Workplace interventions for smoking cessation
Abstract
Background
The workplace has potential as a setting through which large groups of people can be reached to encourage smoking cessation.
Objectives
1. To categorize workplace interventions for smoking cessation tested in controlled studies and to determine the extent to which they help workers to stop smoking.
2. To collect and evaluate data on costs and cost effectiveness associated with workplace interventions.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register (July 2013), MEDLINE (1966 ‐ July 2013), EMBASE (1985 ‐ June 2013), and PsycINFO (to June 2013), amongst others. We searched abstracts from international conferences on tobacco and the bibliographies of identified studies and reviews for additional references.
Selection criteria
We selected interventions conducted in the workplace to promote smoking cessation. We included only randomized and quasi‐randomized controlled trials allocating individuals, workplaces, or companies to intervention or control conditions.
Data collection and analysis
One author extracted information relating to the characteristics and content of all kinds of interventions, participants, outcomes and methods of the studies, and a second author checked them. For this update we have conducted meta‐analyses of the main interventions, using the generic inverse variance method to generate odds ratios and 95% confidence intervals.
Main results
We include 57 studies (61 comparisons) in this updated review. We found 31 studies of workplace interventions aimed at individual workers, covering group therapy, individual counselling, self‐help materials, nicotine replacement therapy, and social support, and 30 studies testing interventions applied to the workplace as a whole, i.e. environmental cues, incentives, and comprehensive programmes. The trials were generally of moderate to high quality, with results that were consistent with those found in other settings. Group therapy programmes (odds ratio (OR) for cessation 1.71, 95% confidence interval (CI) 1.05 to 2.80; eight trials, 1309 participants), individual counselling (OR 1.96, 95% CI 1.51 to 2.54; eight trials, 3516 participants), pharmacotherapies (OR 1.98, 95% CI 1.26 to 3.11; five trials, 1092 participants), and multiple intervention programmes aimed mainly or solely at smoking cessation (OR 1.55, 95% CI 1.13 to 2.13; six trials, 5018 participants) all increased cessation rates in comparison to no treatment or minimal intervention controls. Self‐help materials were less effective (OR 1.16, 95% CI 0.74 to 1.82; six trials, 1906 participants), and two relapse prevention programmes (484 participants) did not help to sustain long‐term abstinence. Incentives did not appear to improve the odds of quitting, apart from one study which found a sustained positive benefit. There was a lack of evidence that comprehensive programmes targeting multiple risk factors reduced the prevalence of smoking.
Authors' conclusions
1. We found strong evidence that some interventions directed towards individual smokers increase the likelihood of quitting smoking. These include individual and group counselling, pharmacological treatment to overcome nicotine addiction, and multiple interventions targeting smoking cessation as the primary or only outcome. All these interventions show similar effects whether offered in the workplace or elsewhere. Self‐help interventions and social support are less effective. Although people taking up these interventions are more likely to stop, the absolute numbers who quit are low.
2. We failed to detect an effect of comprehensive programmes targeting multiple risk factors in reducing the prevalence of smoking, although this finding was not based on meta‐analysed data.
3. There was limited evidence that participation in programmes can be increased by competitions and incentives organized by the employer, although one trial demonstrated a sustained effect of financial rewards for attending a smoking cessation course and for long‐term quitting. Further research is needed to establish which components of this trial contributed to the improvement in success rates.
4. Further research would be valuable in low‐income and developing countries, where high rates of smoking prevail and smoke‐free legislation is not widely accepted or enforced.
PICO
Plain language summary
Is the workplace an effective setting for helping people to stop smoking
Background
The workplace appears to be a useful setting for helping people to stop smoking. Large groups of smokers are available who can easily be reached and helped, using proven methods. It is also in the employers’ interests to improve the health of their workforce. Recent changes introducing anti‐smoking laws in many developed countries may have eased the pressure to demonstrate the value of work‐based programmes. The situation in developing countries still requires that such methods be tested and proved in those communities. We reviewed the evidence about workplace programmes to help employees stop smoking, and any information about their costs and benefits.
Study characteristics
For this updated review (first published in 2003), we searched for randomized and quasi‐randomized controlled trials, comparing the success rates of those in a work‐based stop‐smoking programme with those not involved in a work‐based stop‐smoking programme. The comparison could be between people within a single worksite, or between one or more worksites randomized to a stop‐smoking programme or to no programme (cluster‐randomized). The study had to include adults (over 18), and could be in any language and reported in any format, published or not. It had to report the numbers stopping smoking for at least six months.
Results
We searched for studies in July 2013, and identified ten new trials that fitted our criteria, making a total for this update of 61 comparisons across 57 included studies. We grouped them into two broad categories: those aimed at helping individual smokers, and those that targeted the workplace environment as a whole. The first group includes such methods as individual or group counselling, self help, nicotine replacement therapy (NRT) and other medications, help from workmates or other staff, and helping quitters to stay smoke‐free. The second group includes environmental cues (posters, reminders), financial or material incentives, and comprehensive smoking cessation or health promotion programmes. The review found that programmes based on group behaviour therapy (eight trials; 1309 participants), on individual counselling (eight trials; 3516 participants), on medications (five trials; 1092 participants), and on several interventions combined (six trials; 5018 participants) helped people to stop smoking. The chances of stopping smoking using these methods are about the same in the workplace as they are in other settings. This review found that the following do not help people to stop smoking when delivered in the workplace: self‐help methods, support from friends, family and workmates, relapse prevention programmes, environmental cues, or comprehensive programmes aimed at changing several high‐risk behaviours. Results were mixed for incentives, with one high‐quality trial finding a clear benefit for incentives while the remaining five did not.
Quality of the evidence
Earlier studies tended to be less well‐conducted and reported than recent ones. Fewer than one in five studies randomized their study population by an acceptable method. Two‐thirds of the studies checked the accuracy of those who said they had quit by testing their breath, blood or urine. The results were generally in line with findings from other reviews of those ways of quitting in any setting. The 'Summary of findings' table shows that the trials were generally rated as being of moderate to high quality, further confirming the strength of our findings. Future research might examine what features of the large incentives trial made it more successful than other trials in that group. It would also be helpful to have more trials from developing and low‐income countries, where smoking rates remain high and anti‐smoking laws are not widely enforced.
Authors' conclusions
Summary of findings
| Smoking cessation interventions for the workplace | ||||||
| Patient or population: Employees who smoke | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Smoking cessation interventions | |||||
| Group therapy | 51 per 1000 | 84 per 1000 | OR 1.71 | 1309 | ⊕⊕⊕⊝ | |
| Individual counselling | 61 per 1000 | 113 per 1000 | OR 1.96 | 3516 | ⊕⊕⊕⊝ | |
| Self‐help interventions | 45 per 1000 | 52 per 1000 | OR 1.16 | 1906 | ⊕⊕⊕⊕ | |
| Pharmacological interventions | 77 per 1000 | 142 per 1000 | OR 1.98 | 1092 | ⊕⊕⊕⊕ | Limiting to NRT only (4 studies) reduced OR to 1.81 (1.07 to 3.08). |
| Incentives | 73 per 1000 | 113 per 1000 | OR 1.60 | 1928 | ⊕⊕⊕⊝ | |
| Multiple interventions | 63 per 1000 | 95 per 1000 | OR 1.55 | 5018 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 11 +outlier (Glasgow 1994); removing this study reduced the OR to 1.62 (0.98 to 2.66). | ||||||
Background
Most adults spend about a third of their day in a workplace environment. The workplace is therefore a setting through which large groups of smokers can potentially be reached by health promotion (Gruman 1993).
Description of the condition
It is estimated that in 2007 approximately 60% of Americans were covered by health insurance plans sponsored through their or their spouse's workplace (DeNaves‐Walt 2008). Milani 2009 estimates that in the US nearly 60% of after‐tax profits are spent on corporate health benefits, with 80% of such expenses incurred by 10% to 20% of the workforce. US employers have a vested interest in improving and maintaining the health and performance of their workforce.
National smoke‐free policies introduced in the UK in 2006‐7 are complemented by government guidelines on how to implement the policies and how best to assist employees with smoking cessation during this process (NICE 2007). Recommendations include summaries of interventions of proven efficacy, and encouragement for employers conducting smoking cessation programmes within working hours. The cost‐effectiveness of such implementation has also been tested (NICE 2006).
Description of the intervention
Work‐based smoking cessation programmes are similar to those deployed in other institutions (e.g. hospitals, colleges, schools) or in the community. They include behavioural or pharmacological interventions, or a combination of both, and comprehensive health promotion initiatives, targeting individuals or cohorts of workers. Proven cessation strategies can be conducted opportunistically on workplace premises and in working hours.
How the intervention might work
There are several advantages to the traditional workplace as a setting for smoking cessation:
-
It provides access to a large number of people who make up a relatively stable population.
-
It has the potential for higher participation rates than non‐workplace environments.
-
It may encourage sustained peer‐group support and positive peer pressure.
-
It provides a particular opportunity to target young men, who traditionally have low general practitioner consultation rates and are thus less likely to benefit from opportunistic health promotion activity in primary care.
-
Occupational health staff may be on hand to give professional support.
-
The employee generally is not required to travel to the programme or to dedicate their own personal time to it.
However, all of these assumptions are based on a model of the workplace that is rapidly changing. Recent research, included in this update, highlights the increasingly transient and volatile nature of the modern workforce, with younger employees changing jobs and locations more frequently than earlier generations (see for example Mishra 2010; Okechukwu 2009).
Why it is important to do this review
During the life of this review (first published ten years ago), smoke‐free legislation has fundamentally altered working conditions in many developed countries. The attention of the tobacco control community is now shifting to areas where anti‐tobacco initiatives are still in their infancy. We include new studies in this update which test such approaches in India, Malaysia and China, with varying levels of success.
Objectives
The specific objectives of this updated review were:
1. To categorize workplace interventions for smoking cessation tested in controlled studies, and to determine the extent to which they help workers to stop smoking.
2. To collect and evaluate data on costs and cost effectiveness associated with workplace interventions.
Methods
Criteria for considering studies for this review
Types of studies
We include randomized and quasi‐randomized controlled trials, allocating individuals, workplaces or companies to intervention or control conditions.
Types of participants
Adults over 18 years of age, in employment, who smoked.
Types of interventions
In earlier versions of this review, we divided the interventions into those aimed at helping individual smokers to quit, and those aimed at the workplace as a whole. Although we still include both types of interventions, previous versions of this review also included bans and restrictions as interventions aimed at the workplace as a whole. These are now covered by a companion review (Callinan 2010), and we no longer include them here.
We include any workplace interventions aimed at smoking cessation. These include programmes designed to exclusively target smoking behaviour or to target multiple lifestyle risk behaviours. The studies aim mainly to assess the effects of cessation programmes for individual workers who smoke. They test a range of interventions, including individual and group counselling, self‐help materials, pharmacological therapy, social and environmental support, incentives, and comprehensive programmes. They are usually aimed at individuals who seek help rather than at the workforce as a whole. We also include studies that test relapse prevention interventions in association with worksite‐based smoking cessation programmes.
Types of outcome measures
The main outcome was employee smoking behaviour (cessation rates for programmes and workplace prevalence data), for a minimum of six months. We preferred prolonged or continuous abstinence to point prevalence, and biochemically verified abstinence to self‐report, but did not exclude studies on the basis of their chosen outcomes. We excluded studies with less than six months follow‐up.
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Review Group Specialized Register, which includes studies identified by systematic electronic searches of multiple databases, handsearching of specialist journals and conference proceedings, and grey literature (i.e. conference proceedings and unpublished reports not normally covered by most electronic indexing systems). At the time of the search, the Register included the results of searches of the Cochrane Central Register of Controlled Trials (CENTRAL), issue 12, 2012; MEDLINE (via OVID) to update 20130607; EMBASE (via OVID) to week 201324; and PsycINFO (via OVID) to update 20130610. See the Tobacco Addiction Group module in The Cochrane Library for full search strategies and a list of other resources searched. See Appendix 1 for the search terms used to search the Register for this review. These searches include terms to identify controlled trials. We also conducted ad hoc searches of MEDLINE, EMBASE and PsycINFO using topic‐related keywords without design limits. These searches were updated on 2nd July 2013. Search terms included 'worksite*' or 'workplace*' as keyword or free‐text terms, and 'work' or 'occupational health' as keywords. We list the search strategies in Appendix 2. The results of these searches were cross‐checked against references in the identified papers and previous reviews and meta‐analyses.
We also contacted authors of studies for additional information where necessary.
Data collection and analysis
Selection of studies
One author (KC) prescreened reports for relevance, i.e. studies that might be included, or for useful background, and obtained full‐text copies of candidate studies for inclusion. Both authors then independently assessed them against the inclusion criteria, resolving discrepancies by discussion. We noted reasons for the non‐inclusion of studies, and incorporated those into the Characteristics of excluded studies table.
Data extraction and management
One author (KC) extracted data and a second author (TL) checked them. Where possible, we extracted data on quit rates using the number randomized as the denominator, making the assumption that those lost to follow‐up (or not reported) continued to smoke.
Assessment of risk of bias in included studies
For this update, we have evaluated all the included studies (past and new) for risks of bias, using the Cochrane 'Risk of bias' assessment tool (Cochrane Handbook, Chapter 8). We have assessed the randomization process (sequence generation and allocation concealment; selection bias), blinding (performance and detection bias), incomplete outcome reporting (attrition bias), and selective outcome reporting (reporting bias).
Dealing with missing data
We contacted authors where possible to fill gaps in the data. We counted participants lost to follow‐up or unaccounted for as continuing smokers, and where possible included them in the denominator on an intention‐to‐treat basis.
Data synthesis
For this update, we have pooled data for the main categories of intervention, using the generic inverse variance (GIV) method (derived from the log odds), to produce Mantel‐Haenszel odds ratios (ORs), using a fixed‐effect model with 95% confidence intervals (CIs). We have used the intraclass correlation coefficient reported by Martinson 1999 (ICC for percentage quit smoking in worksite) to obtain an adjusted estimate of the effect size for the studies that were cluster‐randomized. This represents a departure from the Cochrane Tobacco Addiction Group's preferred analysis method of risk ratios, in order to accommodate the ICC calculations where necessary.
We include a 'summary of findings Table for the main comparison' for the main results.
We also include a glossary of tobacco‐related terms (Appendix 3).
Results
Description of studies
We found 57 studies meeting the inclusion criteria. Ten of these are new for this update (Bergstrom 2008; Prochaska 2008; Milani 2009; Okechukwu 2009; Volpp 2009; Hishida 2010; Mayer 2010; Mishra 2010; Groeneveld 2011; Noor 2011). Detailed information about each is shown in the table Characteristics of included studies. We assigned the individual studies to one or more of the categories of intervention. Four studies (Omenn 1988; Windsor 1988; Gomel 1993a; Tanaka 2006) contribute to more than one category. For this update, we identified eight additional studies that did not meet the inclusion criteria, and we also now exclude four trials that were originally included (Kornitzer 1987; Jeffery 1988; Burling 1989; Burling 2000). This gives a total for this update of 105 excluded studies, brief details of which are listed in the table Characteristics of excluded studies. We also identified two ongoing studies (NCT01124110 2012; NTR8148 2012), and one Chinese study (Gao 2010) for which we currently have insufficient information to include it.
A number of studies evaluated interventions aimed at the individual, usually without any attempt to target or modify the workplace as a whole. The types of intervention were diverse, including intensive behavioural interventions, self‐help materials, pharmacological treatments, and social support.
Other studies included environmental support for not smoking, incentives for not smoking, and multicomponent programmes aimed primarily at smoking cessation, or at improving employees' general health, including their smoking status.
GROUP 1: INTERVENTIONS AIMED AT THE INDIVIDUAL TO PROMOTE SMOKING CESSATION
1. Intensive behavioural interventions: Group counselling
We found 10 randomized controlled trials that reported 6‐ to 24‐month quit rates for individuals receiving group‐based behavioural interventions (Glasgow 1984; Frank 1986; Klesges 1987; Omenn 1988; DePaul 1994; Razavi 1999; Schröter 2006; Gunes 2007; Mayer 2010; Mishra 2010). Some of these studies compared an intensive intervention, typically including group support meetings, with a less intensive intervention such as provision of self‐help materials, or with a waiting list control. Some compared variations of group programmes, or the additional impact of incentives.
Three studies evaluated ways to improve the results of group programmes:
-
Glasgow 1984 compared three versions of a controlled smoking programme in 36 employees: abrupt reduction; gradual reduction; or gradual reduction plus feedback on nicotine consumption with one pre‐ and two post‐tests. Smoking reduction was defined as an outcome for this study, targeting nicotine content (brand smoked), number of cigarettes smoked daily, and percentage of each cigarette smoked. Participants could choose either cessation or reduction as their desired outcome. The participation rate was not reported, though an 8% attrition rate was reported.
-
Frank 1986 assigned 63 participants to one of three treatments: four hypnotic sessions with a booster; two hypnotic sessions; or two hypnotic and two behavioural sessions with a booster. A follow‐up group of 15 later recruits received four hypnotic sessions and a booster session. The participation rate was not reported. The study lacked a no‐treatment control group.
-
Klesges 1987 tested the effect of competitions on cessation rates in 136 smokers from eight workplaces. The workplace was the unit of randomization (cluster‐randomized) but with individuals as the unit of analysis. The participation rate was not reported but was estimated at 28% across all eight participating workplaces. The drop‐out rate from treatment was 7% overall, with no difference across conditions.
DePaul 1994) compared three interventions; self‐help alone, self‐help with incentive payments for days abstinent, and intensive group support with incentive payments, cognitive behavioural strategies, and maintenance manuals.
Two group‐counselling trials (Razavi 1999; Mayer 2010) targeted recent ex‐smokers, to reinforce abstinence and reduce relapse rates. Three other trials (Klesges 1987; Omenn 1988: Schröter 2006) also included a relapse prevention component, alongside a cessation intervention.
-
Razavi 1999 randomized abstainers (98.6% of those eligible) who had completed a non‐randomized cessation programme, to test the efficacy of two relapse prevention programmes. Participants were assigned to a psychologist‐run support group (PG), or an ex‐smoker‐run support group (SG) or a 'no formal support' group (NG), and were assessed at 12 months. Participants in the PG and SG groups attended monthly meetings, where cessation support was given, and weight, blood pressure, pulse and concomitant medical problems were monitored. At the end of nine months, participants completed a Brief Symptoms Inventory and a Life Events Scale. All who were participants at three months were followed up until 12 months post‐treatment.
-
Mayer 2010 randomized successful quitters following a smoking cessation programme in a number of Belgian companies to either work‐based group counselling or to proactive phone counselling to minimize relapse rates. The cohorts were assessed after nine months for long‐term relapse‐free quit rates.
Omenn 1988 recruited smokers at a single workplace. Participants with a preference for a group format were randomized to one of two smoking cessation programmes (Multiple Component Programme or Relapse Prevention Programme) or to a 'self help only' condition (American Cancer Society Quitter's Guide). Those not interested in group support were randomized to a manual‐based version of the same Multiple Component Programme or Relapse Prevention Programme, or the same Guide. The participation rate was 11%. This study also contributes data to the self‐help group.
Schröter 2006 offered a relapse prevention programme in four German workplaces, compared with a standard behavioural programme. Nicotine replacement therapy was also provided if requested. Sessions rather than individuals were randomized, and the programme lasted eight weeks, with follow‐up at one year.
Gunes 2007, set in a Turkish textile factory, primarily measured movement through stages of change in two matched cohorts of male smokers. The intervention group received a three‐week behavioural programme based on the American Lung Association's Seven Steps to a Smoke‐Free Life. Smoking status was assessed at the six‐month follow‐up.
Mishra 2010 is a study set in four Indian call centres, each of which hosted a different level of intervention. The control site distributed self‐help materials to all employees on the hazards of tobacco, methods of quitting, and where to go for practical help. The first intervention site (which we use in this review) conducted health education sessions for all employees, followed by focus‐group discussions for tobacco users in groups of 7 to 10. The second intervention site replicated this but added one‐to‐one behavioural therapy, and the third intervention site added the offer of bupropion based on needs assessment. The researchers followed up all tobacco users in person or by phone to 12 months. It is notable that high staff turnover in this population meant that 52.4% of baseline tobacco users had changed jobs after 12 months.
2. Intensive behavioural interventions: Individual counselling
We found eight studies that investigated individual counselling, in most cases given by a physician (Cambien 1981; Li 1984; Gomel 1993a; Kadowaki 2000; Lang 2000), in Terazawa 2001 by trained nursing staff, in Groeneveld 2011 by either an occupational physician or an occupational nurse, and in Windsor 1988 by a 'health educator'.
Two years post‐intervention, Cambien 1981 followed up the first 1292 participants in a cluster‐randomized controlled trial, the Paris Cardiovascular Risk Factor Prevention Trial, conducted in 160 sections of a civil service administration. They measured the effects of physician advice, information leaflets and physical monitoring on diet, alcohol and cigarette consumption in young men (25 to 35 years of age). We focus here on the 610 participants in the smoking cessation components. The intervention participants received either three or four tailored counselling sessions, depending on whether their baseline assessment showed them to be at low or at high risk of coronary disease. The control group received only baseline and follow‐up assessments.
Li 1984 studied asbestos‐exposed male smokers undergoing screening in a mandated programme for naval shipyard workers. The workers were categorized as having normal or abnormal pulmonary status on the basis of a chest X‐ray and pulmonary function tests. They were then randomly assigned within pulmonary function test categories to receive either a simple warning or three to five minutes of behavioural cessation counselling from the physician who gave them the results of their pulmonary tests. The participation rate is reported as 84.6%. The study did not have a no‐treatment control group.
Windsor 1988 studied the incremental effectiveness of a skills training programme with social support enhancement and monetary incentives to a self‐help manual. A health educator provided training in cessation skills in a structured 20‐ to 30‐minute one‐to‐one session; one group received this training to supplement the self‐help manual, and another group received both interventions plus monetary incentives. This study also contributes data to the incentives group.
Gomel 1993a randomized 28 Sydney (Australia) ambulance stations to four intervention groups (without a no‐treatment control), in an attempt to reduce cardiovascular risk factors. The HRA (Health Risk Assessment) group received measurements and risk assessments, including body mass index, blood pressure, cholesterol, smoking status, percentage of body fat, and aerobic capacity. We focus here on the 128 smokers who participated. Those assessed as being at high risk were referred to their own family physician, but received no direct support from the intervention programme. The RFE (Risk Factor Education) group received a similar assessment, but were given standard advice, through written and video material. The BC (Behavioural Counselling) group, after the standard assessment, were offered up to six counselling sessions in risk reduction, together with a manual on behaviour change. The fourth group (BCI, Behavioural Counselling and Incentives) received the same programme as the BC group, together with an incentive scheme which gave individuals the chance to win AUD 40 for achieving risk reduction targets at three and six months, plus a prize of AUD 1000 for the station which achieved the highest percentage of successful participants at six‐month follow‐up. The participation rate was 88% (431 participants, including 128 smokers). This study also contributes data to the incentives group.
Kadowaki 2000 evaluated the effectiveness of a smoking cessation intervention in all male smokers in a Japanese radiator manufacturing factory. Participants in the intervention group received individual counselling by a doctor, and those who signed a Smoking Cessation Declaration underwent a five‐month intervention. Subjects in the control group received equivalent delayed intervention after four months. Randomization was by individual smoker.
Lang 2000 compared the effects of a workplace intervention by the occupational physician, offering simple advice on smoking cessation for five to ten minutes, with a more active strategy of advice including a quit date and extra support. For both strategies, the medical team was composed of a physician and whenever possible a nurse, who would reinforce the physician's advice. Both the randomization and the analysis were by workplace.
Terazawa 2001 randomized 228 smokers presenting for routine occupational health checks in a Japanese factory; 117 were allocated to the intervention condition, and 111 to the control. All participants completed a baseline questionnaire and had carbon monoxide (CO) and urinary metabolites measured to verify their level of smoking. Intervention group smokers also received a 15‐ to 20‐minute counselling session from a nurse trained in cessation methods, and those who were prepared to set a quit date received four follow‐up phone calls to support their quit attempt. Control subjects received the baseline screening and usual care. All participants were re‐assessed at six and twelve months follow‐up.
Groeneveld 2011 targeted individuals at elevated risk for cardiac illness in a cohort of Dutch male construction workers. The participants could choose to address either nutrition and physical activity or smoking cessation, and we focus for this review on the 115 intervention smokers and the 123 control smokers who attempted to quit smoking. The programme offered three face‐to‐face 45‐ to 60‐minute sessions of motivational interviewing (MI) counselling, and four 15‐ to 30‐minute phone calls with an occupational physician or nurse. All participants also received brochures on physical activity, healthy eating, smoking, and cardiovascular disease. The relevant outcome was self‐reported smoking cessation at six and 12 months.
3. Self‐help interventions
We found six studies that examined self‐help interventions (Omenn 1988; Sutton 1988a; Sutton 1988b; Sutton 1988c; Sutton 1988d; Hishida 2010). A variety of approaches were tested and included short videos (Sutton 1988a; Sutton 1988b; Sutton 1988c; Sutton 1988d), self‐help manuals (Omenn 1988), and information about genotyping (Hishida 2010).
The Omenn 1988 study is described in the Group Counselling section. We consider only the self‐help arms for this category.
Sutton (Sutton 1988a; Sutton 1988b; Sutton 1988c; Sutton 1988d), in a series of four randomized controlled studies in four UK companies, evaluated a minimal smoking intervention programme based on the use of motivational videotapes. In the videotape studies groups of smokers (n = 603) were randomly assigned to watch one of several different videotapes. They were followed up along with non‐participants (n = 1015) at three months and again at one year.
Hishida 2010 was set in a Tokyo bank, and allocated smokers to genotyping or to assessment only by alternate months of routine occupational health checks. All smokers received a booklet indicating that particular genotypes appeared to be at increased risk of smoking‐related cancers; those in the intervention cohort who agreed to be genotyped received their results three months later, without specific cessation advice. All participants were assessed at 12 months. The primary outcome was movement through the stages of change, but smoking status by allocated group and by genotype were also collected.
4. Pharmacological therapy
Five studies investigated pharmacological therapy in the workplace (Sutton 1987; Sutton 1988e; Kornitzer 1995; Rodriguez 2003; Noor 2011). Seven other included studies (Razavi 1999; Schröter 2006; Tanaka 2006; Sorensen 2007; Okechukwu 2009; Bergstrom 2008; Mayer 2010) also included NRT as part of their intervention, but not as the component being tested. Mishra 2010 added bupropion to its most intensive intervention arm, but only 10 of the 24 smokers who were offered bupropion took up the option.
Kornitzer 1995 evaluated the effects of adding nicotine gum to smokers already using the nicotine patch in a double‐blind placebo‐controlled randomized trial. The effect of the nicotine patch against placebo patch in both groups receiving placebo nicotine gum was also assessed.
Sutton 1987 evaluated the effectiveness of a brief treatment for smoking using nicotine chewing gum in a large retailing company in London, UK. The study was randomized with a two‐group pre‐test/post‐test design. In total 270 of 334 cigarette smokers who expressed interest were invited to take part in the programme, which consisted of two individual consultations two weeks apart and a prescription for 2 mg Nicorette gum with recommendations for its use. The remaining 64 smokers constituted a no‐intervention control group.
Sutton 1988e evaluated the effect of offering brief individual treatment based on nicotine chewing gum to a randomly chosen sample in one company (n = 161) still smoking at the three‐month follow up to a previous video intervention (Sutton 1988d). The treatment course was administered by occupational health nurses and consisted of four short consultations over a 12‐week period.
Rodriguez 2003 delivered a combined intervention of individual structured counselling with nicotine patches in an open (non‐blinded) randomized controlled trial conducted in three Spanish worksites. Intervention participants (115 people) were graded by Fagerstrom score and treated with appropriate levels of nicotine replacement therapy for up to 12 weeks. Progress, withdrawal symptoms and adverse events were monitored over the 12‐month trial period. Control group smokers (103 people) received brief, sporadic and unstructured advice, usually at their annual occupational health check.
Noor 2011, set in two Malaysian towns, offered a herbal compound (Viva QS) or placebo tablets to male smokers employed in 11 worksites and attending a mobile smoking cessation clinic. Participants were contacted by phone at two and four weeks, to assess progress and adverse events, and were given brief counselling. Outcomes were 7‐day point prevalence and continuous abstinence from weeks 4 to 24, verified by expired CO. All participants, whether claiming abstinence or not, were checked face‐to‐face at 24 weeks. Fifty‐four urine samples for cotinine were collected at week 24, but not analysed.
5. Social support for not smoking
Two studies evaluated social support as an increment to other cessation strategies (Malott 1984; Glasgow 1986). Social support, in this context, refers to the support of a 'significant other', for example a spouse, a workmate or a close friend.
Malott 1984 randomly assigned 24 smokers to controlled smoking or a controlled smoking plus partner support intervention. Both studies defined smoking reduction as one of their outcomes, targeting nicotine content (brand smoked), number of cigarettes smoked daily, and percentage of each cigarette smoked. Participants could choose either cessation or reduction as their desired outcome. The participation rate was not reported.
Glasgow 1986 recruited 29 smokers who were assigned to small groups and were then randomly allocated to a basic programme or basic programme plus social support. The participation rate was not reported.
GROUP 2: INTERVENTIONS AIMED AT THE WORKSITE AS A WHOLE
6. Environmental support for not smoking
We found three studies that reported environmental or institutional support programmes (Dawley 1991; Erfurt 1991; Hymowitz 1991). Tanaka 2006 also included environmental components, as one part of a complex intervention programme (see Comprehensive programmes for details).
Dawley 1991 evaluated a small study of workplace smoking control in two comparable oil refineries. One company was randomly assigned to an environmental programme of smoking control, discouragement, and cessation, while the other company received only a smoking cessation programme. Humorous anti‐smoking posters emphasizing the benefits of quitting smoking were distributed throughout the intervention workplace and were changed every two weeks. Three weeks after the initiation of the smoking discouragement programme at one refinery, a group smoking cessation programme was begun at both plants. The participation rate was not reported.
Erfurt 1991 compared the effects of four interventions: (1) wellness screening, (2) wellness screening plus health education, (3) 1 and 2, plus follow‐up counselling, and (4) 1, 2 and 3 plus peer support groups, buddy systems, health promotion classes, and plant‐wide activities.
Hymowitz 1991 evaluated the effect of an enriched environment on the impact of a group quit‐smoking programme in six workplaces. Two hundred and fifty‐two smokers participated in the group quit smoking programmes; 131 at the full programme sites (group plus physician counselling plus workplace health promotion) and 121 at the group‐only sites (group cessation programme). The participation rate was not reported.
7. Incentives
We found six studies of incentives with comparison groups and quit rates (Rand 1989; Windsor 1988; Glasgow 1993; Gomel 1993a; Hennrikus 2002; Volpp 2009). A number of other included studies (Klesges 1987; DePaul 1989; DePaul 1994; Sutton 1988a to Sutton 1988d; Tanaka 2006) used incentives as an aid to cessation or reduction, but not as the intervention being tested.
Rand 1989 examined the relative contribution of a contingent payment (up to USD 200) and workplace CO monitoring to the long‐term maintenance of smoking abstinence. Forty‐seven hospital employees who had abstained from smoking for five days were randomly assigned to one of three follow‐up groups: contingent payment and frequent monitoring (n = 17), non‐contingent payment with frequent monitoring (n = 16), or contingent payment with infrequent monitoring (n = 14).
Windsor 1988 studied the incremental effectiveness of skills training with social support enhancement and monetary incentives to a self‐help manual. The participants were randomized to four groups in a two‐by‐two factorial pre‐test/post‐test design. The monetary incentive was a USD 25 payment to the employee after six weeks of abstinence. An additional USD 25 incentive was awarded at the end of six months abstinence.
Glasgow 1993 evaluated the impact of a year‐long incentive‐based workplace cessation programme (the HIP program). Nineteen workplaces were randomized to incentive or no‐incentive conditions. Smokers were paid USD 10 each time they were confirmed abstinent by CO validation at monthly meetings over the year‐long programme. In addition, each month at each workplace abstinent smokers were also eligible to win a lottery prize (which ranged from USD 5 to USD 20) and grand prize lotteries during the final month of the programme. All identified smokers in the workplace were considered as participants for the study, whether or not they participated in the intervention. Analyses were conducted at both the workplace and individual level and using both self‐reported and biochemically validated cessation as endpoints. There was a participation rate of 23% in the incentive group.
Gomel 1993a, in a cluster‐randomized study of 28 Australian ambulance stations, included an incentives component in its four‐way comparison study to reduce cardiovascular risk factors. This trial is described above, under the individual counselling section.
The SUCCESS Project (Hennrikus 2002) compared three programme options (telephone counselling, group sessions, or a choice of either), each offered with and without incentives for recruitment and cessation. Four workplaces were assigned to each of the six options, and were surveyed at baseline, and again at 12 and 24 months. Incentive site smokers were paid for signing up to a programme (USD 10), for completing it (USD 20) and for 30 days abstinence (USD 20). Successful quitters were entered into a prize draw, to win up to USD 500. A sample of quitters at 24 months were also paid USD 25 if they supplied saliva for cotinine measurement.
Volpp 2009, set in a multinational company in the US, randomized smokers to information about local smoking cessation services versus the same information combined with stepped financial rewards for completing a smoking cessation course and for sustained cessation. Up to USD 750 were available to each intervention smoker for long‐term (12‐month) cessation biochemically confirmed. Participants were also rewarded for completing a smoking cessation course, for complying with assessments and for supplying confirmatory samples. The primary outcome was validated abstinence at 12 or 18 months (depending on earlier success). Assessments were conducted and rewards given some months after completion of the cessation programme.
8. Comprehensive programmes
We classify these trials into two groups:
(a) Programmes which used a combination of types of intervention to support the primary aim of the trial, i.e. to quit smoking.
(b) Programmes which used a mix of interventions to reduce a number of different high‐risk behaviours, including smoking.
a) Multiple intervention smoking cessation programmes
We found seven trials which have smoking cessation as their primary or only outcome, but use a combination of interventions to address it (DePaul 1987; Willemsen 1998; DePaul 1989; Sorensen 1993; Shimizu 1999; Tanaka 2006; Okechukwu 2009).
DePaul 1987 randomized workplaces to self‐help materials in conjunction with televised cessation programmes versus the same materials and programmes plus group or individual counselling at the workplace.
In DePaul 1989, the basic design was as for DePaul 1987, but enhanced with monthly booster sessions, and with successful quitters and up to five of their family and co‐workers entered in a lottery at the end of the intervention period and at one‐year follow‐up.
Sorensen 1993 examined the effectiveness of a multi‐component smoking cessation programme. The three‐month intervention included consultation for employers on the adoption of a non‐smoking policy (90‐minute consultation), training for nonsmokers (a one‐hour class) to provide assistance to smokers attempting to quit, and cessation classes for smokers (three one‐hour behavioural cessation classes). Eight workplaces were randomized to two groups (intervention/no intervention) with one and two post‐tests. Although the workplace was the unit of randomization, analyses were conducted using the individual as the unit of analysis. The participation rate was reported as 12% of smokers and 3.7% of nonsmokers. The attrition rate was not reported. No data were available for individual smoking cessation.
A Dutch study (Willemsen 1998) compared a comprehensive smoking cessation intervention of self‐help manuals, group courses, a mass media campaign, and smoking policies with a minimal intervention of self‐help manuals only. Eight workplaces (four matched pairs) participated in the study. The 'bogus pipeline' procedure was used to improve the validity of self reports of smoking status. This means that subjects are informed that their self reports can be biochemically verified, although the test is not necessarily performed. Respondents who claimed they were nonsmokers at the 14‐month follow‐up were asked to co‐operate with biochemical validation of their smoking status.
A Japanese study (Shimizu 1999), available only as an abstract, examined the effectiveness of a multicomponent smoking cessation programme (intensive education, group lectures and individual counselling) compared to a waiting‐list control group of smokers. The participation rate was not reported.
The HIPOP‐OHP Study (Tanaka 2006) was a Japanese multicomponent intervention to reduce cardiovascular risk factors, including smoking, in six intervention sites matched to six control sites. The study concentrated mostly on blue‐collar workers. The six‐week cessation programme was offered five times over 36 months, and included information brochures on stages of change, four counselling sessions and NRT if requested. It was integrated with an intra‐site publicity campaign (posters, newsletters, web site), designation of smoking areas, and an award for successful abstainers. Participants were assessed at 12, 24, and 36 months. The participation rate was 9% of smokers across the six sites.
Okechukwu 2009 targeted apprentices in the US building trades, with a four‐month multi‐pronged programme based on group counselling, supplemented by free NRT, self‐help materials and environmental cues (posters, support materials). The smoking cessation component was integrated with training in work‐related toxic hazards. The outcome was self‐reported abstinence at least six months from the end of the intervention.
b) Multiple‐outcome comprehensive workplace programmes
Thirteen studies evaluated multiple‐outcome workplace programmes, i.e. targeting several health indicators, including smoking, for risk reduction (Kornitzer 1980; Shi 1992; Glasgow 1995; Sorensen 1996; Sorensen 1998; Emmons 1999; Nilsson 2001; Campbell 2002; Sorensen 2002; Sorensen 2007; Bergstrom 2008; Prochaska 2008; Milani 2009).
In the Belgian Heart Disease Prevention Project, Kornitzer 1980 cluster‐randomized 30 paired Belgian factories to intervention or control conditions, with all male workers aged 40 to 59 eligible to take part. All intervention participants were screened for cardiovascular risk factors (blood pressure, serum cholesterol, weight, smoking and physical activity), and were given written advice to reduce their risks. The screening results were also passed on to participants' family and workplace doctors. The two deciles with the highest risk score were ranked as the high risk group, and additionally received six‐monthly physician advice and testing. At the environmental level, anti‐smoking posters were regularly displayed, and each intervention factory held a conference on the dangers of tobacco use. A five per cent sample of the intervention group were re‐assessed annually. In the 15 control factories a random 10% sample were fully assessed at baseline, and then followed throughout the trial. Within that group a 20% high‐risk group was identified and compared throughout with their intervention counterparts. The participation rate was 83.7% (n = 16,230).
The HealthWise Stepped Intervention Study (Shi 1992) allocated nine North Californian worksites belonging to Pacific Gas & Electric to four intervention levels. The seven sites allocated to levels 1 to 3 were randomly assigned, while the two smallest sites were allocated to Intervention level 4, in order to minimize the running costs of the trial. The trial lacked a no‐treatment control site. The interventions ranged incrementally from Health Risk Assessments (HRAs) at the start and finish of the trial with a bimonthly health newsletter at Level 1, through the addition of a Health Resource Centre and self‐care books at Level 2 sites, behavioural workshops and a social support team at Level 3, to an environmental smoking policy and a case management programme for the high‐risk group (the 15% with the highest overall risk score) at Level 4. Outcomes were measured by cross‐sectional HRAs at baseline and at two‐year follow‐up, and included smoking, drinking and speeding. The participation rate was 69% at baseline and 48% at follow‐up.
The 'Take Heart' study (Glasgow 1995) evaluated the short‐term effects of a low‐intensity heart disease risk reduction programme, targeting smoking, dietary intake and cholesterol. Twenty‐six workplaces with between 125 and 750 employees were randomly assigned to early or delayed intervention. Early intervention consisted of an 18‐month multi‐faceted programme that featured an employee steering committee and a menu approach to intervention activities tailored to each site.
The Working Well Trial (Sorensen 1996) used a randomized matched‐pair design, with the workplace as the unit of assignment and analysis in 108 workplaces, with an average of 316 workers per site. The intervention targeted individuals and the workplace environment, and included dietary habits (all four study centres) as well as smoking (three of the four centres). Each centre also addressed one additional risk factor; these included occupational exposure to carcinogens, exercise, cancer screening, and smokeless tobacco.
Nested within the Working Well Trial, and based at the Massachusets study centre, was the WellWorks Study (Sorensen 1998), a randomized matched‐pair trial in 24 workplaces. The two‐year intervention, aimed at changing dietary and smoking habits, integrated health promotion and health protection through joint worker‐management participation in programme planning and implementation, consultation on workplace changes, and educational programmes targeting health behaviour change, including smoking cessation. This study particularly addressed differences in behaviour change between white‐collar and blue‐collar workers.
Another study within the Working Well Trial was the Working Healthy Project (Emmons 1999). The Brown University study centre developed an extended programme within its 26 worksites (reduced eventually to 22), similar in aims and scope to the parent trial but including physical activity as a target objective, and following a cohort rather than assessment by cross‐sectional surveys. The control sites received a minimal self‐help programme of two smoking cessation courses and one each of nutrition and exercise, for those sites that wished to implement them.
A Swedish study (Nilsson 2001) reported the effects of a long‐term comprehensive programme of lifestyle interventions, including smoking cessation, to reduce risk factors for cardiovascular disease. This randomized controlled trial for at‐risk public sector employees also targeted body mass index, diastolic blood pressure, heart rate, low‐density lipoprotein and cholesterol. The intervention group received individual counselling as well as 16 annual group sessions, using lectures, discussions, videos and outdoor activities; the control group received standard oral and written advice about cardiovascular risk reduction at the beginning of the trial, and nothing subsequently. Smoking point prevalence was assessed at 12‐ and at 18‐months follow‐up.
The Health Works for Women trial (Campbell 2002) developed a two‐pronged approach to helping rural blue‐collar women workers to improve their diet and physical activity levels, and to stop smoking. The programme was a combination of tailored 'magazines' at baseline and at six months, personalized for the characteristics and preferences of each participant, and social support at work from volunteer 'natural helpers'. The smoking intervention was incompletely delivered, however, as no lay helpers were willing to be trained to deliver the personal support. The control group received a minimal intervention (one personalized magazine) at six months, with no offer of social support. Randomization was by worksite. The participation rate was 73% at baseline.
Based on the WellWorks Study, WellWorks‐2 (Sorensen 2002) was a block‐randomized controlled trial of 15 workplaces, all handling hazardous chemicals. The intervention and aims of the study were very similar to the original WellWorks Project, being primarily health promotion and protection, but follow‐up was only to six months. Like its parent project, WellWorks‐2 targeted differences between white‐ and blue‐collar workers, and concentrated on smoking and nutrition; an additional outcome of interest in this study was changes in perceived hazard exposure.
The Tools for Health study (Sorensen 2007) targeted American construction workers, on the basis that they represented a transient population who tended not to benefit from workplace occupational health provision. Six hundred and seventy‐four workers, contacted through their trade union, completed a baseline survey on their smoking and their consumption of fruit and vegetables. The intervention consisted of tailored phone‐based counselling (up to four calls over three months), mailed tailored feedback, six targeted mailings of educational materials, and NRT for those who requested it. At six months, 582 participants (including 188 smokers) completed the follow‐up survey, giving an attrition rate of 13.6%.
Another Swedish study (Bergstrom 2008) allocated four companies in the paper, steel and truck manufacturing industries to a 3½‐year comprehensive lifestyle programme to reduce risks of COPD, asthma and cardiovascular disease, and to target neck and back pain, alcohol, and absenteeism. The whole programme was offered three times over the course of the study. 'High‐risk' individuals were offered inpatient rehabilitation courses based on behavioural approaches, while those deemed to be at moderate risk were given smoking cessation information and the offer of NRT by medical staff at the occupational health service. A 'reference' company acted as the control. Evaluation became problematic, as individual companies often set up their own health promotion activities, including 'stop smoking' support groups.
Prochaska 2008 aimed to compare the effect of three approaches (motivational interviewing, an online transtheoretical model (Prochange Lifestyle Management Programs), and a health risk intervention (HRI)) on four risk factors (inactivity, BMI, stress and smoking) in a worksite sample. We focus here on the smoking interventions, and compare the HRI‐alone arm with the HRI plus motivational interviewing arm. The outcome was progression through the stages of change, but also reported six‐month quit rates, although without end‐of‐study denominators.
Milani 2009 was a six‐month comprehensive cardiovascular risk reduction programme in New Orleans, USA, targeting nutrition, smoking, and physical activity in employees and their spouses. The basis for inclusion was coverage by employer‐sponsored healthcare insurance. Smoking was addressed in the intervention site by referral to a group counselling programme for smoking cessation. Participants who achieved milestones were rewarded with extra vacation days and "other job‐related perks". Outcomes were assessed at one year, including any cost savings to the company.
Risk of bias in included studies
A summary of the 'Risk of bias' assessments is given in Figure 1.
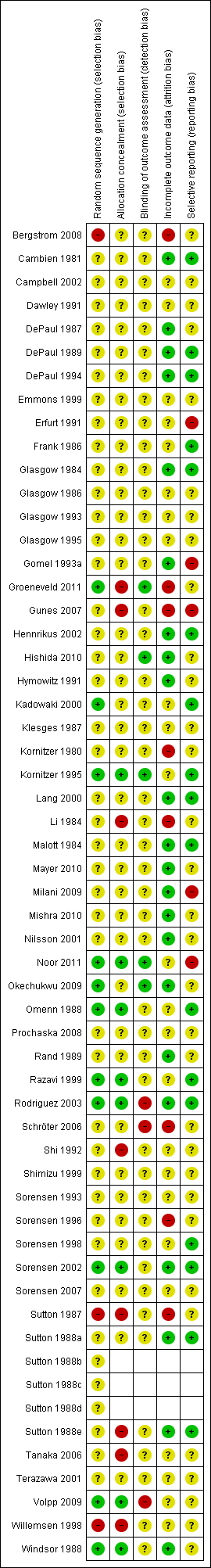
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Empty cells are for multiple studies within a single study report.
Some randomized studies aim to intervene with the workplace as a whole. They use a cluster‐randomized design, allocating entire workplaces to conditions. Such studies should be analysed at the level of the cluster rather than the individual. When workplaces are the unit of allocation, but results are presented for individual quitters, the assumption that outcomes are independent is violated, since people in the same site may be more like one another than expected by chance. If the analysis ignores the clustering, the confidence intervals are likely to be too narrow (Bland 1997). The effect is greater if there are a small number of large clusters. Cluster‐randomized studies with individual outcomes also present problems related to the choice of an appropriate denominator. The number of smokers who attend group meetings or use self‐help materials is considerably smaller than the total number of smokers in a workplace. In cluster‐randomized studies the denominator chosen for the analysis may be all smokers, smokers who express interest in treatment, or those who attend sessions. If the intervention involves individual cessation treatment, then trials focus on the outcome in the group of attenders. If the intervention includes other changes to the workplace environment, for example the introduction of restrictions on smoking, it is reasonable to assess the impact on the smoking workforce as a whole.
Eleven of the included studies (19%) reported randomization procedures in sufficient detail to be rated as adequate for their attempts to control selection bias. The majority of included studies (67%) either did not describe how randomization was performed or reported in insufficient detail to determine whether a satisfactory attempt to control selection bias had been made. Eight studies (14%) either failed to randomize appropriately or did not use a randomized trial design at all. In two studies (Sutton 1987; Sutton 1988e) a few control group subjects were allowed to move into the intervention group. One study (Li 1984) modified its randomization procedure partway through the study and was also obliged to reconstitute its experimental and control groups to accommodate physicians who did not comply with the protocol; Shi 1992 and Gomel 1993a allocated smaller or fewer worksites to the most expensive interventions in order to keep trial costs down. Gomel 1993a also reported some level of contamination between conditions as staff moved from station to station. Schröter 2006 compromised the distinction between the experimental and control group interventions, by applying 'rescue' relapse prevention techniques to members of the standard behavioural group when necessary. Prochaska 2008 reported six‐month quit rates, but without the relevant denominators. Okechukwu 2009 warned that there may have been contamination between intervention and control cohorts, since randomization was by the union training base but apprentices from different bases could sometimes have worked together on the same worksites. Hishida 2010 reported that unexpectedly high cessation rates in the control group may have been associated with concurrent health promotion legislation to prevent passive smoking in the workplace.
Two Japanese studies (Shimizu 1999; Terazawa 2001) are included on the basis of data derived from the abstracts alone.
The 'gold standard' outcome for smoking cessation studies is biochemical validation of self‐reported cessation (i.e. testing of saliva, blood, urine samples or exhaled breath for evidence of recent smoking). This generally results in lower rates of cessation, due not only to people misreporting their smoking, but also to relapsing, or refusing to provide samples for other reasons. Using validation may not change the relative effect of the intervention, since similar levels of misreporting are likely to be seen in the control condition as well, unless no intervention at all is provided to the control. Of the 55 studies in which intervention was provided to individuals, 41 (66%) used some form or combination of biochemical verification procedures for at least one follow‐up point. These included butt counts, carbon monoxide ((CO) in 47% of the included studies), salivary thiocyanate and urinary, blood or salivary cotinine.
In assessing the potential impact of workplace interventions it is important to know the proportion of smokers who can be recruited to different types of intervention, whilst recognizing that some barriers to recruitment to trials may not be relevant to real settings. In some of the studies included here the use of a workplace population would appear to have been largely a matter of convenience for ease of recruitment. These studies have typically not reported on the proportion of the smoking workforce who participated. Where studies have calculated the participation rate we have recorded this in the Characteristics of included studies table. The participation rates in the studies included here ranged from 9% to 88%.
GROUP 1: INTERVENTIONS AIMED AT THE INDIVIDUAL TO PROMOTE SMOKING CESSATION
1. Intensive behavioural interventions: GROUPS
Only two of this group of 10 trials described the method of randomization in sufficient detail to exclude the possibility of allocation bias: in Omenn 1988 allocation was based on randomized assignment lists, while Razavi 1999 used random numbers concealed by a label. Four studies (Klesges 1987; DePaul 1994; Mayer 2010; Mishra 2010) used cluster randomization, and Gunes 2007 assembled matched cohorts of intervention and control participants, based on shifts worked, i.e. quasi‐cluster‐randomized. All of the trials except Schröter 2006, and Gunes 2007 used biochemical validation of self‐reported smoking status. Two studies (Omenn 1988; DePaul 1994) used saliva cotinine, one study (Frank 1986) used saliva thiocyanate and one (Klesges 1987) used both saliva thiocyanate and expired air carbon monoxide. Two studies (Glasgow 1984; Mishra 2010) used expired air CO alone, and two studies (Razavi 1999; Mayer 2010) used a combination of expired CO and urinary cotinine.
2. Intensive behavioural interventions: INDIVIDUAL COUNSELLING
Only two of the eight studies in this group (Kadowaki 2000; Groeneveld 2011) adequately described the method of randomization. Three trials (Cambien 1981; Gomel 1993a; Lang 2000) used cluster randomization. Five studies used CO assessment for validation of self‐reported cessation, with Lang 2000 using partial validation, as several workplace physicians had no access to a carbon monoxide monitor. Gomel 1993a used serum cotinine to validate smoking status at all assessment points, and Windsor 1988 used salivary cotinine testing. Only Groeneveld 2011 relied upon self report, without any biochemical validation.
3. Self‐help interventions
Among this group of six studies, none described the method of randomization. All except Hishida 2010 validated their cessation rates, the Sutton studies with expired air CO, and Omenn 1988 with salivary cotinine.
4. Pharmacological therapy
Three studies described adequate randomization procedures, for a placebo‐controlled double‐blind trial (Kornitzer 1995; Noor 2011) and an open‐label randomized controlled trial (Rodriguez 2003). The remaining two studies allowed movement between the intervention and control groups (Sutton 1987; Sutton 1988e). All five studies validated self reports of cessation, using expired air CO to verify smoking status. Noor 2011 collected end‐of‐trial cotinine samples from all participants, whether abstinent or not, but failed to analyse the urine samples because of technical problems at the laboratory.
5. Social support for not smoking
Neither study (Malott 1984; Glasgow 1986) gave randomization details or participation rates. Self‐reported cessation was validated in both studies using expired air CO and quantity of cigarette butts. The Glasgow study also monitored saliva cotinine.
GROUP 2: INTERVENTIONS AIMED AT THE WORKPLACE AS A WHOLE:
6. Environmental support for not smoking
Three of the four studies in this group employed a clustered design, with Tanaka 2006 allocating sites non‐randomly to intervention or control in a matched‐pair design. Two of the cluster‐randomized trials (Dawley 1991; Hymowitz 1991) analysed by individual, while the third (Erfurt 1991) used the workplace as the unit of analysis. There was no biochemical validation of self‐reported cessation in Erfurt 1991 or Tanaka 2006. Dawley 1991 reported validation by urinary cotinine and Hymowitz 1991 by expired air CO.
7. Incentives
Details of Gomel 1993a are reported under the Individual Counselling heading.
Windsor 1988 described randomization using a computer‐generated assignment system in numbered envelopes. Glasgow 1993 was described as cluster‐randomized with both the workplace and the individual used as the units of analysis. Rand 1989 gave no details of randomization. The SUCCESS Project (Hennrikus 2002) was described as a 3x2 factorial study, with workplaces randomly assigned to the six treatment options, but stratified by gender and education level. No details of randomization were offered. Volpp 2009 randomized in permuted blocks of four, stratified by site, income and level of smoking (more or less than 40 per day). Assignments were concealed in this trial until after allocation, but not thereafter. All six studies reported biochemical validation, using saliva thiocyanate (Windsor 1988; Hennrikus 2002; Volpp 2009), serum cotinine (Gomel 1993a), urine if on NRT (Volpp 2009), or carbon monoxide (Rand 1989) and carbon monoxide plus cotinine (Glasgow 1993).
8. Comprehensive approach
No details of randomization were given, apart from Okechukwu 2009, who used a random number generator. Bergstrom 2008, Tanaka 2006 and Willemsen 1998 allocated the worksites non‐randomly to intervention or control status. Fourteen of the 20 studies employed a cluster‐randomized design, while Nilsson 2001 aggregated its participants from four public sector workplaces within the same district. Non‐validated self‐reported smoking cessation was recorded in 14 studies, partial or complete saliva cotinine validation in two studies (DePaul 1987; DePaul 1989), and CO in Shimizu 1999 and Campbell 2002. Sorensen 1993 at six months and Willemsen 1998 at four months collected saliva cotinines, but did not test them (the 'bogus pipeline' principle). Willemsen 1998 did, however, collect and test cotinine samples for 52% of self‐reported quitters at the 14‐month follow‐up.
Effects of interventions
See: Summary of findings for the main comparison Smoking cessation interventions for the workplace
Smoking cessation/reduction
For this update, we have conducted meta‐analyses of the main classes of intervention. Where there was more than one intervention arm, we have compared the control group (minimal or no intervention) with the next simplest treatment. Although this may occasionally underestimate the trial's true efficacy over multiple interventions, we avoid the risks of overstating the effect of the treatment or of tipping a result into significance by forcing a binary comparison that does not reflect the true findings of the trial. Studies treated selectively in this way include DePaul 1994, Erfurt 1991, Glasgow 1984, Kornitzer 1995, Omenn 1988, Rand 1989, Razavi 1999, Sutton 1988a, Sutton 1988b, Sutton 1988c, Sutton 1988d, Windsor 1988, Prochaska 2008 and Mishra 2010. Analysable data were not available for Hennrikus 2002 (incentives, Analysis 2.2), for Sorensen 1993 (multiple intervention programmes for smoking cessation; Analysis 2.3) or for 10 of the 13 comprehensive programme trials targeting multiple risk factors (therefore not meta‐analysed).
We have also produced a Results table (Analysis 3.1), which gives details of types of participants, follow‐up, smoking outcomes and validation of cessation.
GROUP I: INTERVENTIONS AIMED AT THE INDIVIDUAL TO PROMOTE SMOKING CESSATION
1. Intensive behavioural intervention: GROUP COUNSELLING
Eight trials (1309 participants) of worksite‐based group behavioural therapy interventions for smoking cessation demonstrated a benefit for the counselling programmes, with an odds ratio (OR) of 1.71 (95% confidence interval (CI) 1.05 to 2.80; Analysis 1.1.1). There was no statistical heterogeneity (I² = 0%).
Within this category, two trials of relapse prevention using group‐based therapy (484 participants) found no benefit for the programmes: OR 1.15 (95% CI 0.80 to 1.65); Analysis 1.1.2.
Glasgow 1984 showed that at six months one‐third of participants in the gradual condition were abstinent compared to no participants in the abrupt condition. However, in this small sample the result was not statistically significant. This study also targeted smoking reduction as a valid outcome, and 47% of participants stated that they wished to reduce their consumption. Reducers were found to have been successful for each of the target behaviours as they addressed them, without compensatory increases in the other two behaviours. Achieved reductions were statistically significant (P values from 0.001 to < 0.02). Mean reduction in nicotine content was 50%, in percentage of each cigarette smoked 34% and in number of cigarettes smoked 28%. Carbon monoxide (CO) levels were 28% lower on average, suggesting that participants were not compensating for the behavioural changes. All but one participant improved on at least two measures, and 46% on all four variables. At six‐month follow‐up, reducers maintained all the changes except for percentage of the cigarette smoked, with both abrupt and gradual plus feedback participants relapsing on this measure (P < 0.05).
Frank 1986, testing combinations of behavioural support and hypnotic sessions, showed no long‐term differences between any treatment variants.
Klesges 1987, testing both a relapse prevention component and a competition in a factorial design, failed to detect evidence for a long‐term benefit of either. At the immediate post‐test, the competition intervention resulted in higher quit rates (39% versus 16%, P < 0.004) but these differences were minimal at six months (12% versus 11%, NS). The six‐month differences for relapse prevention were in the expected direction but not statistically significant (15% versus 8%), although the competition appeared to increase short‐term quit rates.
Omenn 1988 showed non‐significant trends towards higher quit rates for groups than for self‐help controls. The three Group arms achieved 12‐month validated quit rates of 16% for the multiple component arm, 18% for the relapse prevention arm and 8% for the minimal treatment arm (NS).
In DePaul 1994 at 12 months, the self‐help participants achieved a sustained abstinence rate of 5.1%, the incentives participants 11%, and the group participants 31.2% (P < 0.01).
Schröter 2006 found that participants in the standard behavioural (SB) programme were more successful than those who received relapse prevention (RP) support (21.1% continuously abstinent at 12 months versus 12.2%). They speculated that this unexpected finding might be attributable to the emphasis in the RP group on the likelihood of failure, but also noted that SB participants had received relapse prevention 'rescue' support when necessary, which may have compromised the separation between the two interventions.
Gunes 2007 reported a non‐significant difference in the six‐month quit rate between the intervention and control groups (6% versus 2%, P = 0.14). The primary outcome of interest for this study was movement through stages of change, and for this measure the intervention group achieved significantly lower numbers in 'pre‐contemplation' and higher numbers in the 'preparation' stages at six months, but this did not translate into higher quit rates within the time scale of the trial.
Mishra 2010 demonstrated a benefit for counselling and focus groups over self‐help materials alone at 12 months, with an intervention quit rate of 20% compared to 6% in the control group (P = 0.03). The two incrementally more intensive arms (+ individual counselling, and + bupropion if wished) achieved similar results, with 12‐month quit rates of 19% (P = 0.06) and 20% (P = 0.03) respectively.
For the two relapse prevention studies, Mayer 2010 did not detect an advantage at nine months for the proactive phone counselling (57.5% remained quit) over the worksite‐based group programme (61.7% remained quit, P = 0.552). Predictors of higher abstinence included a lower BSI‐GSI score, lower levels of urinary cotinine, and having to pay EUR 50 for the programme.
The Belgian relapse prevention study (Razavi 1999) found differences between psychologist‐supported quitters (43.7% still abstinent at 12 months), ex‐smoker‐supported quitters (37.5%) and no formal support quitters (35.5%), but these did not reach statistical significance.
2. Intensive behavioural interventions: INDIVIDUAL COUNSELLING
Eight trials (3516 participants) of worksite‐based individual counselling interventions demonstrated a benefit for the counselling programmes, with an OR of 1.96 (95% CI 1.51 to 2.54; Analysis 1.2). There was minimal statistical heterogeneity (I² = 24%).
Cambien 1981 found that at two‐year follow up 21.4% (65/304) of smokers in the intervention group had quit, compared with 13.4% (41/306) in the control group. Although the descriptive forest plot suggests that this result was statistically significant, the authors report that it was not. The result does not take account of the 195 participants lost to follow‐up, and the authors observe that those lost to follow‐up from the intervention group were significantly heavier smokers than the follow‐up attenders (P < 0.01) or the control participants.
Li 1984 found that at 11 months smokers given behavioural counselling from a physician were more likely to remain abstinent (8.4%) than those with a minimal warning (3.6%, P < 0.05). Prolonged abstinence rates did not differ between participants with abnormal lung function tests (3.7%) and normal lung function tests (5.9%).
Windsor 1988: As the two incentive arms of the trial did not detect any significant benefit for the payment schemes, the authors collapsed the incentive and no‐incentive groups together in the analysis to test the efficacy of adding counselling and social support to self‐help materials. This comparison yielded a cessation rate of 5.8% (11/190) at 12 months for the combined self‐help groups, compared with 14.4% (27/188) for the self help with counselling and social support combined groups (P < 0.001).
Gomel 1993a did not find significant differences in continuous abstinence rates between any of the four groups (HRA, RFE, BC and BCI) at six or 12 months. However, when the authors pooled the HRA group with RFE (n = 68 smokers) and BC group with BCI (n = 60 smokers) to test the efficacy of the counselling component, they detected statistically significant differences in abstinence rates. At six months, the combined HRA/RFE group had a continuous abstinence rate of 1%, compared with 10% for the BC/BCI pooled group (Fisher's Exact Test P = 0.05); 12‐month rates were 0% and 7% respectively (P = 0.05). The authors report that contamination between the intervention groups and low participation rates among the RFE stations meant that the effect size of the whole trial may have been compromised.
Kadowaki 2000 found cessation rates of 12.9% and 3.1% in the intervention and control groups respectively (P = 0.003). Among those who succeeded in quitting 48.6% maintained cessation at 18‐month follow‐up. Overall the cessation rate was 8.4% after 22 months and the prevalence of male smokers had decreased from 62.9 to 56.7% (P = 0.038).
Lang 2000 found point prevalence quit rates of 18.4% in the intensive group compared to 13.5% in the minimal intervention group at one year (P = 0.03). Self‐reported sustained cessation of six months and more was reported in 6.1% of the intervention group compared with 4.6% of the comparison group (P = 0.26).
Terazawa 2001 detected a point prevalence cessation rate of 11.1% (13/117) at 12 months in the intervention group, compared with 1.8% (2/111) in the control group. Twelve‐month continuous abstinence rates were 6.8% (8/117) and 0.9% (1/111) respectively (Fisher's Exact 2‐tailed Test P = 0.04 [our calculation]). Only 25 of the 117 counselled smokers in the intervention group agreed to make a quit attempt and would therefore have received the four follow‐up phone calls.
The HIPOP‐OHP study (Tanaka 2006) detected a steady rise in quit rates in both the intervention and control worksites over the 36‐month assessment period. They report final quit rates of 12.1% in the intervention sites versus 9.4% for the controls (P = 0.021), but this is based only on those who responded at both baseline and at final follow‐up. An intention‐to‐treat analysis yields quit rates of 8.9% and 7.0% respectively (P = 0.046 [our calculation]).
Groeneveld 2011 found that a statistically significant benefit at six months (31.3% versus 13.4%) was not sustained to 12 months (23.7% versus 19.5%; P = 0.45). The intervention was more successful in older participants (> 45 years) at both time points.
3. Self‐help programmes
Six trials (1906 participants) of worksite‐based self‐help interventions demonstrated no clear benefit for the self‐help programmes, with an OR of 1.16 (95% CI 0.74 to 1.82; Analysis 1.3). There was no statistical heterogeneity (I² = 0%).
Omenn 1988, discussed earlier in the group counselling section, reported that the self‐help arms achieved 12‐month validated cessation rates of 9% for the multiple component arm, 11% for the relapse prevention arm, and 6% for the minimal treatment arm (NS).
Video studies
The four studies of minimal video interventions with control groups (Sutton 1988a; Sutton 1988b; Sutton 1988c; Sutton 1988d) failed to detect a difference in validated abstinence rates between the video groups, although the second study (Sutton 1988b) detected a difference between the video groups and the non‐participant group (P < 0.05). This study, however, included younger smokers who smoked more heavily than participants in the other three studies. Another finding of the first of the studies (Sutton 1988a) was of more smokers trying to stop in the intervention group than in the control group (P < 0.05), but in that study the 'control' video concerned seatbelts, whereas the 'control' videos in the other three studies all related in some way to tobacco.
Genotyping information studies
Hishida 2010 found a higher quit rate among the controls (baseline information, plus observation only, 8.0%) at 12 months than in the intervention group (baseline information, then genotyped, 5.8%; P = 0.34). Although the authors offer concurrent anti‐smoking legislation as a possible confounder to account for this, it is not clear why that would have impacted the control group more heavily than the intervention group. Comparisons by genotype also detected no significant differences, with quit rates of 5.6% and 7.1% in the LS and SS (high‐risk) groups respectively versus 4.9% (P = 0.723) in the LL (low‐risk) group.
4. Pharmacological therapy
Five trials (1092 participants) of worksite‐based pharmacological interventions demonstrated a benefit for the treatment programmes, with an OR of 1.98 (95% CI 1.26 to 3.11; Analysis 1.4). There was mild statistical heterogeneity (I² = 19%).
Sutton 1987 reported one‐year continuous abstinence rates of 12% among those allocated to nicotine gum and 2% among the control group (no P value given). If an intention‐to‐treat analysis (i.e. based on all randomized participants) is performed on these data, the quit rate drops to 7.8% for the intervention group at 12 months.
Sutton 1988e reported validated one‐year abstinence rates of 22% in those receiving nicotine gum compared with 2% in the control group (P < 0.001). An intention‐to‐treat analysis of these data would yield an intervention quit rate of 10.1% (8/79). The more rigorous 'complete' abstinence rates (i.e. no smoking of any kind up to follow‐up assessment) are 6.3% (5/79) for the intervention group and 2.4% (2/82) for the controls.
In Kornitzer 1995 the three treatment groups (Group 1: active nicotine patch and active gum; Group 2: active nicotine patch and placebo gum; Group 3: placebo patch and placebo gum) achieved 12‐month abstinence rates in Group 1, 2 and 3 of 18.1%, 12.7% and 13.3% respectively (P = 0.19). ORs comparing Groups 1 and 2 at 12 months (OR 1.47, CI 0.76 to 2.78, P = 0.125), and comparing Groups 2 and 3 (OR 0.96, no further details) were not significant. Time to relapse was longer in Group 1 compared with the other two groups (P = 0.04).
Rodriguez 2003 detected a 12‐month CO‐validated continuous abstinence rate of 20.2% (23/114) in the intervention group, compared with 8.7% (9/103) among the controls. This gave an OR of 2.58 (95% CI 1.13 to 5.90, P = 0.025). These results are based on an intention‐to‐treat analysis, except for one death in the intervention group.
Noor 2011 found a higher rate of continuous abstinence (weeks 4 to 24) in the intervention than in the control group; 25.3% versus 12.5%, P = 0.04. Although point prevalence abstinence was biochemically validated, the continuous abstinence rates were not.
5. Social support for not smoking
Two trials (53 participants) of worksite‐based social support interventions demonstrated no benefit for the programmes, with an OR of 0.69 (95% CI 0.18 to 2.62; Analysis 1.5). There was no statistical heterogeneity (I² = 0%).
Two studies of social support (Malott 1984; Glasgow 1986) found no difference with the addition of this component to a basic programme of group counselling and support. Both studies also defined smoking reduction as an outcome of interest, in which participants could choose to attempt either complete cessation or reduction of smoking. In the earlier study (Malott 1984) the authors note that among non‐abstainers, at six months follow‐up the Controlled Smoking (CS) Group daily consumption of nicotine was 0.52 mg compared with Controlled Smoking + Partner Support (CS+PS) Group's consumption of 0.45 mg. Average number of cigarettes per day at six months follow‐up was CS:21.5, compared with CS+PS: 20.1. In both conditions, participants relapsed on number of cigarettes smoked (P < 0.05). In addition, CS participants relapsed on nicotine content (P < 0.05), and CS+PS relapsed on percentage of cigarette smoked (P < 0.01). Neither group relapsed on CO levels, and non‐abstinent smokers in both groups were smoking less at follow‐up than they had been before treatment.
In Glasgow 1986 no differences in outcome were detected between the two groups of reducers (Basic Programme [BP] and Basic Programme + Social Support [BP+SS]). Both groups at six months had achieved reductions in nicotine (BP: 0.90 to 0.49; BP+SS: 0.78 to 0.49, P < 0.05 for both). Number of cigarettes per day was reduced in both groups (BP: 20.5 to 18.3; BP+SS: 27.7 to 24.4), but was statistically significantly higher than at immediate post‐test. The same pattern applied to percentage of each cigarette smoked, although the BP+SS group six‐month rate was still lower (P < 0.05) than pre‐test levels (BP: 83.3 to 74.8; BP+SS: 89.0 to 81.2). Carbon monoxide levels followed the same pattern, while saliva thiocyanate levels were higher at six‐month follow‐up than at baseline. As with cessation, this study offered no evidence that social support enhanced sustained reduction.
GROUP 2: INTERVENTIONS AIMED AT THE WORKSITE AS A WHOLE
6. Environmental support for not smoking
Four trials (3851 participants) of worksite‐based environmental support demonstrated no benefit for the environmental programmes, with an OR of 1.00 (95% CI 0.60 to 1.65; Analysis 2.1). There was no statistical heterogeneity (I² = 0%).
In Dawley 1991 at five months the abstinence rate at the environmental intervention site was twice that of the cessation‐only site (43% versus 21%, no P value given).
Erfurt 1991 compared the effects of four interventions: (1) wellness screening; (2) wellness screening plus health education; (3) as 2, plus follow‐up counselling; and (4) as 3, plus peer support groups, buddy systems, health promotion classes, and plant‐wide activities. In each group there was a reduction in the prevalence of smoking over three years, and the smoking prevalence at three years was lower for interventions 3 and 4 compared with interventions 1 and 2 (P < 0.01), although this difference depended on combining the 1985 smokers with the then ex‐smokers. Interventions 3 and 4 recorded slightly higher quit rates (20.3% and 18.9% respectively) than interventions 1 and 2 (17.1% and 17.6% respectively) among employees who were smoking at baseline, but the difference was not statistically significant, and may have been compromised by differences in baseline.
Hymowitz 1991 failed to detect an effect of environmental support. Twelve‐month quit rates were 22% for physician counselling and group support alone, and 18% for the same support with an 'enriched milieu'.
Tanaka 2006 found that quit rates in both groups rose steadily over 36 months of follow‐up. Six‐month sustained abstinence at 36 months (ITT analysis) was 8.9% for the intervention sites versus 7.0% for the controls (P = 0.0467).
7. Incentives
Five trials (1928 participants) of worksite‐based incentive interventions demonstrated a benefit for payment or reward schedules, with an OR of 1.60 (95% CI 1.12 to 2.30; Analysis 2.2). There was moderate statistical heterogeneity (I² = 43%). Hennrikus 2002 did not contribute any analysable data to this comparison.
Our analysis of the incentives trials demonstrates a benefit for rewards programmes over the control condition. However, a single study (Volpp 2009) contributed 37% of the weight and all of the statistical significance to this analysis; removing these data reduced the OR to 1.16 (95% CI 0.73 to 1.83). But despite this cautionary note, we observe that Volpp 2009 was the only study to conduct assessments and deliver rewards considerably after the cessation programmes, when success rates might have been expected to have dissipated. This was a large study conducted and reported to high standards, and delivering robust findings.
Windsor 1988 failed to detect an effect of monetary incentives on quit rates, with 6/95 achieving continuous cessation in the self‐help group at 12 months compared with 5/95 in the self help plus incentives group. The corresponding rates for the counselling groups were 18/94 and 9/94. If anything, the incentive component appeared to have a negative impact.
Rand 1989 found that contingent payments delayed but did not necessarily prevent relapse to smoking. The study failed to detect an effect on relapse of monitoring and feedback of CO rates.
Glasgow 1993 failed to detect a difference between incentive and no‐incentive conditions across 19 workplaces. There were no statistically significant differences in self‐reported cessation rates at one year (12.9% for incentives versus 12% for control) or at two years (18% for incentives versus 15.5% for control).
The SUCCESS Project (Hennrikus 2002; not included in the meta‐analysis) found that programme recruitment was higher in the incentive sites (22% vs 12%, P = 0.0054), but that this did not translate to higher cessation rates. Although the authors suggest that telephone counselling appeared to be at least as effective as group programmes, the two types of support seem to have been offered at different levels of intensity, with drop‐outs from group programmes not followed up, while telephone counsellors routinely made ten contact attempts per session plus messages or letters to their participants.
Gomel 1993a failed to detect an effect of either individual or group incentives. Rewards appeared to have no effect, or possibly a negative impact, with 3/30 smokers continuously abstinent at 12 months in the BC (counselling only) group compared with 1/30 in the BCI (counselling plus incentives) group, but this difference did not achieve statistical significance. Other outcomes for this trial are covered under the Individual Counselling heading.
Volpp 2009 detected a statistically significant benefit of incentives over control conditions, with 15‐ or 18‐month prolonged abstinence rates of 9.4% versus 3.6% (P = 0.0008) respectively.
The effectiveness of incentives and competitions as an aid to smoking cessation in any setting is covered in another Cochrane review (Cahill 2011).
8. Comprehensive programmes
(a) Multiple interventions for smoking cessation:
Six trials (5018 participants) demonstrated a benefit for comprehensive programmes aimed at smoking cessation, with an OR of 1.55 (95% CI 1.13 to 2.13; Analysis 2.3.1). There was some heterogeneity (I² = 30%). Sorensen 1993 did not contribute analysable data to this comparison.
The initial De Paul study (DePaul 1987) achieved 12‐month sustained cessation rates of 6% for the group participants versus 2% for the self‐help participants (NS), with both arms achieving a 19% point prevalence rate. It reported a non‐significant trend towards higher quit rates for groups than for self‐help controls.
Willemsen 1998 failed to detect an effect of a comprehensive programme. The six‐month sustained abstinence rates were 8% in the comprehensive workplaces and 7% in the minimal‐treatment workplaces. Among the medium to heavy smokers, prolonged abstinence rates were 9% for the comprehensive programme and 4% for the minimal programme.
At 12 months, point prevalence for the group participants in DePaul 1989 was 26%, compared with 16% for non‐group participants (P < 0.06), with sustained abstinence rates of 11% and 3% respectively (P < 0.05).
Sorensen 1993 (not included in the meta‐analysis) demonstrated that at six‐month follow‐up, 12% of smokers in the intervention group reported quitting, compared to 9% in the control group (P < 0.05), with cessation predicted by co‐worker requests not to smoke.
A small study (Shimizu 1999) of a multicomponent programme including group and individual counselling did not detect a statistically significant difference, with quit rates of 19% in the intervention group and 7% in the control group.
Tanaka 2006 detected a six‐month sustained abstinence rate at 36 months of 8.9% (self‐reported) for the intervention sites versus 7.0% for the control sites (P = 0.0467). Quit rates in both groups rose steadily over the three‐year course of outcome measurement.
Okechukwu 2009 found that a significantly higher one‐month quit rate in the intervention group had dissipated to a non‐significant difference by six months, at 9% versus 7.2% (P = 0.48). These results were not biochemically validated, in order to avoid any perceived association with 'drug‐testing', and because such tests might have been confounded by work‐related toxins.
(b) Comprehensive interventions targeting several risk factors, including smoking
Since only three trials (Glasgow 1995; Prochaska 2008; Milani 2009; 383 participants) of the 13 (> 78,000 participants) in this group offered data for meta‐analysis, we decided against pooling these findings, as they are likely to be unrepresentative and misleading. We report the results here narratively, and in the Results Table Analysis 3.1.
Kornitzer 1980 detected a decline in smoking prevalence of 18.7% in the high‐risk intervention group at two‐year follow‐up, compared with a 12.2% drop in the high‐risk control group (P < 0.05). A five per cent sample of all the intervention participants demonstrated a prevalence decline of 12.5% over two years, which was very close to the 10% sample control group's decline of 12.6% (non‐significant). The authors speculate that the lack of face‐to‐face counselling (available only to the high‐risk intervention group) may have been a significant factor in the failure of the anti‐smoking campaign. Stepwise multiple discriminant function analysis among the high‐risk groups identified fewer cigarettes smoked at baseline, more previous quit attempts and the residential area of the country as significant predictors of quitting among the intervention group, and higher education and more previous quit attempts among the controls. The significant differences between intervention and control high‐risk groups gradually disappeared over the subsequent four years of the study, due to a combination of less intensive intervention activity and a spontaneous rise in the control group's quit rate in line with secular trends.
The HealthWise Stepped Intervention Study (Shi 1992) noted a decline in smoking prevalence at two‐year follow‐up in all four intervention levels (nine worksites). Smoking reduced in Level 1 sites by 34%, from 18% to 12%, in Level 2 sites by 18%, from 17% to 14%, in Level 3 sites by 35%, from 24% to 15%, and in Level 4 sites by 44%, from 14% to 8%. All differences were statistically significant at P < 0.01 level, except for the Level 2 decline which was significant at the 0.1 level. Outcomes were measured by cross‐sectional surveys rather than cohort analysis, with relatively low participation rates of 69% at baseline and 48% at follow‐up.
The 'Take Heart' study (Glasgow 1995) reported that the early and delayed intervention groups did not differ on changes in smoking rates, dietary intake or cholesterol levels. Despite documented implementation of the intervention, there were no short‐term improvements beyond secular trends also observed in control workplaces. Glasgow 1997 (an excluded study) also reported the results of 'Take Heart II' which was non‐randomized but with a matched quasi‐experimental study design similar to the first 'Take Heart' trial, plus updated menu and added guidance for employee steering committees and implementation. The authors reported that there were no statistically significant differences in smoking prevalence and smoking cessation between intervention and control workplaces.
The Working Well Trial (Sorensen 1996) reported a non‐significant 1.53% difference between intervention and control workplaces in six‐month smoking cessation rates. Smoking prevalence declined in intervention sites (from 24.5% to 21.2%) and in control sites (from 25.8% to 21.8%) (NS).
The WellWorks Study (Sorensen 1998) nested within the Working Well Trial, was a randomized controlled trial, with similar aims to its parent trial, but combining health promotion and health protection interventions, and also targeting outcome differences by job category. Six‐month smoking abstinence rates were 15% in the intervention workplaces, and 9% in the control workplaces (P = 0.123). We have not used the first analyses for this study, published in 1996, since these did not include results from the control workplaces.
At three‐year follow‐up, the Working Healthy Project (Emmons 1999) did not detect significant differences between either the seven‐day point prevalence quit rates (intervention 25.6% versus control 21.8%) or the six‐month continuous abstinence quit rates (intervention 8.0% versus control 8.1%).
The Swedish trial of cardiovascular risk reduction (Nilsson 2001) detected a decline in smoking prevalence in the intervention group from 65% to 37% at 12 months, compared with a non‐significant decline in the control group from 65% to 63%. Prevalence at 18 months was 40% for the intervention group and 59% for the control group, and this difference influenced the decrease in the mean risk score from 10.3 (standard deviation (SD) 1.5) to 9.0 (SD 2.2, P = 0.042).
The intervention arm of the Health Works for Women trial (Campbell 2002) had a higher smoking prevalence at baseline (30%) than the control arm (22%), but only 9% of the intervention participants (26% of the current smokers) chose to concentrate their efforts on quitting. Both groups reduced their prevalence rate by about 3% at 18‐month follow‐up. The intervention for smokers was incomplete, as no lay helpers were willing to be trained to support the smokers trying to quit. It is therefore not possible to draw any meaningful inferences from the lack of a detectable difference between the two arms of the trial.
The WellWorks‐2 Trial (Sorensen 2002) did not detect a significant difference in point prevalence rates at six months between intervention and control workplaces (reductions of 4.1% and 1.6% respectively). Cohort analysis failed to detect an effect in overall quit rates between intervention (11.3%) and control workplaces (7.5%, OR 1.57, P = 0.17).
Sorensen 2007 detected a significant increase in the six‐month quit rate in the intervention group compared with the controls (19% versus 8%, P = 0.03). This analysis was restricted to the 188 smokers who completed both the baseline and follow‐up surveys, although the authors reported that an intention‐to‐treat (ITT) analysis also found a statistically significant benefit of the intervention. The ITT data were 19 quitters from 125 baseline smokers in the intervention group (15%), compared with 7 quitters from 106 baseline smokers in the control group (7%, P = 0.04).
Bergstrom 2008 evaluated a 3½‐year comprehensive programme at ten time points, including smoking cessation support by the occupational health services for those assessed as being at moderate risk, and referral to inpatient rehabilitation or inpatient smoking cessation for those at high risk. All four intervention companies showed a decline in smoking prevalence compared with the reference (control) company; Companies 1, 2 and 4 demonstrated a statistically significant decline (‐0.60 (P < 0.05), ‐0.92 (P < 0.01) and ‐0.45 (P < 0.05) respectively), while the decline in Company 3 (‐0.09) was non‐significant. Sensitivity analyses to assess white‐ and blue‐collar workers separately found no meaningful differences between the subgroups.
The primary outcome for Prochaska 2008 was movement through the stages of change in response to three workplace interventions targeting inactivity, BMI, stress and smoking. The comparison of health risk intervention (HRI) alone versus HRI + motivational interviewing (MI) for smoking cessation indicated a non‐significant benefit for the MI arm, with an abstinence rate at six months of 34.6% versus 16.7%. The third arm (HRI + transtheoretical model) achieved a rate of 21.1%. We were unable to establish the distribution of the 101 reported to be 'at criterion' at six months (i.e. deemed to be in the action stage, which for smoking was defined as achieving point prevalence abstinence). We have therefore applied an intention‐to‐treat principle, using the baseline smokers as the denominators for our assessment of success rates.
In a personal communication, Milani 2009 reported six‐month point prevalence cessation rates of 18.2% (6/33) for the intervention group, and 0% (0/31) for the control group (P = 0.08). The primary outcome for this trial was cost savings to the employer of a systematic health promotion programme for their insured work force.
Economic analysis
There is limited literature on the costs of implementing workplace smoking control programmes. Only six of the studies identified for this review (Windsor 1988; DePaul 1989; Erfurt 1991; DePaul 1994; Tanaka 2006; Milani 2009) reported cost data. All except the Japanese HIPOP‐OHP study were conducted in the USA.
Windsor 1988 found that material costs to deliver the programme plus lost employee time to participate produced a total programme cost of approximately USD 50 per employee. The cost to implement the programme for combined groups 1 (brief advice and self‐help materials) and 3 (as 1 with monetary awards) was approximately USD 9500 (USD 50 x 190 per combined intervention group). The estimated savings to the University for Groups 1 and 3 with a 5.8% quit rate (9 employee quitters at USD 1000) was about USD 9000. From a cost‐to‐benefit ratio perspective the estimated savings observed from combined groups 2 (as 1 with self help, further counselling, buddy selection and contract) and 4 (as 2 with monetary rewards for cessation) was the same as for groups 1 and 3 (USD 9500). The observed quit rate of 15% (27 employee quitters at USD 1000) produced an estimated saving of approximately USD 27,000. The researchers suggested that reducing the estimated savings by 50% (for example, USD 500 per employee per year instead of USD 1000) still led to estimated savings of USD 13,500, 40% above the estimated cost of USD 9500. The cost‐to‐benefit ratio for the most effective methods (groups 2 and 4) was approximately 2 to 1.
DePaul 1989 showed that in the Group condition (media, self‐help manuals, groups and incentives) 44 participants had quit at the 12‐month follow‐up and for the Non‐group condition 26 had quit smoking. Incentives and supplies cost approximately USD 21,000 for the Group intervention, so each Group quitter cost USD 477. Supplies for the Non‐group cost about USD 2000, so each quitter cost USD 77.
Erfurt 1991 found that the annual direct cost per employee for post‐screening interventions was USD 2.97 for site 1 (control site), USD 17.68 for site 2 (health education), USD 30.96 for site 3 (health education plus follow‐up counselling), and USD 38.31 for site 4 (health education, follow‐up counselling plus plant organization for health promotion). For engaging employees into treatment or programme participation, sites 3 and 4 were approximately 10 times more cost‐effective than site 2. Also, for reducing risks and relapse prevention, sites 3 and 4 were five to six times more cost‐effective than site 2. At sites 3 and 4 the total direct cost per percentage of risks reduced and relapse prevented was less than one dollar (USD 0.67 and USD 0.74, respectively) per employee per year.
The last of the DePaul studies, DePaul 1994, summarized the cost implications of all three De Paul studies. The total cost for each intervention was Self‐help USD 4717, Incentives USD 6992 and Group USD 26,867. Costs per quitter (12‐month point prevalence to continuous quit rate) were Self‐help USD 225 ‐ 1179, Incentives USD 250 ‐ 699 and Group USD 455 ‐ 790. The cost of the programme offered to the public (50,000 self‐help manuals and newspaper supplements) was USD 62,500. If 5% to 15% of the recipients of self‐help materials could quit smoking, the cost would range from USD 8 to USD 25 per quitter. With the television series costing about USD 20,000, if only 5% of smokers who watched it managed to quit, the cost per quitter would be USD 3.
The HIPOP‐OHP Study (Tanaka 2006) calculated that the intervention delivered 36.3 additional quitters, in a period when intervention and control sites all reported a steady rise in the cessation rate. Based on materials and opportunity costs, each quitter was estimated to have cost JPY 70,080 (95% CI JPY 32,800 to JPY 532,200). This converts to USD 707 at November 2013 rates. The authors report that this is comparable with the cost per quitter in companies with a smoke‐free policy (USD 799), and lower than the cost of a smoker who quits without NRT through primary care or smoking specialist services (USD 2100 to 7900).
Milani 2009 presents the findings of their trial as an illustration to the employer of potential savings from an effective healthcare intervention in the workplace. They comment that in the US nearly 60% of after‐tax profits are spent on corporate health benefits, with 80% of such expenses incurred by 10% to 20% of the workforce. Before the intervention, total medical claim costs averaged USD 2981 per participant, but in the 12 months after the intervention declined in the intervention group to an average of USD 1539 per participant (P = 0.002), compared with USD 2522 per participant in the control group (P = NS). The authors report that "For every dollar invested in worksite intervention, $6 was realized in health care savings".
Absenteeism
One study of a comprehensive lifestyle intervention (Nilsson 2001) reported on mean number of sick days over the last four months of the first year of the trial. Mean sick days taken by the intervention group fell from 6.0 to 2.9 (P = 0.03), while the mean sick days taken by the control group for that period rose from 4.5 to 7.4 (P = 0.04). However, smoking was only one of several behaviours targeted in this trial, and the contribution of reduced smoking prevalence to absentee rates could not be separately estimated.
Discussion
Workplace interventions are heterogeneous. Although the workplace may offer particular opportunities for recruitment to programmes, many of the interventions tested in workplace studies are not specific to this setting. This is particularly true of interventions aimed at helping individuals to stop smoking. The effects of smoke‐free policies and restrictions within the workplace are covered in a companion review (Callinan 2010).
Summary of main results
This updated review (latest search July 2013) identified 57 randomized or quasi‐randomized trials (61 comparisons), with later trials tending to be of higher quality for both conduct and reporting. We report the main findings in the 'summary of findings Table for the main comparison'. Ten trials of group behavioural therapy (1793 participants), eight trials of individual counselling (3516 participants), five trials of pharmacological interventions (1092 participants) and six trials of multiple interventions targeting smoking cessation (5018 participants) all found clear evidence in support of such worksite‐based programmes. Work‐based incentives programmes (five trials, 1928 participants) also demonstrated benefits, but this analysis was dominated by one large trial with positive findings that were not sustained when it was removed from the calculation. Self‐help interventions, social support programmes, relapse prevention programmes and environmental cues did not demonstrate significant benefits when offered in the workplace. Although we identified 13 studies of comprehensive intervention programmes for multiple risk factors including smoking, we were unable to extract sufficient data for meta‐analysis. Although there is a strong theoretical rationale for approaches that integrate smoking cessation with comprehensive health promotion and protection programmes in the workplace, formal studies of such approaches have failed to show that they significantly decrease overall prevalence of smoking.
In addition to effectiveness, it is clearly important for employers to consider the economic aspects of introducing smoking programmes in their workplaces. These issues are infrequently addressed in the studies included in this review, and those studies which do discuss the economic implications are difficult to compare. The absolute figures quoted vary across time and across countries, and the methods of calculating costs differ from one study to the next. Some studies calculate a cost per quitter from among the smokers only, while others use the entire workforce as the denominator. These approaches also take no account of smokers who are not enrolled in the programme, but who are nonetheless reached and affected by the programme's publicity, or by friends and family who participate. Given that the quitters among them may have been influenced by the presence of the programme, they might reasonably be counted among the programme's successes. Furthermore, it is inappropriate to base the calculations simply on the programme costs, without reference to other direct costs such as occupied space that could have been used differently, donated or discounted time and resources, and avoidance of future healthcare expenditure on continuing smokers. Some studies risk an over‐simplified approach to the analysis, calculating the costs per quitter in the intervention group without reference to the costs per quitter in the control or pre‐policy group. The intervention costs should be reckoned as incremental to those incurred by the control group, which can be seen as demonstrating the background or placebo rate.
Overall completeness and applicability of evidence
In this third update of our review, we are confident that rigorous ad hoc and routine searching have ensured that we have identified the key research to inform the review question. Previous iterations have been dominated by US‐based trials, through a combination of funding resources and the material interests of employers who usually cover health insurance costs for their staff. It is notable that for this update we have been able to include a few recent trials set in developing countries; this arena is likely to become increasingly important as the big tobacco companies shift their focus to these areas, while smoking rates in high‐income countries stabilise or decline.
Agreements and disagreements with other studies or reviews
In drawing conclusions about the effectiveness of these interventions we have placed the findings of the workplace studies in the context of what is known from systematic reviews that include non‐workplace studies. Although the results of some of the individual studies considered in this review have inconclusive findings, most are consistent with the findings from other systematic reviews. On this basis, we conclude that there is strong evidence for an effect of group therapy, of individual counselling, of pharmacological treatments, and of multiple interventions for smoking cessation. The Cochrane review of group behaviour therapy (Stead 2005) showed that such programmes increase the likelihood of quitting, approximately doubling the odds of quitting (odds ratio (OR) 2.20, 95% confidence interval (CI) 1.72 to 2.81 compared with other formats; 13 studies; risk ratio (RR) converted to OR). This compares with the OR in this review for the group behavioural studies of 1.71 (95% CI 1.05 to 2.80). Only one included study (DePaul 1994) is common to that review and the relevant comparison in this update, making the two bodies of evidence relatively independent of each other. The Cochrane review of individual counselling (Lancaster 2005a) identified 18 trials in workplaces and other settings, with only one study (Windsor 1988) in common with this review. The OR for successful smoking cessation in the individual counselling review was 1.53 (95% CI 1.31 to 1.80; RR converted to OR), compared with the individual counselling OR in this review of 1.96 (95% CI 1.51 to 2.54). Pharmacological interventions (predominantly NRT) were also successful when mediated through the workplace, which was compatible with results in other settings (OR 1.78; 95% CI 1.68 to 1.88; 119 trials, Stead 2012; RR converted to OR), compared with the pharmacological interventions OR in this review of 1.98 (95% CI 1.26 to 3.11); the same analysis restricted only to NRT trials delivered an OR of 1.81 (95% CI 1.07 to 3.08). Self‐help interventions conducted in the workplace generally did not deliver enhanced rates of smoking cessation (OR 1.16; 95% CI 0.74 to 1.82), which again is consistent with the findings of the Cochrane self‐help review (OR 1.08; 95% CI 0.98 to 1.19; 32 studies; Lancaster 2005b, RR converted to OR).
A particular attraction of the workplace is that it provides a route of access for communicating about smoking and offering help to stop. However, participation rates are often low. A number of studies considered methods for increasing participation. This review found limited evidence that participation in programmes can be increased by competitions and incentives organized by the employer (see also Cahill 2011). One limitation of the existing evidence is that most studies were conducted in stable workplace settings which are becoming less common, as workers are increasingly mobile (for example, in the construction and transport industries) or on short‐term contracts (as in many modern service industries). The assumption that the workplace is a good place for recruitment is only valid for certain types of workplace.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Empty cells are for multiple studies within a single study report.
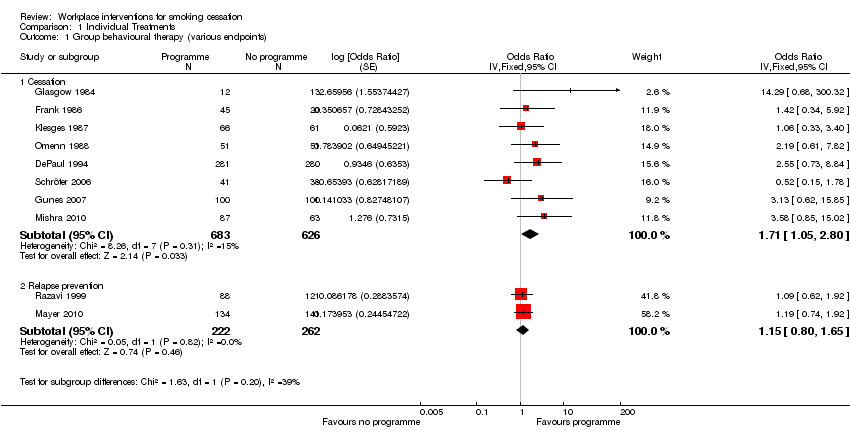
Comparison 1 Individual Treatments, Outcome 1 Group behavioural therapy (various endpoints).

Comparison 1 Individual Treatments, Outcome 2 Individual counselling (various endpoints).

Comparison 1 Individual Treatments, Outcome 3 Any self‐help intervention (various endpoints).
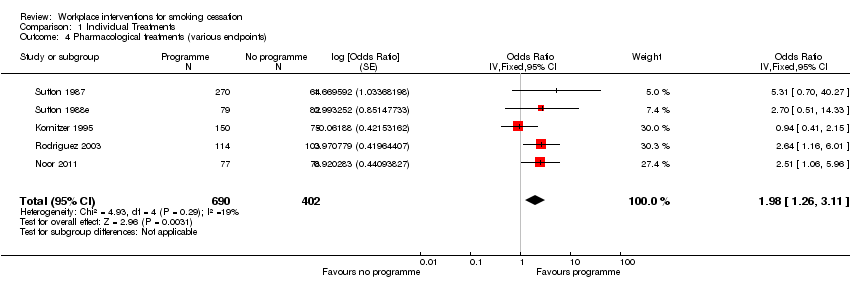
Comparison 1 Individual Treatments, Outcome 4 Pharmacological treatments (various endpoints).

Comparison 1 Individual Treatments, Outcome 5 Social support (various endpoints).

Comparison 2 Worksite Treatments, Outcome 1 Environmental support (various endpoints).

Comparison 2 Worksite Treatments, Outcome 2 Incentives (various endpoints).
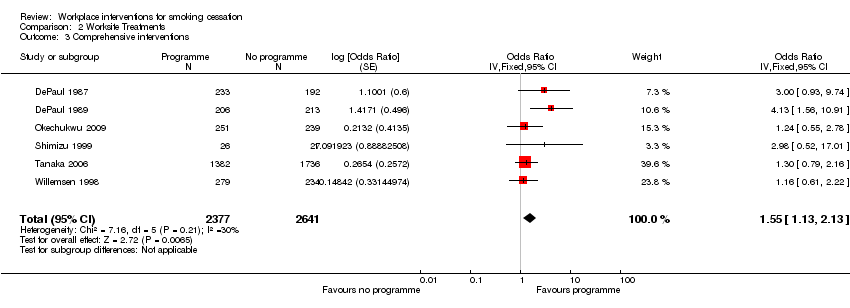
Comparison 2 Worksite Treatments, Outcome 3 Comprehensive interventions.
| Study | Baseline/follow‐up | Smoking outcome | Validated ? |
| Bergstrom 2008 | About 881 smokers across 4 companies risk‐assessed and given SC classes and NRT; about 98 controls assessed only | No MA data, but logistic regression results over 10 timepoints for 3½ years. Significant reductions in gradient relative to controls in Companies 1 (‐0.6), 2 (‐0.92) and 4 (‐0.45), not in Company 3 (‐0.09). | No indication of validation (all long‐term data collected by post or phone) |
| Cambien 1981 | 304 intervention smokers recalled at 2 yrs, and 306 control smokers. 195 participants lost to follow up, proportion of smokers not reported | 21.4% of intervention smokers quit, vs 13.4% of control smokers. Point prevalence at 2 yrs, not a significant difference | Validation by blood CO levels |
| Campbell 2002 | 538 women in 9 worksites (4 exp, 5 control) completed all surveys (282 I, 256 C) to 18m. | No raw data given for smoking, but prevalence went down by around 3% in both groups. No significant differences, and no p values. | Self‐report on all outcomes, no biochemical validation |
| Dawley 1991 | 2 US oil refineries randomized to eenviornmental anti‐smoking campaign + SC programme, versus SC programme alone. | 7/16 smokers in the comprehensive programme site had quit at 5m, versus 3/14 at the SC only site. | Self report only, no biochemical validation |
| DePaul 1987 | 425 smokers in 43 corporations, randomised to group support programmes or self‐help alone programmes | 6% vs 2% continuously abstinent (NS), 19% in both groups were abstinent at 12 months point prevalence. | Partial validation by salivary cotinine, with family and colleague report |
| DePaul 1989 | 419 smokers in 38 worksites, randomised to experimental programme (206) and comparison programme (213). The attrition rate was 17% for Group worksites and 29% for Non Group worksite participants, so correcting the data for attrition would increase the apparent efficacy of the Group condition. | At the company level of analysis the 12 month point prevalence quit rates were Group 26% vs No Group 16% (p<0.06); continuous abstinence rates were 11% (Group) vs 3% (No Group) (p<0.05). | Partial validation by salivary cotinine, with family and colleague report |
| DePaul 1994 | 844 smokers in 61 worksites, randomised to Self‐help [SH] (289), Incentives [I] (281) or Group support [G] (283). | 12 month quit rates for sustained abstinence were 5.1% (n=79) SH, 11% (n=91) I, 31.2% (n=109) G (p<0.01). An Intention to Treat analysis, taking account of attrition, would further favour the intervention groups. | Validation by salivary cotinine at 6 months, and CO<9ppm at 12 months |
| Emmons 1999 | 2055 workers (28% smokers) completed all surveys from 22 worksites, and constituted the cohort. | At 3 yr final follow up, 8.0% of the intervention smokers had quit for 6m, and 8.1% of the control smokers. 25.6% and 21.8% respectively claimed 7‐day PP. Differences were non‐significant | Self‐report, with no biochemical validation |
| Erfurt 1991 | Four sites were assessed at baseline; Site 1 had 1096 smokers (45%), Site 2 598 (44%), Site 3 844 (41%) and Site 4 834 (44%). | Participation was affected by the intervention: 5% in Site 1, 9% in Site 2, 53% in Site 3 and 58% in Site 4. | Self‐report only, not biochemically validated |
| Frank 1986 | 48 smokers initially randomised to three groups, with varying levels of hypnosis, booster and self‐management training. A 4th group (15 smokers) was later recruited, with Group 2 interventions applied more intensively. | No difference between the groups for smoking cessation 6 months after treatment, regardless of the frequency, length between sessions, or addition of behavioural methods. Quit rate was 20% for all groups, based on Intention to Treat. | Salivary cotinine measured at 3 months, but self‐report only at 6 months |
| Glasgow 1984 | 36 employees, randomised to abrupt reduction (13), gradual reduction (12) and gradual reduction + feedback (11). | At 6 months up to one third in the gradual condition were abstinent compared to no subjects in the abrupt condition (NS). | CO<10 ppm at 6 months, weighing of cigarette butts |
| Glasgow 1986 | 29 employees randomised to Basic Programme (13) or Basic Programme + Social Support (16). | Consistent with previous findings, supportive social interactions were not related to treatment outcome.3/13 in the Basic Programme had quit at 6 months, and 3/16 in the Basic + Social Support Group (NS). | Self report, weighing of cigarette butts, CO monitoring and salivary thiocyanate |
| Glasgow 1993 | 19 worksites, random allocation to Incentive programme (474 smokers) or No Incentive programme (623 smokers). | At 2 year follow‐up 49/344 (14%) were abstinent in the Incentives group, and 49/426 (12%) in the No incentives group (NS). Intention to Treat analysis would give more conservative quit rates | CO monitoring and salivary cotinine |
| Glasgow 1995 | 26 worksites, randomised to early or delayed interventions. 1222 employees were followed up at all assessment points to 2 years. years. | Comprehensive programme; a 26% rate of cessation was noted across both longitudinal cohort groups (NS), and a 30% rate across both cross‐sectional groups (NS). No significant differences were seen between the 2 types of intervention | Self report, not biochemically validated |
| Gomel 1993a | 28 ambulance stations randomized to 4 levels of risk reduction intervention. 128 baseline smokers followed for 1 yr | No significant differences between HRA and RFE groups at any follow‐up point, nor between BC and BCI groups. HRA and RFE groups (68 smokers) were pooled and compared with 60 smokers in pooled BC and BCI groups. Continuous abstinence rates at 6m were 1% for HRA+RFE and 10% for BC+BCI (Fisher's Exact Test P = 0.05); 12m rates were 0% and 7% (P = 0.05). | Serum cotinine validation used. |
| Groeneveld 2011 | 115 intervention smokers got MI + phone counselling, and 123 controls received 'usual care'. | PPA at 6 and 12m. Study used as denominators only those with complete follow‐up data, who had attended at least 5 sessions. On this basis, 6m quit rates were (I) 31.2%, (C) 13.4%, and 12m (I) 23.75, (C) 19.5. Our ITT analysis gives 6m: (I) 21.7%, (C) 8.9%: 12m: (I) 16.5%, (C) 13%. | No biochemical validation |
| Gunes 2007 | 200 smokers randomized to 7‐step behavioural programme or no intervention, followed for 6m | Main outcome was movement through stages of change. Smoking cessation at 6m was (I): 6/100, (C) 2/100. | Probably no validation (not mentioned) |
| Hennrikus 2002 | 24 worksites, randomised to 6 programmes, 4 worksites in each programme. 2402 smokers were surveyed at baseline and at 12 and 24 months. 85.5% response rate at 12 months, and 81.7% at 24. | 407 (17%) smokers signed up to programmes. 15.4% at 12 months and 19.4% at 24 months reported themselves as non‐smokers. | Self‐report, validated by family member or friend. |
| Hishida 2010 | 257 smokers genotyped, given self‐help booklet; 276 controls assessment only. | At 12m, 15/257 intervention smokers had quit, versus 22/276 control smokers. Intervention smokers at genetically higher risk of smoking‐related cancers (5.6% and 7.1%) did not perform differently from the low‐risk smokers (4.9%, P = 0.723). Possible confounding by legislation preventing passive smoking. | Self report only, not verified. |
| Hymowitz 1991 | Six worksites randomised to Full Programme or Group‐only interventions. Participation was 50% in the Full Programme sites, and 44% at Group‐only (NS). | At 12 months, 23/131 (18%) in the Full Programme arm had quit, while 27/121 (22%) in the Group‐only arm had quit (NS). | Self‐report and expired CO < 8 ppm. |
| Kadowaki 2000 | 263 male employees randomised to intervention (132) or control (131). | Quit rates 17/132 (Intervention), 4/131 (Control) at 5‐month follow‐up (p=0.003). Male smoking decreased from 62.9% to 56.7% (p=0.04). | Expired CO<9 ppm at baseline, 5 months and 12 months, and a urine test at 12 months |
| Klesges 1987 | 8 Oregon worksites (127 participating smokers), randomized to group behavioural intervention, + either a 2x2 competition component or a 2x2 relapse prevention component | At 6m, 15/127 had quit; Competition sites 12%, no‐competition 11%. | CO < 10 ppm and saliva thiocyanate |
| Kornitzer 1980 | 30 Belgian factories (16,230 men) randomized to intervention (risk assessment, physician and written advice) or control (assessment only). tested at 2 yrs. | High risk intervention group (n = 1268) reduced prevalence by 18.7% (84.5% to 68.7%), and high risk control group (n = 202) reduced by 12.2% (80.8% to 70.9%). P < 0.05. | Self report only, no biochemical validation. |
| Kornitzer 1995 | 374 employees randomised to Group 1(149, active patch + active gum), Group 2(150, active patch + placebo gum) or Group 3 (75, placebo patch + placebo gum) | At 12 months, abstinence in Group 1 was 18.1% (NS), in Group 2 12.7% (NS) and in Group 3 13.3% (NS). Time to relapse was significantly longer in Group 1 compared with the other 2 groups (p=0.04). | Salivary cotinine at baseline, and expired CO<10 ppm at subsequent checks |
| Lang 2000 | 30 worksite physicians (1095 smokers) were randomised to Group A (504, simple advice) or Group B (591, advice + support and 'contract'). | 2 physicians dropped out post randomisation. | Self‐report, with CO<7 ppm validation on a subset of 231 subjects whose physicians had access to a CO monitor. |
| Li 1984 | 871 employee smokers, randomised to Group 1 (simple warning) or Group 2(brief physician advice), stratified by normal/abnormal lung function. | Counselled workers had an 8.4% abstinence rate at 11 months, compared with 3.6% in the control group (p<0.05). | Expired CO<10 ppm at 11 months follow‐up in all quitters, and in a random sample of 379 continuing smokers |
| Malott 1984 | 24 employees randomised to controlled smoking Group (1) or controlled smoking + partner support Group (2). | Few differences were observed between controlled smoking and controlled smoking plus partner support conditions either during treatment or at the 6‐month follow‐up. 25% of Group 1, and 17% of Group 2 were abstinent at 6 months (NS). | Self‐monitoring, butt counts, expired CO levels |
| Mayer 2010 | 275 abstainers randomized by company to proactive phone counselling (134) or to worksite‐based group counselling (141) for relapse prevention. | No significant differences at 9m: Phone counselling 57.5% and group counselling 61.7% remained abstinent (P = 0.552). | 4‐wk CA assessed by CO < 10 ppm, and by urinary cotinine ≤ 317 ng/ml. |
| Milani 2009 | Two sites chosen; 33 intervention smokers received comprehensive CV risk programme, including referral to SC programme, and 31 controls. | 6/33 intervention smokers quit at 6m, versus 0/31 controls. Cost effectiveness analysis reported. | Not stated, but probably unvalidated. |
| Mishra 2010 | Tobacco users in 4 call centres (63 control, 204 intervention) received increasing levels of support and bupropion; controls got self‐help leaflets. 99% received long‐term counselling. | 6m PA at 12m rates were 4/63 (controls), 17/87 BPO2 (HE sessions + focus groups), 8/43 BPO3 (as BPO2 + individual counselling), and 15/74 BPO4 (as BPO3 + bupropion offered); high turnover (52.4%) but only 1% lost to follow‐up. | CO monitor < 6ppm. |
| Nilsson 2001 | 113 workers randomised to intervention (65) or control (63). | Baseline prevalence for both groups was 65%. At 12 months the intervention group point prevalence rate was 37%, and the control group 63%. At 18 months, the rates were 40% (of 43) and 59% (of 46) respectively. This difference influenced the decrease in mean risk score from 10.3 to 9.0 after 18 months in the intervention group (P = 0.042) | Self‐report, not biochemically validated |
| Noor 2011 | 77 male intervention smokers randomized to 24 wks of Viva QS tablets + brief counselling; 78 male control smokers randomized to placebo tablets. Attrtition was 1 intervention and 3 controls at start of study. | At wk 24 all pts had face‐to‐face meeting at workplace for smoking status. Intervention 20‐wk CA quit rate 19/75, controls 9/72. Similar AEs (sore throat: I 28.8%, C 36.6%; dry mouth: I 17.8%, C 16.9%). | All pts cotinine‐ (< 50 ng/ml) and CO‐ (< 8 ppm) tested at 24 wks, whether claiming abstinence or not. 54 24‐wk urine samples not analysed, so only CO reliable. |
| Okechukwu 2009 | 251 intervention smokers (4 sites) and 239 control smokers (6 sites) had 4m programme of counselling, self help, NRT and environmental cues. Attrition at 6m post‐programme was I 2%, C 1.7%. | At 6m, 22/245 intervention and 17/235 controls had PA quit. 2/10 sites had delayed assessment (8m and 9m). | Not verified (fear of 'drug‐testing, + possibility of industrial toxin contamination). |
| Omenn 1988 | 402 employee smokers randomised within their preference for group or self‐help programmes, to 3 programmes, MCP (1), RPP (2) or MTP (3). | Self‐reported quit rates similar across all three group preference conditions but more missing saliva samples in self‐help so validated rates lower. | Salivary cotinine at 12 months < 35 ng/ml |
| Prochaska 2008 | 48 smokers randomized to the HRI group, 40 to the MI group, and 48 to the TTM group. Attrition was 25.7% at 6m (distribution NK) | PPA at 6m ("at criteria") was estimated at 8/48 for HRI alone, 14/40 for HRI + MMI, 10/48 for HRI + TTM; 6m denominators not reported (no reply from authors), so we have applied 6m quit rates to baseline smoking rates. | Not stated, but probably no validation. |
| Rand 1989 | 47 employees randomised to contingent payment/frequent CO monitoring group (17), non‐contingent payment/frequent CO monitoring (16), non‐contingent payment/ infrequent monitoring (14). | Contingent payment combined with frequent CO monitoring delayed but did not ultimately prevent participant relapse to smoking by the end of the six month follow‐up. Contingent payment group had CO value at or less than 11 ppm significantly longer than the other two groups (p=0.03). CO monitoring alone had no effect on abstinence. | Expired CO monitoring <12 ppm |
| Razavi 1999 | 344 post‐cessation abstainers randomised to psychologist support (135), ex‐smoker support (88), or no formal support (121), | 12 months abstinence rates were 59/135 (43.7%) in the PG group; 33/88 (37.5%) in the SG group; 43/121 (35.5%) in no support group (NS). | Expired CO and urinary cotinine. Unvalidated self‐report (higher) were also given. |
| Rodriguez 2003 | 218 smokers randomized to counselling + NRT (115) or minimal sporadic advice (103) in 3 Bilabao (Spain) worksites | 12 months continuous abstinence rates were 23/114 (20.2%) for the intervention group, vs 9/103 (8.7%) in the control group (P = 0.025). | Expired CO <+ 10 ppm |
| Schröter 2006 | 38 smokers assigned to standard behavioural (SB) programme, 41 to relapse prevention (RP) programme. | 12m continuous abstinence rates were 8/38 (21.1%) for SB, and 5/41 (12.2%) for RP. | Self‐reported, no biochemical validation |
| Shi 1992 | 2887 workers (533 smokers) across 9 Californian sites, partially randomized to 4 intervention levels. No non‐intervention control group | 2 yr cross‐sectional survey of 1998 workers (250 smokers); Prevalence declined by 34% from 18% to 12% in Level 1 (p < 0.1); by 18% from 17% to 14% in Level 2 (p < 0.1); by 35% from 24% to 15% in level 3 (p < 0.01); by 44% from 14% to 8% in Level 4 (p < 0.01) | Self‐reported PP at HRA, not biochemically validated |
| Shimizu 1999 | 53 volunteer employee smokers, randomised to intervention and control groups. | After the 5 months of intervention, smoking cessation rate in the intervention group (19.2%) tended to be higher than that in the control group (7.4%), (NS). | Expired CO monitoring |
| Sorensen 1993 | Eight worksites, randomised to intervention (1885 workers) or comparison (1479 workers). | Analysis of all smokers, not just participants. | Self‐report only. |
| Sorensen 1996 | 108 matched worksites (>28,000 workers), randomised to intervention or control conditions, though Florida center sites did not target smoking, leaving smoking outcomes available in only 84 worksites. | Worksite was the unit of allocation and analysis. Baseline smoking data were not reported in detail. | Self‐reported, no biochemical validation |
| Sorensen 1998 | Cohort analysis (2658 employees) of a randomised controlled study of 12 matched pairs of worksites. | PP abstinence for the 6 months prior to 2‐year follow‐up was 15% for intervention group and 9% for control group (p=0.123) | Self‐reported, no biochemical validation |
| Sorensen 2002 | Cross‐sectional analysis (9019 at baseline [80%] and 7327 [65%] ) at six months follow‐up, plus cohort analysis of 5156 employees who responded to both surveys (embedded cohort of 436 smokers). | At six months, point prevalence in the HP/OHS sites fell from 20.4% to 16.3%, and in the HP sites from 18.6% to 17%. | Self‐reported, no biochemical validation |
| Sorensen 2007 | Baseline participants 674 workers, (354 Int/ 320 Cont). | 7‐day self‐reported PPA at 6m: Int: 19/101 (19%), Cont: 7/87 (8%) (P=0.03). | Self‐reported, no biochemical validation |
| Sutton 1987 | 270/334 interested smokers invited to nicotine gum cessation programme; the uninvited 64 represented a control group. 172 (64%) of invitees attended the 1st consultation, 163 the 2nd. | 12% (20/172) of those who attended the intervention course were abstinent at 12 months, compared with 1% (1/98) of those who did not accept the invitation, and 2% (1/64) of the control group; p values not given. | Expired CO<11 ppm |
| Sutton 1988a | Video programme (smoking, plus seat‐belt advice) was offered to all employees. 77 employees were randomised to DFF video (33) or seatbelt (44=control) videos. | Abstinence rates (DFF: 3%, SB [control] 0%) were not significantly different from each other at 12 months follow‐up, There was no significant difference in validated abstinence between the video groups and the non‐participant group. | Expired CO<11 ppm. |
| Sutton 1988b | 150 employees (smokers only) participated. 46 watched the DFF video, 50 watched a confidence‐boosting version of the DFF video, and 54 (control group) watched LTK video. | Abstinence rates (DFF: 11%, DFF+C 8%, LTK [control] 9%) were higher than in the other 3 studies, but not significantly different from each other | Expired CO<11 ppm. |
| Sutton 1988c | 197 employees (smokers only) participated. 56 watched the DFF video, 67 watched a less gory version of the DFF video, and 74 (control group) watched the TW video. | Abstinence rates (DFF: 4%, DFF‐G 3%, TW [control] 4%) were not significantly different from each other. | Expired CO<11 ppm. |
| Sutton 1988d | 179 employees (smokers only) participated. 62 watched the DFF video, 59 watched SL video, and 58 (control group) watched TW video. | Abstinence rates (DFF: 3%, SL 2%, Tw [control] 5%) were not significantly different from each other at 12 months follow‐up. There was no significant difference in validated abstinence artes between the video groups and the non‐participant group. | Expired CO<11 ppm. |
| Sutton 1988e | Fourth study (D) of the video studies groups provided a nested RCT. 161 continuing smokers at 3‐month follow‐up were randomised to intervention (79) or control (82). | 22% (7/32) of attenders in the intervention group were abstinent at 12 months, compared with 2% (1/47) of the non‐attending invitees, and compared with 2% (2/82)of the control group (p<0.001). | Expired CO<11 ppm. |
| Tanaka 2006 | Six intervention sites matched to 6 control sites; Of 1017 intervention smokers who completed baseline and 36m follow‐up, 125 participated in cessation campaign, and 79 accepted counselling + NRT. | 6m sustained abstinence at 36m ITT analysis was 8.9% (123/1382) intervention vs 7.0% (121/1736) control (P = 0.0467). Quit rates in both groups rose steadily over 36m. | No biochemical confirmation |
| Terazawa 2001 | 228 smokers randomized to intervention (117) or control (111). 25 smokers in the intervention group made a supported quit attempt | PP 11.1% (13/117) in the intervention group at 12m, compared with 1.8% (2/111) controls. Continuous abstinence 6.8% (8/117) intervention, compared with 0.9% (1/111) controls. Fisher's Exact test 2‐tailed P = 0.04 | Probably validated by expired CO |
| Volpp 2009 | 878 smokers randomized to intervention (436) or control (442); all got information about local SC programmes, and intervention also got stepped rewards for cessation efforts and goals. Attrition (losses + withdrawals) by 18m was 29% intervention and 24% controls. | At 15m or 18m 41/436 intervention and 16/442 control had quit (PA). | Confirmed by cotinine in saliva or urine. |
| Willemsen 1998 | Four intervention worksites matched to 4 control sites (minimal self‐help), giving 498 smokers who completed baseline survey and enrolled in programmes. | Overall sustained abstinence quit rates at 6 months were 8% (9% for heavy smokers) in the comprehensive group, and 7% (4% for heavy smokers) in the minimal group (no p values given) | Self‐report, plus baseline Fagerstrom score. |
| Windsor 1988 | 387 smokers randomly assigned to four groups, in a 2x2 factorial pre‐/post‐test design. | As monetary incentives made no difference, groups 1&3 were compared with 2&4. Sustained abstinence at 1 year was 5.8% (11/190) in the self‐help only groups, and 14.4% (27/188) in the self‐help + counselling groups (p<0.001). | Baseline salivary cotinine, and follow‐up salivas at 6 weeks, 6 months and 1 year. |
Comparison 3 Results of included studies, Outcome 1 Results of included studies.
| Smoking cessation interventions for the workplace | ||||||
| Patient or population: Employees who smoke | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Smoking cessation interventions | |||||
| Group therapy | 51 per 1000 | 84 per 1000 | OR 1.71 | 1309 | ⊕⊕⊕⊝ | |
| Individual counselling | 61 per 1000 | 113 per 1000 | OR 1.96 | 3516 | ⊕⊕⊕⊝ | |
| Self‐help interventions | 45 per 1000 | 52 per 1000 | OR 1.16 | 1906 | ⊕⊕⊕⊕ | |
| Pharmacological interventions | 77 per 1000 | 142 per 1000 | OR 1.98 | 1092 | ⊕⊕⊕⊕ | Limiting to NRT only (4 studies) reduced OR to 1.81 (1.07 to 3.08). |
| Incentives | 73 per 1000 | 113 per 1000 | OR 1.60 | 1928 | ⊕⊕⊕⊝ | |
| Multiple interventions | 63 per 1000 | 95 per 1000 | OR 1.55 | 5018 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 11 +outlier (Glasgow 1994); removing this study reduced the OR to 1.62 (0.98 to 2.66). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Group behavioural therapy (various endpoints) Show forest plot | 10 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Cessation | 8 | 1309 | Odds Ratio (Fixed, 95% CI) | 1.71 [1.05, 2.80] |
| 1.2 Relapse prevention | 2 | 484 | Odds Ratio (Fixed, 95% CI) | 1.15 [0.80, 1.65] |
| 2 Individual counselling (various endpoints) Show forest plot | 8 | 3516 | Odds Ratio (Fixed, 95% CI) | 1.96 [1.51, 2.54] |
| 3 Any self‐help intervention (various endpoints) Show forest plot | 6 | 1906 | Odds Ratio (Fixed, 95% CI) | 1.16 [0.74, 1.82] |
| 4 Pharmacological treatments (various endpoints) Show forest plot | 5 | 1092 | Odds Ratio (Fixed, 95% CI) | 1.98 [1.26, 3.11] |
| 5 Social support (various endpoints) Show forest plot | 2 | 53 | Odds Ratio (Fixed, 95% CI) | 0.69 [0.18, 2.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Environmental support (various endpoints) Show forest plot | 4 | 3851 | Odds Ratio (Fixed, 95% CI) | 1.00 [0.60, 1.65] |
| 2 Incentives (various endpoints) Show forest plot | 5 | 1928 | Odds Ratio (Fixed, 95% CI) | 1.60 [1.12, 2.30] |
| 3 Comprehensive interventions Show forest plot | 6 | 5018 | Odds Ratio (Fixed, 95% CI) | 1.55 [1.13, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results of included studies Show forest plot | Other data | No numeric data | ||

