Intervenciones en el lugar de trabajo para el abandono del hábito de fumar
Appendices
Appendix 1. Search Strategy for Register
Strategy for Tobacco Addiction Group Specialised Register
via Cochrane Register of Studies (CRS)
#1 workplace*:TI,AB,KY,XKY,MH,EMT
#2 worksite*:TI,AB,KY,XKY,MH,EMT
#3 work:KY,XKY,MH,EMT
#4 occupational health:KY,XKY,MH,EMT
#5 #1 OR #2 OR #3 OR #4
(KY, XKY, MH & EMT are keyword fields)
Appendix 2. Search strategies for electronic databases
Strategies used for update in 2013
Strategy for EMBASE
1. health behavior/
2. health promotion/
3. health education/
4. prevention/ or primary prevention/
5. mass screening/
6. 1 or 2 or 3 or 4 or 5
7. workplace/
8. (smok* or tobacco).mp.
9. 6 and 7 and 8
10. limit 9 to exclude medline journals
11. ("2008" or "2009" or "2010" or "2011" or "2012" or "2013").yr.
12. 10 and 11
Strategy for MEDLINE
1. exp Health Behavior/
2. exp Health Education/
3. Health Promotion/
4. Healthy People Programs/
5. exp Primary Prevention/
6. 1 or 2 or 3 or 4 or 5
7. Work/
8. Workplace/
9. Occupational Health/
10. 7 or 8 or 9
11. (smok* or tobacco).mp.
12. 6 and 10 and 11
13. ("2008" or "2009" or "2010" or "2011" or "2012" or "2013").yr.
14. 12 and 13
Strategy for PsycINFO
1. health behavior/
2. health promotion/
3. health education/
4. health care psychology/
5. prevention/ or preventive medicine/
6. health screening/
7. 1 or 2 or 3 or 4 or 5 or 6
8. occupational health/
9. workplace*.tw.
10. 8 or 9
11. (smok* or tobacco).mp.
12. ("2008" or "2009" or "2010" or "2011" or "2012" or "2013").yr.
13. 7 and 10 and 11 and 12
Appendix 3. Glossary of tobacco‐related terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' |
| Continuous abstinence | Also called 'sustained abstinence' |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Empty cells are for multiple studies within a single study report.
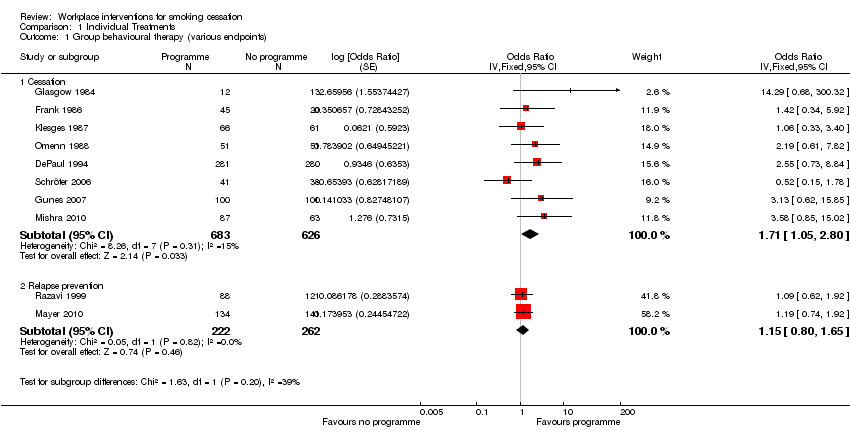
Comparison 1 Individual Treatments, Outcome 1 Group behavioural therapy (various endpoints).

Comparison 1 Individual Treatments, Outcome 2 Individual counselling (various endpoints).

Comparison 1 Individual Treatments, Outcome 3 Any self‐help intervention (various endpoints).
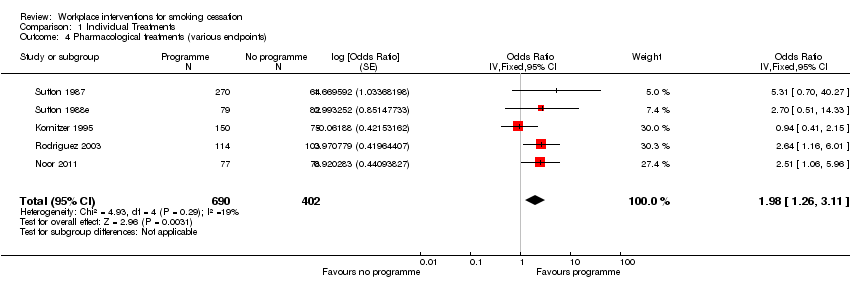
Comparison 1 Individual Treatments, Outcome 4 Pharmacological treatments (various endpoints).

Comparison 1 Individual Treatments, Outcome 5 Social support (various endpoints).

Comparison 2 Worksite Treatments, Outcome 1 Environmental support (various endpoints).

Comparison 2 Worksite Treatments, Outcome 2 Incentives (various endpoints).
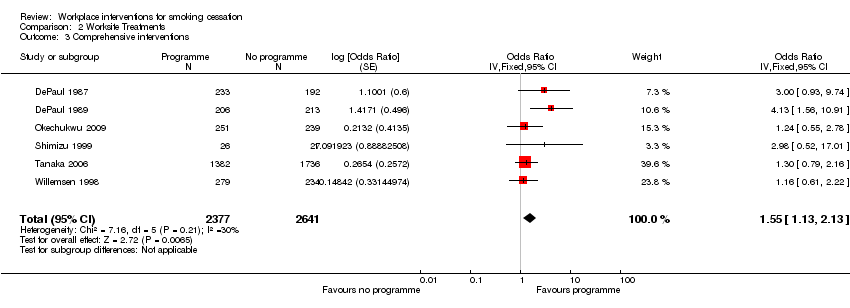
Comparison 2 Worksite Treatments, Outcome 3 Comprehensive interventions.
| Study | Baseline/follow‐up | Smoking outcome | Validated ? |
| Bergstrom 2008 | About 881 smokers across 4 companies risk‐assessed and given SC classes and NRT; about 98 controls assessed only | No MA data, but logistic regression results over 10 timepoints for 3½ years. Significant reductions in gradient relative to controls in Companies 1 (‐0.6), 2 (‐0.92) and 4 (‐0.45), not in Company 3 (‐0.09). | No indication of validation (all long‐term data collected by post or phone) |
| Cambien 1981 | 304 intervention smokers recalled at 2 yrs, and 306 control smokers. 195 participants lost to follow up, proportion of smokers not reported | 21.4% of intervention smokers quit, vs 13.4% of control smokers. Point prevalence at 2 yrs, not a significant difference | Validation by blood CO levels |
| Campbell 2002 | 538 women in 9 worksites (4 exp, 5 control) completed all surveys (282 I, 256 C) to 18m. | No raw data given for smoking, but prevalence went down by around 3% in both groups. No significant differences, and no p values. | Self‐report on all outcomes, no biochemical validation |
| Dawley 1991 | 2 US oil refineries randomized to eenviornmental anti‐smoking campaign + SC programme, versus SC programme alone. | 7/16 smokers in the comprehensive programme site had quit at 5m, versus 3/14 at the SC only site. | Self report only, no biochemical validation |
| DePaul 1987 | 425 smokers in 43 corporations, randomised to group support programmes or self‐help alone programmes | 6% vs 2% continuously abstinent (NS), 19% in both groups were abstinent at 12 months point prevalence. | Partial validation by salivary cotinine, with family and colleague report |
| DePaul 1989 | 419 smokers in 38 worksites, randomised to experimental programme (206) and comparison programme (213). The attrition rate was 17% for Group worksites and 29% for Non Group worksite participants, so correcting the data for attrition would increase the apparent efficacy of the Group condition. | At the company level of analysis the 12 month point prevalence quit rates were Group 26% vs No Group 16% (p<0.06); continuous abstinence rates were 11% (Group) vs 3% (No Group) (p<0.05). | Partial validation by salivary cotinine, with family and colleague report |
| DePaul 1994 | 844 smokers in 61 worksites, randomised to Self‐help [SH] (289), Incentives [I] (281) or Group support [G] (283). | 12 month quit rates for sustained abstinence were 5.1% (n=79) SH, 11% (n=91) I, 31.2% (n=109) G (p<0.01). An Intention to Treat analysis, taking account of attrition, would further favour the intervention groups. | Validation by salivary cotinine at 6 months, and CO<9ppm at 12 months |
| Emmons 1999 | 2055 workers (28% smokers) completed all surveys from 22 worksites, and constituted the cohort. | At 3 yr final follow up, 8.0% of the intervention smokers had quit for 6m, and 8.1% of the control smokers. 25.6% and 21.8% respectively claimed 7‐day PP. Differences were non‐significant | Self‐report, with no biochemical validation |
| Erfurt 1991 | Four sites were assessed at baseline; Site 1 had 1096 smokers (45%), Site 2 598 (44%), Site 3 844 (41%) and Site 4 834 (44%). | Participation was affected by the intervention: 5% in Site 1, 9% in Site 2, 53% in Site 3 and 58% in Site 4. | Self‐report only, not biochemically validated |
| Frank 1986 | 48 smokers initially randomised to three groups, with varying levels of hypnosis, booster and self‐management training. A 4th group (15 smokers) was later recruited, with Group 2 interventions applied more intensively. | No difference between the groups for smoking cessation 6 months after treatment, regardless of the frequency, length between sessions, or addition of behavioural methods. Quit rate was 20% for all groups, based on Intention to Treat. | Salivary cotinine measured at 3 months, but self‐report only at 6 months |
| Glasgow 1984 | 36 employees, randomised to abrupt reduction (13), gradual reduction (12) and gradual reduction + feedback (11). | At 6 months up to one third in the gradual condition were abstinent compared to no subjects in the abrupt condition (NS). | CO<10 ppm at 6 months, weighing of cigarette butts |
| Glasgow 1986 | 29 employees randomised to Basic Programme (13) or Basic Programme + Social Support (16). | Consistent with previous findings, supportive social interactions were not related to treatment outcome.3/13 in the Basic Programme had quit at 6 months, and 3/16 in the Basic + Social Support Group (NS). | Self report, weighing of cigarette butts, CO monitoring and salivary thiocyanate |
| Glasgow 1993 | 19 worksites, random allocation to Incentive programme (474 smokers) or No Incentive programme (623 smokers). | At 2 year follow‐up 49/344 (14%) were abstinent in the Incentives group, and 49/426 (12%) in the No incentives group (NS). Intention to Treat analysis would give more conservative quit rates | CO monitoring and salivary cotinine |
| Glasgow 1995 | 26 worksites, randomised to early or delayed interventions. 1222 employees were followed up at all assessment points to 2 years. years. | Comprehensive programme; a 26% rate of cessation was noted across both longitudinal cohort groups (NS), and a 30% rate across both cross‐sectional groups (NS). No significant differences were seen between the 2 types of intervention | Self report, not biochemically validated |
| Gomel 1993a | 28 ambulance stations randomized to 4 levels of risk reduction intervention. 128 baseline smokers followed for 1 yr | No significant differences between HRA and RFE groups at any follow‐up point, nor between BC and BCI groups. HRA and RFE groups (68 smokers) were pooled and compared with 60 smokers in pooled BC and BCI groups. Continuous abstinence rates at 6m were 1% for HRA+RFE and 10% for BC+BCI (Fisher's Exact Test P = 0.05); 12m rates were 0% and 7% (P = 0.05). | Serum cotinine validation used. |
| Groeneveld 2011 | 115 intervention smokers got MI + phone counselling, and 123 controls received 'usual care'. | PPA at 6 and 12m. Study used as denominators only those with complete follow‐up data, who had attended at least 5 sessions. On this basis, 6m quit rates were (I) 31.2%, (C) 13.4%, and 12m (I) 23.75, (C) 19.5. Our ITT analysis gives 6m: (I) 21.7%, (C) 8.9%: 12m: (I) 16.5%, (C) 13%. | No biochemical validation |
| Gunes 2007 | 200 smokers randomized to 7‐step behavioural programme or no intervention, followed for 6m | Main outcome was movement through stages of change. Smoking cessation at 6m was (I): 6/100, (C) 2/100. | Probably no validation (not mentioned) |
| Hennrikus 2002 | 24 worksites, randomised to 6 programmes, 4 worksites in each programme. 2402 smokers were surveyed at baseline and at 12 and 24 months. 85.5% response rate at 12 months, and 81.7% at 24. | 407 (17%) smokers signed up to programmes. 15.4% at 12 months and 19.4% at 24 months reported themselves as non‐smokers. | Self‐report, validated by family member or friend. |
| Hishida 2010 | 257 smokers genotyped, given self‐help booklet; 276 controls assessment only. | At 12m, 15/257 intervention smokers had quit, versus 22/276 control smokers. Intervention smokers at genetically higher risk of smoking‐related cancers (5.6% and 7.1%) did not perform differently from the low‐risk smokers (4.9%, P = 0.723). Possible confounding by legislation preventing passive smoking. | Self report only, not verified. |
| Hymowitz 1991 | Six worksites randomised to Full Programme or Group‐only interventions. Participation was 50% in the Full Programme sites, and 44% at Group‐only (NS). | At 12 months, 23/131 (18%) in the Full Programme arm had quit, while 27/121 (22%) in the Group‐only arm had quit (NS). | Self‐report and expired CO < 8 ppm. |
| Kadowaki 2000 | 263 male employees randomised to intervention (132) or control (131). | Quit rates 17/132 (Intervention), 4/131 (Control) at 5‐month follow‐up (p=0.003). Male smoking decreased from 62.9% to 56.7% (p=0.04). | Expired CO<9 ppm at baseline, 5 months and 12 months, and a urine test at 12 months |
| Klesges 1987 | 8 Oregon worksites (127 participating smokers), randomized to group behavioural intervention, + either a 2x2 competition component or a 2x2 relapse prevention component | At 6m, 15/127 had quit; Competition sites 12%, no‐competition 11%. | CO < 10 ppm and saliva thiocyanate |
| Kornitzer 1980 | 30 Belgian factories (16,230 men) randomized to intervention (risk assessment, physician and written advice) or control (assessment only). tested at 2 yrs. | High risk intervention group (n = 1268) reduced prevalence by 18.7% (84.5% to 68.7%), and high risk control group (n = 202) reduced by 12.2% (80.8% to 70.9%). P < 0.05. | Self report only, no biochemical validation. |
| Kornitzer 1995 | 374 employees randomised to Group 1(149, active patch + active gum), Group 2(150, active patch + placebo gum) or Group 3 (75, placebo patch + placebo gum) | At 12 months, abstinence in Group 1 was 18.1% (NS), in Group 2 12.7% (NS) and in Group 3 13.3% (NS). Time to relapse was significantly longer in Group 1 compared with the other 2 groups (p=0.04). | Salivary cotinine at baseline, and expired CO<10 ppm at subsequent checks |
| Lang 2000 | 30 worksite physicians (1095 smokers) were randomised to Group A (504, simple advice) or Group B (591, advice + support and 'contract'). | 2 physicians dropped out post randomisation. | Self‐report, with CO<7 ppm validation on a subset of 231 subjects whose physicians had access to a CO monitor. |
| Li 1984 | 871 employee smokers, randomised to Group 1 (simple warning) or Group 2(brief physician advice), stratified by normal/abnormal lung function. | Counselled workers had an 8.4% abstinence rate at 11 months, compared with 3.6% in the control group (p<0.05). | Expired CO<10 ppm at 11 months follow‐up in all quitters, and in a random sample of 379 continuing smokers |
| Malott 1984 | 24 employees randomised to controlled smoking Group (1) or controlled smoking + partner support Group (2). | Few differences were observed between controlled smoking and controlled smoking plus partner support conditions either during treatment or at the 6‐month follow‐up. 25% of Group 1, and 17% of Group 2 were abstinent at 6 months (NS). | Self‐monitoring, butt counts, expired CO levels |
| Mayer 2010 | 275 abstainers randomized by company to proactive phone counselling (134) or to worksite‐based group counselling (141) for relapse prevention. | No significant differences at 9m: Phone counselling 57.5% and group counselling 61.7% remained abstinent (P = 0.552). | 4‐wk CA assessed by CO < 10 ppm, and by urinary cotinine ≤ 317 ng/ml. |
| Milani 2009 | Two sites chosen; 33 intervention smokers received comprehensive CV risk programme, including referral to SC programme, and 31 controls. | 6/33 intervention smokers quit at 6m, versus 0/31 controls. Cost effectiveness analysis reported. | Not stated, but probably unvalidated. |
| Mishra 2010 | Tobacco users in 4 call centres (63 control, 204 intervention) received increasing levels of support and bupropion; controls got self‐help leaflets. 99% received long‐term counselling. | 6m PA at 12m rates were 4/63 (controls), 17/87 BPO2 (HE sessions + focus groups), 8/43 BPO3 (as BPO2 + individual counselling), and 15/74 BPO4 (as BPO3 + bupropion offered); high turnover (52.4%) but only 1% lost to follow‐up. | CO monitor < 6ppm. |
| Nilsson 2001 | 113 workers randomised to intervention (65) or control (63). | Baseline prevalence for both groups was 65%. At 12 months the intervention group point prevalence rate was 37%, and the control group 63%. At 18 months, the rates were 40% (of 43) and 59% (of 46) respectively. This difference influenced the decrease in mean risk score from 10.3 to 9.0 after 18 months in the intervention group (P = 0.042) | Self‐report, not biochemically validated |
| Noor 2011 | 77 male intervention smokers randomized to 24 wks of Viva QS tablets + brief counselling; 78 male control smokers randomized to placebo tablets. Attrtition was 1 intervention and 3 controls at start of study. | At wk 24 all pts had face‐to‐face meeting at workplace for smoking status. Intervention 20‐wk CA quit rate 19/75, controls 9/72. Similar AEs (sore throat: I 28.8%, C 36.6%; dry mouth: I 17.8%, C 16.9%). | All pts cotinine‐ (< 50 ng/ml) and CO‐ (< 8 ppm) tested at 24 wks, whether claiming abstinence or not. 54 24‐wk urine samples not analysed, so only CO reliable. |
| Okechukwu 2009 | 251 intervention smokers (4 sites) and 239 control smokers (6 sites) had 4m programme of counselling, self help, NRT and environmental cues. Attrition at 6m post‐programme was I 2%, C 1.7%. | At 6m, 22/245 intervention and 17/235 controls had PA quit. 2/10 sites had delayed assessment (8m and 9m). | Not verified (fear of 'drug‐testing, + possibility of industrial toxin contamination). |
| Omenn 1988 | 402 employee smokers randomised within their preference for group or self‐help programmes, to 3 programmes, MCP (1), RPP (2) or MTP (3). | Self‐reported quit rates similar across all three group preference conditions but more missing saliva samples in self‐help so validated rates lower. | Salivary cotinine at 12 months < 35 ng/ml |
| Prochaska 2008 | 48 smokers randomized to the HRI group, 40 to the MI group, and 48 to the TTM group. Attrition was 25.7% at 6m (distribution NK) | PPA at 6m ("at criteria") was estimated at 8/48 for HRI alone, 14/40 for HRI + MMI, 10/48 for HRI + TTM; 6m denominators not reported (no reply from authors), so we have applied 6m quit rates to baseline smoking rates. | Not stated, but probably no validation. |
| Rand 1989 | 47 employees randomised to contingent payment/frequent CO monitoring group (17), non‐contingent payment/frequent CO monitoring (16), non‐contingent payment/ infrequent monitoring (14). | Contingent payment combined with frequent CO monitoring delayed but did not ultimately prevent participant relapse to smoking by the end of the six month follow‐up. Contingent payment group had CO value at or less than 11 ppm significantly longer than the other two groups (p=0.03). CO monitoring alone had no effect on abstinence. | Expired CO monitoring <12 ppm |
| Razavi 1999 | 344 post‐cessation abstainers randomised to psychologist support (135), ex‐smoker support (88), or no formal support (121), | 12 months abstinence rates were 59/135 (43.7%) in the PG group; 33/88 (37.5%) in the SG group; 43/121 (35.5%) in no support group (NS). | Expired CO and urinary cotinine. Unvalidated self‐report (higher) were also given. |
| Rodriguez 2003 | 218 smokers randomized to counselling + NRT (115) or minimal sporadic advice (103) in 3 Bilabao (Spain) worksites | 12 months continuous abstinence rates were 23/114 (20.2%) for the intervention group, vs 9/103 (8.7%) in the control group (P = 0.025). | Expired CO <+ 10 ppm |
| Schröter 2006 | 38 smokers assigned to standard behavioural (SB) programme, 41 to relapse prevention (RP) programme. | 12m continuous abstinence rates were 8/38 (21.1%) for SB, and 5/41 (12.2%) for RP. | Self‐reported, no biochemical validation |
| Shi 1992 | 2887 workers (533 smokers) across 9 Californian sites, partially randomized to 4 intervention levels. No non‐intervention control group | 2 yr cross‐sectional survey of 1998 workers (250 smokers); Prevalence declined by 34% from 18% to 12% in Level 1 (p < 0.1); by 18% from 17% to 14% in Level 2 (p < 0.1); by 35% from 24% to 15% in level 3 (p < 0.01); by 44% from 14% to 8% in Level 4 (p < 0.01) | Self‐reported PP at HRA, not biochemically validated |
| Shimizu 1999 | 53 volunteer employee smokers, randomised to intervention and control groups. | After the 5 months of intervention, smoking cessation rate in the intervention group (19.2%) tended to be higher than that in the control group (7.4%), (NS). | Expired CO monitoring |
| Sorensen 1993 | Eight worksites, randomised to intervention (1885 workers) or comparison (1479 workers). | Analysis of all smokers, not just participants. | Self‐report only. |
| Sorensen 1996 | 108 matched worksites (>28,000 workers), randomised to intervention or control conditions, though Florida center sites did not target smoking, leaving smoking outcomes available in only 84 worksites. | Worksite was the unit of allocation and analysis. Baseline smoking data were not reported in detail. | Self‐reported, no biochemical validation |
| Sorensen 1998 | Cohort analysis (2658 employees) of a randomised controlled study of 12 matched pairs of worksites. | PP abstinence for the 6 months prior to 2‐year follow‐up was 15% for intervention group and 9% for control group (p=0.123) | Self‐reported, no biochemical validation |
| Sorensen 2002 | Cross‐sectional analysis (9019 at baseline [80%] and 7327 [65%] ) at six months follow‐up, plus cohort analysis of 5156 employees who responded to both surveys (embedded cohort of 436 smokers). | At six months, point prevalence in the HP/OHS sites fell from 20.4% to 16.3%, and in the HP sites from 18.6% to 17%. | Self‐reported, no biochemical validation |
| Sorensen 2007 | Baseline participants 674 workers, (354 Int/ 320 Cont). | 7‐day self‐reported PPA at 6m: Int: 19/101 (19%), Cont: 7/87 (8%) (P=0.03). | Self‐reported, no biochemical validation |
| Sutton 1987 | 270/334 interested smokers invited to nicotine gum cessation programme; the uninvited 64 represented a control group. 172 (64%) of invitees attended the 1st consultation, 163 the 2nd. | 12% (20/172) of those who attended the intervention course were abstinent at 12 months, compared with 1% (1/98) of those who did not accept the invitation, and 2% (1/64) of the control group; p values not given. | Expired CO<11 ppm |
| Sutton 1988a | Video programme (smoking, plus seat‐belt advice) was offered to all employees. 77 employees were randomised to DFF video (33) or seatbelt (44=control) videos. | Abstinence rates (DFF: 3%, SB [control] 0%) were not significantly different from each other at 12 months follow‐up, There was no significant difference in validated abstinence between the video groups and the non‐participant group. | Expired CO<11 ppm. |
| Sutton 1988b | 150 employees (smokers only) participated. 46 watched the DFF video, 50 watched a confidence‐boosting version of the DFF video, and 54 (control group) watched LTK video. | Abstinence rates (DFF: 11%, DFF+C 8%, LTK [control] 9%) were higher than in the other 3 studies, but not significantly different from each other | Expired CO<11 ppm. |
| Sutton 1988c | 197 employees (smokers only) participated. 56 watched the DFF video, 67 watched a less gory version of the DFF video, and 74 (control group) watched the TW video. | Abstinence rates (DFF: 4%, DFF‐G 3%, TW [control] 4%) were not significantly different from each other. | Expired CO<11 ppm. |
| Sutton 1988d | 179 employees (smokers only) participated. 62 watched the DFF video, 59 watched SL video, and 58 (control group) watched TW video. | Abstinence rates (DFF: 3%, SL 2%, Tw [control] 5%) were not significantly different from each other at 12 months follow‐up. There was no significant difference in validated abstinence artes between the video groups and the non‐participant group. | Expired CO<11 ppm. |
| Sutton 1988e | Fourth study (D) of the video studies groups provided a nested RCT. 161 continuing smokers at 3‐month follow‐up were randomised to intervention (79) or control (82). | 22% (7/32) of attenders in the intervention group were abstinent at 12 months, compared with 2% (1/47) of the non‐attending invitees, and compared with 2% (2/82)of the control group (p<0.001). | Expired CO<11 ppm. |
| Tanaka 2006 | Six intervention sites matched to 6 control sites; Of 1017 intervention smokers who completed baseline and 36m follow‐up, 125 participated in cessation campaign, and 79 accepted counselling + NRT. | 6m sustained abstinence at 36m ITT analysis was 8.9% (123/1382) intervention vs 7.0% (121/1736) control (P = 0.0467). Quit rates in both groups rose steadily over 36m. | No biochemical confirmation |
| Terazawa 2001 | 228 smokers randomized to intervention (117) or control (111). 25 smokers in the intervention group made a supported quit attempt | PP 11.1% (13/117) in the intervention group at 12m, compared with 1.8% (2/111) controls. Continuous abstinence 6.8% (8/117) intervention, compared with 0.9% (1/111) controls. Fisher's Exact test 2‐tailed P = 0.04 | Probably validated by expired CO |
| Volpp 2009 | 878 smokers randomized to intervention (436) or control (442); all got information about local SC programmes, and intervention also got stepped rewards for cessation efforts and goals. Attrition (losses + withdrawals) by 18m was 29% intervention and 24% controls. | At 15m or 18m 41/436 intervention and 16/442 control had quit (PA). | Confirmed by cotinine in saliva or urine. |
| Willemsen 1998 | Four intervention worksites matched to 4 control sites (minimal self‐help), giving 498 smokers who completed baseline survey and enrolled in programmes. | Overall sustained abstinence quit rates at 6 months were 8% (9% for heavy smokers) in the comprehensive group, and 7% (4% for heavy smokers) in the minimal group (no p values given) | Self‐report, plus baseline Fagerstrom score. |
| Windsor 1988 | 387 smokers randomly assigned to four groups, in a 2x2 factorial pre‐/post‐test design. | As monetary incentives made no difference, groups 1&3 were compared with 2&4. Sustained abstinence at 1 year was 5.8% (11/190) in the self‐help only groups, and 14.4% (27/188) in the self‐help + counselling groups (p<0.001). | Baseline salivary cotinine, and follow‐up salivas at 6 weeks, 6 months and 1 year. |
Comparison 3 Results of included studies, Outcome 1 Results of included studies.
| Smoking cessation interventions for the workplace | ||||||
| Patient or population: Employees who smoke | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Smoking cessation interventions | |||||
| Group therapy | 51 per 1000 | 84 per 1000 | OR 1.71 | 1309 | ⊕⊕⊕⊝ | |
| Individual counselling | 61 per 1000 | 113 per 1000 | OR 1.96 | 3516 | ⊕⊕⊕⊝ | |
| Self‐help interventions | 45 per 1000 | 52 per 1000 | OR 1.16 | 1906 | ⊕⊕⊕⊕ | |
| Pharmacological interventions | 77 per 1000 | 142 per 1000 | OR 1.98 | 1092 | ⊕⊕⊕⊕ | Limiting to NRT only (4 studies) reduced OR to 1.81 (1.07 to 3.08). |
| Incentives | 73 per 1000 | 113 per 1000 | OR 1.60 | 1928 | ⊕⊕⊕⊝ | |
| Multiple interventions | 63 per 1000 | 95 per 1000 | OR 1.55 | 5018 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 11 +outlier (Glasgow 1994); removing this study reduced the OR to 1.62 (0.98 to 2.66). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Group behavioural therapy (various endpoints) Show forest plot | 10 | Odds Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Cessation | 8 | 1309 | Odds Ratio (Fixed, 95% CI) | 1.71 [1.05, 2.80] |
| 1.2 Relapse prevention | 2 | 484 | Odds Ratio (Fixed, 95% CI) | 1.15 [0.80, 1.65] |
| 2 Individual counselling (various endpoints) Show forest plot | 8 | 3516 | Odds Ratio (Fixed, 95% CI) | 1.96 [1.51, 2.54] |
| 3 Any self‐help intervention (various endpoints) Show forest plot | 6 | 1906 | Odds Ratio (Fixed, 95% CI) | 1.16 [0.74, 1.82] |
| 4 Pharmacological treatments (various endpoints) Show forest plot | 5 | 1092 | Odds Ratio (Fixed, 95% CI) | 1.98 [1.26, 3.11] |
| 5 Social support (various endpoints) Show forest plot | 2 | 53 | Odds Ratio (Fixed, 95% CI) | 0.69 [0.18, 2.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Environmental support (various endpoints) Show forest plot | 4 | 3851 | Odds Ratio (Fixed, 95% CI) | 1.00 [0.60, 1.65] |
| 2 Incentives (various endpoints) Show forest plot | 5 | 1928 | Odds Ratio (Fixed, 95% CI) | 1.60 [1.12, 2.30] |
| 3 Comprehensive interventions Show forest plot | 6 | 5018 | Odds Ratio (Fixed, 95% CI) | 1.55 [1.13, 2.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Results of included studies Show forest plot | Other data | No numeric data | ||

