Drenaje linfático manual para el linfedema posterior al tratamiento del cáncer de mama
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT Parallel groups design; participants in control group given the option to cross over at three months N = (A/R) 42/44 TYPE OF LYMPHEDEMA: Arm LE DEFINITION FOR INCLUSION: 200 mL and/or 2 cm excess arm volume but < 30% Excess volume Note: Publication reports: "participants with severe lymphedema, defined as a difference in arm volume exceeding 30%, were not included but were offered DLT, including compression bandaging. However, if they declined to receive this more extensive treatment, they were allowed to participate in the study." PARTICIPANTS RECRUITED FROM: An outpatient lymphedema clinic in an oncology department COUNTRY: Denmark | |
| Participants | INCLUSION CRITERIA: Limb volume < 30% swelling (mild to moderate). One or more symptoms of LE (numbness, tightness, stiffness, pain, aching, heaviness or other kinds of discomfort). Post surgery at least 4 m. EXCLUSION CRITERIA: Evidence of recurrence. Bilateral breast cancer. LE treatment in the previous 3 m. Severe LE (arm volume > 30%). | |
| Interventions | INTERVENTION n = 20 MLD + custom‐made sleeve‐and‐glove garment providing 32‐40 mmHg compression (Jobst‐Elvarex, compression class 2, Beiersdorf, Sweden), education in lymphedema exercise and skin care Plus 8 Vodder sessions over 2 w about 1 h/session (ts = 8) plus education in self‐massage. CONTROL n = 22 CDT alone: Custom‐made sleeve‐and‐glove providing 32‐40 mmHg compression (Jobst‐Elvarex, compression class 2, Beiersdorf, Sweden), education in lymphedema exercises and skin care. Note: The compression therapy in both groups is reported in two stages: "For the first couple of treatment weeks, our follow‐up used decreasing sizes of Jobst compression garments to reduce the edema. Then measurements were taken for a custom‐made compression garment. In general, the garments were replaced every 2‐6 months to maintain the proper amount of compression." | |
| Outcomes |
FOLLOW‐UP TIMES: Follow‐up:1, 3, 6, 9, 12 m from baseline, so first follow‐up was 2 w post‐tx REPORTED FINDINGS: LE volume (water displacement and circumference) Volumetry: 3 months: No statistically significant between‐groups difference: 60% (95% CI: 43% to 78%) reduction in standard therapy group; 48% (95% CI: 32% to 65%) in standard therapy plus MLD. 12 months: both groups combined showed a 66% reduction from baseline P =< 0.001. QoL (EORTC QLQ‐C30 Cancer Questionnaire): results not reported Subjective Sensations: Shoulder mobility: "Both groups obtained a significant reduction in limb volume, a decrease in discomfort and an increased joint mobility during treatment." | |
| Notes | DROPOUTS AND WITHDRAWALS: 2 patients were dropped – one had a recurrence and one was less than 4 months post‐treatment. ADVERSE EVENTS: None reported, unable to determine if assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Study design’ section, “The patients were randomly assigned to receive standard therapy or…” Also, the subtitle of the article is “A randomized study”. However, there are no details about the method of randomization. (According to Chapter 8 of the CH, a judgment of unclear is used for the following: “Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’… A simple statement such as ‘we randomly allocated’ or ‘using a randomized design’ is often insufficient to be confident that the allocation sequence was genuinely randomized…If there is doubt, then the adequacy of sequence generation should be considered to be unclear.”) |
| Allocation concealment (selection bias) | Unclear risk | There is not enough information to determine the adequacy of concealment. |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding of participants or personnel. Unclear, for outcome assessor: The ‘Discussion’ section (2nd paragraph) states the following: “One person (LA), an experienced and certified lymphotherapist…carried out all treatments in the present study.” However, the article does not report whether LA also did the outcomes assessments. |
| Incomplete outcome data (attrition bias) | Low risk | Adequate: Results (1st paragraph): “Forty‐four patients were randomized, but 2 patients, one in each treatment group, were subsequently found not to be eligible (one was found to have lymphedema caused by a local recurrence and one was randomized less than 4 months after surgery).” However, these 2 ineligible patients are unlikely to contribute to incomplete outcome data. Section 8.12.1 of The Cochrane Handbook has the following guidance about this: “ Some exclusions of participants may be justifiable, in which case they need not be considered as leading to missing outcome data (Fergusson 2002). For example, participants who are randomized but are subsequently found not to have been eligible for the trial may be excluded, as long as the discovery of ineligibility could not have been affected by the randomized intervention, and preferably on the basis of decisions made blinded to assignment. ” Besides these 2 ineligible patients, there was one other dropout before the 3 month cross‐over point (‘Results’ 1st paragraph): “This allowed data to be obtained on 42 patients at 1 month, on 41 patients at 3 months…” In the section ‘Statistical methods’: “The effect of treatment was analyzed by intention to treat.” Because the publication does not state how imputations were done for any missing data, it is not clear if this was truly intention‐to‐treat. However, whether or not a true intention‐to‐treat analysis was used is largely not relevant because there was only 1 dropout (other than the 2 exclusions) prior to the cross‐over at 3 months. |
| Selective reporting (reporting bias) | Unclear risk | Unclear – “The women also completed the EORTC QLQ‐C30 questionnaire for breast cancer, but these data are not reported in the present study.” Because these data were collected but not reported, this raises the question of selective reporting. |
| Adherence with Treatment Sessions and Home Program | Unclear risk | Treatment visits: Unclear (not reported) Exercises: Adequate: ‘Results’ (last paragraph): “There was no difference between groups in the compliance of the patients concerning use of compression sleeves or performance of arm exercise. The analysis showed that the effect of treatment on lymphedema was significantly related to the use of compression sleeves in both groups (P < 0.001). This effect was constant over time.” Because there were no differences between the two groups in the use of compression sleeves or exercises, this criterion was rated as ‘adequate’ (i.e., a low risk of bias) |
| Methods | Parallel group design, quasi‐randomized N = (A/R) 24/28 Arm LE TYPE OF LYMPHEDEMA: Arm. LE DEFINITION FOR INCLUSION: >10% arm volume difference between abnormal and contralateral arm. PATIENTS RECRUITED FROM: Lymphedema Unit, University Hospital, Lund. COUNTRY: Sweden. | |
| Participants | INCLUSION CRITERIA: No history of LE before breast cancer surgery. EXCLUSION CRITERIA: Previous contralateral breast disease or intercurrent disease affecting the swollen arm. Difficulties in participating in the study such as dementia. Complete resolution LE after compression sleeve treatment. | |
| Interventions | INTERVENTION: W 1 and 2: Standard compression sleeve in daytime. W 3 and 4: MLD + compression sleeve (Vodder technique 45 m/d for 2 w) (ts = 10). CONTROL: W 1 and 2: Standard compression sleeve in daytime. W3 and 4: Pneumatic pump 2 h/d at 40‐60 mmHg. | |
| Outcomes |
FOLLOW‐UP TIMES: Immediate after W2 and Immediate after W4. REPORTED FINDINGS: Volume outcomes: Part I Compression sleeve only – both groups had 7% reduction, or 49 ml in LE. P = 0.05. Part II – MLD group had 15% reduction, or 75 mL. P < 0.001. SPC group had 7% reduction, or 28 mL. P = 0.003 Shoulder mobility : "In test 2 [after two weeks of compression sleeve], there was reduced arm mobility compared to the unaffected contralateral arm in the total group. Treatment with MLD or SPC did not change arm mobility from test 2 to test 3." Isometric muscle strength : "Mean + SD for the total group in test 2 for shoulder flexion on the affected side was 7.5 + 1.8 kg, for abduction 7.0 + 1.7 kp, for adduction 5.8 + 1.6 kp, for gripping force 36.7 + 13.2 kp/cm2. No significant changes over time were seen for any of these in the two groups at test 3." Sensations: "During part I, a significant decrease in feeling of tension (P=0.004) and heaviness (P=0.01) in the arm was found in the total group. In Part II, only the MLD group showed a further decrease of tension (P=0.01) and heaviness (P=0.008). In a separate analysis, the data were stratified to exclude patients who had scored 100 (no discomfort) on the scales in test 2. The results revealed the significance to be greater but still only for MLD as regards to tension and heaviness. There was no significant difference between the two groups in Part II." Part II – MLD group showed additional decrease in tension ( P = 0.01) and additional decrease in heaviness (P = 0.008) | |
| Notes | DROPOUTS AND WITHDRAWALS: 4 participants were excluded from study completion. (1 resolved completely after W1 and 2 compression sleeve; 2 had breast cancer recurrence; 1 had erysipelas, 1 could not adhere to measurement protocol). ADVERSE EVENTS: None reported. Contact with author indicated there were no other adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Clinical population’ section states: “After written and informed consent, the patients were randomly allocated to either MLD or SPC therapy for two weeks (Part II).” Per author: Shuffling cards. |
| Allocation concealment (selection bias) | Low risk | Per author: Sequentially numbered, opaque, sealed envelopes were done ahead of enrolment and the next in sequence opened when a person enrolled. |
| Blinding (performance bias and detection bias) | High risk | Given the design, the participants could not be blinded. Per author: Outcomes assessor was not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Adequate: ‘Clinical population’ section: “Any patients who after Part I did not fulfil the criteria of lymphedema (21) were excluded from Part II. After written and oral consent, the patients were randomly allocated to either MLD or SPC therapy for two weeks (Part II)…Two patients in each group were dropped during Part II; two because of recurrent breast cancer, and one because of erysipelas during the period of treatment and one who was unable to participate in repeated measuring. Demographics of the remaining 24 women are shown in Table 1.” Because the number of dropouts are small, evenly balanced across groups, and unlikely to be related to treatment assignment or treatment outcome, there is low risk of bias for incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Adequate: No evidence of selective outcome reporting, as all relevant outcomes (e.g., limb volume measurement, shoulder mobility, muscle strength, subjective assessment) are reported. Although limb volume measurement was only measured using the water displacement method, this method has high accuracy and reliability and it is unlikely that different results would be obtained by measuring the participants’ arm circumferences. |
| Adherence with Treatment Sessions and Home Program | Low risk | Per author: Attendance was not a problem. Participants attended almost all the sessions. |
| Methods | Parallel groups design, quasi‐randomized N = (A/R) 38/40 TYPE OF LYMPHEDEMA: Arm. LE DEFINITION FOR INCLUSION: LE Def: >10% excess arm volume difference between affected and contralateral arm. PATIENTS RECRUITED FROM: Department of Surgery, University Hospital, Lund. COUNTRY: Sweden. | |
| Participants | INCLUSION CRITERIA: No history of LE before breast cancer surgery. Undergone axillary node dissection. EXCLUSION CRITERIA: Previous contralateral breast disease or intercurrent disease affecting the swollen arm. Difficulties in participating in the study such as dementia. Complete resolution LE after elastic compression sleeve treatment. | |
| Interventions | INTERVENTION: W 1 and 2: CB alone. W3: CB + MLD: Vodder technique, 45 m/d for 5 d (ts = 5). CONTROL: W1‐3: CB alone with low‐stretch compression bandaging. Bandage changed every 2nd day. | |
| Outcomes |
FOLLOW‐UP TIME: Immediate after W2; Immediate after W3 REPORTED FINDINGS: Volumetry: At test 2 [after only compression bandaging for 2 weeks] there was a188 ml (26%) (P =< 0.001) in both groups compared to baseline At test 3 [after MLD or no MLD} There was a 20ml (4%) P =< 0.8) further reduction in the CB group There was a 47 ml (11%) (P =< 0.001) in the CB+MLD Subjective sensations: "There were no differences in mean score between the two groups in test 1 [baseline]. From test 1 to test 3, [with‐in group analysis] a decreased feeling of pain (P=0.03) heaviness and tensions (both P<0.001) was found in the CB+MLD group. Decreased pain P=.03 heaviness/tension P=<.001 both) in CB+MLD group only. In the CB group, [within‐groups analysis] the feeling of heaviness (P=0.006) and tension (P<0.001) was decreased. There were no significant differences between the two groups at test 3." | |
| Notes | DROPOUTS AND WITHDRAWALS: Two participants were dropped from the study. One for numbness/weakness during bandaging; one unable to participate in serial measurements ADVERSE EVENTS: None reported. Communication with author indicated there were no other adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate (high risk of bias): ‘Clinical population’ section: “After written and oral information and approval by the patients, they were allocated to either CB treatment alone (CB group) or to CB in combination with MLD (CB + MLD group). The series was determined so that the patients were consecutively numbered and the patients with even numbers were included in the CB group and those with odd numbers in the CB + MLD group.” ‘Discussion’ section: “The patients in this study were allocated consecutively (i.e., not randomly but alternatively) to the two treatment groups when they were referred to the Lymphedema Unit. The patients were referred from many different clinics and the severity or the incoming order sequence was not influenced by any referring doctor.” The randomization appeared to create roughly comparable groups: ‘Clinical population’ section: “Other characteristics of which there was no difference between the groups are presented in Table 1.” |
| Allocation concealment (selection bias) | High risk | Alternate assignment, according to Cochrane Handbook, is not adequately concealed. |
| Blinding (performance bias and detection bias) | High risk | Participants were not able to be blinded due to different treatments Per author: Outcomes assessor was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Adequate: ‘Clinical population’ section: “In this prospective study, 40 consecutive women [were included for Part I]…After written and oral information and approval by the patients, they were allocated to either CB treatment alone (CB) or to CB in combination with MLD (CB + MLD group)…After 2 weeks (Part I) MLD was added to the CB treatment in 17 of the patients for 5 days for another week (Part II), whereas the other 18 patients continued with CB alone…Two patients in the CB group were dropped during Part I; one because of feelings of numbness and weakness in the arm during bandaging and one who was unable to participate in serial measurements. [These two patients were assumedly not randomized, because the randomization occurred at the end of Part I.] The mean +/‐ SD (range) age of the remaining 38 women was 64 +/‐12 (37‐83) years in the CB group (n=18) and 58 +/‐12 (41‐80) years in the CB + MLD group (n=20).” The tables in the Results section indicated that there were 18 participants in CB group and 20 in CB + MLD group. Thus, there is a small discrepancy in the numbers of participants included in Part II: it is not clear from the text copied above whether there were 18 and 20 analyzed in the two groups or whether there were 18 and 17 (for CB and CB+MLD respectively). It was also not clear how many patients were randomized and the reasons for the dropouts. However, even under the worst case scenario, if all 38 patients who completed Part I (i.e., the 40 patients who started minus the 2 patients who dropped out during Part I) were randomized and 35 were included in the analysis (i.e., 18 + 17), these 3 dropouts out of 38 randomized would still be a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Adequate: No evidence of selective outcome reporting, as the most important and expected outcomes were reported (i.e., limb volume measurement, subjective assessment). Although limb volume measurement was only measured using the water displacement method, this method has high accuracy and reliability and it is unlikely that different results would be obtained by measuring the participants’ arm circumferences. |
| Adherence with Treatment Sessions and Home Program | Low risk | Per author: Attendance was not a problem. Participants attended almost all the sessions. Exercises: NA – The study did not use an exercise intervention |
| Methods | RCT, Parallel groups design N = (A/R) 45/50 TYPE OF LYMPHEDEMA: Arm. LE DEFINITION FOR INCLUSION: LE Def: 150 mL excess arm volume. PATIENTS RECRUITED FROM: Rehabilitation Center at the Cross Center Institute. COUNTRY: Canada. | |
| Participants | INCLUSION CRITERIA: Unilateral breast surgery including an axillary node dissection following BC diagnosis. No LE treatment within last 6 m. No one was excluded who was wearing a compression sleeve for maintenance. However, to control for any potential treatment effect from the sleeve, a minimum 4‐m wait period was observed. EXCLUSION CRITERIA: Metastatic or local recurrence. Undergoing radiotherapy or chemotherapy, infection in affected limb. Hypertension, heart disease, renal insufficiency, and venous thrombosis. | |
| Interventions | INTERVENTION: MLD + compression bandaging: MLD Vodder 45 m 5d/w for 4 w (ts = 20) bandages changed daily. CONTROL: Compression bandaging: for 4 w Short stretch bandages with gradient pressure, gauze to fingers and hand, foam1/2 cm wrapped around arm and hand. Worn continuously until next tx. Bandages changed daily. | |
| Outcomes |
| |
| Notes | DROPOUTS AND WITHDRAWALS: Five participants did not complete the study. One developed a skin reaction; one withdrew due to elbow discomfort; one left for a family illness and two quit due to dissatisfaction with treatment response. ADVERSE EVENTS: See above. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: ‘Methods and Materials: Settings and Participants’ section: “A physical therapist was responsible for screening subjects for eligibility and the referring physician was contacted for approval. Subjects were randomized to one of the two treatment groups by use of a computer‐generated code.” The baseline characteristics of the patients, reported in Table 1, shows no significant difference between groups for all variables, suggesting the randomization created two comparable groups. |
| Allocation concealment (selection bias) | Low risk | Adequate: “The allocation sequence was concealed from research personnel involved in screening, scheduling and enrolling participants.” |
| Blinding (performance bias and detection bias) | Low risk | Adequate: “Two independent assessors (IA), blinded to the subjects’ treatment assignment, administered the outcome measurements. The independent assessors were qualified physical therapists familiar with, and trained in, the measurement procedure.” |
| Incomplete outcome data (attrition bias) | Low risk | Adequate: ‘Results’ section (2nd paragraph): “A total of 45 subjects completed the study. Two subjects withdrew as a result of adverse events. One subject in the MLD/CB group withdrew after she developed a skin reaction to the bandaging. One subject in the CB group withdrew in the second week of treatment due to discomfort in the elbow region from the constant CB. Three other subjects in the CB group withdrew from the study: one due to illness of a family member and two as a result of dissatisfaction with treatment response. One subject in the MLD/CB group, though completing the study, was excluded from analysis for the water displacement volumetry as an error was found in the recording of the arm volume. Figure 2 presents the flow of participants through each stage of the study.” |
| Selective reporting (reporting bias) | Low risk | Adequate: No evidence of selective outcome reporting. “Both water displacement volumetry and measurement of circumference were used to assess lymphedema volume…” In addition, the last paragraph of the Discussion section explicitly states that range of motion and subjective outcomes were not assessed as part of this study. |
| Adherence with Treatment Sessions and Home Program | Low risk | Adequate: Per author: Treatment sessions were well attended. There was no home program. |
| Methods | RCT, Parallel groups design N = (A/R) 27/28 TYPE OF LYMPHEDEMA: Arm. LE DEFINITION FOR INCLUSION: > 20% excess arm volume. PATIENTS RECRUITED FROM: New referrals to lymphedema clinic, Worthington Hospital. COUNTRY: England. | |
| Participants | INCLUSION CRITERIA: Breast cancer treatment including surgery to axilla, radiotherapy to breast and axilla. No previous LE tx except support hosiery. No active disease. EXCLUSION CRITERIA: None stated. | |
| Interventions | INTERVENTION: MLD + CB: LeDuc standardized protocol, 90 m/d for 2 w (ts = 10) CONTROL: SLD + CB: 30 m/d for 2 w | |
| Outcomes | Arm volume AS % reduction (circumference measured by tape measure). FOLLOW‐UP TIMES: No follow‐up post 2 week treatment. REPORTED FINDINGS: Limb volume (per cent change), circumference by tape measure. 33.8% reduction MLD ; 22% SLD | |
| Notes | DROPOUTS AND WITHDRAWALS: One patient refused to return for week two of treatment. ADVERSE EVENTS: None reported, unable to determine if assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: ‘Consent and randomization’ section: “They would be allocated at random by a computer program to one of two treatments…Twenty‐eight women consented to inclusion in the study. Randomisation performed using a computer‐generated code, and trial data were managed by a non‐clinical researcher attached to Worthing Nursing Development Unit” |
| Allocation concealment (selection bias) | Unclear risk | Unclear: From the description above, it looks like allocation concealment may have been used (i.e., “trial data were managed by a non‐clinical researcher”), but it is not certain. The 1st paragraph of the ‘Results’ states the following: “Characteristics of the two groups are listed in Table 3. No significant difference was found for any characteristic between the two groups.” Thus, the randomization likely created two comparable groups. |
| Blinding (performance bias and detection bias) | High risk | Inadequate: “All treatments for all patients were carried out by the same lymphoedema specialist nurse (LS), who had received MLD training…To maintain reliability, in this study all [outcome] measurements for all patients were carried out by the same lymphoedema specialist nurse (LS).” |
| Incomplete outcome data (attrition bias) | Low risk | Adequate: ‘Results’ 2nd paragraph: “One participant in the SLD group failed to complete the full treatment course; she attended for treatment for five days, but was unwilling to return to the hospital for the remainder of the intensive treatment period. No data from this patient were included in the analyses presented below.” The analysis presented included 27 of the 28 patients randomized, so it is assumed that only this one patient was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Adequate: ‘Abstract’: “The sole outcome measure was percentage change in excess limb volume (PCEV) following treatment.” ‘Patients and methods: Outcome measures’: “A recent review of the accuracy of assessment methods for lymphoedematous limbs concluded that this ‘tape measure’ method of measurement is accurate and reliable when used by a single operator using a good technique (Stanton et al. 2000).” Thus, because the limb volume was the sole outcome, and also because the ‘tape measure’ method is an accurate way to assess limb volume (and would likely give the same results as the water displacement method), there is a low risk of selective outcome reporting. |
| Adherence with Treatment Sessions and Home Program | Low risk | Treatment visits: Unclear: The publication only described the adherence with the treatment course for the one loss to follow‐up (described above under ‘Incomplete outcome data’), and there is no mention of adherence of the other patients. Exercises: Unclear: ‘Discussion’ 2nd to last paragraph: “Thirdly, the behavior of study participants with regard to arm exercises was not monitored in this study; we do not know if all participants carried out the exercises as prescribed, and so can reach no conclusions regarding the contribution made by exercise to reduction in PCEV. It would be advisable to include some monitoring procedure, such as a patient self‐completed diary in any future work.” |
| Methods | RCT Cross‐over design N = (A/R) 29/31 TYPE OF LYMPHEDEMA: Arm and trunk. LE DEFINITION FOR INCLUSION: > 10% excess volume. PATIENTS RECRUITED FROM: Lymphedema clinic at a large cancer hospital. COUNTRY: United Kingdom. | |
| Participants | INCLUSION CRITERIA; Unilateral breast cancer‐related LE for more than 3 months, two limb volume measurements > 10% excess volume, > 1 year post cancer treatment. Clinically detectable trunk swelling. EXCLUSION CRITERIA: Using diuretics/other edema‐influencing drugs. Active cancer. | |
| Interventions | INTERVENTION: MLD + elastic sleeve (n = 15) MLD: Vodder sessions, standardized protocol, Vodder technique, for 3 weeks at 45 m/d for 5 d (t = 15 s), 6‐week washout, then 3 weeks of SLD. CONTROL: SLD + elastic sleeve (n = 16) for 3 weeks at 20 m/d, kept diaries of compliance. 6‐week washout, then 3 weeks of MLD. Per author: 'Fitted elastic sleeves' were standard sleeves, not custom made. | |
| Outcomes |
FOLLOW‐UP TIMES: Immediate after W3, W9, W12. REPORTED FINDINGS: Within groups: MLD reduction of 71 mL P = 0.013; SLD reduction of 30 mL P = 0.08 Trunk volume (MLD only) .23 mm reduction P = 0.04 Deltoid Skin Thickness (MLD only) 15mm reduction P = 0.03 QoL (MLD only) 7.2 EMCT P = 0.006, dyspnea ‐4.6 P = 0.04, sleep disturbance ‐9.2 P = 0.03 | |
| Notes | DROPOUTS AND WITHDRAWALS: Two participants were excluded from study completion. One developed a herpes infection and the other developed a chest infection ADVERSE EFFECTS: None reported. Communication with author indicated there were no other adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate: Per author: The central research office for the hospital randomized according to what I think was a random number table. |
| Allocation concealment (selection bias) | Low risk | Adequate: Per author: " I had to telephone a central research office for the hospital and they assigned according to their method. Randomization could not be altered." |
| Blinding (performance bias and detection bias) | High risk | Inadequate: ‘Assessments and outcome measures’: The principal researcher (A.F.W.) undertook all the measurements and did not provide any of the MLD treatments… ‘Discussion’: “Although the researcher did not provide the MLD treatments or take part in the randomization process, she was aware, at each measurement point, of what treatments had been provided and, thus, may have unintentionally biased the data. Subjects were also aware of which treatment intervention they were receiving at each point during the trial.” Subjective outcomes (e.g., quality of life) were also measured in this trial, and because the patients, who were not blinded, assessed these outcomes, there was also no blinding for the subjective outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Adequate: As described in the first paragraph of the ‘Results’ section, there were only 2 dropouts out of 31 participants randomized, and both dropouts occurred after the cross‐over, so there were no dropouts for the analysis used in this review. |
| Selective reporting (reporting bias) | Low risk | Adequate: ‘Discussion’: “The study used a variety of outcome measures. The method of measuring and calculating limb volume in this study has been shown to be reliable, particularly when used in a consistent manner by the same operator (Stanton et al., 2000).” All expected outcomes were reported, including arm volume and subjective outcomes. Arm volume was measured in several ways, including the ‘tape measure’ method, which is reliable and would likely give same results as other methods of measurement. |
| Adherence with Treatment Sessions and Home Program | Low risk | Treatment visits: Per author: "The sessions were really well attended given that there were 15 in total. I think very few women were unable to attend‐ apart from the 2 who had to drop out." Exercises: NA: No reporting of an exercise intervention in this trial. However, “The SLD was taught by the researcher and therapists and performed by subjects for 20 min each day during the SLD period…Their technique was monitored weekly during the study and each participant kept a diary recording the areas covered and time taken each day for SLD.” Data set of diary data shows high adherence with SLD. |
BC = breast cancer
CB = compression bandaging
CDT = complex decongestive therapy
CI = confidence interval
DLT = decongestive lymphatic therapy
LE = lymphedema
MLD = manual lymphatic drainage
QoL = quality of life
RCT = randomized controlled trial
SD = standard deviation
SLD = simple lymphatic drainage (self‐massage)
SPC = sequential pneumatic compression
d = day or days
w = week or weeks
m = month or months
min = minutes
ts = total sessions
tx = treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Evaluates compression therapy not MLD | |
| Applies electric not manual lymph drainage | |
| MLD not given as an standard intervention per communication with author | |
| MLD not given as an standard intervention per communication with author | |
| Prevention trial | |
| Trial altered the remedial exercises between groups not just the MLD treatment | |
| Review | |
| Gives MLD to both groups in order to evaluate compression therapy | |
| MLD not the massage technique used per communication with the author | |
| MLD given to both groups | |
| Self‐massage not MLD used as intervention | |
| No control group | |
| Cohort study | |
| MLD appears not to be the physiotherapy technique used but author could not be located. | |
| No control group | |
| Review | |
| Used elastic bandages for compression therapy instead of non elastic bandages | |
| MLD given to both groups | |
| MLD given to both groups | |
| Prevention trial | |
| Applies electric not manual lymph drainage | |
| Allocation method not report and author could not be contacted | |
| Prevention trial |
MLD = manual lymphatic drainage
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Manual lymphatic drainage therapy in patients with breast cancer related lymphoedema |
| Methods | Randomized, parallel group design |
| Participants | "A randomized, controlled clinical trial in 58 women with post‐mastectomy lymphoedema. The therapy will be administered daily for four weeks and the patient’s condition will be assessed one, three and six months after treatment." |
| Interventions | "The control group includes 29 patients with standard treatment (skin care, exercise and compression measures, bandages for |
| Outcomes | "The primary outcome parameter is volume reduction of the affected arm after treatment, expressed as a percentage. Secondary outcome parameters include: duration of lymphoedema reduction and improvement of the concomitant symptomatology (degree of pain, sensation of swelling and functional limitation in the affected extremity, subjective feeling of being physically less attractive and less feminine, difficulty looking at oneself naked and dissatisfaction with the corporal image)." |
| Starting date | Unknown |
| Contact information | ClinicalTrials (NCT): NCT01152099 |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymphedema Volume (Excess volume remaining in limb after treatment) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
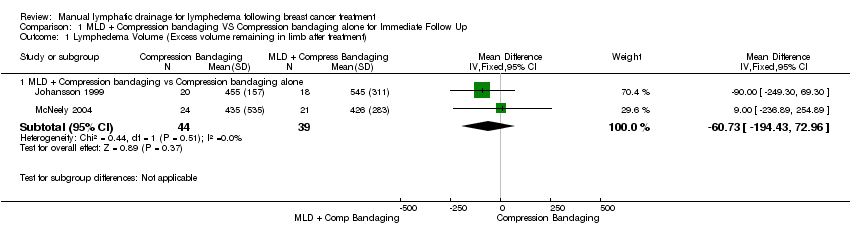
| Analysis 1.1  Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 1 Lymphedema Volume (Excess volume remaining in limb after treatment). | ||||
| 1.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐60.73 [‐194.43, 72.96] |
| 2 Volume reduction in mL Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
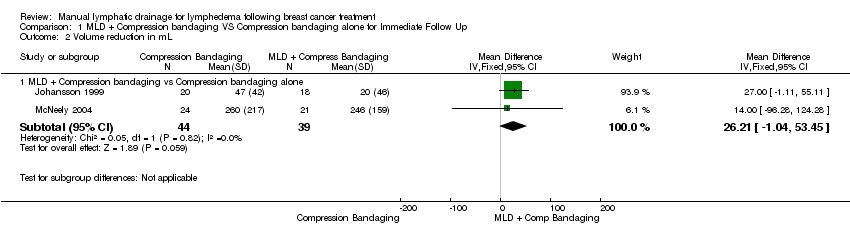
| Analysis 1.2  Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 2 Volume reduction in mL. | ||||
| 2.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 26.21 [‐1.04, 53.45] |
| 3 Per cent change Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 3 Per cent change. | ||||
| 3.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 7.11 [1.75, 12.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymphedema volume (excess volume remaining in limb after treatment) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 1 Lymphedema volume (excess volume remaining in limb after treatment). | ||||
| 1.1 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐230.0 [‐450.84, ‐9.16] |
| 1.2 MLD + Compression sleeve vs Intermittent Sequential Pneumatic Pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 122.0 [‐57.59, 301.59] |
| 2 Volume reduction in mL Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 2 Volume reduction in mL. | ||||
| 2.1 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐90.54, 110.54] |
| 2.2 MLD + Compression sleeve vs Intermittent sequential pneumatic pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 47.0 [15.25, 78.75] |
| 3 Per cent change Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 3 Per cent change. | ||||
| 3.1 MLD + Compression bandaging vs SLD + Compression bandaging | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [‐2.47, 26.07] |
| 3.2 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐14.11, 9.31] |
| 3.3 MLD + Compression sleeve vs. Intermittent Sequential Pneumatic Pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.75, 16.75] |
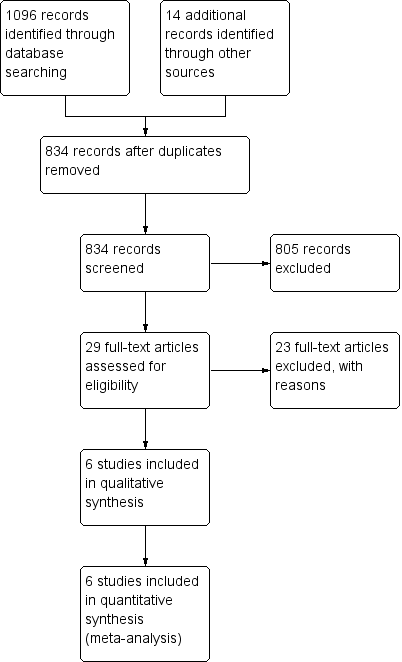
Study flow diagram for review.
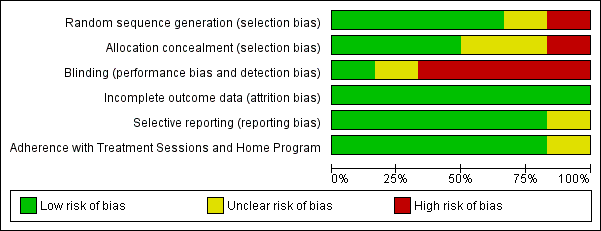
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, outcome: 1.1 Lymphedema Volume (Excess volume remaining in limb after treatment).

Forest plot of comparison: 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, outcome: 1.2 Volume reduction in mL.

Forest plot of comparison: 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, outcome: 1.3 Per cent change.

Forest plot of comparison: 2 MLD + Compression therapy vs Other treatment + Compression therapy, outcome: 2.1 Lymphedema volume (excess volume remaining in limb after treatment).

Forest plot of comparison: 2 MLD + Compression therapy vs Other treatment + Compression therapy, outcome: 2.2 Volume reduction in mL.

Forest plot of comparison: 2 MLD + Compression therapy vs Other treatment + Compression therapy, outcome: 2.3 Per cent change.
Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 1 Lymphedema Volume (Excess volume remaining in limb after treatment).
Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 2 Volume reduction in mL.

Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 3 Per cent change.

Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 1 Lymphedema volume (excess volume remaining in limb after treatment).

Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 2 Volume reduction in mL.

Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 3 Per cent change.
| Volumetric Outcome | Definition / Explanation | Formula | Other terms for this outcome in the included studies |
| Lymphedema Volume | The excess volume in the limb. The volume is measured in milliliters (mL). It is called ‘lymphedema volume’ because it is the amount of the limb volume that is attributed to lymphedema. Lymphedema volume is NOT the total volume of the limb. To calculate the lymphedema volume, you have to compare the affected limb to the unaffected limb by subtracting. In this review, we are interested in the lymphedema volume (or excess volume) that remains in the limb after treatment. | Post‐treatment total volume of the affected arm minus post‐treatment total volume of the unaffected arm. | Lymphedema volume has also been called absolute lymphedema volume, post‐intervention volume (McNeely 2004), and excess limb volume (Williams 2002) |
| Volume Reduction | An estimate of how much the limb has been reduced (in ml) presumably from the treatment. | Lymphedema volume at baseline minus the lymphedema volume after treatment. OR Excess volume before treatment minus the excess volume after treatment. | Volume reduction has also been called the mean lymphedema volume reduction (Johansson 1998; Johansson 1999) and mean change lymphedema volume (McNeely 2004) |
| Per cent Reduction | The decrease in excess volume relative to the amount of excess volume at baseline. Both the lymphedema volume and the volume reduction are considered absolute values not relative values. However, when absolute values are used, a person with a large excess limb volume might get a 2% reduction, but the amount can look large because the beginning volume was large. By contrast, a person with a small beginning volume, can get a 30% reduction, and it can look small in absolute terms. Thus, it is valuable to have a third way to think about lymphedema outcomes, and that is to look at the per cent change because that is a relative value. | Difference Test A – Difference Test B ____________________________________ x 100 Difference Test A Where difference is the affected arm volume minus the unaffected arm volume (McNeely 2004) Another way to think of per cent reduction is this formula: Excess volume at baseline – Excess volume post‐treatment ______________________________________________x100 Excess volume at baseline | Per cent Reduction has also been called the percentage lymphedema reduction (Johansson 1998; Johansson 1999), per cent change, per cent reduction in lymphedema volume (McNeely 2004), percentage change in excess limb volume (Sitzia 2002) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymphedema Volume (Excess volume remaining in limb after treatment) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐60.73 [‐194.43, 72.96] |
| 2 Volume reduction in mL Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 26.21 [‐1.04, 53.45] |
| 3 Per cent change Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 7.11 [1.75, 12.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymphedema volume (excess volume remaining in limb after treatment) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐230.0 [‐450.84, ‐9.16] |
| 1.2 MLD + Compression sleeve vs Intermittent Sequential Pneumatic Pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 122.0 [‐57.59, 301.59] |
| 2 Volume reduction in mL Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐90.54, 110.54] |
| 2.2 MLD + Compression sleeve vs Intermittent sequential pneumatic pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 47.0 [15.25, 78.75] |
| 3 Per cent change Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 MLD + Compression bandaging vs SLD + Compression bandaging | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [‐2.47, 26.07] |
| 3.2 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐14.11, 9.31] |
| 3.3 MLD + Compression sleeve vs. Intermittent Sequential Pneumatic Pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.75, 16.75] |

