Drenaje linfático manual para el linfedema posterior al tratamiento del cáncer de mama
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003475.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 mayo 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer de mama
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
-
Draft the protocol: KJ, LB, DH, JE
-
Study selection: KJ, RW, DH, JE
-
Extract data from studies: TB, LB, DH, EM, MM, JE
-
Enter data into RevMan: EM, JE
-
Perform ratings of MLD treatments KJ, CT, MM, DK
-
Carry out the analysis: EM, JE
-
Interpret the analysis:TB, LB, DH, EM, MM, JE, KJ, AW, RW
-
Draft the final review: TB, LB, EM, JE, MM, KJ, CT, AW, RW
-
Disagreement resolution: JE
-
Update the review: This is the first version.
Sources of support
Internal sources
-
None, Other.
External sources
-
NCCAM‐NIH USA, USA.
Eric Manheimer was partially funded by grant number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM) of the US National Institutes of Health. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the US National Institutes of Health.
-
NCCAM‐NIH USA, USA.
Jeanette Ezzo was partially funded by grant R24 AT001293 from NCCAM (National Center for Complementary and Alternative Medicine) of the US National Institutes of Health.
Declarations of interest
None known.
Acknowledgements
We would like to express our appreciation to the Cochrane Breast Cancer Group editorial base and to Dr. John Sitzia, who provided additional information on his trial included in this review. We would also like to express appreciation to the Cochrane Complementary Medicine Field for providing funding for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 May 21 | Manual lymphatic drainage for lymphedema following breast cancer treatment | Review | Jeanette Ezzo, Eric Manheimer, Margaret L McNeely, Doris M Howell, Robert Weiss, Karin I Johansson, Ting Bao, Linda Bily, Catherine M Tuppo, Anne F Williams, Didem Karadibak | |
| 2002 Jan 21 | Complete decongestive therapy for lymphedema following breast cancer treatment | Protocol | Doris M Howell, Jeanette Ezzo, Linda Bily, Karin Johansson | |
Differences between protocol and review
In our protocol, we proposed to examine the trials pertaining to CDT and to MLD. However, since that protocol was written, another Cochrane review (Preston 2004) has examined CDT. Therefore, our focus is on MLD.
Also, when we wrote the protocol, some assessment methods such as tissue dielectric constant and bioimpedance were nonexistent or in their infancy. We have opted to include them as acceptable means of assessing LE in order to reflect the state of the art practice; however, it should be noted that even though we permitted these assessments, none of the included trials used them.
Finally, there are outcomes which appear in this review that do not appear in the protocol. Again, this is because since the time of the protocol submission, outcomes in this area have continued to evolve. We have elected to include these additional outcomes for the sake of comprehensiveness not only for this review, but so that anyone doing research in this field, could see in a thumbnail sketch using this review ALL the outcomes that had been used in prior trials. The category under secondary outcomes entitled "other" captures these outcomes that have not been prespecified in the protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans;
PICO
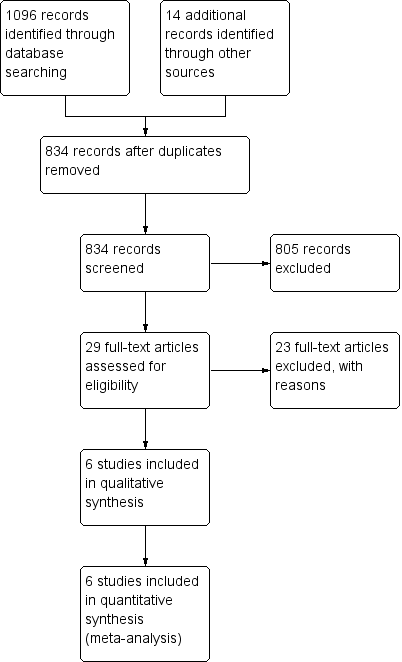
Study flow diagram for review.
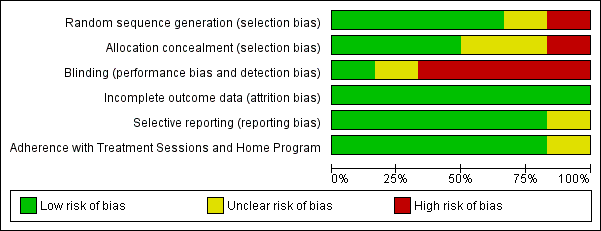
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, outcome: 1.1 Lymphedema Volume (Excess volume remaining in limb after treatment).

Forest plot of comparison: 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, outcome: 1.2 Volume reduction in mL.

Forest plot of comparison: 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, outcome: 1.3 Per cent change.

Forest plot of comparison: 2 MLD + Compression therapy vs Other treatment + Compression therapy, outcome: 2.1 Lymphedema volume (excess volume remaining in limb after treatment).

Forest plot of comparison: 2 MLD + Compression therapy vs Other treatment + Compression therapy, outcome: 2.2 Volume reduction in mL.

Forest plot of comparison: 2 MLD + Compression therapy vs Other treatment + Compression therapy, outcome: 2.3 Per cent change.
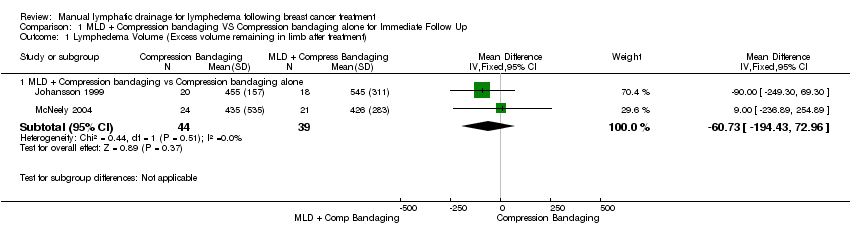
Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 1 Lymphedema Volume (Excess volume remaining in limb after treatment).
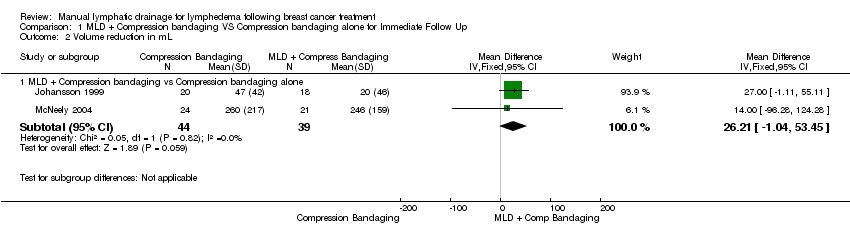
Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 2 Volume reduction in mL.

Comparison 1 MLD + Compression bandaging VS Compression bandaging alone for Immediate Follow Up, Outcome 3 Per cent change.

Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 1 Lymphedema volume (excess volume remaining in limb after treatment).

Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 2 Volume reduction in mL.

Comparison 2 MLD + Compression therapy vs Other treatment + Compression therapy, Outcome 3 Per cent change.
| Volumetric Outcome | Definition / Explanation | Formula | Other terms for this outcome in the included studies |
| Lymphedema Volume | The excess volume in the limb. The volume is measured in milliliters (mL). It is called ‘lymphedema volume’ because it is the amount of the limb volume that is attributed to lymphedema. Lymphedema volume is NOT the total volume of the limb. To calculate the lymphedema volume, you have to compare the affected limb to the unaffected limb by subtracting. In this review, we are interested in the lymphedema volume (or excess volume) that remains in the limb after treatment. | Post‐treatment total volume of the affected arm minus post‐treatment total volume of the unaffected arm. | Lymphedema volume has also been called absolute lymphedema volume, post‐intervention volume (McNeely 2004), and excess limb volume (Williams 2002) |
| Volume Reduction | An estimate of how much the limb has been reduced (in ml) presumably from the treatment. | Lymphedema volume at baseline minus the lymphedema volume after treatment. OR Excess volume before treatment minus the excess volume after treatment. | Volume reduction has also been called the mean lymphedema volume reduction (Johansson 1998; Johansson 1999) and mean change lymphedema volume (McNeely 2004) |
| Per cent Reduction | The decrease in excess volume relative to the amount of excess volume at baseline. Both the lymphedema volume and the volume reduction are considered absolute values not relative values. However, when absolute values are used, a person with a large excess limb volume might get a 2% reduction, but the amount can look large because the beginning volume was large. By contrast, a person with a small beginning volume, can get a 30% reduction, and it can look small in absolute terms. Thus, it is valuable to have a third way to think about lymphedema outcomes, and that is to look at the per cent change because that is a relative value. | Difference Test A – Difference Test B ____________________________________ x 100 Difference Test A Where difference is the affected arm volume minus the unaffected arm volume (McNeely 2004) Another way to think of per cent reduction is this formula: Excess volume at baseline – Excess volume post‐treatment ______________________________________________x100 Excess volume at baseline | Per cent Reduction has also been called the percentage lymphedema reduction (Johansson 1998; Johansson 1999), per cent change, per cent reduction in lymphedema volume (McNeely 2004), percentage change in excess limb volume (Sitzia 2002) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymphedema Volume (Excess volume remaining in limb after treatment) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐60.73 [‐194.43, 72.96] |
| 2 Volume reduction in mL Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 26.21 [‐1.04, 53.45] |
| 3 Per cent change Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 MLD + Compression bandaging vs Compression bandaging alone | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | 7.11 [1.75, 12.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymphedema volume (excess volume remaining in limb after treatment) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐230.0 [‐450.84, ‐9.16] |
| 1.2 MLD + Compression sleeve vs Intermittent Sequential Pneumatic Pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 122.0 [‐57.59, 301.59] |
| 2 Volume reduction in mL Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐90.54, 110.54] |
| 2.2 MLD + Compression sleeve vs Intermittent sequential pneumatic pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 47.0 [15.25, 78.75] |
| 3 Per cent change Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 MLD + Compression bandaging vs SLD + Compression bandaging | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.80 [‐2.47, 26.07] |
| 3.2 MLD + Compression sleeve vs SLD + Compression sleeve | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐14.11, 9.31] |
| 3.3 MLD + Compression sleeve vs. Intermittent Sequential Pneumatic Pump + Compression sleeve | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐0.75, 16.75] |

