Campos electromagnéticos para el tratamiento de la osteoartritis
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003523.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 diciembre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud musculoesquelética
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Dr. Shasha Li and Bo Yu performed the bibliographic searches, identified the studies, assessed their methodological quality, extracted the data and produced the first draft of the review. Dr. Chengqi He and Dr. Dong Zhou assessed the methodological quality of the studies, checked the extracted data and commented on all the draft manuscripts. Jennifer Hulme and Dr. Qi Zhuo helped to perform the bibliographic searches, identified the studies, assessed their methodological quality and extracted the data.
Sources of support
Internal sources
-
Chinese Cochrane Centre, Chinese EBM Centre, INCLEN CERTC in West China Hospital, Sichuan University, China.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
The authors wish to thank Louise Falzon (CMSG Trial Search Co‐ordinator) for developing the search strategy. Thanks also to the Cochrane Musculoskeletal Group (CMSG) editorial team and Elizabeth Tanjong Ghogomu (Assistant Managing Editor, Cochrane Musculoskeletal Group) for their helpful comments and suggestions for revisions. The authors also thank Professor Taixiang Wu and Guanjian Liu (Chinese Cochrane Centre, China) for their helpful comments and guidelines on the preparation of this review. We acknowledge the work of the author team for the first version of the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 14 | Electromagnetic fields for treating osteoarthritis | Review | Shasha Li, Bo Yu, Dong Zhou, Chengqi He, Qi Zhuo, Jennifer M Hulme | |
| 2002 Jan 21 | Electromagnetic fields for the treatment of osteoarthritis | Review | Jennifer M Hulme, Vivian Welch, Rob de Bie, Maria Judd, Peter Tugwell | |
Differences between protocol and review
The major outcomes were changed to pain, physical function, radiographic joint structure changes, health‐related quality of life measure, number of patients experiencing any adverse event, patients who withdrew because of adverse events and patients experiencing any serious adverse events on the recommendation of the CMSG. 'Risk of bias' assessment, 'Summary of findings' table and GRADE quality assessment were presented.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO
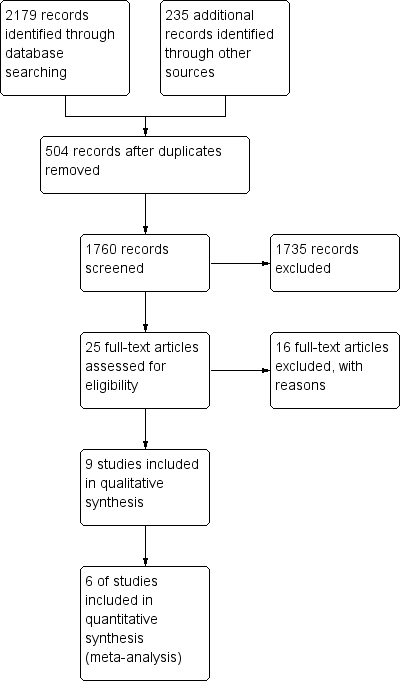
Study flow diagram.
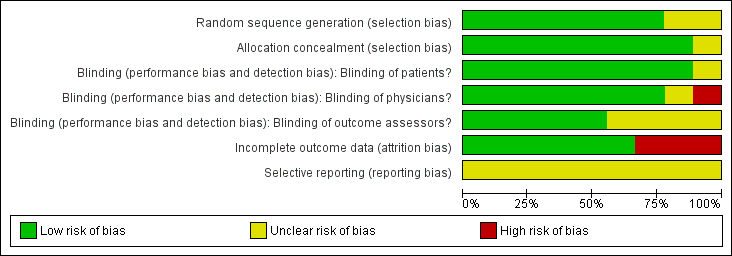
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
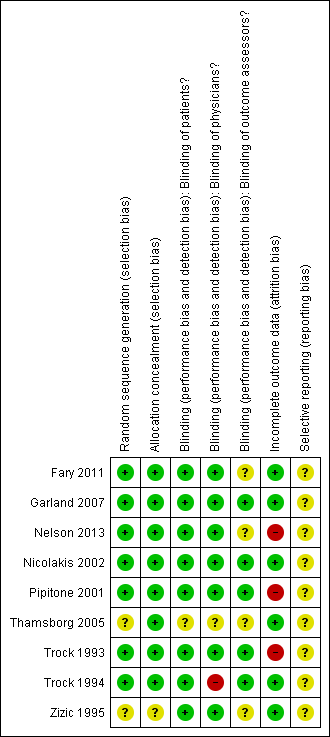
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
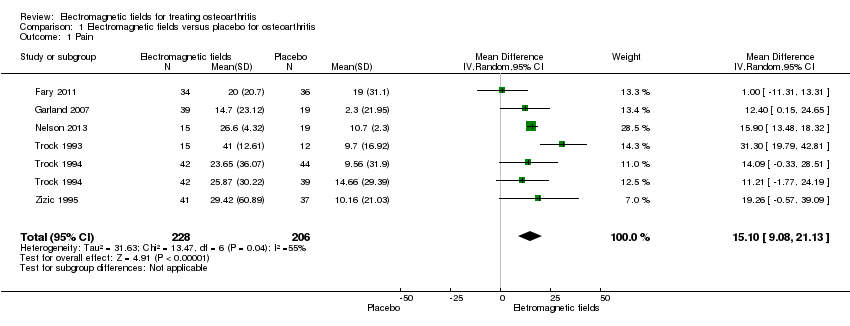
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 1 Pain.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 2 Physical function.
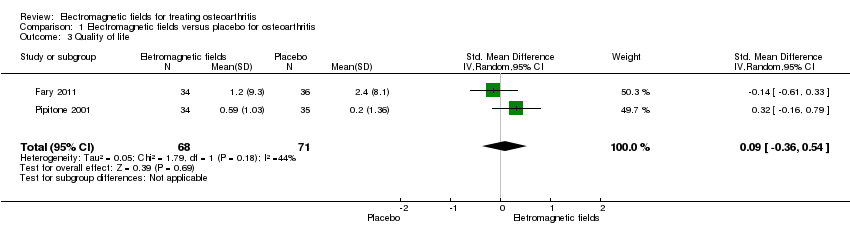
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 3 Quality of life.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 4 Number of patients experiencing any adverse event.
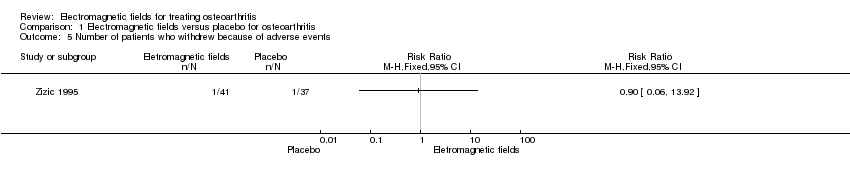
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 5 Number of patients who withdrew because of adverse events.
| Electromagnetic field treatment compared to placebo for the treatment of osteoarthritis | ||||||
| Patient or population: patients with osteoarthritis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Electromagnetic field treatment | |||||
| Pain Scale from: 0 to 100 (Higher scores mean worse pain) | The mean change in pain in the control groups was 10.7 | The mean change in pain in the intervention groups was | 434 | ⊕⊕⊕⊝ | MD 15.10 (95% CI 9.08 to 21.13) Absolute risk difference: 15% (95% CI 9.08% to 21.13%) Relative per cent change: 21.03% (95% CI 12.65% to 29.43%) NNT: 2 (95% CI 1 to 6) | |
| Physical function WOMAC function Scale from: 0 to 100 (Higher scores mean more severe limitation) | The mean change in physical function in the control groups was | The mean change in physical function in the intervention groups was | 197 | ⊕⊕⊝⊝ | MD 4.55 (95% CI ‐2.23 to 11.32) Absolute risk difference: 4.55% (95% CI ‐2.23% to 11.32%) Relative per cent change: 268% (95% CI ‐131% to 666%) NNT: not statistically significant | |
| Quality of life SF‐36 item Scale from: 0 to 100 (Lower scores mean worse quality) Follow‐up: mean 16 weeks | The mean change in quality of life in the control groups was | The mean change in quality of life in the intervention groups was | 145 | ⊕⊕⊕⊝ | SMD 0.09 (95% CI ‐0.36 to 0.54) Absolute risk difference: 1% (95% CI ‐2.92% to 4.37%) Relative per cent change: 30.38% (95% CI ‐121.5% to 182.25%) NNT: not statistically significant | |
| Radiographic progression Bone scintigraphic examinations Follow‐up: mean 2.5 months | See comment | See comment | Not estimable | 78 | See comment | No related data were available |
| Number of patients experiencing any adverse event Follow‐up: mean 1 month | 167 per 1000 | 195 per 1000 | RR 1.17 | 288 | ⊕⊕⊕⊝ | Absolute risk difference: 3% (95% CI ‐6% to 12%) Relative per cent change: 17% (95% CI ‐28% to 92%) NNT: not statistically significant |
| Number of patients who withdrew because of adverse events Follow‐up: mean 6 months | 27 per 1000 | 24 per 1000 (2 to 376) | RR 0.90 (0.06 to 13.92) | 78 | ⊕⊕⊝⊝ | Only 1 study: 1 participant withdrew from each group because of adverse skin reactions unrelated to the therapy |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded for moderate heterogeneity (I2 = 55%); unclear risk for random sequence generation (Zizic 1995), allocation concealment (Zizic 1995), blinding of outcome assessors (Fary 2011; Nelson 2013; Zizic 1995), selective reporting (all six studies) and high risk for incomplete outcome data (Zizic 1995). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 6 | 434 | Mean Difference (IV, Random, 95% CI) | 15.10 [9.08, 21.13] |
| 2 Physical function Show forest plot | 3 | 197 | Mean Difference (IV, Random, 95% CI) | 4.55 [‐2.23, 11.32] |
| 3 Quality of life Show forest plot | 2 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| 4 Number of patients experiencing any adverse event Show forest plot | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.72, 1.92] |
| 5 Number of patients who withdrew because of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

