Estrategias para el tratamiento de la disfunción sexual inducida por la medicación antidepresiva
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, multicentreD (37 sites), parallel group, 4‐week duration | |
| Participants | 288 adults (ages 22‐67 years) randomised (84 men, 204 women). Inclusion criteria: sexually active at least once a week; sexual dysfunction treatment‐emergent; ASEX score 19 or more; stable dosage of fluoxetine or paroxetine for at least 8 weeks prior to screening and for 3 months. Exclusion criteria: HAM‐D score of 12 or more | |
| Interventions | 1. VML‐670 (300 μg once daily) or, 2. Placebo daily | |
| Outcomes | Assessed with following scales: ASEX, CGI, HAM‐D | |
| Notes | 37 UK primary care sites VML‐670 is a 5‐HT1A receptor agonist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | All study personnel and participants were blinded to treatment assignment for duration of the study |
| Blinding of participants and personnel (performance bias) | Low risk | All study personnel and participants were blinded to treatment assignment for duration of the study |
| Blinding of outcome assessment (detection bias) | Low risk | All study personnel and participants were blinded to treatment assignment for duration of the study |
| Incomplete outcome data (attrition bias) | Low risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, 2 period cross‐over design, 2 weeks of active treatment | |
| Participants | 12 men (aged 18‐65 years) randomised Inclusion criteria: In remission from panic disorder; treated with clomipramine; ejaculatory delay or anorgasmia (no participants with erectile dysfunction) | |
| Interventions | 1. Bethanecol chloride 20 mg as needed (taken 45 minutes before sexual intercourse on up to 2 occasions in each 2‐week period), or 2. Placebo | |
| Outcomes | Changes in VAS sexual function scale | |
| Notes | Trial performed in Brazil Bethanecol has mixed central and peripheral cholinergic and adrenergic effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm, multicentred (3 sites), 4‐weeks duration | |
| Participants | 55 adults (aged 18‐45 years) randomised (48 women, 7 men). | |
| Interventions | 1. Bupropion SR 150 mg (once daily for 3 days, increasing to twice daily if tolerated) in addition to SSRI, or 2. Placebo twice daily, in addition to SSRI | |
| Outcomes | CSFQ, HAM‐D | |
| Notes | USA sites Bupropion is thought to act by dual inhibition of norepinephrine and dopamine reuptake | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm trial, 6‐week duration | |
| Participants | 41 adults (aged 18‐60 years) randomised (17 men, 24 women). Inclusion criteria: stable dose of fluoxetine, paroxetine, citalopram or sertraline for at least 6 weeks; sexual side effects which participants believed were temporally related to the antidepressant use; ASEX score of at least 15 | |
| Interventions | 1. Bupropion SR 150 mg once daily for six weeks, in addition to current SSRI, or 2. Placebo for six weeks, in addition to current SSRI | |
| Outcomes | ASEX, HAM‐D, Beck Depression Inventory | |
| Notes | Trial performed in USA Bupropion is thought to act by dual inhibition of norepinephrine and dopamine reuptake | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | High risk | No outcome data reported for secondary measures |
| Methods | Parallel group trial, 12 weeks of treatment | |
| Participants | 20 adults (3 men, 17 women) Inclusion criteria: depression in remission (HAM‐D < 10); SSRI‐induced sexual dysfunction for at least 4 weeks (no sexual dysfunction before antidepressant, clear temporal relationship between antidepressant and dysfunction) | |
| Interventions | 1. Maca root (Lepidium meyenii) extract 3.0 g/day, or 2. Maca root (L meyenii) extract 1.5 g/day | |
| Outcomes | ASEX, MGH‐SFQ, HAM‐D, HAM‐A | |
| Notes | Trial performed in USA Maca, also known as “Peruvian Ginseng,” is a plant traditionally used for medicinal purposes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Parallel group trial, 12‐week duration | |
| Participants | 54 men (aged 23‐74 years) randomised. Inclusion criteria: sexual dysfunction emerged during antidepressant (SRI) treatment; dysfunction continued for 4 weeks or more | |
| Interventions | 1. Tadalafil 20 mg, or 2. Placebo Tadalafil or placebo taken 1 h before sexual activity, and not more than once daily | |
| Outcomes | IIEF; SEP diary; Global Assessment Questions | |
| Notes | Trial performed in Turkey Tadalafil is a phosphodiesterase type 5 inhibitor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States 'randomly assigned', but no details provided on method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Participants, physicians and data collators were unaware of the treatment assignment of the patients' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Participants, physicians and data collators were unaware of the treatment assignment of the patients' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Participants, physicians and data collators were unaware of the treatment assignment of the patients' |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | All outcomes described |
| Methods | Double‐blind, parallel‐arm trial, 6‐week duration | |
| Participants | 142 men (aged 27‐74 years) randomised. Inclusion criteria: SSRI‐associated erectile dysfunction (Sexual Health Inventory for Men score > 20); no erectile dysfunction prior to antidepressant; DSM‐IV major depressive disorder currently in remission (HAM‐D < 10); taking SRI for 8 weeks or more, and stable dose for 4 weeks | |
| Interventions | 1. Sildenafil 50 mg daily initially then variable dose later (25‐100 mg depending on efficacy and tolerability), or 2. Placebo | |
| Outcomes | IIEF, Erectile Dysfunction Inventory of Treatment Satifisaction, Global Efficacy Questionnaire, HAM‐D, Beck Anxiety Inventory | |
| Notes | Centres in USA, Germany, UK, Canada Sildenafil is a phosphodiesterase inhibitor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a computer algorithm of random permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm, multicentreD (9 sites) trial, 10‐week duration | |
| Participants | 75 adults (aged 18‐65 years) randomised (34 women, 38 men). | |
| Interventions | 1‐week washout period when sertraline treatment suspended, then 7‐10 day placebo lead in, then: 1. Nefazodone 100 mg twice daily increasing to 200 mg after 1 week, or 2. Sertraline 50 mg once daily increasing to 100 mg after 1 week and placebo at night | |
| Outcomes | Physician's rating of sexual dysfunction, modified version of Rush‐Presbyterian Sexual Function Inventory, HAM‐D, CGI (for depressive symptoms) | |
| Notes | Trial performed in USA, 9 sites | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of sequence generation method provided |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Unclear risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Unclear risk | Three of 75 participants randomised excluded from efficacy analyses |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm trial, 8‐week duration | |
| Participants | 23 men (aged 30‐64 years). Inclusion criteria: clinically recovered mood or anxiety disorder; SSRI associated sexual dysfunction (SSRI or venlafaxine); medications stable for 4 weeks prior and during study | |
| Interventions | 1. Sildenafil 50‐100 mg once daily for 8 weeks, or 2. Placebo for 8 weeks | |
| Outcomes | IIEF, ASEX, Erectile Dysfunction Inventory of Treatment Satifsaction, Rigiscan | |
| Notes | Multicentred Sildenafil is a phosphodiesterase inhibitor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported (abstract, not full publication) |
| Methods | Cross‐over design, 2 sequential 3‐week trials | |
| Participants | 33 participants (11 men, 22 women). Inclusion criteria: sexual dysfunction after starting SRI treatment; DSM‐IV major depression, bipolar disorder, or obsessive compulsive disorder; fluoxetine or sertraline taken for at least 8 weeks prior to study entry | |
| Interventions | 1. Yohimbine 5.4 mg three times daily, or 2. Placebo three times daily | |
| Outcomes | 4‐point scales for libido, orgasm, erection (men), mood, anxiety, sleep disturbance, gastrointestinal distress, flushing | |
| Notes | Trial performed in USA Yohimbine blocks alpha‐2 adrenoceptors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear whether more than 33 people randomised |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm trial, 14‐day duration | |
| Participants | 12 women (age range unclear) randomised. Inclusion criteria: antidepressant‐induced sexual dysfunction; past diagnosis of depression (by MINI neuropsychiatric interview); no change in psychotropic treatment in previous 2 months; no comorbid psychiatric or medical disorder; no past history of sexual dysfunction | |
| Interventions | 1. Granisetron (dose not specified), or 2. Placebo | |
| Outcomes | FSFSQ, ASEX, CGI ratings of depressive symptoms | |
| Notes | Trial performed in South Africa. Granisetron is a 5‐HT3 antagonist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of random sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm, single‐centre trial, 2‐month duration | |
| Participants | 37 adults randomised (27 men, 10 women). | |
| Interventions | 1. Ginkgo biloba (EGb761) 120 mg/day increasing to 160 mg/ day after 2 weeks, and increasing to 240 mg/day after 4 weeks, or 2. Placebo | |
| Outcomes | Investigator‐developed questionnaire, Beck Depression Inventory (Korean version), State‐Trait Anxiety Inventory | |
| Notes | Trial performed in South Korea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random number table' |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm trial, 3‐week duration | |
| Participants | 31 adults randomised (breakdown by gender unknown). | |
| Interventions | 1. Bupropion SR 150 mg daily, or 2. Placebo | |
| Outcomes | ASEX, HAM‐D, UKU side effects rating scale | |
| Notes | Trial performed in the USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, cross‐over design, 8‐week duration | |
| Participants | 29 women randomised. Inclusion criteria: SSRI for depression; at least 10 weeks treatment; treatment otherwise successful; sexual dysfunction onset not less than 1 week and not more than 3 months after beginning SSRI; sexual dysfunction distinctly different from any noticed during depressed phase | |
| Interventions | 1. Ephedrine 50 mg once daily, or 2. Placebo Treatments to be taken approximately 1 h before sexual activity | |
| Outcomes | BISF‐W, Beck Depression Inventory | |
| Notes | Trial performed in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation method provided |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Low risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm, multicentred trial (3 sites), 8 weeks of treatment | |
| Participants | 61 women (aged 50 years or younger) randomised. | |
| Interventions | 1. Buspirone 10 mg twice daily increasing to 15 mg in addition to fluoxetine, or 2. Amantadine 50 mg once daily increasing to 50 mg twice daily in addition to fluoxetine, or 3. Placebo twice daily, in addition to fluoxetine | |
| Outcomes | Interviewer Rating of Sexual Function, Participant‐rated VAS for sexual function, Clinician‐rated global impression and participant‐rated global impression, HAM‐D, Beck Depression Inventory, State‐Trait Anxiety Inventory | |
| Notes | Trial performed in USA. Buspirone is a 5HT‐1A agonist Amantadine is thought to increase dopamine availability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of sequence generation method provided |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Patients and efficacy raters were blinded to treatment assignment and to the criteria for study entry and dose adjustments' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Patients and efficacy raters were blinded to treatment assignment and to the criteria for study entry and dose adjustments' |
| Blinding of outcome assessment (detection bias) | Low risk | Patients and efficacy raters were blinded to treatment assignment and to the criteria for study entry and dose adjustments |
| Incomplete outcome data (attrition bias) | Unclear risk | Data analysed from 57 out of total of 61 randomised |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, parallel‐arm. multicentred trial (12 centres), 6‐week treatment period | |
| Participants | 148 women randomised. | |
| Interventions | 1. Mirtazapine 15 mg once daily increasing to 30 mg in addition to fluoxetine, or 2. Yohimbine 5.4 mg once daily increasing to 10.8 mg in addition to fluoxetine, or 3. Olanzapine 2.5 mg once daily increasing to 5 mg in addition to fluoxetine, or 4. Placebo, in addition to fluoxetine. Medications taken daily 1‐2 h before sexual activity | |
| Outcomes | Participant‐assessment of sexual function, Daily diary VAS, Kinsey Ratings of Sexual Function ‐ computer‐assisted structured interview | |
| Notes | Trial performed in USA. Mirtazapine is a 5HT2 and alpha‐2 adrenergic antagonist. Yohimbine is an alpha‐2 adrenergic antagonist. Olanzapine is a 5HT2 antagonist (and dopamine antagonist) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data on sequence generation method provided |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, cross‐over design, 6‐week duration | |
| Participants | 20 adults randomised (2 men, 18 women). | |
| Interventions | 1. Granisetron 1‐2 mg, as required, in addition to SSRI, or 2. Placebo, in addition to SSRI. Medication taken 1‐2 h prior to sexual activity | |
| Outcomes | SSES, HAM‐D | |
| Notes | Trial performed in USA. Granisetron is a 5‐HT3 antagonist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of sequence generation method provided |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | 'Double blind' |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Double‐blind, randomised, placebo‐controlled, multicentred trial, 8‐week duration | |
| Participants | 98 women randomised, aged 18‐50 years. Includsion criteria: substance‐induced sexual dysfunction to DSM‐IV criteria; major depressive disorder, in remission; taking antidepressant with serotonin reuptake inhibition action for at least 8 weeks; persistent sexual dysfunction for at least 4 weeks | |
| Interventions | 1. Sildenafil, flexible dose between 50 mg and 100mg, or 2. Placebo Medication taken 1‐2 h before sexual activity, not more than once daily | |
| Outcomes | CGI scale, Sexual Function Questionnaire, ASEX, University of New Mexico Sexual Function Inventory ‐ female version | |
| Notes | Trial performed in USA ‐ 7 centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used an unrestricted computer‐generated randomisation schedule using SPSS version 10, restriction to this randomisation was that the groups had to be of equal size |
| Allocation concealment (selection bias) | Low risk | The randomisation schedule was given to an independent pharmacy. Medications were sealed in sequentially‐numbered identical containers according to allocation sequence |
| Blinding (performance bias and detection bias) | Low risk | All study personnel and participants were blinded to treatment assignment for the duration of the study |
| Blinding of participants and personnel (performance bias) | Low risk | All study personnel and participants were blinded to treatment assignment for the duration of the study |
| Blinding of outcome assessment (detection bias) | Low risk | All study personnel and participants were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Multicentred, double‐blind, placebo‐controlled trial, 8‐week duration, followed by single‐blind, open‐label 8‐week extension | |
| Participants | 150 women. Inclusion criteria: major depressive disorder in remission; serotonergic reuptake inhibitor‐associated female sexual dysfunction; no pre‐existing sexual dysfunction; 38 weeks stable dose SRI; HAM‐D score < 10; significant sexual dysfunction by CGI‐SF | |
| Interventions | 1. Sildenafil (50‐100 mg flexible dose), or 2. Matching placebo | |
| Outcomes | CGI‐SF, HAM‐D | |
| Notes | Limited information, no outcome data available from end of randomised phase. Outcomes reported after further open‐label sildenafil treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‐ |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Unclear risk | ‐ |
| Blinding of participants and personnel (performance bias) | Unclear risk | ‐ |
| Blinding of outcome assessment (detection bias) | Unclear risk | ‐ |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Multicentred (3 centres), double‐blind, parallel‐arm trial, 6‐week duration | |
| Participants | 90 men (aged 18‐55 years) randomised. | |
| Interventions | 1. Sildenafil 50 mg, as required, increasing to 100 mg, as required, in addition to SSRI, or 2. Placebo in addition to SSRI | |
| Outcomes | IIEF, ASEX, MGH‐SFQ, HAM‐D | |
| Notes | Trial performed in USA ‐ 3 centres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Developed an unrestricted computer‐generated randomisation schedule using SPSS version 10 |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule was given to an independent pharmacy. Medications were sealed in sequentially‐numbered, identical containers according to allocation sequence |
| Blinding (performance bias and detection bias) | Low risk | All study personnel and patents were blinded to treatment for the duration of the study |
| Blinding of participants and personnel (performance bias) | Low risk | All study personnel and patents were blinded to treatment for the duration of the study |
| Blinding of outcome assessment (detection bias) | Low risk | All study personnel and patents were blinded to treatment for the duration of the study |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Parallel‐group design, 12‐week randomised phase | |
| Participants | 234 men (aged 25‐50 years). Inclusion criteria: major depressive disorder currently in remission; stable dose of SSRI for at least 6 months; new sexual dysfunction for at least 4 weeks | |
| Interventions | 1. Bupropion SR 150 mg twice daily, or 2. Placebo | |
| Outcomes | CGI‐SF, IIEF, ASEX, Erectile Dysfunction Inventory of Treatment Satisfaction (patient and partner versions), HAM‐D and HAM‐A | |
| Notes | Trial performed in Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation number for assignment to treatment group was determined using random permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | 'Double blind' |
| Blinding of participants and personnel (performance bias) | Low risk | 'Double blind' |
| Blinding of outcome assessment (detection bias) | Low risk | Investigator was not involved in the recruitment procedure and did not know the randomisation list. Seals were broken after the trial |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Parallel‐group design, 12 weeks of randomised treatment after 1 week placebo run‐in | |
| Participants | 218 women (aged 25‐45). Inclusion criteria: SSRI‐induced sexual dysfunction; first episode of major depressive disorder in remission | |
| Interventions | 1. Bupropion SR 150 mg twice daily, or 2. Placebo | |
| Outcomes | CGI‐SF, Female Sexual Function Index, HAM‐D | |
| Notes | Trial performed in Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Methods | Triple‐blind, parallel‐arm design. 12 weeks of treatment after 1 week run‐in without treatment | |
| Participants | 24 adults randomised (14 men, 10 women), aged 18‐65 years. Inclusion criteria: taking antidepressant for at least 2 weeks and experiencing sexual problems as a consequence | |
| Interventions | 1. Ginkgo biloba (LI‐156) 240 mg daily, or 2. Placebo daily | |
| Outcomes | Changes in sexual dysfunction scale, ASEX, University of Mexico Sexual Function Inventory | |
| Notes | Trial performed in the UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | ‐ |
| Blinding (performance bias and detection bias) | Unclear risk | Allocation blinded to participant, investigator and statistician |
| Blinding of participants and personnel (performance bias) | Unclear risk | Allocation blinded to participant, investigator and statistician |
| Blinding of outcome assessment (detection bias) | Unclear risk | ‐ |
| Incomplete outcome data (attrition bias) | Low risk | ‐ |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
Abbreviations
< = less than
> = more than
ASEX = Arizona Sexual Experiences scale
BISF‐W = Brief Index of Sexual Functioning for Women
CGI = Clinical Global Impression scale
CGI‐SF = Clinical Global Impression scale for Sexual Function
CSFQ = Changes in Sexual Functioning Questionnaire
DSM‐III‐R = Diagnostic and Statistical Manual of American Psychiatric Association, third edition, revised
DSM‐IV = Diagnostic and Statistical Manual of American Psychiatric Association, fourth edition
FSFSQ = Feiger Sexual Function and Satisfaction Questionnaire
h = hour(s)
HAM‐A = Hamilton rating scale for anxiety
HAM‐D = Hamilton rating scale for depression
IIEF = International Index of Erectile Function
MGH‐SFQ = Massachusetts General Hospital‐Sexual Function Questionnaire
MINI = Mini‐Mental State Examination
SEP diary = Sexual Encounter Profile diary
SR = sustained release
SRI = Serotonin Reuptake Inhibitor
SSES = Sexual Side Effects Scale
SSRI = Selective Serotonin Reuptake Inhibitor
VAS = Visual Analogue Scale
UKU = Udvalg fur Kliniske Undersogelser
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Non‐randomised design | |
| Not established as antidepressant‐induced sexual dysfunction: depressed men, also hypogonadal (low or low‐normal testosterone levels) | |
| Non‐randomised design | |
| Non‐randomised design | |
| Non‐randomised design | |
| Not established as antidepressant‐induced sexual dysfunction: changes in sexual dysfunction ratings during treatment for resistant depression | |
| Non‐randomised design | |
| Not established as antidepressant‐induced sexual dysfunction: changes in sexual dysfunction ratings during treatment for resistant depression | |
| Not a study of sexual dysfunction effects (safety evaluation) | |
| Review article | |
| Pooled analysis of subgroup data from multiple individual studies; participants taking antidepressant medication, but not established as antidepressant‐induced sexual dysfunction | |
| Non‐randomised design | |
| Not established as antidepressant‐induced sexual dysfunction: changes in sexual dysfunction ratings during treatment for resistant depression | |
| Non‐randomised design | |
| Non‐randomised design | |
| Pooled analysis of subgroup data from multiple individual studies; participants taking antidepressant medication, but not established as antidepressant‐induced sexual dysfunction | |
| Not established as antidepressant‐induced sexual dysfunction: persistent erectile dysfunction after depression was brought into remission with or without antidepressant medication | |
| Non‐randomised design | |
| Non‐randomised design |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized allocation |
| Participants | Women, aged 18‐50 years. Inclusion criteria: mild or remitted depressive disorder; SSRI or SNRI; decreased sexual desire and distress present for at least 4 weeks |
| Interventions | 1. Flibanserin, or 2. Placebo |
| Outcomes | |
| Notes |
| Methods | Random allocation |
| Participants | 38 women. Inclusion criteria: major depression; stabilized on fluoxetine 40 mg/day for a minimum of 6 weeks; had experienced subjective feeling of sexual dysfunction |
| Interventions | 1. Saffron (30 mg/daily), for 4 weeks, or 2. Placebo for 4 weeks |
| Outcomes | FSFI |
| Notes |
| Methods | Randomised allocation |
| Participants | Women, aged 45‐65 years. Inclusion criteria: post‐menopause; persistent depression despite SSRI treatment |
| Interventions | 1. Testosterone cream, or 2. Placebo |
| Outcomes | |
| Notes | Study title 'Testosterone Antidepressant Augmentation in Women' |
| Methods | Randomised, double‐blind, placebo‐controlled study |
| Participants | 36 men Inclusion criteria: major depressive disorder; depressive symptoms stabilized on fluoxetine; subjective complaints of sexual impairment |
| Interventions | 1. Saffron (15 mg twice per day) for 4 weeks, or 2. Placebo for 4 weeks |
| Outcomes | IIEF scale |
| Notes |
Abbreviations
IIEF = International Index of Erectile Function
SSRI = Selective Serotonin Reuptake Inhibitor
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Trazodone for SSRI‐SD in Civilian Administration Division of Beitou Armed Forces Hospital |
| Methods | Randomized, placebo‐control. cross‐over design, 6‐week duration |
| Participants | Inclusion criteria: participants 20‐65 years of age; receiving SSRI treatment for > 4 weeks; minimal dose of fluoxetine, paroxetine, and citalopram 20 mg/day; minimal dose of fluvoxamine and sertraline 50 mg/day; and minimal dose of escitalopram 10 mg/day; developing sexual dysfunction based on the definition of ASEX Chinese version |
| Interventions | 1. Trazodone 50 mg/day titrated to 100 mg/day over 1 week, then maintained, or 2. Placebo |
| Outcomes | Primary outcomes: differences between trazodone and placebo in the ASEX Chinese version at the end of week 6. Secondary outcomes assessed include: difference between trazodone and placebo in the CGI scale, 10‐point VAS, HAM‐D, and HAM‐A at the end of week 6. Relationships between 5‐HT2A polymorphism and changes in ASEX Chinese version also evaluated. |
| Starting date | 2010 |
| Contact information | Kuo‐Tung Chiang, MD |
| Notes |
| Trial name or title | A double‐blind, placebo‐controlled study of maca root for the treatment of antidepressant‐induced sexual dysfunction in women |
| Methods | Randomized. 12‐week duration |
| Participants | Women, 18‐80 years |
| Interventions | 1. Maca root 3 g/day, or 2. Placebo |
| Outcomes | Decrease in baseline ASEX and MGH‐Sexual Dysfunction scores |
| Starting date | 2007 |
| Contact information | Christina Dording, MD, Massachussetts General Hospital |
| Notes |
| Trial name or title | Treatment of sexual dysfunction secondary to antidepressant pharmacotherapy: a double‐blind comparison of Requip (Ropinirole) vs placebo in patients taking SSRI antidepressants |
| Methods | Randomized, cross‐over trial, 6‐ week duration |
| Participants | Inclusion criteria: male or female outpatients, 18‐65 years old; currently taking fluoxetine, sertraline, paroxetine, citalopram, or escitalopram at a stable dosage within the ranges specified for 1 month or longer; required dosage range: (Prozac (fluoxetine) 20‐80 mg/day; Celexa (citalopram) 20‐60 mg/day; Lexapro (escitalopram) 10‐30 mg/day; Zoloft (sertraline) 50‐200 mg/day; Paxil (paroxetine) 20‐60 mg/day; Paxil CR (paroxetine CR) 25‐75 mg/day; currently responding to SSRI antidepressant treatment, as indicated by a score of 15 or less on the HAM‐D 24‐item at screening and baseline, and (b) CGI‐Severity score of 2 or less at baseline; meets DSM‐IV criteria for Substance‐Induced Sexual Dysfunction, with impairment of desire, arousal, or orgasm; currently involved in an intimate relationship that includes sexual contact; agree to use double‐barrier contraception during sexual intercourse during the course of the study (women only); agree to let the study team contact the physician who prescribed the SSRI medication to inform him/her of patient's participation in the current study |
| Interventions | 1. Ropinirole 1 mg extended release formulation given once/day to a maximum of 4/day 2. placebo |
| Outcomes | Primary outcomes: IIEF, SFSQ |
| Starting date | 2006 |
| Contact information | David J Hellerstein, MD St. Luke's Roosevelt Hospital Center and NY State Psychiatric Institute |
| Notes |
| Trial name or title | Ginkgo Biloba: Antidepressant‐Induced Sexual Dysfunction |
| Methods | Parallel‐group design |
| Participants | 36 women (age 18‐65 years). Inclusion criteria: stabilised on antidepressant medication and free of a current Axis I disorder |
| Interventions | 1. Ginkgo biloba extract 200 mg for 8 weeks, or 2. Placebo for 8 weeks |
| Outcomes | Daily participant diary, participant‐rating scales, blind independent evaluator ratings, vaginal photoplethysmography |
| Starting date | June 2002 |
| Contact information | Cindy Meston, University of Texas at Austin |
| Notes | Outcomes for those with antidepressant‐associated sexual dysfunction not yet published |
| Trial name or title | A randomised, double‐blind, parallel‐group, active‐controlled, flexible‐dose study evaluating the effect of Lu AA21004 vs escitalopram on sexual functioning in adults with well‐treated major depressive disorder experiencing selective serotonin reuptake inhibitor‐induced sexual dysfunction |
| Methods | Randomised; parallel‐group design; blinding applied to subjects, caregivers, investigators, outcomes assessors, 8‐week treatment duration |
| Participants | Estimated enrolment: 440, aged 18‐55 years; male and female participants. Inclusion criteria: treated for last 8 weeks, or more, with SSRI monotherapy (only citalopram, paroxetine, or sertraline allowed) prescribed to treat a major depressive episode, according to the DSM‐IV‐TR criteria; depression currently stable; subject has a CGI Scale‐Severity of Illness Scale (CGI‐S) score of ≤ 3; currently experiencing treatment‐emergent sexual dysfunction (defined as a CSFQ‐14 total score ≤ 41 for women and ≤ 47 for men); considered to be attributable to the current SSRI monotherapy; suitable for a switch in medication |
| Interventions | 1. Lu AA21004 up to 20 mg daily, or 2. Escitalopram up to 20 mg daily |
| Outcomes | Primary outcome: change from baseline in the CSFQ‐14 Total Score |
| Starting date | June 2011 |
| Contact information | Contact: Takeda Study Registration Call Center |
| Notes | 62 study locations in USA and Canada Lu AA21004 is also known as vortioxetine |
| Trial name or title | Lybrido(s) and SSRIs @Home: a double‐blind, randomised, cross‐over, placebo‐controlled study to investigate the subjective and physiological efficacy and safety of Lybrido and Lybridos in the domestic setting in healthy women with female sexual dysfunction in combination with SSRI use. ‐ @HOME |
| Methods | Cross‐over design in which a placebo regime (duration 4 weeks), the Lybrido regime (duration 4 weeks), and the Lybridos regime (duration 4 weeks) are separated by a 1‐ to 4‐week washout period |
| Participants | Target sample size: 40 Inclusion criteria: women 21‐70 years old with hypoactive sexual desire disorder (comorbidity with other sexual dysfunctions e.g. Female Sexual Arousal Disorder (FSAD) allowed) or SSRI‐induced sexual dysfunctioning, or both; at least 3 months use of an SSRI; SSRI must be on a stable dose for at least 6 weeks |
| Interventions | 1. Lybrido [combination testosterone and sildenafil], or 2. Lybridos [combination testosterone and buspirone], or 3. Placebo |
| Outcomes | Unclear |
| Starting date | January 2010 |
| Contact information | Emotional Brain bv, Louis Armstrongweg 78 1311 RL Almere The Netherlands |
| Notes |
Abbreviations
≤ = less than or equal to
> = more than
ASEX = Arizona Sexual Experiences scale
CGI = Clinical Global Impression scale
CGI‐SF = Clinical Global Impression scale for Sexual Function
CSFQ = Changes in Sexual Functioning Questionnaire
DSM‐IV = Diagnostic and Statistical Manual of American Psychiatric Association, fourth edition
DSM‐IV‐TR = Diagnostic and Statistical Manual of American Psychiatric Association, fourth edition, text revision
FSFI = Female sSexual Function Index
GAFS = Global Assessment of Functioning Scale
HAM‐D = Hamilton rating scale for depression
IIEF = International Index of Erectile Function
MGH = Massachusetts General Hospital
SFSQ = Sexual Function Satisfaction Questionnaire
SNRI = Serotonin‐Norepinephrine Reuptake Inhibitor
SSRI = Selective Serotonin Reuptake Inhibitor
VAS = Visual Analogue Scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint International Index of Erectile Function (IIEF) scores Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Sildenafil vs placebo, Outcome 1 Endpoint International Index of Erectile Function (IIEF) scores. | ||||
| 1.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 19.36 [15.00, 23.72] |
| 1.2 Erectile function (questions 1‐5,15) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [7.39, 12.61] |
| 1.3 Question 3: ability to achieve erection | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [0.65, 1.44] |
| 1.4 Question 4: ability to maintain erection | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [0.78, 1.59] |
| 1.5 Intercourse satisfaction (questions 6, 7, 8) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [2.48, 4.52] |
| 1.6 Orgasmic function (questions 9, 10) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.36, 3.64] |
| 1.7 Sexual desire (questions 11, 12) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.38, 1.38] |
| 1.8 Overall satisfaction (questions 13,14) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.86, 2.74] |
| 2 Endpoint Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Sildenafil vs placebo, Outcome 2 Endpoint Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores. | ||||
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint Clinical Global Impression ‐ Sexual Function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Sildenafil vs placebo, Outcome 3 Endpoint Clinical Global Impression ‐ Sexual Function. | ||||
| 4 Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint Show forest plot | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.33, 0.58] |
| Analysis 1.4  Comparison 1 Sildenafil vs placebo, Outcome 4 Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint. | ||||
| 4.1 Males | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| 4.2 Females | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.62] |
| 5 Endpoint Arizona Sexual Experience Scale (ASEX) total scores Show forest plot | 3 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.65 [‐3.86, ‐1.44] |
| Analysis 1.5  Comparison 1 Sildenafil vs placebo, Outcome 5 Endpoint Arizona Sexual Experience Scale (ASEX) total scores. | ||||
| 5.1 Males | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 5.2 Females | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.24, 1.24] |
| 6 Males: endpoint Arizona Sexual Experience Scale scores Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Sildenafil vs placebo, Outcome 6 Males: endpoint Arizona Sexual Experience Scale scores. | ||||
| 6.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 6.2 Sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.08, ‐0.12] |
| 6.3 Arousal | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.06, ‐0.14] |
| 6.4 Erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.66, ‐0.74] |
| 6.5 Orgasm (ability) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.90, ‐0.90] |
| 6.6 Orgasm (satisfaction) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.58, ‐0.42] |
| 7 Endpoint MGH‐Sexual Functioning Questionnaire scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Sildenafil vs placebo, Outcome 7 Endpoint MGH‐Sexual Functioning Questionnaire scores. | ||||
| 7.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Sildenafil vs placebo, Outcome 8 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint. | ||||
| 9 Dropouts Show forest plot | 4 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.41, 1.14] |
| Analysis 1.9  Comparison 1 Sildenafil vs placebo, Outcome 9 Dropouts. | ||||
| 10 Endpoint Hamilton Rating Scale for Depression score Show forest plot | 2 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.94, 0.07] |
| Analysis 1.10  Comparison 1 Sildenafil vs placebo, Outcome 10 Endpoint Hamilton Rating Scale for Depression score. | ||||
| 11 Loss of remission: Hamilton Rating Scale for Depression score > 9 Show forest plot | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.09] |
| Analysis 1.11  Comparison 1 Sildenafil vs placebo, Outcome 11 Loss of remission: Hamilton Rating Scale for Depression score > 9. | ||||
| 12 Global Efficacy Questionnaire (questions 1 & 2) Show forest plot | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.90, 3.35] |
| Analysis 1.12  Comparison 1 Sildenafil vs placebo, Outcome 12 Global Efficacy Questionnaire (questions 1 & 2). | ||||
| 12.1 Improvement in erections | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [1.67, 3.73] |
| 12.2 Improvement in ability to have sexual intercourse | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.71, 3.80] |
| 13 Global efficacy questionnaire (question 3) Show forest plot | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.65, 1.75] |
| Analysis 1.13  Comparison 1 Sildenafil vs placebo, Outcome 13 Global efficacy questionnaire (question 3). | ||||
| 13.1 Question 3: Frequency of erection that allowed satisfactory sexual intercourse | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.65, 1.75] |
| 14 Endpoint Sexual Function Questionnaire (SFQ) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 Sildenafil vs placebo, Outcome 14 Endpoint Sexual Function Questionnaire (SFQ). | ||||
| 14.1 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Arousal‐sensation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Arousal‐lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.5 Enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.6 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.7 Partner | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 UNM Sexual Function Inventory Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.15  Comparison 1 Sildenafil vs placebo, Outcome 15 UNM Sexual Function Inventory. | ||||
| 15.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Sexual arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.5 Ability to reach orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Females: endpoint Arizona Sexual Experience Scale scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.16  Comparison 1 Sildenafil vs placebo, Outcome 16 Females: endpoint Arizona Sexual Experience Scale scores. | ||||
| 16.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Orgasm (satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.6 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global Assessment Questions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Tadalafil vs placebo, Outcome 1 Global Assessment Questions. | ||||
| 1.1 Has the treatment you have been taking improved your erectile function? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.5 [3.03, 43.67] |
| 1.2 Has the treatment improved your ability to engage in sexual activity? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.5 [3.03, 43.67] |
| 2 Endpoint Sexual Encounter Profile (SEP) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Tadalafil vs placebo, Outcome 2 Endpoint Sexual Encounter Profile (SEP). | ||||
| 2.1 Question 2: Were you able to insert your penis into your partner's vagina? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.68, 8.01] |
| 2.2 Question 3: Did your erection last long enough for you to have successful intercourse? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 2.3 Question 4: Were you satisfied with the hardness of your erection? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 2.4 Question 5: Were you satisfied overall with this sexual experience? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Tadalafil vs placebo, Outcome 3 Dropouts. | ||||
| 3.1 Overall | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3.2 Lack of efficacy | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3.3 Adverse effects | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint scale total scores Show forest plot | 3 | 482 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.40, 1.81] |
| Analysis 3.1  Comparison 3 Bupropion vs placebo, Outcome 1 Endpoint scale total scores. | ||||
| 1.1 International Index of Erectile Function (IIEF) | 1 | 227 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.76 [1.45, 2.07] |
| 1.2 Female Sexual Function Index (FSFI) | 1 | 213 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.73 [1.41, 2.05] |
| 1.3 Changes in Sexual Functioning Questionnaire (CSFQ) | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.11, 1.12] |
| 2 Response (as defined by study) Show forest plot | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 31.77 [10.10, 99.89] |
| Analysis 3.2  Comparison 3 Bupropion vs placebo, Outcome 2 Response (as defined by study). | ||||
| 2.1 50% reduction Arizona Sexual Experiences Scale (ASEX) | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.09, 4.41] |
| 2.2 Clinical Global Impression (CGI‐SF) 2‐point improvement | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 186.73 [11.74, 2969.23] |
| 3 Endpoint International Index of Erectile Function (IIEF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Bupropion vs placebo, Outcome 3 Endpoint International Index of Erectile Function (IIEF). | ||||
| 3.1 Total score | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 20.10 [17.14, 23.06] |
| 3.2 Erectile function | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 9.30 [8.18, 10.42] |
| 3.3 Orgasmic function | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [2.15, 3.25] |
| 3.4 Sexual desire | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.76, 2.44] |
| 3.5 Intercourse satisfaction | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 3.6 [3.00, 4.20] |
| 3.6 Overall satisfaction | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [1.99, 3.21] |
| 4 Endpoint Female Sexual Function Index score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Bupropion vs placebo, Outcome 4 Endpoint Female Sexual Function Index score. | ||||
| 4.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Endpoint Changes in Sexual Functioning Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Bupropion vs placebo, Outcome 5 Endpoint Changes in Sexual Functioning Questionnaire score. | ||||
| 5.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Desire/interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Desire/frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Arousal/excitement | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Completion/orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Dropouts Show forest plot | 5 | 579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.72] |
| Analysis 3.6  Comparison 3 Bupropion vs placebo, Outcome 6 Dropouts. | ||||
| 7 Endpoint Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Bupropion vs placebo, Outcome 7 Endpoint Hamilton Rating Scale for Depression score. | ||||
| 8 Endpoint Clinical Global Impression (CGI ‐ SF) Show forest plot | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐1.87, ‐1.61] |
| Analysis 3.8  Comparison 3 Bupropion vs placebo, Outcome 8 Endpoint Clinical Global Impression (CGI ‐ SF). | ||||
| 8.1 Male | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.80, ‐1.20] |
| 8.2 Female | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐1.95, ‐1.65] |
| 9 Endpoint ASEX Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.9  Comparison 3 Bupropion vs placebo, Outcome 9 Endpoint ASEX. | ||||
| 9.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Ability to reach orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Satisfaction with orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Endpoint EDITS (participant) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.10  Comparison 3 Bupropion vs placebo, Outcome 10 Endpoint EDITS (participant). | ||||
| 10.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Expectations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Likelihood of continuing | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Confidence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 Partner satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.7 Partner desire to continue treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.8 Naturalness of achieving erection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.9 Naturalness of erection hardness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.10 Quickness of achieving erection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.11 Duration that erection lasts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.12 Ease of use | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Endpoint EDITS (partner) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.11  Comparison 3 Bupropion vs placebo, Outcome 11 Endpoint EDITS (partner). | ||||
| 11.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Expectations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Sexual desirability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Participant's feelings about continuing treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Duration that erection lasts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐emergence of antidepressant‐induced sexual dysfunction (physician rated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Nefazodone vs sertraline, Outcome 1 Re‐emergence of antidepressant‐induced sexual dysfunction (physician rated). | ||||
| 1.1 Week 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Endpoint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall degree of sexual satisfaction (participant rated) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Nefazodone vs sertraline, Outcome 2 Overall degree of sexual satisfaction (participant rated). | ||||
| 2.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Last rating recorded | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Nefazodone vs sertraline, Outcome 3 Dropouts. | ||||
| 3.1 Overall | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Nefazodone vs sertraline, Outcome 4 Hamilton Rating Scale for Depression score. | ||||
| 4.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint sexual function ratings (investigator questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Ginkgo biloba vs placebo, Outcome 1 Endpoint sexual function ratings (investigator questionnaire). | ||||
| 1.1 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Erection maintenance time | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Orgasm frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Satisfaction to orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Sexual Dysfunction Scale (investigator developed) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Ginkgo biloba vs placebo, Outcome 2 Sexual Dysfunction Scale (investigator developed). | ||||
| 2.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Ginkgo biloba vs placebo, Outcome 3 Dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change from baseline on Sexual Side Effects Scale (SSES) total score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Granisetron vs placebo, Outcome 1 Change from baseline on Sexual Side Effects Scale (SSES) total score. | ||||
| 2 Endpoint Feiger Sexual Function and Satisfaction Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Granisetron vs placebo, Outcome 2 Endpoint Feiger Sexual Function and Satisfaction Questionnaire score. | ||||
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Item 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Item 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Item 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Item 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Item 5 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Item 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint Arizona Sexual Experience Scale (ASEX) score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Granisetron vs placebo, Outcome 3 Endpoint Arizona Sexual Experience Scale (ASEX) score. | ||||
| 3.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Orgasm (satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Granisetron vs placebo, Outcome 4 Dropouts. | ||||
| 5 Recurrence of mood symptoms Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 66.75] |
| Analysis 6.5  Comparison 6 Granisetron vs placebo, Outcome 5 Recurrence of mood symptoms. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Absence of sexual dysfunction at end point Show forest plot | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.86, 1.77] |
| Analysis 7.1  Comparison 7 VML‐670 vs placebo, Outcome 1 Absence of sexual dysfunction at end point. | ||||
| 2 'Improved' or 'much improved' on Clinical Global Impression Show forest plot | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| Analysis 7.2  Comparison 7 VML‐670 vs placebo, Outcome 2 'Improved' or 'much improved' on Clinical Global Impression. | ||||
| 3 Change in Arizona Sexual Experiences Scale (ASEX) item scores Show forest plot | 1 | 1264 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.19, 0.05] |
| Analysis 7.3  Comparison 7 VML‐670 vs placebo, Outcome 3 Change in Arizona Sexual Experiences Scale (ASEX) item scores. | ||||
| 3.1 How strong is your sexual drive? | 1 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 3.2 How easily are you sexually aroused? | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.47, 0.07] |
| 3.3 Females: how easily does your vagina become moist or wet? | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 3.4 Males: can you easily get and keep an erection? | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.80, 0.00] |
| 3.5 How easily can you reach orgasm? | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 3.6 Are your orgasms satisfying? | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 4 Dropouts Show forest plot | 1 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.93] |
| Analysis 7.4  Comparison 7 VML‐670 vs placebo, Outcome 4 Dropouts. | ||||
| 4.1 Total | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.68] |
| 4.2 Attributed to adverse effects | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.75, 7.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient‐rated visual analogue scales Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Buspirone vs placebo, Outcome 1 Change in patient‐rated visual analogue scales. | ||||
| 1.1 Overall function (total of interest, lubrication, orgasm, pleasure) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐38.33, 44.53] |
| 1.2 Mood | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐7.61, 9.21] |
| 1.3 Energy | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐3.88, 14.48] |
| 1.4 Interest/desire | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐7.99, 13.39] |
| 1.5 Lubrication | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [‐3.65, 23.45] |
| 1.6 Orgasm | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐6.3 [‐19.98, 7.38] |
| 1.7 Pleasure | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐15.53, 9.13] |
| 1.8 Discomfort | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐3.15, 17.15] |
| 2 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 Buspirone vs placebo, Outcome 2 Dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visual analogue scale of orgasmic function ‐ best score achieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Bethanecol vs placebo, Outcome 1 Visual analogue scale of orgasmic function ‐ best score achieved. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 Olanzapine vs placebo, Outcome 1 Change in patient rated assessment of sexual function. | ||||
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.2  Comparison 10 Olanzapine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales). | ||||
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.3  Comparison 10 Olanzapine vs placebo, Outcome 3 Dropouts due to adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Mirtazapine vs placebo, Outcome 1 Change in patient rated assessment of sexual function. | ||||
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.2  Comparison 11 Mirtazapine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales). | ||||
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint modified Kinsey Structured Interview Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.19, 1.01] |
| Analysis 11.3  Comparison 11 Mirtazapine vs placebo, Outcome 3 Endpoint modified Kinsey Structured Interview. | ||||
| 3.1 Sexual satisfaction | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.19, 1.01] |
| 4 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.4  Comparison 11 Mirtazapine vs placebo, Outcome 4 Dropouts. | ||||
| 4.1 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 Yohimbine vs placebo, Outcome 1 Change in patient rated assessment of sexual function. | ||||
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.2  Comparison 12 Yohimbine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales). | ||||
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.3  Comparison 12 Yohimbine vs placebo, Outcome 3 Dropouts. | ||||
| 3.1 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient‐rated visual analogue scales Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 13.1  Comparison 13 Amantadine vs placebo, Outcome 1 Change in patient‐rated visual analogue scales. | ||||
| 1.1 Overall function (total of interest, lubrication, orgasm, pleasure) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐29.02, 55.02] |
| 1.2 Mood | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 8.1 [1.23, 14.97] |
| 1.3 Energy | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 12.7 [5.30, 20.10] |
| 1.4 Interest/desire | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐7.96, 9.76] |
| 1.5 Lubrication | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐5.74, 21.54] |
| 1.6 Orgasm | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐11.91, 16.91] |
| 1.7 Pleasure | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐11.28, 14.68] |
| 1.8 Discomfort | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐11.25, 13.85] |
| 2 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.2  Comparison 13 Amantadine vs placebo, Outcome 2 Dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint Brief Index of Sexual Functioning for Women (BISF‐W) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 14.1  Comparison 14 ephedrine vs placebo, Outcome 1 Endpoint Brief Index of Sexual Functioning for Women (BISF‐W). | ||||
| 1.1 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual arousability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Lack of vaginal lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Orgasm ability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm intensity/pleasure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Sexual dissatisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint Arizona Sexual Experiences Scale (ASEX) score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 15.1  Comparison 15 Maca root: high vs low dose, Outcome 1 Endpoint Arizona Sexual Experiences Scale (ASEX) score. | ||||
| 1.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Question 1: Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Endpoint MGH‐SFQ Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 15.2  Comparison 15 Maca root: high vs low dose, Outcome 2 Endpoint MGH‐SFQ. | ||||
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Item a: Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.60, 3.74] |
| Analysis 15.3  Comparison 15 Maca root: high vs low dose, Outcome 3 Dropouts. | ||||
| 4 Endpoint ratings of psychiatric symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 15.4  Comparison 15 Maca root: high vs low dose, Outcome 4 Endpoint ratings of psychiatric symptoms. | ||||
| 4.1 Hamilton Depression Rating Scale | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐7.98, 2.98] |
| 4.2 Hamilton Anxiety Rating Scale | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.15, 2.35] |

Study flow diagram
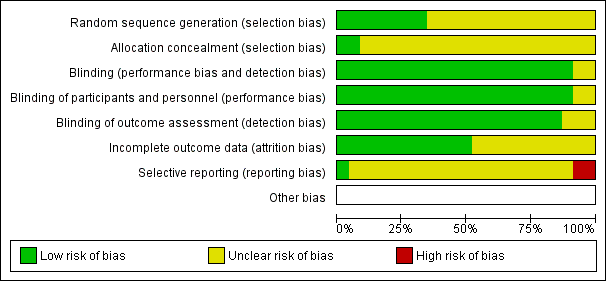
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1: sildenafil vs placebo, outcome: 1.1 endpoint International Index of Erectile Function (IIEF) scores

Forest plot of comparison 1: sildenafil vs placebo, outcome: 1.5 endpoint Arizona Sexual Experience Scale (ASEX) total scores
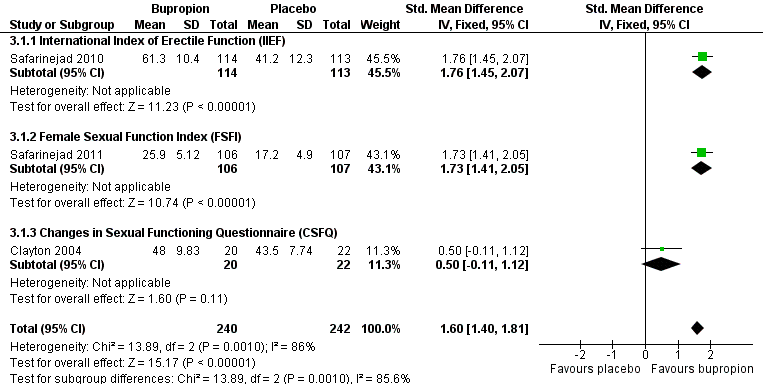
Forest plot of comparison 3: bupropion vs placebo, outcome: 3.1 endpoint scale total scores
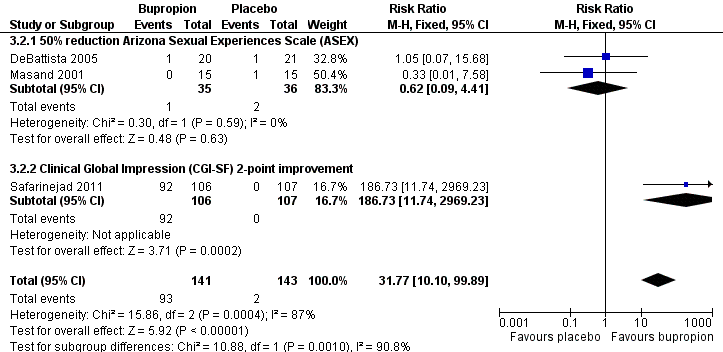
Forest plot of comparison 3: bupropion vs placebo, outcome: 3.2 response (as defined by study)

Comparison 1 Sildenafil vs placebo, Outcome 1 Endpoint International Index of Erectile Function (IIEF) scores.
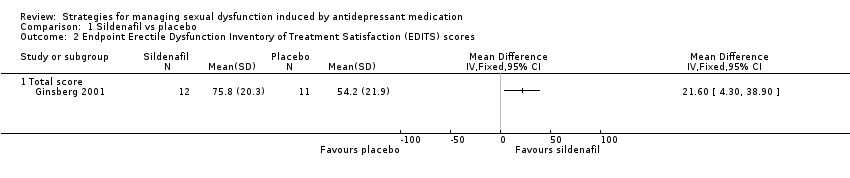
Comparison 1 Sildenafil vs placebo, Outcome 2 Endpoint Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores.
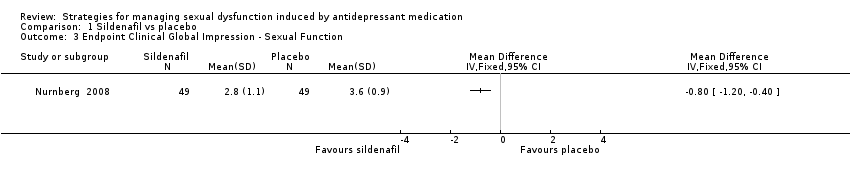
Comparison 1 Sildenafil vs placebo, Outcome 3 Endpoint Clinical Global Impression ‐ Sexual Function.
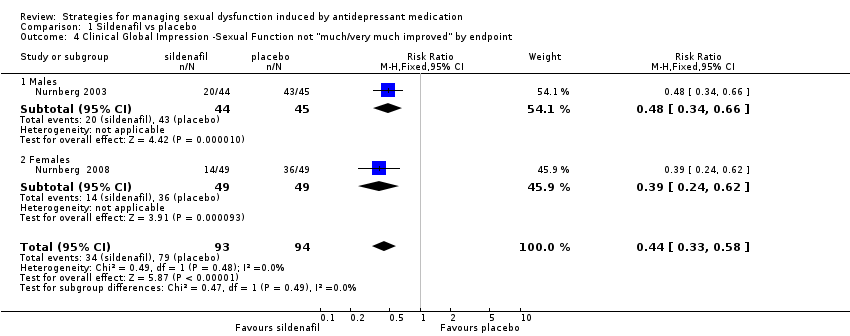
Comparison 1 Sildenafil vs placebo, Outcome 4 Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint.
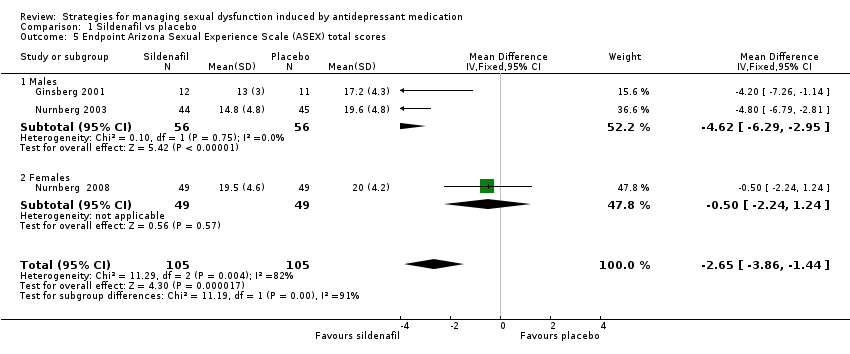
Comparison 1 Sildenafil vs placebo, Outcome 5 Endpoint Arizona Sexual Experience Scale (ASEX) total scores.

Comparison 1 Sildenafil vs placebo, Outcome 6 Males: endpoint Arizona Sexual Experience Scale scores.
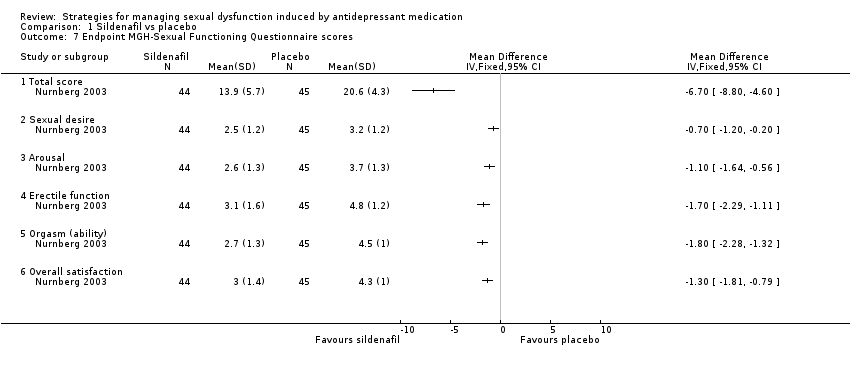
Comparison 1 Sildenafil vs placebo, Outcome 7 Endpoint MGH‐Sexual Functioning Questionnaire scores.

Comparison 1 Sildenafil vs placebo, Outcome 8 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint.

Comparison 1 Sildenafil vs placebo, Outcome 9 Dropouts.
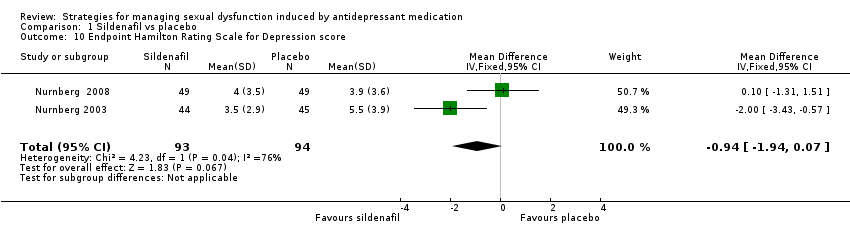
Comparison 1 Sildenafil vs placebo, Outcome 10 Endpoint Hamilton Rating Scale for Depression score.
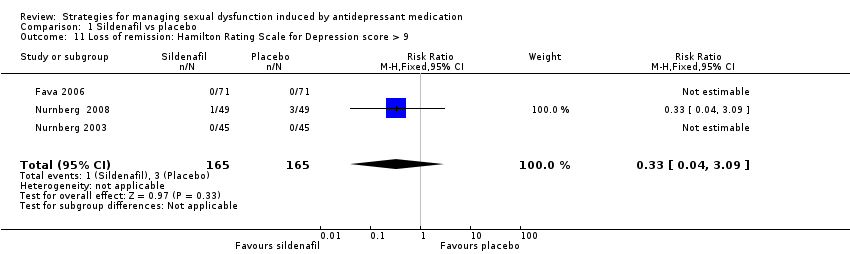
Comparison 1 Sildenafil vs placebo, Outcome 11 Loss of remission: Hamilton Rating Scale for Depression score > 9.

Comparison 1 Sildenafil vs placebo, Outcome 12 Global Efficacy Questionnaire (questions 1 & 2).

Comparison 1 Sildenafil vs placebo, Outcome 13 Global efficacy questionnaire (question 3).

Comparison 1 Sildenafil vs placebo, Outcome 14 Endpoint Sexual Function Questionnaire (SFQ).

Comparison 1 Sildenafil vs placebo, Outcome 15 UNM Sexual Function Inventory.

Comparison 1 Sildenafil vs placebo, Outcome 16 Females: endpoint Arizona Sexual Experience Scale scores.
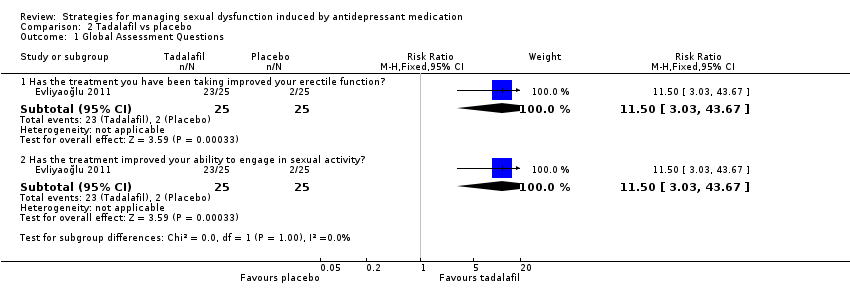
Comparison 2 Tadalafil vs placebo, Outcome 1 Global Assessment Questions.

Comparison 2 Tadalafil vs placebo, Outcome 2 Endpoint Sexual Encounter Profile (SEP).
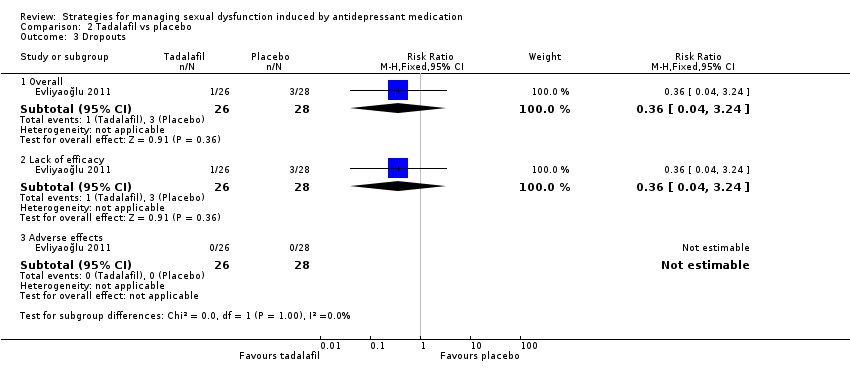
Comparison 2 Tadalafil vs placebo, Outcome 3 Dropouts.

Comparison 3 Bupropion vs placebo, Outcome 1 Endpoint scale total scores.
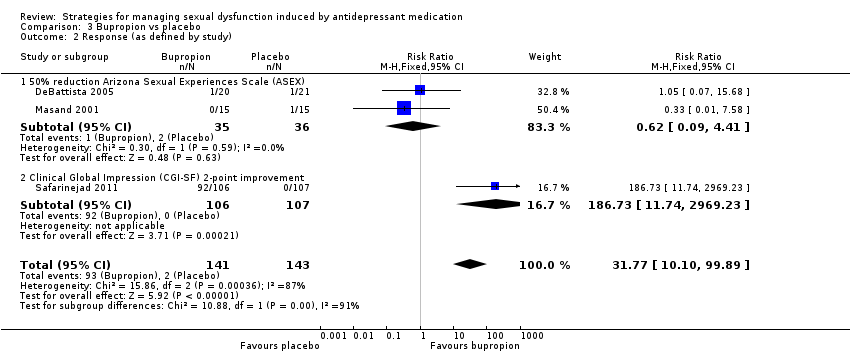
Comparison 3 Bupropion vs placebo, Outcome 2 Response (as defined by study).

Comparison 3 Bupropion vs placebo, Outcome 3 Endpoint International Index of Erectile Function (IIEF).
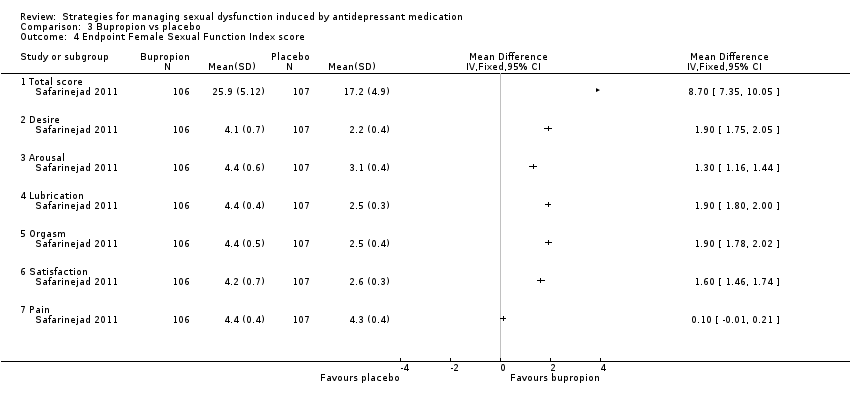
Comparison 3 Bupropion vs placebo, Outcome 4 Endpoint Female Sexual Function Index score.

Comparison 3 Bupropion vs placebo, Outcome 5 Endpoint Changes in Sexual Functioning Questionnaire score.
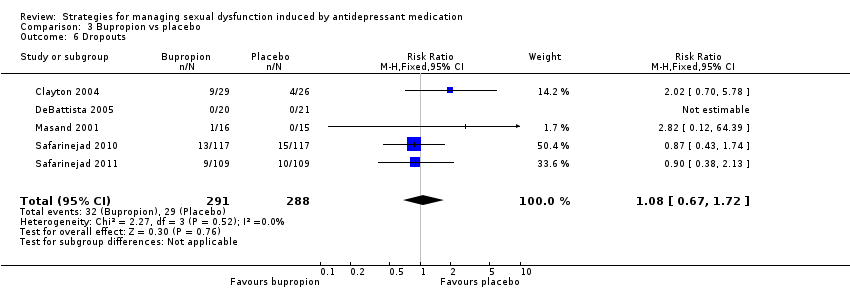
Comparison 3 Bupropion vs placebo, Outcome 6 Dropouts.
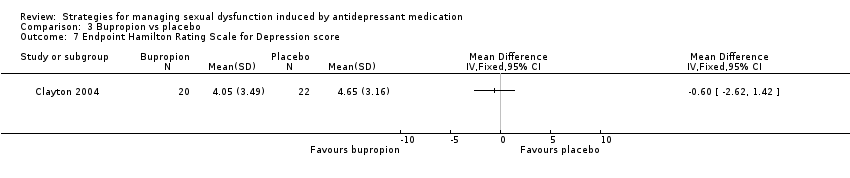
Comparison 3 Bupropion vs placebo, Outcome 7 Endpoint Hamilton Rating Scale for Depression score.

Comparison 3 Bupropion vs placebo, Outcome 8 Endpoint Clinical Global Impression (CGI ‐ SF).

Comparison 3 Bupropion vs placebo, Outcome 9 Endpoint ASEX.
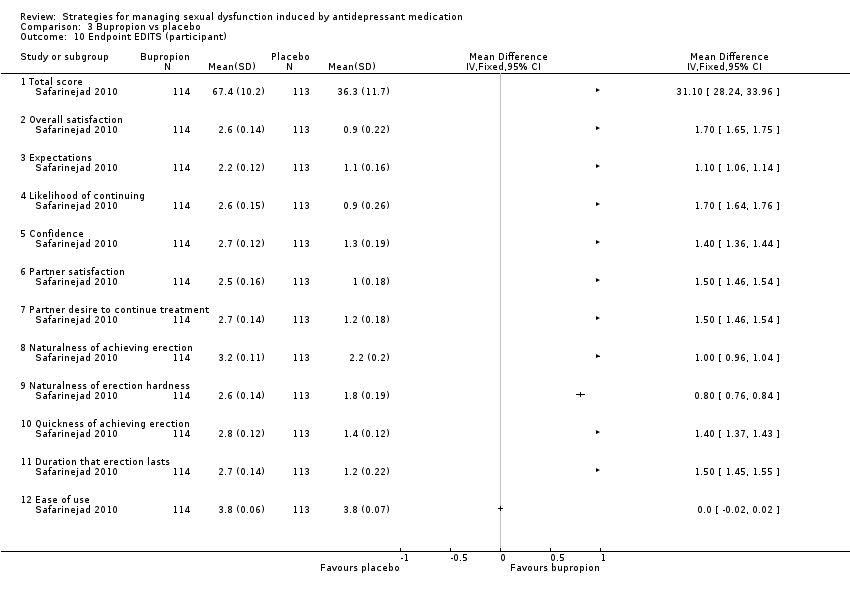
Comparison 3 Bupropion vs placebo, Outcome 10 Endpoint EDITS (participant).

Comparison 3 Bupropion vs placebo, Outcome 11 Endpoint EDITS (partner).
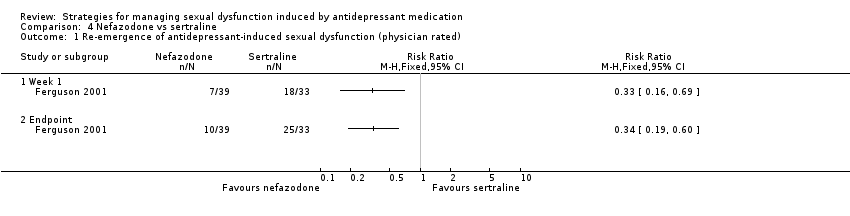
Comparison 4 Nefazodone vs sertraline, Outcome 1 Re‐emergence of antidepressant‐induced sexual dysfunction (physician rated).

Comparison 4 Nefazodone vs sertraline, Outcome 2 Overall degree of sexual satisfaction (participant rated).

Comparison 4 Nefazodone vs sertraline, Outcome 3 Dropouts.
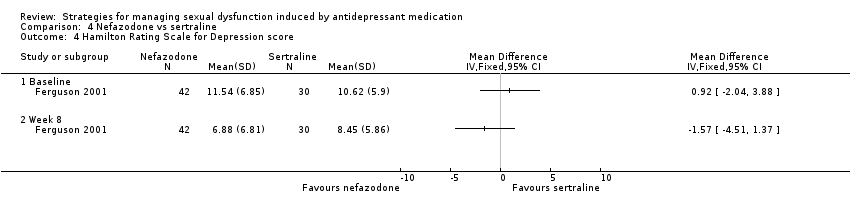
Comparison 4 Nefazodone vs sertraline, Outcome 4 Hamilton Rating Scale for Depression score.

Comparison 5 Ginkgo biloba vs placebo, Outcome 1 Endpoint sexual function ratings (investigator questionnaire).

Comparison 5 Ginkgo biloba vs placebo, Outcome 2 Sexual Dysfunction Scale (investigator developed).

Comparison 5 Ginkgo biloba vs placebo, Outcome 3 Dropouts.

Comparison 6 Granisetron vs placebo, Outcome 1 Change from baseline on Sexual Side Effects Scale (SSES) total score.
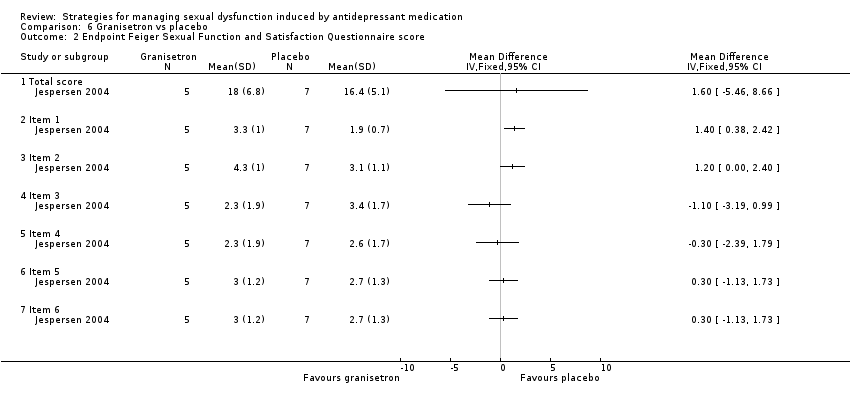
Comparison 6 Granisetron vs placebo, Outcome 2 Endpoint Feiger Sexual Function and Satisfaction Questionnaire score.
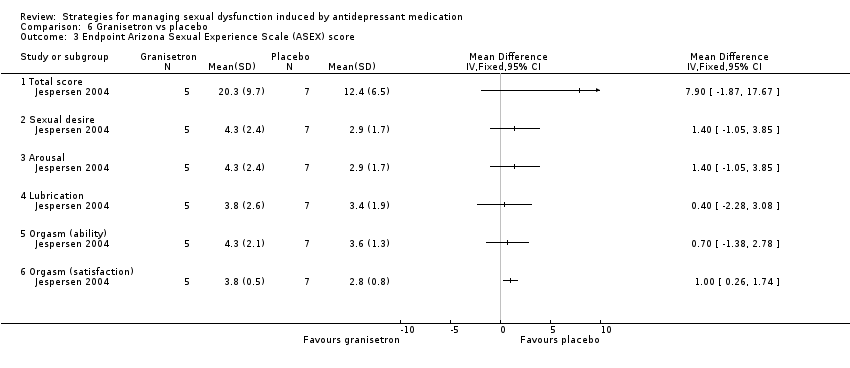
Comparison 6 Granisetron vs placebo, Outcome 3 Endpoint Arizona Sexual Experience Scale (ASEX) score.

Comparison 6 Granisetron vs placebo, Outcome 4 Dropouts.

Comparison 6 Granisetron vs placebo, Outcome 5 Recurrence of mood symptoms.
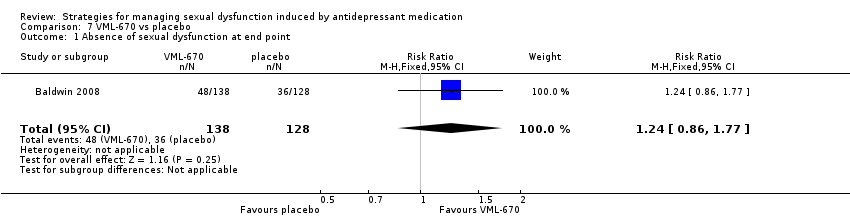
Comparison 7 VML‐670 vs placebo, Outcome 1 Absence of sexual dysfunction at end point.

Comparison 7 VML‐670 vs placebo, Outcome 2 'Improved' or 'much improved' on Clinical Global Impression.
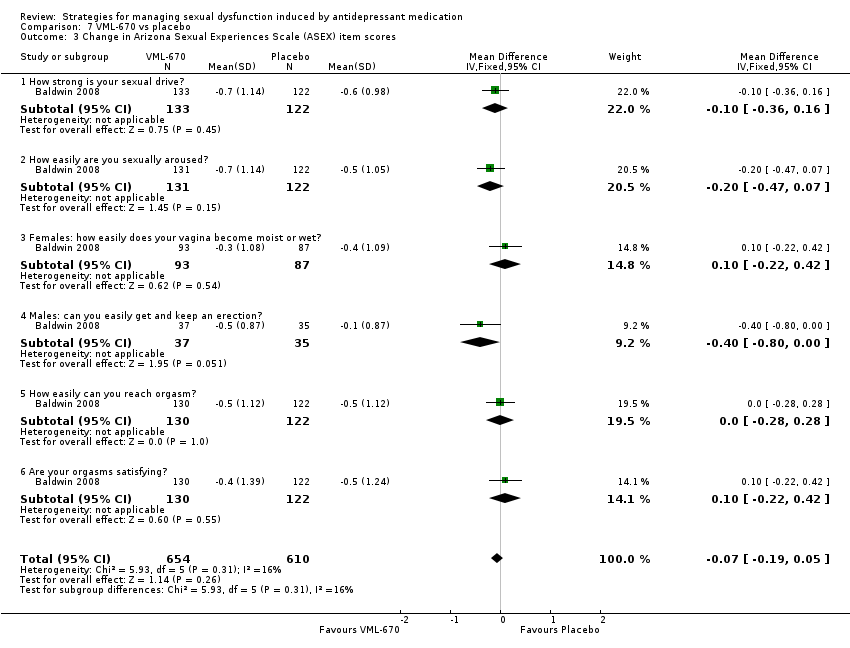
Comparison 7 VML‐670 vs placebo, Outcome 3 Change in Arizona Sexual Experiences Scale (ASEX) item scores.
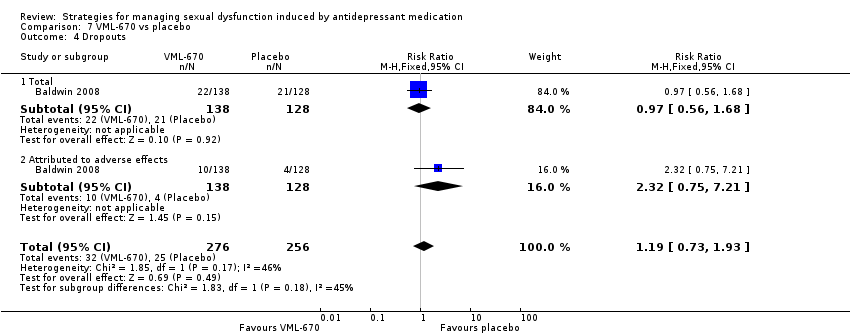
Comparison 7 VML‐670 vs placebo, Outcome 4 Dropouts.

Comparison 8 Buspirone vs placebo, Outcome 1 Change in patient‐rated visual analogue scales.
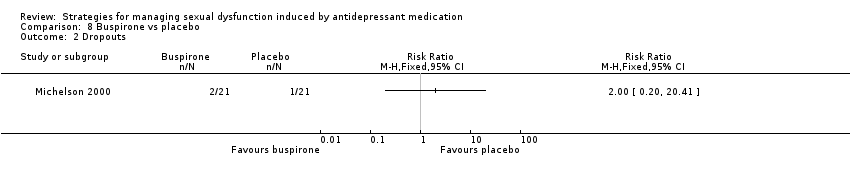
Comparison 8 Buspirone vs placebo, Outcome 2 Dropouts.
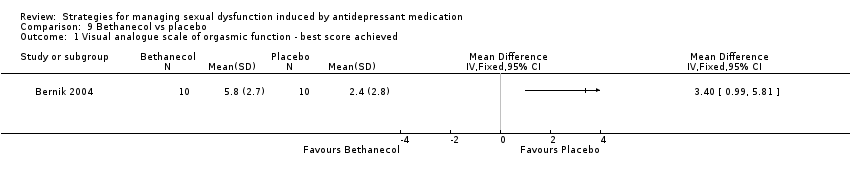
Comparison 9 Bethanecol vs placebo, Outcome 1 Visual analogue scale of orgasmic function ‐ best score achieved.
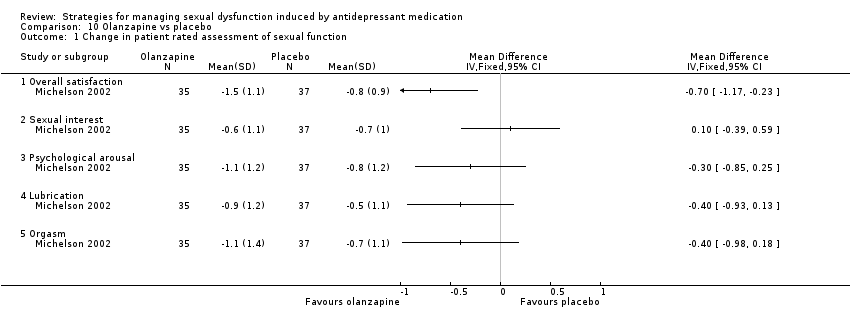
Comparison 10 Olanzapine vs placebo, Outcome 1 Change in patient rated assessment of sexual function.
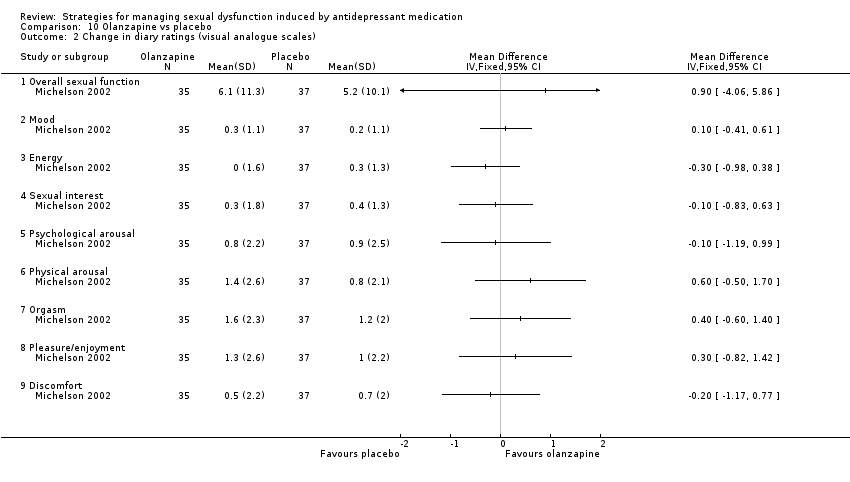
Comparison 10 Olanzapine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales).
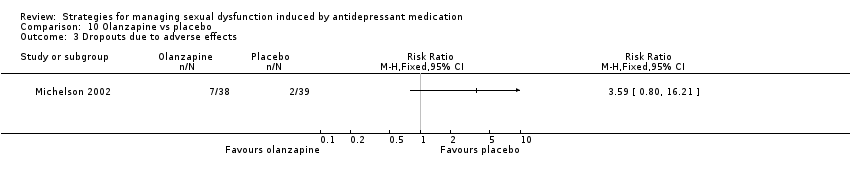
Comparison 10 Olanzapine vs placebo, Outcome 3 Dropouts due to adverse effects.

Comparison 11 Mirtazapine vs placebo, Outcome 1 Change in patient rated assessment of sexual function.
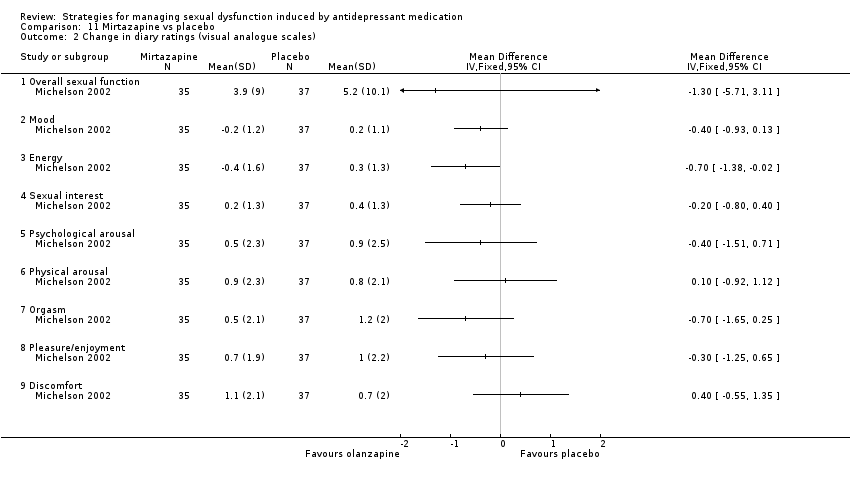
Comparison 11 Mirtazapine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales).

Comparison 11 Mirtazapine vs placebo, Outcome 3 Endpoint modified Kinsey Structured Interview.

Comparison 11 Mirtazapine vs placebo, Outcome 4 Dropouts.
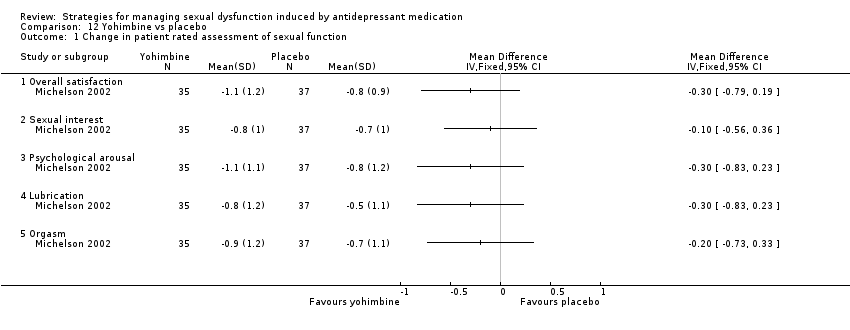
Comparison 12 Yohimbine vs placebo, Outcome 1 Change in patient rated assessment of sexual function.
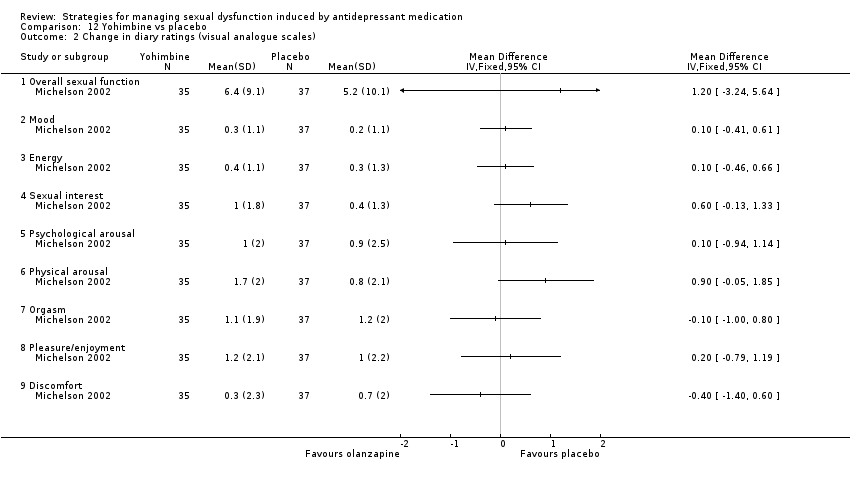
Comparison 12 Yohimbine vs placebo, Outcome 2 Change in diary ratings (visual analogue scales).

Comparison 12 Yohimbine vs placebo, Outcome 3 Dropouts.
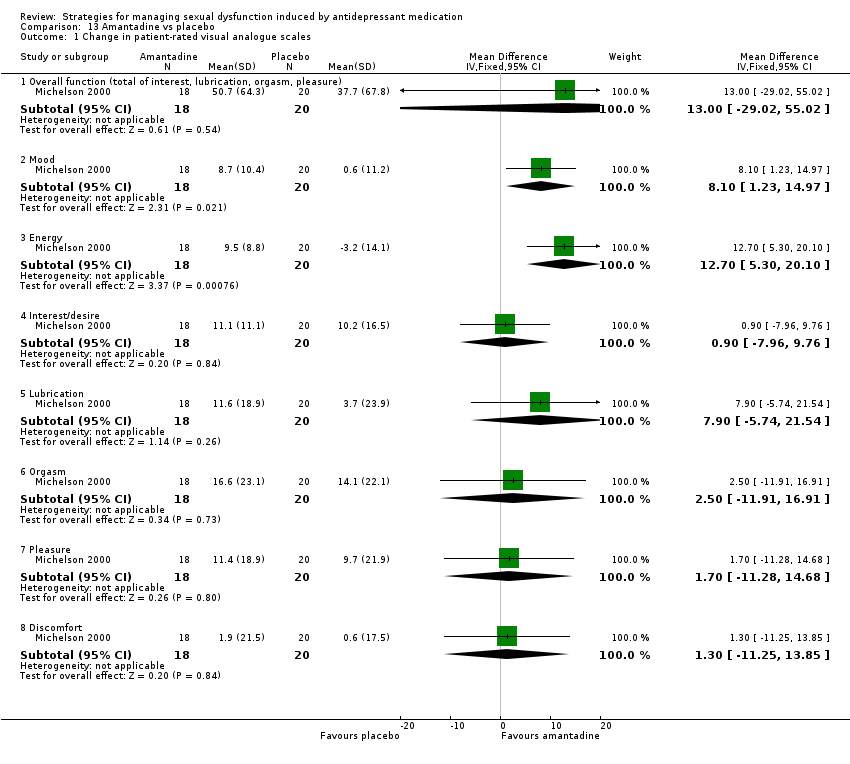
Comparison 13 Amantadine vs placebo, Outcome 1 Change in patient‐rated visual analogue scales.
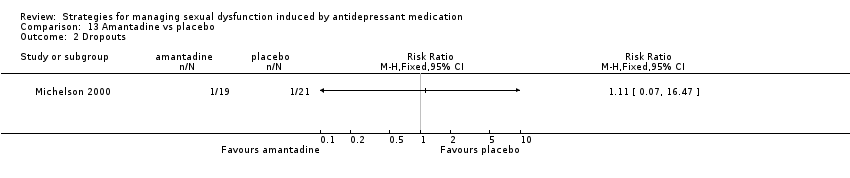
Comparison 13 Amantadine vs placebo, Outcome 2 Dropouts.

Comparison 14 ephedrine vs placebo, Outcome 1 Endpoint Brief Index of Sexual Functioning for Women (BISF‐W).

Comparison 15 Maca root: high vs low dose, Outcome 1 Endpoint Arizona Sexual Experiences Scale (ASEX) score.
Comparison 15 Maca root: high vs low dose, Outcome 2 Endpoint MGH‐SFQ.
Comparison 15 Maca root: high vs low dose, Outcome 3 Dropouts.

Comparison 15 Maca root: high vs low dose, Outcome 4 Endpoint ratings of psychiatric symptoms.
| Sildenafil compared with placebo for antidepressant‐induced sexual dysfunction | ||||||
| Patient or population: people with antidepressant‐induced sexual dysfunction Settings: outpatient Intervention: sildenafil Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil | |||||
| Endpoint International Index of Erectile Function (IIEF) total scores (The IIEF is a self‐report measure with 15 questions examining erectile function, orgasmic function, sexual desire, and intercourse satisfaction. Maximum possible score 75) | The mean IIEF score ranged across control groups from | The mean IIEF score in the intervention groups was | 112 men | ⊕⊕⊕⊕ | ||
| Endpoint International Index of Erectile Function (IIEF) scores ‐ question 3: ability to achieve erection (Maximum score 5) | The mean score in control groups was 3.1 | The mean score in the intervention groups was | 231 men | ⊕⊕⊕⊕ | ||
| Endpoint International Index of Erectile Function (IIEF) scores ‐ intercourse satisfaction (questions 6, 7, 8) (Maximum score 15) | The mean score in the control group was 7.2 | The mean score in the intervention group was | 89 men | ⊕⊕⊕⊝ | ||
| Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint | Male population | RR 0.44 (0.33 to 0.58) | 187 | ⊕⊕⊕⊝ | ||
| 956 per 1000 | 459 per 1000 | |||||
| Female population | ||||||
| 735 per 1000 | 287 per 1000 | |||||
| Dropouts (People leaving the trial early) | Low risk population | RR 0.68 (0.41 to 1.14) | 353 | ⊕⊕⊕⊕ | ||
| 90 per 1000 | 61 per 1000 | |||||
| Medium risk population | ||||||
| 250 per 1000 | 170 per 1000 | |||||
| High risk population | ||||||
| 360 per 1000 | 245 per 1000 | |||||
| Global Efficacy Questionnaire (questions 1 & 2) (Questions assessing improvement attributed to medication compared to having no treatment at all) | Improvement in erections | RR 2.50 (1.67 to 3.73) and RR 2.55 (1.71 to 3.80) | 284 men | ⊕⊕⊕⊝ | ||
| 282 per 1000 | 705 per 1000 | |||||
| Improvement in ability to have sexual intercourse | ||||||
| 282 per 1000 | 719 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The evidence for effects based on single trials is rated as moderate quality since further trial data may well change the estimate. | ||||||
| Bupropion compared with placebo for antidepressant‐induced sexual dysfunction | ||||||
| Patient or population: people with antidepressant‐induced sexual dysfunction Settings: outpatients Intervention: bupropion (doses of 150 mg daily and 150 mg twice daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Bupropion | |||||
| Endpoint scale total scores (150 mg twice daily dose) (Scales of sexual functioning. As studies used different scales to assess sexual functioning, differences are expressed as standardised mean differences (SMD)) | The mean value with this analysis is in effect zero | The mean score in the intervention groups was 1.60 higher (1.40 to 1.81) | 482 | ⊕⊕⊕⊝ | ||
| 50% reduction in score on the Arizona Sexual Experiences Scale (ASEX) (150 mg once daily dose) (The ASEX is a 5‐item self‐report inventory of sexual function) | Lower risk population | RR 0.62 (0.09 to 4.41) | 71 | ⊕⊕⊕⊝ | ||
| 47 per 1000 | 29 per 1000 | |||||
| Higher risk population | ||||||
| 67 per 1000 | 41 per 1000 | |||||
| Dropouts (People leaving the trial early) | Lower risk population | RR 1.08 (0.67 to 1.72) | 579 | ⊕⊕⊕⊕ | ||
| 90 per 1000 | 97 per 1000 | |||||
| Higher risk population | ||||||
| 150 per 1000 | 162 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Unexplained inconsistency in effects between studies reduces confidence in this effect | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint International Index of Erectile Function (IIEF) scores Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 19.36 [15.00, 23.72] |
| 1.2 Erectile function (questions 1‐5,15) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [7.39, 12.61] |
| 1.3 Question 3: ability to achieve erection | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [0.65, 1.44] |
| 1.4 Question 4: ability to maintain erection | 2 | 231 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [0.78, 1.59] |
| 1.5 Intercourse satisfaction (questions 6, 7, 8) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [2.48, 4.52] |
| 1.6 Orgasmic function (questions 9, 10) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.36, 3.64] |
| 1.7 Sexual desire (questions 11, 12) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.38, 1.38] |
| 1.8 Overall satisfaction (questions 13,14) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.86, 2.74] |
| 2 Endpoint Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint Clinical Global Impression ‐ Sexual Function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Clinical Global Impression ‐Sexual Function not "much/very much improved" by endpoint Show forest plot | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.33, 0.58] |
| 4.1 Males | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.66] |
| 4.2 Females | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.62] |
| 5 Endpoint Arizona Sexual Experience Scale (ASEX) total scores Show forest plot | 3 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.65 [‐3.86, ‐1.44] |
| 5.1 Males | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 5.2 Females | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.24, 1.24] |
| 6 Males: endpoint Arizona Sexual Experience Scale scores Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 6.2 Sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.08, ‐0.12] |
| 6.3 Arousal | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.06, ‐0.14] |
| 6.4 Erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.66, ‐0.74] |
| 6.5 Orgasm (ability) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.90, ‐0.90] |
| 6.6 Orgasm (satisfaction) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.58, ‐0.42] |
| 7 Endpoint MGH‐Sexual Functioning Questionnaire scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Dropouts Show forest plot | 4 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.41, 1.14] |
| 10 Endpoint Hamilton Rating Scale for Depression score Show forest plot | 2 | 187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.94, 0.07] |
| 11 Loss of remission: Hamilton Rating Scale for Depression score > 9 Show forest plot | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.09] |
| 12 Global Efficacy Questionnaire (questions 1 & 2) Show forest plot | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.90, 3.35] |
| 12.1 Improvement in erections | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [1.67, 3.73] |
| 12.2 Improvement in ability to have sexual intercourse | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.71, 3.80] |
| 13 Global efficacy questionnaire (question 3) Show forest plot | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.65, 1.75] |
| 13.1 Question 3: Frequency of erection that allowed satisfactory sexual intercourse | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.2 [0.65, 1.75] |
| 14 Endpoint Sexual Function Questionnaire (SFQ) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Arousal‐sensation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Arousal‐lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.5 Enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.6 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.7 Partner | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 UNM Sexual Function Inventory Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Sexual arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.5 Ability to reach orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Females: endpoint Arizona Sexual Experience Scale scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Orgasm (satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.6 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global Assessment Questions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Has the treatment you have been taking improved your erectile function? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.5 [3.03, 43.67] |
| 1.2 Has the treatment improved your ability to engage in sexual activity? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.5 [3.03, 43.67] |
| 2 Endpoint Sexual Encounter Profile (SEP) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Question 2: Were you able to insert your penis into your partner's vagina? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.68, 8.01] |
| 2.2 Question 3: Did your erection last long enough for you to have successful intercourse? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 2.3 Question 4: Were you satisfied with the hardness of your erection? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 2.4 Question 5: Were you satisfied overall with this sexual experience? | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.78, 46.29] |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Overall | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3.2 Lack of efficacy | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.24] |
| 3.3 Adverse effects | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint scale total scores Show forest plot | 3 | 482 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.60 [1.40, 1.81] |
| 1.1 International Index of Erectile Function (IIEF) | 1 | 227 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.76 [1.45, 2.07] |
| 1.2 Female Sexual Function Index (FSFI) | 1 | 213 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.73 [1.41, 2.05] |
| 1.3 Changes in Sexual Functioning Questionnaire (CSFQ) | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.11, 1.12] |
| 2 Response (as defined by study) Show forest plot | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 31.77 [10.10, 99.89] |
| 2.1 50% reduction Arizona Sexual Experiences Scale (ASEX) | 2 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.09, 4.41] |
| 2.2 Clinical Global Impression (CGI‐SF) 2‐point improvement | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 186.73 [11.74, 2969.23] |
| 3 Endpoint International Index of Erectile Function (IIEF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Total score | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 20.10 [17.14, 23.06] |
| 3.2 Erectile function | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 9.30 [8.18, 10.42] |
| 3.3 Orgasmic function | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [2.15, 3.25] |
| 3.4 Sexual desire | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.76, 2.44] |
| 3.5 Intercourse satisfaction | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 3.6 [3.00, 4.20] |
| 3.6 Overall satisfaction | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [1.99, 3.21] |
| 4 Endpoint Female Sexual Function Index score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Endpoint Changes in Sexual Functioning Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Desire/interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Desire/frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Arousal/excitement | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Completion/orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Dropouts Show forest plot | 5 | 579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.72] |
| 7 Endpoint Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Endpoint Clinical Global Impression (CGI ‐ SF) Show forest plot | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐1.87, ‐1.61] |
| 8.1 Male | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐1.80, ‐1.20] |
| 8.2 Female | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐1.95, ‐1.65] |
| 9 Endpoint ASEX Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Ability to reach orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Satisfaction with orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Endpoint EDITS (participant) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Expectations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Likelihood of continuing | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Confidence | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 Partner satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.7 Partner desire to continue treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.8 Naturalness of achieving erection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.9 Naturalness of erection hardness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.10 Quickness of achieving erection | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.11 Duration that erection lasts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.12 Ease of use | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Endpoint EDITS (partner) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Expectations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Sexual desirability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Participant's feelings about continuing treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Duration that erection lasts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Re‐emergence of antidepressant‐induced sexual dysfunction (physician rated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Week 1 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Endpoint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall degree of sexual satisfaction (participant rated) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Last rating recorded | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Overall | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint sexual function ratings (investigator questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Erection maintenance time | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Orgasm frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Satisfaction to orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Sexual Dysfunction Scale (investigator developed) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Week 12 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change from baseline on Sexual Side Effects Scale (SSES) total score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Endpoint Feiger Sexual Function and Satisfaction Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Item 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Item 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Item 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Item 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Item 5 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Item 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint Arizona Sexual Experience Scale (ASEX) score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Orgasm (satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Recurrence of mood symptoms Show forest plot | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 66.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Absence of sexual dysfunction at end point Show forest plot | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.86, 1.77] |
| 2 'Improved' or 'much improved' on Clinical Global Impression Show forest plot | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| 3 Change in Arizona Sexual Experiences Scale (ASEX) item scores Show forest plot | 1 | 1264 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.19, 0.05] |
| 3.1 How strong is your sexual drive? | 1 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 3.2 How easily are you sexually aroused? | 1 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.47, 0.07] |
| 3.3 Females: how easily does your vagina become moist or wet? | 1 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 3.4 Males: can you easily get and keep an erection? | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.80, 0.00] |
| 3.5 How easily can you reach orgasm? | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 3.6 Are your orgasms satisfying? | 1 | 252 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 4 Dropouts Show forest plot | 1 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.93] |
| 4.1 Total | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.56, 1.68] |
| 4.2 Attributed to adverse effects | 1 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.75, 7.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient‐rated visual analogue scales Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall function (total of interest, lubrication, orgasm, pleasure) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐38.33, 44.53] |
| 1.2 Mood | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐7.61, 9.21] |
| 1.3 Energy | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐3.88, 14.48] |
| 1.4 Interest/desire | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐7.99, 13.39] |
| 1.5 Lubrication | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [‐3.65, 23.45] |
| 1.6 Orgasm | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐6.3 [‐19.98, 7.38] |
| 1.7 Pleasure | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐15.53, 9.13] |
| 1.8 Discomfort | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐3.15, 17.15] |
| 2 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visual analogue scale of orgasmic function ‐ best score achieved Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endpoint modified Kinsey Structured Interview Show forest plot | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.19, 1.01] |
| 3.1 Sexual satisfaction | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.19, 1.01] |
| 4 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient rated assessment of sexual function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in diary ratings (visual analogue scales) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Mood | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Energy | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Sexual interest | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Psychological arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Physical arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Pleasure/enjoyment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Attributed to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in patient‐rated visual analogue scales Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall function (total of interest, lubrication, orgasm, pleasure) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐29.02, 55.02] |
| 1.2 Mood | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 8.1 [1.23, 14.97] |
| 1.3 Energy | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 12.7 [5.30, 20.10] |
| 1.4 Interest/desire | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐7.96, 9.76] |
| 1.5 Lubrication | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐5.74, 21.54] |
| 1.6 Orgasm | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐11.91, 16.91] |
| 1.7 Pleasure | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐11.28, 14.68] |
| 1.8 Discomfort | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐11.25, 13.85] |
| 2 Dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint Brief Index of Sexual Functioning for Women (BISF‐W) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Sexual arousability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Lack of vaginal lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Orgasm ability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Orgasm intensity/pleasure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Sexual dissatisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endpoint Arizona Sexual Experiences Scale (ASEX) score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Question 1: Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Endpoint MGH‐SFQ Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Item a: Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dropouts Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.60, 3.74] |
| 4 Endpoint ratings of psychiatric symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Hamilton Depression Rating Scale | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐7.98, 2.98] |
| 4.2 Hamilton Anxiety Rating Scale | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.15, 2.35] |

