Fármacos para la prevención de la deshidratación de los eritrocitos en personas con enfermedad de células falciformes
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, placebo‐controlled, randomised controlled trial. | |
| Participants | 145 participants from India with SS disease, aged over 5 years. 15 participants were lost to follow up. | |
| Interventions | Zinc (oral 220 mg tds) or placebo (identical in appearance). Participants were seen weekly, follow up was for 1.5 years and the data were analysed at the end of the follow‐up period. | |
| Outcomes | Sickle related crises: vaso‐occlusive; mixed; haemolytic; sequestration; and aplastic. Days in hospital and working days lost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
IV: intravenous
SCD: sickle cell disease
SS: sickle cell anaemia
tds: three times daily
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT of megestrol acetate. There is no good recent evidence to suggest that sex hormones prevent dehydration of the sickle red cells. | |
| Trial of poloxamer 188 ‐ inappropriate mechanism of action for inclusion in this review. | |
| Trial of isoxsurpine in treatment of sickle cell crises ‐ rather than prevention. | |
| RCT of piracetam. There is a lack of evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the cell membrane. | |
| Pharmacokinetic study of ICA 17043, with no measure of clinical outcomes of interest. | |
| RCT of tucaresol. Prevents sickling by shifting the oxygen affinity, not through red cell dehydration. | |
| RCT of cetiedil citrate. Used in treatment rather than prevention of crises. | |
| RCT of pentoxifylline. There is no evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the red cell membrane. | |
| RCT of cetiedil citrate. Used in treatment rather than prevention of crises. | |
| RCT of ticlopidine. Mainly an inhibitor of platelet action, rather than anti‐dehydration. | |
| RCT of Depo‐Provera and Microgynon. There is no good evidence to suggest that sex hormones prevent dehydration of the sickle red cells. | |
| RCT of medroxyprogesterone acetate. There is no good recent evidence to suggest that sex hormones prevent dehydration of the sickle red cells | |
| RCT of pentoxifylline. There is no evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the red cell membrane. | |
| RCT of urea. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of the sickle red cells. | |
| RCT of ketoprofen for treatment, rather than prevention, of vaso‐occlusive crises. | |
| RCT of steroids. There is no good recent evidence to suggest that sex hormones prevent dehydration of the sickle red cells. | |
| Risk analysis using data from a previous RCT, in which morphine was administered in treatment, rather than prevention, of acute chest syndrome. | |
| RCT of ticlopidine. Mainly an inhibitor of platelet action, rather than anti‐dehydration. | |
| RCT of a phenothiazine. Used in treatment rather than prevention of crises. | |
| Pharmacokinetic study of aspartame, with no measure of outcomes of interest. | |
| RCT of pentoxifylline. There is no evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the red cell membrane. | |
| RCT of 12C79, which prevents sickling primarily by increasing oxygen affinity, with possible secondary effects on the potassium‐chloride co‐transport channels in the red cell membrane. | |
| Trial of aspirin. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of the sickle red cells. | |
| Study not randomised, looks at promazine chloride. | |
| Study of diflusinal, anti‐sickling effects not due to red cell dehydration. | |
| Phase II RCT of N‐Acetylcysteine for prevention of sickle cell related vaso‐occlusion and formation of dense cells. There is insufficient evidence that N‐Acetylcysteine acts via red cell dehydration to include this study in the review. | |
| RCT of pentoxifylline. There is no evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the cell membrane. | |
| RCT of piracetam. There is a lack of evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the cell membrane. | |
| RCT of pentoxifylline. There is no evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the cell membrane. | |
| Pharmacokinetic study of diltiazem, with no measure of outcomes of interest. | |
| RCT of ticlopidine. Mainly an inhibitor of platelet action, rather than anti‐dehydration. | |
| RCT of pentoxifylline. There is no evidence that the anti‐sickling effect of the drug is due to a reduction in water loss through the cell membrane. | |
| Non‐randomised ex‐vivo study of sodium cromoglicate, an anti‐sickling agent, majority of patients also taking hydroxyurea. | |
| RCT of urea. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of the sickle red cells. | |
| RCT of urea. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of the sickle red cells. | |
| RCT of urea. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of the sickle red cells. | |
| RCT of Niprisan, a naturally occurring compound. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of red cells. | |
| Trial of aspirin. There is no evidence that any anti‐sickling action is due to a reduction in dehydration of the sickle red cells. |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Administration of magnesium aspartate in people with HbS/b‐Thal. |
| Methods | |
| Participants | Adults with sickle cell disease or beta‐thalassaemia in Greece. |
| Interventions | Magnesium (aspartate). |
| Outcomes | RBC magnesium, mean cell haemoglobin concentration, reticulocytes, dense red blood cells. |
| Starting date | 1999 |
| Contact information | Thalassaemia Unit, First Department of Medicine, University of Athens, Laikon Hospital, Athens, Greece. |
| Notes |
RBC: red blood cell
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Anti‐sickling drug versus placebo, Outcome 1 Mortality. | ||||
| 2 Number of pain crises | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Number of other serious sickle‐related complications Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Anti‐sickling drug versus placebo, Outcome 3 Number of other serious sickle‐related complications. | ||||
| 3.1 Overall | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Red cell dehydration | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mean cell volume | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Mean cell haemoglobin concentration | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Full blood count (1) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Haemoglobin (g/dl) | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 HbF (%) | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Full blood count (2) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 White blood cell count (x10e9/l) | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Anti‐sickling drug versus placebo, Outcome 7 Quality of life measures. | ||||
| 7.1 Hospital stay per crisis (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Loss of work days/crisis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Number of hospitalisations per year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Other sickle related events | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse drug reactions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

Comparison 1 Anti‐sickling drug versus placebo, Outcome 1 Mortality.
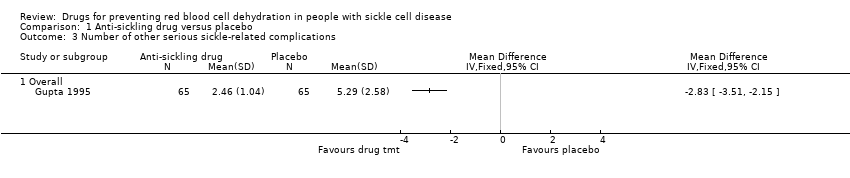
Comparison 1 Anti‐sickling drug versus placebo, Outcome 3 Number of other serious sickle‐related complications.

Comparison 1 Anti‐sickling drug versus placebo, Outcome 7 Quality of life measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of pain crises | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Number of other serious sickle‐related complications Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Overall | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Red cell dehydration | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mean cell volume | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Mean cell haemoglobin concentration | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Full blood count (1) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Haemoglobin (g/dl) | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 HbF (%) | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Full blood count (2) | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 White blood cell count (x10e9/l) | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life measures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Hospital stay per crisis (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Loss of work days/crisis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Number of hospitalisations per year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Other sickle related events | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse drug reactions | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

