Strategies for managing sexual dysfunction induced by antidepressant medication
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double blind, parallel arm, multicentre (3 sites). | |
| Participants | 55 adults randomised (48 women, 7 men). | |
| Interventions | Bupropion SR 150mg twice daily or placebo twice daily, in addition to SSRI | |
| Outcomes | Changes in Sexual Functioning Questionnaire. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double‐blind, parallel arm | |
| Participants | 42 adults randomised. | |
| Interventions | Bupropion SR 150mg once daily or placebo; in addition to current SSRI | |
| Outcomes | Sexual dysfunction measures unclear, Hamilton Rating Scale for Depression, Beck Depression Inventory | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm, multicentre (9 sites). | |
| Participants | 75 adults randomised (34 women, 38 men). | |
| Interventions | Nefazodone 100mg twice daily increasing to 200mg after one week or sertraline 50mg once daily increasing to 100mg after one week and placebo at night | |
| Outcomes | Physician's rating of sexual dysfunction. Hamilton Rating Scale for Depression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm. 8 weeks. (Further 8 week single blind period) | |
| Participants | 23 men (ages 30‐64). clinically recovered mood or anxiety disorder and SRI associated sexual dysfunction | |
| Interventions | Sildenafil 50‐100mg once daily or placebo for 8 weeks. After 8 weeks, all participants received sildenafil 50‐100mg. | |
| Outcomes | International Index of Erectile Function. Arizona Sexual Experience Scale. Erectile Dysfunction Inventory of Treatment Satifsaction. Rigiscan. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double‐blind, parallel arm. 14 days. | |
| Participants | 12 women randomised. antidepressant‐induced sexual dysfunction, past diagnosis of depression. No change in psychotropic treatment in the last 2 months, no comorbid psychiatric or medical disorder, no past history of sexual dysfunction | |
| Interventions | granisetron (dose not specified) or placebo | |
| Outcomes | Feiger Sexual Function and Satisfaction Questionnaire. Arizona Sexual Experience Scale. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm, single centre. 2 months. | |
| Participants | 37 adults randomised (10 women, 27 men). | |
| Interventions | Ginkgo biloba 120mg /day increasing to 160mg / day after two weeks, and increasing to 240mg / day after four weeks, or placebo. | |
| Outcomes | Investigator‐developed questionnaire. Beck Depression Inventory (Korean version). State and Trait Anxiety Inventory. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm, multicentre (12 centres). | |
| Participants | 47 adults from randomised group (27 women, 20 men). | |
| Interventions | Buspirone 20‐60mg od or placebo, in addition to fixed dose usual SSRI | |
| Outcomes | Udvalg fur Kliniske Undersogelser side effects rating scale. Clinical Global Impression. | |
| Notes | Subanalysis of a larger study (n=119) exploring the efficacy of buspirone as add‐on treatment for patients not responding to SSRI alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm. | |
| Participants | 31 adults randomised (breakdown by gender unknown). | |
| Interventions | Bupropion SR 150 mg or placebo, in addition to SSRI | |
| Outcomes | Arizona Sexual Experiences Scale. Hamilton Rating Scale for Depression, Udvalg fur Kliniske Undersogelser side effects rating scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, crossover design. 8 weeks | |
| Participants | 29 women randomised. SSRI for depression, at least 10 weeks treatment, treatment otherwise successful, sexual dysfunction distinctly different from any noticed during depressed phase. | |
| Interventions | Ephedrine 50mg once daily or placebo | |
| Outcomes | Brief Index of Sexual Functioning for Women. Beck Depression Inventory. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm, multicentre (3 sites). | |
| Participants | 67 women randomised. | |
| Interventions | Buspirone 10mg twice daily increasing to 15mg, or amantadine 50mg once daily increasing to 50mg twice daily, or placebo twice daily, in addition to fluoxetine | |
| Outcomes | Interviewer Rating of Sexual Function. Patient rated visual analogue scales for sexual function. Clinician rated global impression. Patient rated global impression. Hamilton Rating Scale for Depression. Beck Depression Inventory. State‐Trait Anxiety Inventory. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm. Multicentre (12 centres) | |
| Participants | 148 women randomised. | |
| Interventions | Mirtazapine 15mg once daily increasing to 30mg, or yohimbine 5.4mg once daily increasing to 10.8mg, or olanzapine 2.5mg once daily increasing to 5mg, or placebo, in addition to fluoxetine. | |
| Outcomes | Patient assessment of sexual function. Daily diary visual analog scales. Kinsey Ratings of Sexual Function ‐ computer assisted structured interview. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, crossover design. | |
| Participants | 38 adults randomised (18 women, 2 men, 18 not described). | |
| Interventions | Granisetron 1‐2mg as required or placebo, in addition to SSRI. | |
| Outcomes | Sexual Side Effects Scale. Hamilton Rating Scale for Depression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm. | |
| Participants | 98 men, from randomised group. | |
| Interventions | Sildenafil 5 ‐ 200mg once daily or placebo once daily | |
| Outcomes | International Index of Erectile Function. | |
| Notes | Retrospective subanalysis of 10 phase II/III trials (total n=3414) analysing outcomes of those also taking SSRI (n=98). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind, parallel arm. | |
| Participants | 90 men randomised. | |
| Interventions | Sildenafil 50mg as required increasing to 100mg as required or placebo. in addition to SSRI | |
| Outcomes | International Index of Erectile Function. Arizona Sexual Experiences Scale. Massachusetts General Hospital‐Sexual Function Questionnaire. Hamilton Rating Scale for Depression. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Double blind. Multicentre (174 centres). 12 weeks. | |
| Participants | 111 men randomised. Receiving antidepressants | |
| Interventions | tadalafil 10 mg or 20 mg or placebo | |
| Outcomes | International Index of Erectile Dysfunction. Sexual Encounter Profile diary. Global Assessment Question. | |
| Notes | retrospective subanalysis of 11 trials (involving 2102 men) analysing outcomes of those receiving antidepressants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
SSRI ‐ Selective Serotonin Reuptake Inhibitor; SRI ‐ Serotonin Reuptake Inhibitor; DSM ‐ Diagnostic and Statistical Manual of American Psychiatric Association
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Non‐randomised | |
| Case series | |
| Case Series | |
| Case Series | |
| Case Series | |
| Review article | |
| Non‐randomised | |
| Case series | |
| Case series | |
| Not antidepressant induced sexual dysfunction | |
| Case series | |
| Non‐randomised |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Ginkgo biloba: antidepressant‐induced sexual dysfunction |
| Methods | |
| Participants | 110 women (age 18‐65) stabilised on antidepressant medication and free of a current Axis I disorder |
| Interventions | 8 weeks of 200mg Ginkgo biloba extract or placebo |
| Outcomes | daily patient diary, patient‐rating scales, blind independent evaluator ratings, vaginal photoplethysmography |
| Starting date | June 2002 |
| Contact information | Alessandra Rellini, MA (512) 232‐4805 [email protected] |
| Notes | estimated completion date September 2004 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 re‐emergence antidepressant induced sexual dysfunction (physician rated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 nefazodone vs sertraline, Outcome 1 re‐emergence antidepressant induced sexual dysfunction (physician rated). | ||||
| 1.1 week one | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 endpoint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 overall degree of sexual satisfaction (patient rated) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 nefazodone vs sertraline, Outcome 2 overall degree of sexual satisfaction (patient rated). | ||||
| 2.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 last rating recorded | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 nefazodone vs sertraline, Outcome 3 dropouts. | ||||
| 3.1 overall | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 due to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 nefazodone vs sertraline, Outcome 4 Hamilton Rating Scale for Depression score. | ||||
| 4.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 endpoint International Index of Erectile Function scores Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 sildenafil vs placebo, Outcome 1 endpoint International Index of Erectile Function scores. | ||||
| 1.1 total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 19.36 [15.00, 23.72] |
| 1.2 erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [7.39, 12.61] |
| 1.3 orgasmic function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.36, 3.64] |
| 1.4 sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.38, 1.38] |
| 1.5 intercourse satisfaction | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [2.48, 4.52] |
| 1.6 overall satisfaction | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.86, 2.74] |
| 1.7 ability to acheive erection | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.71, 1.60] |
| 1.8 ability to maintain erection | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [0.81, 1.73] |
| 1.9 ejaculation frequency | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [0.01, 1.55] |
| 1.10 orgasm frequency | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.04, 1.58] |
| 1.11 desire frequency | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.46, 0.66] |
| 2 endpoint Arizona Sexual Experience Scale scores Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 sildenafil vs placebo, Outcome 2 endpoint Arizona Sexual Experience Scale scores. | ||||
| 2.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 2.2 Sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.08, ‐0.12] |
| 2.3 Arousal | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.06, ‐0.14] |
| 2.4 Erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.66, ‐0.74] |
| 2.5 Orgasm (ability) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.90, ‐0.90] |
| 2.6 Orgasm (satisfaction) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.58, ‐0.42] |
| 3 endpoint MGH‐Sexual Functioning Questionnaire scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 sildenafil vs placebo, Outcome 3 endpoint MGH‐Sexual Functioning Questionnaire scores. | ||||
| 3.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 endpoint Erectile Dysfunction Inventory of Treatment Satisfaction scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 sildenafil vs placebo, Outcome 4 endpoint Erectile Dysfunction Inventory of Treatment Satisfaction scores. | ||||
| 5 Clinical Global Impression ‐Sexual Function not "much/very much improved" by 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 sildenafil vs placebo, Outcome 5 Clinical Global Impression ‐Sexual Function not "much/very much improved" by 6 weeks. | ||||
| 6 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 sildenafil vs placebo, Outcome 6 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint. | ||||
| 7 dropouts Show forest plot | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.86] |
| Analysis 2.7  Comparison 2 sildenafil vs placebo, Outcome 7 dropouts. | ||||
| 8 endpoint Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 sildenafil vs placebo, Outcome 8 endpoint Hamilton Rating Scale for Depression score. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 less than 50% improvement in Arizona Sexual Experiences Scale at 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 bupropion vs placebo, Outcome 1 less than 50% improvement in Arizona Sexual Experiences Scale at 3 weeks. | ||||
| 2 less than 25% improvement in Arizona Sexual Experiences Scale at 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 bupropion vs placebo, Outcome 2 less than 25% improvement in Arizona Sexual Experiences Scale at 3 weeks. | ||||
| 3 mean Changes in Sexual Functioning Questionnaire desire/frequency score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 bupropion vs placebo, Outcome 3 mean Changes in Sexual Functioning Questionnaire desire/frequency score. | ||||
| 3.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dropouts Show forest plot | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.78, 5.72] |
| Analysis 3.4  Comparison 3 bupropion vs placebo, Outcome 4 dropouts. | ||||
| 5 mean Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 bupropion vs placebo, Outcome 5 mean Hamilton Rating Scale for Depression score. | ||||
| 5.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 no remission of sexual dysfunction symptoms at four weeks Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.01] |
| Analysis 4.1  Comparison 4 buspirone vs placebo, Outcome 1 no remission of sexual dysfunction symptoms at four weeks. | ||||
| 1.1 male | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.74] |
| 1.2 female | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.05] |
| 2 change in patient‐rated 'overall function' (sexual) visual analogue scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 buspirone vs placebo, Outcome 2 change in patient‐rated 'overall function' (sexual) visual analogue scale. | ||||
| 3 dropouts Show forest plot | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.32, 13.59] |
| Analysis 4.3  Comparison 4 buspirone vs placebo, Outcome 3 dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change from baseline on Sexual Side Effects Scale score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 granisetron vs placebo, Outcome 1 change from baseline on Sexual Side Effects Scale score. | ||||
| 2 Feiger Sexual Function and Satisfaction Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 granisetron vs placebo, Outcome 2 Feiger Sexual Function and Satisfaction Questionnaire score. | ||||
| 2.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Arizona Sexual Experience Scale score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 granisetron vs placebo, Outcome 3 Arizona Sexual Experience Scale score. | ||||
| 3.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 granisetron vs placebo, Outcome 4 dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in International Index of Erectile Function scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 tadalafil vs placebo, Outcome 1 change in International Index of Erectile Function scores. | ||||
| 1.1 erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 failure to report improvement in erections at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 tadalafil vs placebo, Outcome 2 failure to report improvement in erections at trial endpoint. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 olanzapine vs placebo, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function). | ||||
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 olanzapine vs placebo, Outcome 2 change in 'overall sexual function' (diary ratings). | ||||
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 olanzapine vs placebo, Outcome 3 dropouts due to adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 mirtazapine vs placebo, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function). | ||||
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 mirtazapine vs placebo, Outcome 2 change in 'overall sexual function' (diary ratings). | ||||
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 mirtazapine vs placebo, Outcome 3 dropouts due to adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 yohimbine vs placebo, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function). | ||||
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 yohimbine vs placebo, Outcome 2 change in 'overall sexual function' (diary ratings). | ||||
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.3  Comparison 9 yohimbine vs placebo, Outcome 3 dropouts due to adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in patient‐rated 'overall function' (sexual) visual analogue scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 amantadine vs placebo, Outcome 1 change in patient‐rated 'overall function' (sexual) visual analogue scale. | ||||
| 2 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.2  Comparison 10 amantadine vs placebo, Outcome 2 dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 sexual function ratings at week 8 (questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Ginkgo biloba vs placebo, Outcome 1 sexual function ratings at week 8 (questionnaire). | ||||
| 1.1 sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 erection maintenance time | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 orgasm frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 satisfaction to orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.2  Comparison 11 Ginkgo biloba vs placebo, Outcome 2 dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Brief Index of Sexual Functioning for Women at end of treatment phase Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 ephedrine vs placebo, Outcome 1 Brief Index of Sexual Functioning for Women at end of treatment phase. | ||||
| 1.1 sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 sexual arousability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 lack of vaginal lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 orgasm ability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 orgasm intensity/pleasure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 sexual dissatisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in patient‐rated 'overall function' (sexual) visual analogue scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.1  Comparison 13 amantadine vs buspirone, Outcome 1 change in patient‐rated 'overall function' (sexual) visual analogue scale. | ||||
| 2 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.2  Comparison 13 amantadine vs buspirone, Outcome 2 dropouts. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 14.1  Comparison 14 olanzapine vs mirtazapine, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function). | ||||
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 14.2  Comparison 14 olanzapine vs mirtazapine, Outcome 2 change in 'overall sexual function' (diary ratings). | ||||
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 14.3  Comparison 14 olanzapine vs mirtazapine, Outcome 3 dropouts due to adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 15.1  Comparison 15 olanzapine vs yohimbine, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function). | ||||
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 15.2  Comparison 15 olanzapine vs yohimbine, Outcome 2 change in 'overall sexual function' (diary ratings). | ||||
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 15.3  Comparison 15 olanzapine vs yohimbine, Outcome 3 dropouts due to adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 16.1  Comparison 16 mirtazapine vs yohimbine, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function). | ||||
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 16.2  Comparison 16 mirtazapine vs yohimbine, Outcome 2 change in 'overall sexual function' (diary ratings). | ||||
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 16.3  Comparison 16 mirtazapine vs yohimbine, Outcome 3 dropouts due to adverse effects. | ||||

Comparison 1 nefazodone vs sertraline, Outcome 1 re‐emergence antidepressant induced sexual dysfunction (physician rated).

Comparison 1 nefazodone vs sertraline, Outcome 2 overall degree of sexual satisfaction (patient rated).

Comparison 1 nefazodone vs sertraline, Outcome 3 dropouts.
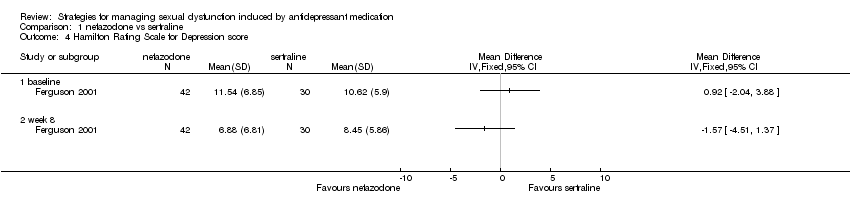
Comparison 1 nefazodone vs sertraline, Outcome 4 Hamilton Rating Scale for Depression score.
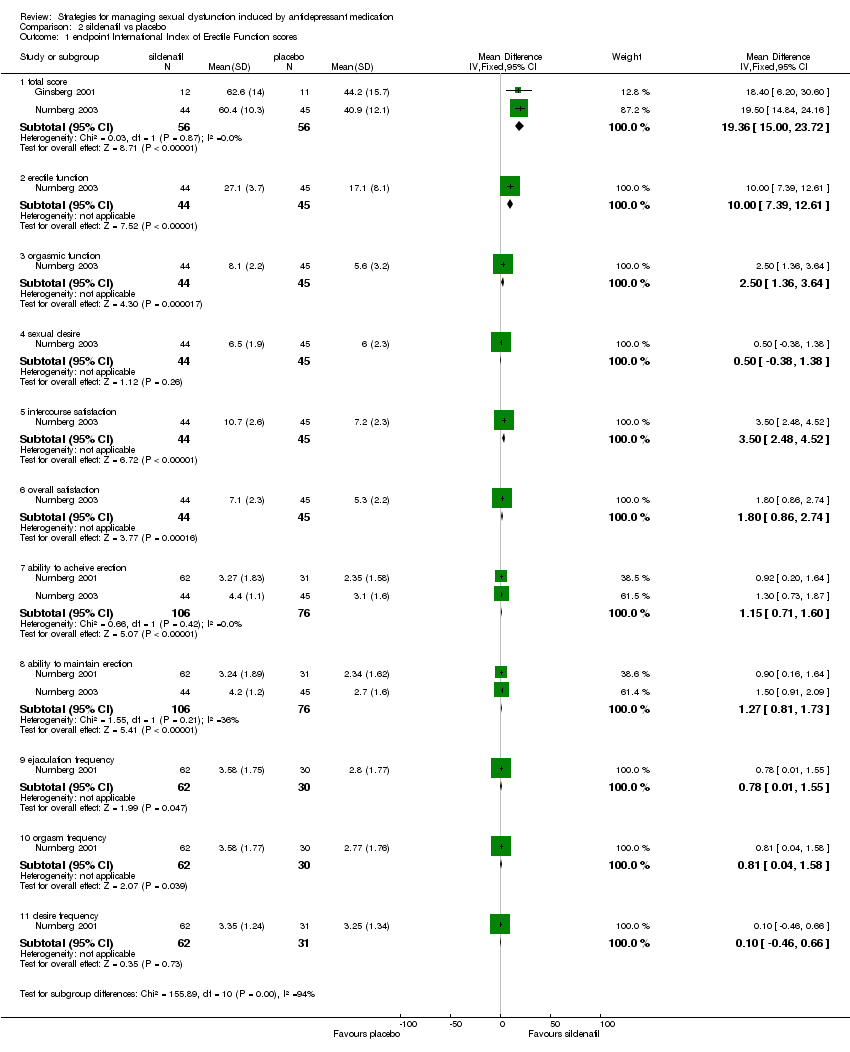
Comparison 2 sildenafil vs placebo, Outcome 1 endpoint International Index of Erectile Function scores.

Comparison 2 sildenafil vs placebo, Outcome 2 endpoint Arizona Sexual Experience Scale scores.

Comparison 2 sildenafil vs placebo, Outcome 3 endpoint MGH‐Sexual Functioning Questionnaire scores.

Comparison 2 sildenafil vs placebo, Outcome 4 endpoint Erectile Dysfunction Inventory of Treatment Satisfaction scores.

Comparison 2 sildenafil vs placebo, Outcome 5 Clinical Global Impression ‐Sexual Function not "much/very much improved" by 6 weeks.
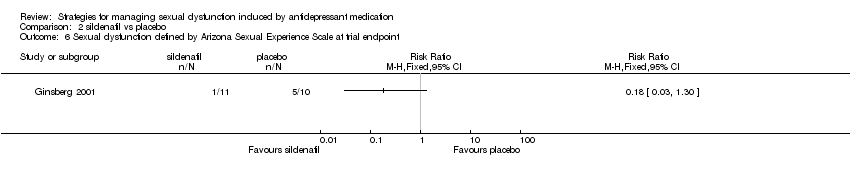
Comparison 2 sildenafil vs placebo, Outcome 6 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint.

Comparison 2 sildenafil vs placebo, Outcome 7 dropouts.

Comparison 2 sildenafil vs placebo, Outcome 8 endpoint Hamilton Rating Scale for Depression score.

Comparison 3 bupropion vs placebo, Outcome 1 less than 50% improvement in Arizona Sexual Experiences Scale at 3 weeks.

Comparison 3 bupropion vs placebo, Outcome 2 less than 25% improvement in Arizona Sexual Experiences Scale at 3 weeks.
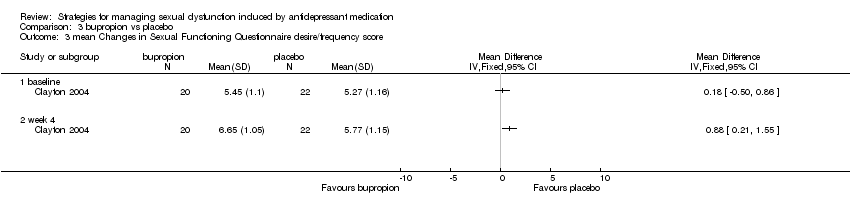
Comparison 3 bupropion vs placebo, Outcome 3 mean Changes in Sexual Functioning Questionnaire desire/frequency score.

Comparison 3 bupropion vs placebo, Outcome 4 dropouts.

Comparison 3 bupropion vs placebo, Outcome 5 mean Hamilton Rating Scale for Depression score.

Comparison 4 buspirone vs placebo, Outcome 1 no remission of sexual dysfunction symptoms at four weeks.
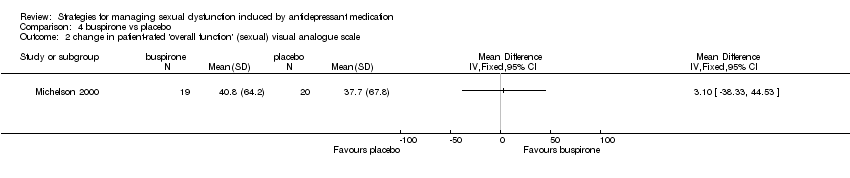
Comparison 4 buspirone vs placebo, Outcome 2 change in patient‐rated 'overall function' (sexual) visual analogue scale.

Comparison 4 buspirone vs placebo, Outcome 3 dropouts.

Comparison 5 granisetron vs placebo, Outcome 1 change from baseline on Sexual Side Effects Scale score.
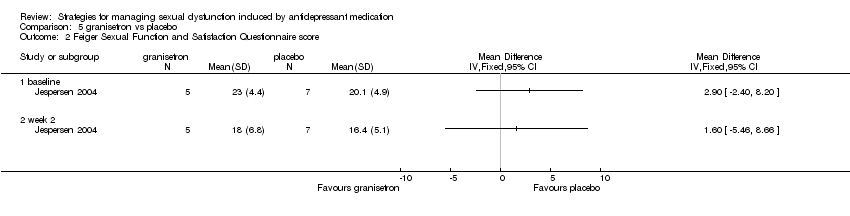
Comparison 5 granisetron vs placebo, Outcome 2 Feiger Sexual Function and Satisfaction Questionnaire score.

Comparison 5 granisetron vs placebo, Outcome 3 Arizona Sexual Experience Scale score.
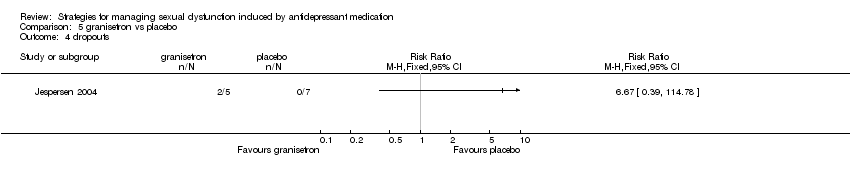
Comparison 5 granisetron vs placebo, Outcome 4 dropouts.

Comparison 6 tadalafil vs placebo, Outcome 1 change in International Index of Erectile Function scores.

Comparison 6 tadalafil vs placebo, Outcome 2 failure to report improvement in erections at trial endpoint.

Comparison 7 olanzapine vs placebo, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function).
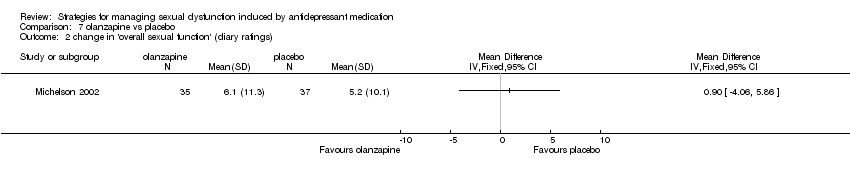
Comparison 7 olanzapine vs placebo, Outcome 2 change in 'overall sexual function' (diary ratings).
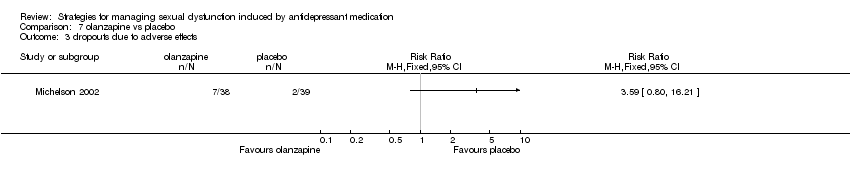
Comparison 7 olanzapine vs placebo, Outcome 3 dropouts due to adverse effects.

Comparison 8 mirtazapine vs placebo, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function).
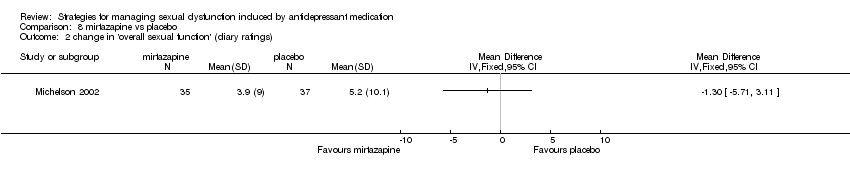
Comparison 8 mirtazapine vs placebo, Outcome 2 change in 'overall sexual function' (diary ratings).

Comparison 8 mirtazapine vs placebo, Outcome 3 dropouts due to adverse effects.

Comparison 9 yohimbine vs placebo, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function).
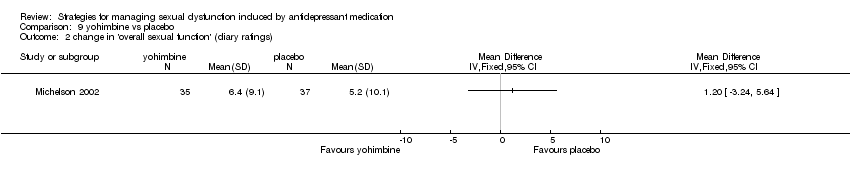
Comparison 9 yohimbine vs placebo, Outcome 2 change in 'overall sexual function' (diary ratings).

Comparison 9 yohimbine vs placebo, Outcome 3 dropouts due to adverse effects.

Comparison 10 amantadine vs placebo, Outcome 1 change in patient‐rated 'overall function' (sexual) visual analogue scale.

Comparison 10 amantadine vs placebo, Outcome 2 dropouts.

Comparison 11 Ginkgo biloba vs placebo, Outcome 1 sexual function ratings at week 8 (questionnaire).

Comparison 11 Ginkgo biloba vs placebo, Outcome 2 dropouts.

Comparison 12 ephedrine vs placebo, Outcome 1 Brief Index of Sexual Functioning for Women at end of treatment phase.

Comparison 13 amantadine vs buspirone, Outcome 1 change in patient‐rated 'overall function' (sexual) visual analogue scale.

Comparison 13 amantadine vs buspirone, Outcome 2 dropouts.

Comparison 14 olanzapine vs mirtazapine, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function).
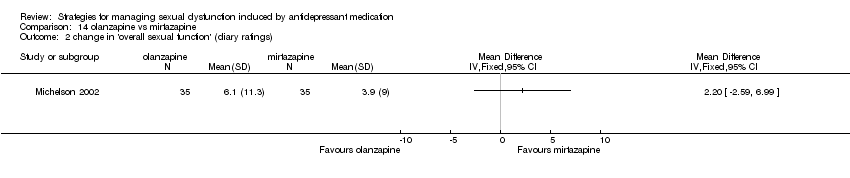
Comparison 14 olanzapine vs mirtazapine, Outcome 2 change in 'overall sexual function' (diary ratings).

Comparison 14 olanzapine vs mirtazapine, Outcome 3 dropouts due to adverse effects.
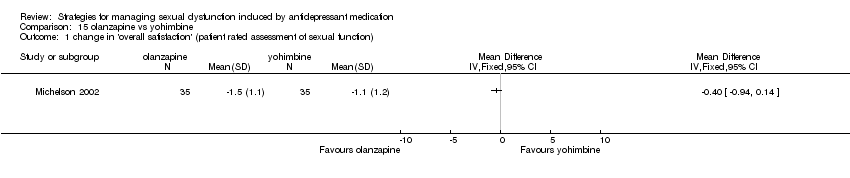
Comparison 15 olanzapine vs yohimbine, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function).

Comparison 15 olanzapine vs yohimbine, Outcome 2 change in 'overall sexual function' (diary ratings).
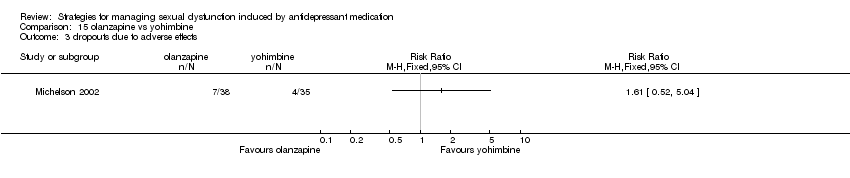
Comparison 15 olanzapine vs yohimbine, Outcome 3 dropouts due to adverse effects.
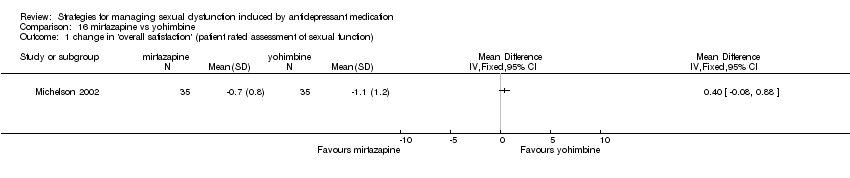
Comparison 16 mirtazapine vs yohimbine, Outcome 1 change in 'overall satisfaction' (patient rated assessment of sexual function).
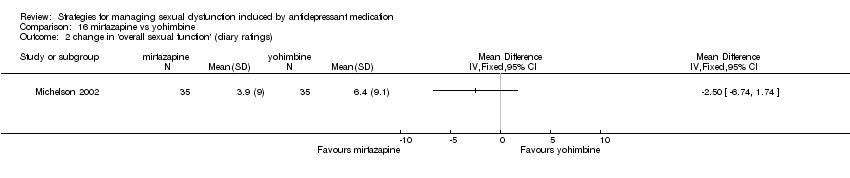
Comparison 16 mirtazapine vs yohimbine, Outcome 2 change in 'overall sexual function' (diary ratings).

Comparison 16 mirtazapine vs yohimbine, Outcome 3 dropouts due to adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 re‐emergence antidepressant induced sexual dysfunction (physician rated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 week one | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 endpoint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 overall degree of sexual satisfaction (patient rated) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 last rating recorded | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 overall | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 due to adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 endpoint International Index of Erectile Function scores Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 19.36 [15.00, 23.72] |
| 1.2 erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [7.39, 12.61] |
| 1.3 orgasmic function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.36, 3.64] |
| 1.4 sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.38, 1.38] |
| 1.5 intercourse satisfaction | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [2.48, 4.52] |
| 1.6 overall satisfaction | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.86, 2.74] |
| 1.7 ability to acheive erection | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 1.15 [0.71, 1.60] |
| 1.8 ability to maintain erection | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [0.81, 1.73] |
| 1.9 ejaculation frequency | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [0.01, 1.55] |
| 1.10 orgasm frequency | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [0.04, 1.58] |
| 1.11 desire frequency | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.46, 0.66] |
| 2 endpoint Arizona Sexual Experience Scale scores Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Total score | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.29, ‐2.95] |
| 2.2 Sexual desire | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.08, ‐0.12] |
| 2.3 Arousal | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.06, ‐0.14] |
| 2.4 Erectile function | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.66, ‐0.74] |
| 2.5 Orgasm (ability) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐1.90, ‐0.90] |
| 2.6 Orgasm (satisfaction) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.58, ‐0.42] |
| 3 endpoint MGH‐Sexual Functioning Questionnaire scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Total score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Arousal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Orgasm (ability) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 endpoint Erectile Dysfunction Inventory of Treatment Satisfaction scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Clinical Global Impression ‐Sexual Function not "much/very much improved" by 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Sexual dysfunction defined by Arizona Sexual Experience Scale at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 dropouts Show forest plot | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.86] |
| 8 endpoint Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 less than 50% improvement in Arizona Sexual Experiences Scale at 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 less than 25% improvement in Arizona Sexual Experiences Scale at 3 weeks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 mean Changes in Sexual Functioning Questionnaire desire/frequency score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dropouts Show forest plot | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.78, 5.72] |
| 5 mean Hamilton Rating Scale for Depression score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 week 4 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 no remission of sexual dysfunction symptoms at four weeks Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.01] |
| 1.1 male | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.74] |
| 1.2 female | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.31, 1.05] |
| 2 change in patient‐rated 'overall function' (sexual) visual analogue scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts Show forest plot | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.32, 13.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change from baseline on Sexual Side Effects Scale score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Feiger Sexual Function and Satisfaction Questionnaire score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Arizona Sexual Experience Scale score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 week 2 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in International Index of Erectile Function scores Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 erectile function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 failure to report improvement in erections at trial endpoint Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in patient‐rated 'overall function' (sexual) visual analogue scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 sexual function ratings at week 8 (questionnaire) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 overall sexual function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 erection maintenance time | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 orgasm frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 satisfaction to orgasm | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Brief Index of Sexual Functioning for Women at end of treatment phase Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 sexual desire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 sexual arousability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 lack of vaginal lubrication | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 orgasm ability | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 orgasm intensity/pleasure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 sexual dissatisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in patient‐rated 'overall function' (sexual) visual analogue scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 dropouts Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 'overall satisfaction' (patient rated assessment of sexual function) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 change in 'overall sexual function' (diary ratings) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 dropouts due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

