Ketamina como coadyuvante de los opiáceos para el dolor por cáncer
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised. Double‐blind, but blinding procedure not described. Placebo control. Parallel group Study duration 5 days | |
| Participants | Inpatients aged 18 or older, palliative care patients with refractory chronic pain (BPI average pain score ≥3 despite ongoing treatment with opioids and co‐analgesics at predefined dose levels) secondary to cancer or its treatment N= 185, 93 randomised to ketamine group, 92 randomised to placebo Mean age in ketamine arm: 63 years. Mean age in placebo group: 64.3 | |
| Interventions | Participants received either ketamine or placebo (normal saline) as a subcutaneous infusion in a 5‐day schedule, starting at the first dose level (100 mg/24 hrs. If 80% of the study drug had been delivered, and average pain improved by ≥ 2 BPI units, with no more than four breakthrough doses, the dose remained the same. If not, the dose was increased to the next level. | |
| Outcomes | The primary outcome was a positive response, defined as a clinically relevant improvement in pain at the end of the 5‐day study period. A clinically relevant improvement in pain was defined as a reduction in BPI average pain score by ≥ 2 points from baseline in the absence of more than four breakthrough doses of analgesia over the previous 24 hours. Secondary outcomes: pain assessments at days 2‐5. Adverse events. | |
| Notes | Data for patients who discontinued due to reasons unrelated to the intervention were imputed using LOCF. Unclear how data for those who discontinued due to adverse effects or lack of effect were handled. Quality/validity: OPVS: 12 Oxford: 4 Supported by a grant from the Palliative Care Branch, Australian Government Department of Health and Ageing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, process of randomisation is described and adequate. "Each site pharmacy used randomization tables from an independent central registry" |
| Allocation concealment (selection bias) | Low risk | "All non pharmacy study staff, treating clinicians,investigators and participants were unaware of treatment allocation until completion". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as blinded. Blinding procedure not described in final paper but was described in protocol "All syringes will look identical in volume and colour". The authors of this review update felt that blinding could have been compromised due to adverse effects from ketamine. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No specific procedure to check for detection bias. The authors of this review update felt that blinding could have been compromised due to adverse effects from ketamine |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how missing data were imputed |
| Selective reporting (reporting bias) | Unclear risk | Total opioid dose was not reported but was mentioned in the protocol |
| Size | Unclear risk | 50 ‐ 199 participants per treatment arm |
| Methods | Stated to be randomised but procedure not described. Blinded study: Drugs prepared in identical syringes by a person not involved in the test sessions Placebo control Cross‐over | |
| Participants | Cancer patients with neuropathic pain unrelieved by morphine N = 10 per group (cross‐over) Mean age of patients: 57 years | |
| Interventions | Treatment 1: Treatment 2: Treatment 3: KET bolus 0.5 mg/kg (IV) | |
| Outcomes | Pain intensity Results: | |
| Notes | Washout period ( "at least two days") Quality/ validity: Information on funding not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised but procedure not described. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The drugs were prepared in identical syringes by a person not involved in the test sessions". Blinding possibly compromised due to adverse effects from ketamine. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No specific procedure to check for detection bias. Possible bias due to adverse effects from ketamine |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | No problems detected |
| Size | High risk | Fewer than 50 participants in total (cross‐over) |
| Methods | Stated to be randomised but procedure not described. Blinded study: double dummy Study duration: not defined | |
| Participants | Hospitalised patients with terminal cancer pain, treated with opioids N = 20 per group (cross‐over) | |
| Interventions | Treatment 1: | |
| Outcomes | Pain intensity Results: | |
| Notes | No washout period Quality/ validity: Supported by a grant from the National Science Council (NSC), Taipei, Taiwan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised but procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "A doubled dummy technique was used, so that the patient, investigator and nurse were unaware of the dose of morphine and ketamine" |
| Blinding of outcome assessment (detection bias) | Low risk | "A doubled dummy technique was used, so that the patient, investigator and nurse were unaware of the dose of morphine and ketamine". Blinding not compromised by adverse effects from ketamine |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | No problems detected |
| Size | High risk | Fewer than 50 participants in total (cross‐over) |
BPI: Brief Pain Inventory
IT: Intrathecal
IV: Intravenous
KET: Ketamine
kg: Kilo
LOCF:Last observation carried forward
mg: Milligram
Mo: Morphine
N: Number of patients
OPVS: Oxford Pain Validity Scale
PO: Oral
SC: Subcutaneous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Poster abstract | |
| Dropouts leaving one group with fewer than 10 participants | |
| Open‐label study ("pilot work"). Described as placebo‐controlled, but in fact used active control (morphine). Design flaw with fixed maximum baseline morphine dose PO and primary outcome measure: daily consumption of morphine. OPVS score: 1 Oxford Quality Scale score: 2 | |
| Described as placebo‐controlled, but in fact used active control (morphine). Design flaw with fixed baseline dose of morphine ED, fixed maximum daily dose of morphine ED and primary outcome measure: daily consumption of morphine ED. OPVS score: 7 Oxford Quality Scale score: 3 | |
| Dropouts leaving one group with fewer than 10 participants |
ED: epidural
OPVS: Oxford Pain Validity Scale
PO: oral
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Ketamine associated with opioids in refractory cancer pain treatment |
| Methods | Randomised, placebo‐controlled, double‐blind parallel group study |
| Participants | 100 hospitalised adult cancer patients undergoing opioid treatment for refractory pain |
| Interventions | Ketamine versus placebo |
| Outcomes | Daily pain score on 11‐point numerical pain rating scale; patient global impression of change; daily sleep interference score;patient satisfaction; opioid consumption; adverse effects |
| Starting date | May 2007. Completed September 2009 |
| Contact information | Sylvie Rostaing‐Rigattieri, Assistance Publique‐ Hopitaux de Paris, Paris, France |
| Notes | Status 'completed'. Not published. Possibly same study as NCT01326325? |
| Trial name or title | Oral ketamine as an adjuvant to opioids for pain treatment in cancer patients |
| Methods | Randomised, placebo‐controlled, double‐blind study |
| Participants | 50 patients with unbalanced (VAS > 6) chronic cancer‐related pain despite opioid treatment |
| Interventions | Oral ketamine versus placebo |
| Outcomes | Pain reduction (VAS scores), adverse effects |
| Starting date | October 2010 |
| Contact information | Silviu Brill, Tel Aviv Sourasky Medical Center |
| Notes |
| Trial name or title | Ketamine hydrochloride and best pain management in treating cancer patients with neuropathic pain |
| Methods | Randomised, placebo‐controlled, double‐blinded, parallel group study |
| Participants | Adult cancer patients with malignant neuropathic pain |
| Interventions | Addition of ketamine hydrochloride or placebo to "best pain management" |
| Outcomes | Time to treatment failure; difference in pain scores; patient distress; adverse effects |
| Starting date | April 2009 |
| Contact information | Marie T. Fallon, Edinburgh Cancer Centre at Western General Hospital, Edinburgh, UK |
| Notes | Unknown status |
| Trial name or title | Efficacy of low analgesic doses of ketamine associated with opioids in refractory cancer pain treatment |
| Methods | Randomised, placebo‐controlled, double‐blind parallel group study |
| Participants | Adult hospitalised cancer patients with refractory pain undergoing opioid treatment |
| Interventions | IV ketamine versus placebo |
| Outcomes | Per cent reduction average daily pain intensity score; daily average pain intensity score; patient global impression of change; daily sleep interference; opioid consumption; patient satisfaction; adverse effects |
| Starting date | July 2011. Completed February 2013 |
| Contact information | Sylvie Rostaing‐Rigattieri, Center of Evaluation and Treatment of Pain, Saint‐Antoine Hospital, Paris, France |
| Notes | "Completed" study, unpublished. Possibly the same study registered as NCT00484484? |
| Trial name or title | Comparison of oral morphine versus nasal ketamine spray with chitosan in cancer pain outpatients |
| Methods | Randomised, placebo‐control double blind cross‐over study |
| Participants | 34 adult cancer outpatients with opioid base therapy because of pain, or pain breakthrough pain or extreme pain on movement |
| Interventions | 3 treatment arms investigating morphine drops, ketamine nasal spray with chitosan or morphine drops + ketamine nasal spray with chitosan |
| Outcomes | Time to onset of action; median numeric rating scale (NRS) improvement; total dose ketamine or morphine; total opioid dose increase; adverse effects |
| Starting date | February 2015 |
| Contact information | Wilhelm Ruppen, Pain Relief Unit and Anesthesiology, University Hospital Basel, Switzerland |
| Notes |
IV: intravenous
VAS: visual analogue scale
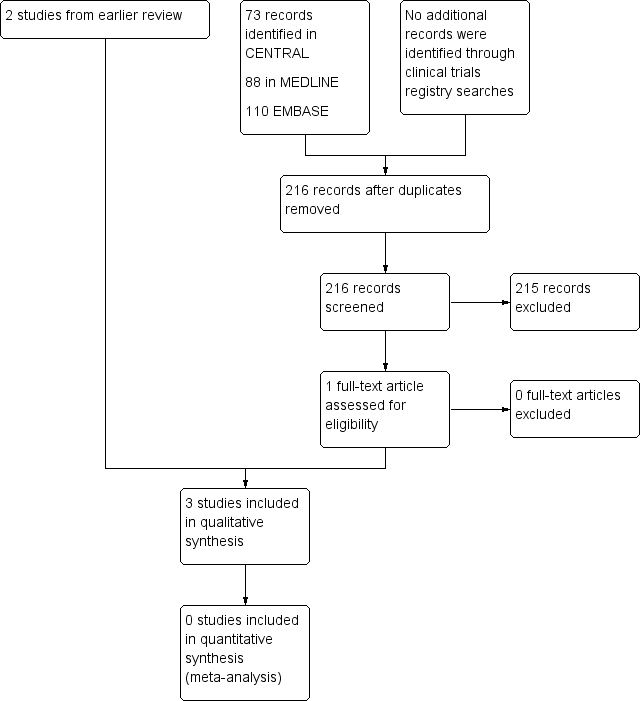
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
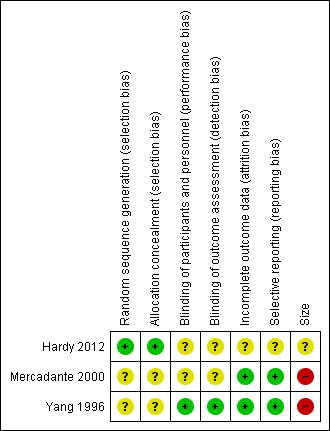
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

