Difference (IV, Random, 95% CI) | ‐10.01 [‐34.23, 14.21] |
| 16.2 6 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐27.40 [‐32.98, ‐21.82] |
| 16.3 24 weeks | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐25.2 [‐30.59, ‐19.81] |
| 17 Number of patients satisfied with their treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Mean investigator assessment of pain to pressure over lateral epicondyle (10 cm VAS) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Number of patients reported pain during treatment Show forest plot | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.48, 2.62] |
| 20 Number of patients reported nausea during treatment Show forest plot | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.89 [2.50, 66.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with successful treatment (defined as reduction in pain of 50% or greater at 3 months) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

